Evaluating Non-Conventional Chitosan Sources for Controlled Release of Risperidone
Abstract
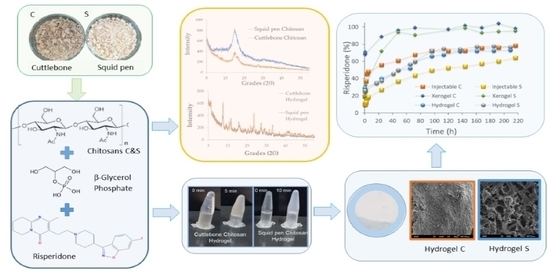
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. β-Chitosan Isolation
2.2.2. Polymer Characterization
2.2.3. Preparation of Hydrogels
2.2.4. Risperidone Load Formulation
2.2.5. Risperidone Release Profile Studies
3. Results
3.1. Chitosan Characterisation
3.2. Chitosan Thermoresponsive Gels
3.3. Chitosan-Based Risperidone Release Formulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurita, K. Controlled Functionalization of the Polysaccharide Chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M.; Harding, D.; Sashiwa, H. Marine Drugs Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Fernando Quiñones-Olvera, L.; Piraino, F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [Green Version]
- Lall, A.; Tamo, A.K.; Doench, I.; David, L.; Nunes De Oliveira, P.; Gorzelanny, C.; Osorio-Madrazo, A. Nanoparticles and Colloidal Hydrogels of Chitosan-Caseinate Polyelectrolyte Complexes for Drug-Controlled Release Applications. Int. J. Mol. Sci. 2020, 21, 5602. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan Hydrogels for Sustained Drug Delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhu, L.; Li, Y.; Zhang, X.; Xu, S.; Yang, G.; Delair, T. Chitosan-Based Colloidal Polyelectrolyte Complexes for Drug Delivery: A Review. Carbohydr. Polym. 2020, 238, 116126. [Google Scholar] [CrossRef] [PubMed]
- Chenite, A.; Buschmann, M.; Wang, D.; Chaput, C.; Kandani, N. Rheological Characterisation of Thermogelling Chitosan/Glycerol-Phosphate Solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar] [CrossRef]
- Rahmanian-Devin, P.; Rahimi, V.B.; Askari, V.R. Thermosensitive Chitosan-β-Glycerophosphate Hydrogels as Targeted Drug Delivery Systems: An Overview on Preparation and Their Applications. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 6640893. [Google Scholar] [CrossRef]
- Amin, S.; Alaa El-Din, B.; Zhifa, S.; Azam, A. Injectable Gel from Squid Pen Chitosan for Bone-Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2016, 4, 1454. [Google Scholar] [CrossRef]
- Ngoenkam, J.; Faikrua, A.; Yasothornsrikul, S.; Viyoch, J. Potential of an Injectable Chitosan/Starch/β-Glycerol Phosphate Hydrogel for Sustaining Normal Chondrocyte Function. Int. J. Pharm. 2010, 391, 115–124. [Google Scholar] [CrossRef]
- Tsai, M.L.; Chang, H.W.; Yu, H.C.; Lin, Y.S.; Tsai, Y.D. Effect of Chitosan Characteristics and Solution Conditions on Gelation Temperatures of Chitosan/2-Glycerophosphate/Nanosilver Hydrogels. Carbohydr. Polym. 2011, 84, 1337–1343. [Google Scholar] [CrossRef]
- Schatzberg, A.F.; Nemeroff, C.B. The American Psychiatric Publishing Textbook of Psychopharmacology; American Psychiatric Pub.: Washington, DC, USA, 2009; ISBN 1585623091. [Google Scholar]
- Salarvand, M.; Ramezani, V.; Salarvand, F.; Darabi, Z.A.; Akrami, M. Improvement of Drug Delivery Properties of Risperidone via Preparation of Fast Dissolution Tablet Containing Nanostructured Microparticles. Iran. J. Pharm. Res. 2021, 20, 183–196. [Google Scholar] [CrossRef]
- Elena de Souza, L.; Eckenstaler, R.; Syrowatka, F.; Beck-Broichsitter, M.; Benndorf, R.A.; Mäder, K. Has PEG-PLGA Advantages for the Delivery of Hydrophobic Drugs? Risperidone as an Example. J. Drug Deliv. Sci. Technol. 2021, 61, 102239. [Google Scholar] [CrossRef]
- Lugasi, L.; Grinberg, I.; Sabag, R.; Madar, R.; Einat, H.; Margel, S. Molecules Proteinoid Nanocapsules as Drug Delivery System for Improving Antipsychotic Activity of Risperidone. Molecules 2020, 25, 4013. [Google Scholar] [CrossRef]
- Bellotti, E.; Contarini, G.; Geraci, F.; Torrisi, S.A.; Piazza, C.; Drago, F.; Leggio, G.M.; Papaleo, F.; Decuzzi, P. Long-Lasting Rescue of Schizophrenia-Relevant Cognitive Impairments via Risperidone-Loaded MicroPlates. Drug Deliv. Transl. Res. 2022. [Google Scholar] [CrossRef]
- Chen, F.; Liu, H.; Wang, B.; Yang, L.; Cai, W.; Jiao, Z.; Yang, Z.; Chen, Y.; Quan, Y.; Xiang, X.; et al. Physiologically Based Pharmacokinetic Modeling to Understand the Absorption of Risperidone Orodispersible Film. Front. Pharmacol. 2020, 10, 1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rukmangathen, R.; Yallamalli, I.M.; Yalavarthi, P.R. Formulation and Biopharmaceutical Evaluation of Risperidone-Loaded Chitosan Nanoparticles for Intranasal Delivery. Drug Dev. Ind. Pharm. 2019, 45, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Pathak, K.; Misra, A. Formulation and Characterization of Nanoemulsion-Based Drug Delivery System of Risperidone. Drug Dev. Ind. Pharm. 2009, 35, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Barmpalexis, P.; Lazaridou, M.; Papageorgiou, G.Z.; Koutris, E.; Karavas, E.; Kostoglou, M.; Bikiaris, D.N. Controlled Release Formulations of Risperidone Antipsychotic Drug in Novel Aliphatic Polyester Carriers: Data Analysis and Modelling. Eur. J. Pharm. Biopharm. 2015, 94, 473–484. [Google Scholar] [CrossRef]
- Silva, A.C.; Amaral, M.H.; González-Mira, E.; Santos, D.; Ferreira, D. Solid Lipid Nanoparticles (SLN)—Based Hydrogels as Potential Carriers for Oral Transmucosal Delivery of Risperidone: Preparation and Characterization Studies. Colloids Surf. B Biointerfaces 2012, 93, 241–248. [Google Scholar] [CrossRef]
- Badshah, A.; Subhan, F.; Rauf, K.; Irfan Bukhari, N.; Shah, K.; Khan, S.; Ahmed, Z.; Khan, I. Development of Controlled-Release Matrix Tablet of Risperidone: Influence of Methocel®- and Ethocel®-Based Novel Polymeric Blend on In Vitro Drug Release and Bioavailability. AAPS PharmSciTech 2011, 12, 525–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.C.; Kumar, A.; Wild, W.; Ferreira, D.; Santos, D.; Forbes, B. Long-Term Stability, Biocompatibility and Oral Delivery Potential of Risperidone-Loaded Solid Lipid Nanoparticles. Int. J. Pharm. 2012, 436, 798–805. [Google Scholar] [CrossRef]
- Nanaki, S.; Barmpalexis, P.; Iatrou, A.; Christodoulou, E.; Kostoglou, M.; Bikiaris, D. Risperidone Controlled Release Microspheres Based on Poly(Lactic Acid)-Poly(Propylene Adipate) Novel Polymer Blends Appropriate for Long Acting Injectable Formulations. Pharmaceutics 2018, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, S.; Faraj, J.A.; Giovagnoli, S.; DeLuca, P.P. Development of Risperidone PLGA Microspheres. J. Drug Deliv. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Arrouze, F.; Desbrieres, J.; Lidrissi Hassani, S.; Tolaimate, A. Investigation of β-Chitin Extracted from Cuttlefish: Comparison with Squid β-Chitin. Polym. Bull. 2021, 78, 7219–7239. [Google Scholar] [CrossRef]
- Galed, G.; Diaz, E.; Goycoolea, F.M.; Heras, A. Influence of N-Deacetylation Conditions on Chitosan Production from α-Chitin. Nat. Prod. Commun. 2008, 3, 1934578X0800300414. [Google Scholar] [CrossRef] [Green Version]
- Muzzarelli, R.A.A.; Rocchetti, R. Determination of the Degree of Acetylation of Chitosans by First Derivative Ultraviolet Spectrophotometry. Carbohydr. Polym. 1985, 5, 461–472. [Google Scholar] [CrossRef]
- Rinaudo, M.; Milas, M.; Le Dung, P. Characterization of Chitosan. Influence of Ionic Strength and Degree of Acetylation on Chain Expansion. Int. J. Biol. Macromol. 1993, 15, 281–285. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, C.; Xue, Y.; Gao, R.; Zhang, X. Determination of the Degree of Deacetylation of Chitin and Chitosan by X-ray Powder Diffraction. Carbohydr. Res. 2005, 340, 1914–1917. [Google Scholar] [CrossRef]
- Cuong, H.N.; Minh, N.C.; van Hoa, N.; Trung, T.S. Preparation and Characterization of High Purity β-Chitin from Squid Pens (Loligo Chenisis). Int. J. Biol. Macromol. 2016, 93, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Local Sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Chen, X.G.; Kong, M.; Liu, C.S.; Cha, D.S.; Kennedy, J.F. Effect of Molecular Weight and Degree of Chitosan Deacetylation on the Preparation and Characteristics of Chitosan Thermosensitive Hydrogel as a Delivery System. Carbohydr. Polym. 2008, 73, 265–273. [Google Scholar] [CrossRef]
- Branca, C.; D’Angelo, G.; Crupi, C.; Khouzami, K.; Rifici, S.; Ruello, G.; Wanderlingh, U. Role of the OH and NH Vibrational Groups in Polysaccharide-Nanocomposite Interactions: A FTIR-ATR Study on Chitosan and Chitosan/Clay Films. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; García-Carvajal, Z.Y.; Jobbágy, M.; Rubio, F.; Yuste, L.; Rojo, F.; Ferrer, M.L.; del Monte, F. Poly(Vinyl Alcohol) Scaffolds with Tailored Morphologies for Drug Delivery and Controlled Release. Adv. Funct. Mater. 2007, 17, 3505–3513. [Google Scholar] [CrossRef] [Green Version]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting Non-Linear Drug Diffusion Data: Utilizing Korsmeyer-Peppas Model to Study Drug Release from Liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Higuchi Equation: Derivation, Applications, Use and Misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the Use of the Weibull Function for the Discernment of Drug Release Mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Kamdem Tamo, A.; Doench, I.; Morales Helguera, A.; Hoenders, D.; Walther, A.; Madrazo, A.O. Biodegradation of Crystalline Cellulose Nanofibers by Means of Enzyme Immobilized-Alginate Beads and Microparticles. Polymers 2020, 12, 1522. [Google Scholar] [CrossRef]
- Ramasamy, P.; Subhapradha, N.; Shanmugam, V.; Shanmugam, A. Extraction, Characterization and Antioxidant Property of Chitosan from Cuttlebone Sepia Kobiensis (Hoyle 1885). Int. J. Biol. Macromol. 2014, 64, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Shushizadeh, M.R.; Pour, E.M.; Zare, A.; Lashkari, Z. Persian Gulf β-Chitin Extraction from Sepia Pharaonis Sp. Cuttlebone and Preparation of Its Derivatives. Bioact. Carbohydr. Diet. Fibre 2015, 6, 133–142. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N.; Velasco, H.; Mecerreyes, D.; Antonio, R.; et al. Polymers Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan/β-Glycerophosphate In Situ Gelling Mucoadhesive Systems for Intravesical Delivery of Mitomycin-C. Int. J. Pharm. 2019, 1, 100007. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Ice-Templated Materials: Sophisticated Structures Exhibiting Enhanced Functionalities Obtained after Unidirectional Freezing and Ice-Segregation-Induced Self-Assembly. Chem. Mater. 2008, 20, 634–648. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Sadrjavadi, K.; Hajialyani, M.; Shokoohinia, Y.; Fattahi, A. Preparation and Characterization of Silk Fibroin Hydrogel as Injectable Implants for Sustained Release of Risperidone. Drug Dev. Ind. Pharm. 2018, 44, 199–205. [Google Scholar] [CrossRef]








| Formulation | Gelation Time, (h) | Site of Gelation | Freeze-Drying |
|---|---|---|---|
| Injectable | 0 | Dialysis Membrane 1 | No |
| Hydrogel | 5 | Eppendorf tube | No |
| Xerogel | 5 | Syringe 1 mL | Yes |
| Property | Cuttlebone Chitosan | Squid Pen Chitosan |
|---|---|---|
| Humidity (%) | 7.76 ± 0.06 | 5.50 ± 0.14 |
| Ash content (%) | 1.65 ± 0.01 | 0.22 ± 0.01 |
| Acetylation Degree (%) | 7.53 ± 0.01 | 5.93 ± 0.01 |
| Intrinsic viscosity (g/dL) | 1.20 ± 0.15 | 6.63 ± 0.11 |
| Mv (kDa) | 16 ± 3 | 153 ± 3 |
| % βGP | % Squid Pen Chitosan | % Cuttlebone Chitosan | ||||
|---|---|---|---|---|---|---|
| 0.5% | 1% | 1.5% | 0.5% | 1% | 1.5% | |
| 5% | - | - | 10 min | - | - | 30 min |
| 10% | - | - | 5 min | - | 10 min | 10 min |
| 15% | - | - | 2 min | - | 5 min | 5 min |
| Model | Equation | Parameters | HSp | HC | ISp | IC |
|---|---|---|---|---|---|---|
| Korsmeyer–Peppas | t | n | 0.45 | 0.354 | 0.291 | 0.145 |
| K | 8.87 | 12.693 | 0.515 | 36.522 | ||
| AIC | 30.53 | 35.975 | 5.840 | 43.500 | ||
| R2 | 0.986 | 0.979 | 0.989 | 0.974 | ||
| Higuchi | KkH | 0.13 | 0.110 | 0.250 | DNF | |
| AIC | 47.36 | 51.885 | 0.828 | |||
| R2 | 0.936 | 0.845 | 111.967 | |||
| Weibul model | Β | 0.005 | 0.005 | 0.004 | 0.779 | |
| Tdtd | 0.18 | 0.159 | 0.304 | 1.989 | ||
| AIC | 72.35 | 71.862 | 111.967 | 111.076 | ||
| R2 | 0.927 | 0.901 | 0.828 | 0.837 | ||
| 1st order | Kk | 0.025 | 0.030 | 0.014 | 0.425 | |
| AIC | 71.080 | 70.828 | 109.631 | 92.884 | ||
| R2 | 0.923 | 0.893 | 0.832 | 0.834 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcinuño, S.; Aranaz, I.; Civera, C.; Arias, C.; Acosta, N. Evaluating Non-Conventional Chitosan Sources for Controlled Release of Risperidone. Polymers 2022, 14, 1355. https://doi.org/10.3390/polym14071355
Garcinuño S, Aranaz I, Civera C, Arias C, Acosta N. Evaluating Non-Conventional Chitosan Sources for Controlled Release of Risperidone. Polymers. 2022; 14(7):1355. https://doi.org/10.3390/polym14071355
Chicago/Turabian StyleGarcinuño, Sara, Inmaculada Aranaz, Concepción Civera, Concepción Arias, and Niuris Acosta. 2022. "Evaluating Non-Conventional Chitosan Sources for Controlled Release of Risperidone" Polymers 14, no. 7: 1355. https://doi.org/10.3390/polym14071355
APA StyleGarcinuño, S., Aranaz, I., Civera, C., Arias, C., & Acosta, N. (2022). Evaluating Non-Conventional Chitosan Sources for Controlled Release of Risperidone. Polymers, 14(7), 1355. https://doi.org/10.3390/polym14071355