RSM Modeling and Optimization of CO2 Separation from High CO2 Feed Concentration over Functionalized Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 6FDA-Durene
2.3. Synthesis of NH2-MIL-125(Ti)
2.4. Fabrication of Dense Membrane
2.5. Binary Gas Separation Measurement
2.6. Optimization of Membrane Separation Performance
3. Results and Discussion
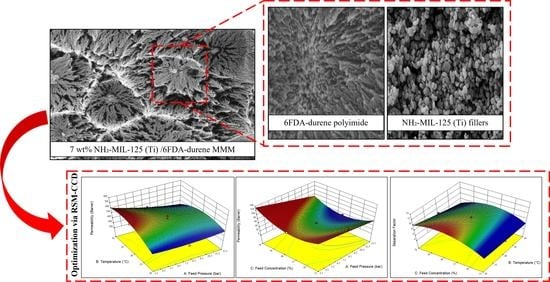
3.1. Characteristics of Membrane
3.2. Central Composite Design (CCD)
3.3. CO2 Permeability
3.4. CH4 Permeability
3.5. CO2/CH4 Separation Factor
3.6. Optimization of CO2/CH4 Separation Performance
3.7. Validation of the Optimum Condition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sukor, N.R.; Shamsuddin, A.H.; Mahlia, T.M.I.; Mat Isa, M.F. Techno-Economic Analysis of CO2 Capture Technologies in Offshore Natural Gas Field: Implications to Carbon Capture and Storage in Malaysia. Processes 2020, 8, 350. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yuan, J.; Li, R.; Zhu, H.; Duan, J.; Guo, Y.; Liu, G.; Jin, W. ZIF-301 MOF/6FDA-DAM polyimide mixed-matrix membranes for CO2/CH4 separation. Sep. Purif. Technol. 2021, 264, 118431. [Google Scholar] [CrossRef]
- Suhaimi, N.H.; Yeong, Y.F.; Jusoh, N.; Chew, T.L.; Bustam, M.A.; Suleman, S. Separation of CO2 from CH4 using mixed matrix membranes incorporated with amine functionalized MIL-125 (Ti) nanofiller. Chem. Eng. Res. Des. 2020, 159, 236–247. [Google Scholar] [CrossRef]
- Peters, L.; Hussain, A.; Follmann, M.; Melin, T.; Hägg, M.B. CO2 removal from natural gas by employing amine absorption and membrane technology—A technical and economical analysis. Chem. Eng. J. 2011, 172, 952–960. [Google Scholar] [CrossRef]
- Alonso, A.; Moral-Vico, J.; Abo Markeb, A.; Busquets-Fite, M.; Komilis, D.; Puntes, V.; Sanchez, A.; Font, X. Critical review of existing nanomaterial adsorbents to capture carbon dioxide and methane. Sci. Total Environ. 2017, 595, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mubashir, M.; Dumee, L.F.; Fong, Y.Y.; Jusoh, N.; Lukose, J.; Chai, W.S.; Show, P.L. Cellulose acetate-based membranes by interfacial engineering and integration of ZIF-62 glass nanoparticles for CO2 separation. J. Hazard. Mater. 2021, 415, 125639. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Role of Amine Type in CO2 Separation Performance within Amine Functionalized Silica/Organosilica Membranes: A Review. Appl. Sci. 2018, 8, 1032. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Cheng, J.; Hou, W.; Yang, X.; Luo, M.; Zhang, H.; Ye, B.; Zhou, J. Mg2(dobdc) crystals adhere to Matrimid matrix membranes bridged by diethylenetriamine (DETA) as an adhesion agent for efficient CO2 separation. Sep. Purif. Technol. 2022, 278, 119635. [Google Scholar] [CrossRef]
- Zagho, M.M.; Hassan, M.K.; Khraisheh, M.; Al-Maadeed, M.A.A.; Nazarenko, S. A review on recent advances in CO2 separation using zeolite and zeolite-like materials as adsorbents and fillers in mixed matrix membranes (MMMs). Chem. Eng. J. Adv. 2021, 6, 100091. [Google Scholar] [CrossRef]
- Baneshi, M.M.; Ghaedi, A.M.; Vafaei, A.; Emadzadeh, D.; Lau, W.J.; Marioryad, H.; Jamshidi, A. A high-flux P84 polyimide mixed matrix membranes incorporated with cadmium-based metal organic frameworks for enhanced simultaneous dyes removal: Response surface methodology. Environ. Res. 2020, 183, 109278. [Google Scholar] [CrossRef]
- Mashhadikhan, S.; Ebadi Amooghin, A.; Moghadassi, A.; Sanaeepur, H. Functionalized filler/synthesized 6FDA-Durene high performance mixed matrix membrane for CO2 separation. J. Ind. Eng. Chem. 2021, 93, 482–494. [Google Scholar] [CrossRef]
- Ahmadipouya, S.; Ahmadijokani, F.; Molavi, H.; Rezakazemi, M.; Arjmand, M. CO2/CH4 Separation by Mixed-Matrix Membranes holding Functionalized NH2-MIL-101(Al) Nanoparticles: Effect of Amino-Silane Functionalization. Chem. Eng. Res. Des. 2021, 176, 49–59. [Google Scholar] [CrossRef]
- Suhaimi, N.H.; Yeong, Y.F.; Ch′ng, C.W.M.; Jusoh, N. Tailoring CO2/CH4 Separation Performance of Mixed Matrix Membranes by Using ZIF-8 Particles Functionalized with Different Amine Groups. Polymer 2019, 11, 2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuah, C.Y.; Li, W.; Samarasinghe, S.A.S.C.; Sethunga, G.S.M.D.P.; Bae, T.-H. Enhancing the CO2 separation performance of polymer membranes via the incorporation of amine-functionalized HKUST-1 nanocrystals. Microporous Mesoporous Mater. 2019, 290, 109680. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on amine and non-amine MIL-53(Al) in Pebax® MH-1657 for CO2 separation. Sep. Purif. Technol. 2018, 200, 177–190. [Google Scholar] [CrossRef]
- Waqas Anjum, M.; Bueken, B.; De Vos, D.; Vankelecom, I.F.J. MIL-125(Ti) based mixed matrix membranes for CO2 separation from CH4 and N2. J. Membr. Sci. 2016, 502, 21–28. [Google Scholar] [CrossRef]
- Guo, X.; Huang, H.; Ban, Y.; Yang, Q.; Xiao, Y.; Li, Y.; Yang, W.; Zhong, C. Mixed matrix membranes incorporated with amine-functionalized titanium-based metal-organic framework for CO2/CH4 separation. J. Membr. Sci. 2015, 478, 130–139. [Google Scholar] [CrossRef]
- Taheri Afarani, H.; Sadeghi, M.; Moheb, A.; Esfahani, E.N. Optimization of the gas separation performance of polyurethane–zeolite 3A and ZSM-5 mixed matrix membranes using response surface methodology. Chin. J. Chem. Eng. 2019, 27, 110–129. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Leng, C.T.; Keong, L.K.; Jusoh, N. Study on the effect of process parameters on CO2/CH4 binary gas separation performance over NH2-MIL-53(Al)/cellulose acetate hollow fiber mixed matrix membrane. Polym. Test. 2020, 81, 106223. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Lau, K.K.; Shariff, A. Transport properties of mixed matrix membranes encompassing zeolitic imidazolate framework 8 (ZIF-8) nanofiller and 6FDA-durene polymer: Optimization of process variables for the separation of CO2 from CH4. J. Clean. Prod. 2017, 149, 80–95. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Cheong, W.L.; Lau, K.K.; Shariff, A. Facile fabrication of mixed matrix membranes containing 6FDA-durene polyimide and ZIF-8 nanofillers for CO2 capture. J. Ind. Eng. Chem. 2016, 44, 164–173. [Google Scholar] [CrossRef]
- Zamidi Ahmad, M.; Navarro, M.; Lhotka, M.; Zornoza, B.; Téllez, C.; Fila, V.; Coronas, J. Enhancement of CO2/CH4 separation performances of 6FDA-based co-polyimides mixed matrix membranes embedded with UiO-66 nanoparticles. Sep. Purif. Technol. 2018, 192, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Sainath, K.; Modi, A.; Bellare, J. CO2/CH4 mixed gas separation using graphene oxide nanosheets embedded hollow fiber membranes: Evaluating effect of filler concentration on performance. Chem. Eng. J. Adv. 2021, 5, 100074. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, C.; Caro, J.; Huang, A. A new UiO-66-NH2 based mixed-matrix membranes with high CO2/CH4 separation performance. Microporous Mesoporous Mater. 2019, 274, 203–211. [Google Scholar] [CrossRef]
- Maghami, S.; Mehrabani-Zeinabad, A.; Sadeghi, M.; Sánchez-Laínez, J.; Zornoza, B.; Téllez, C.; Coronas, J. Mathematical modeling of temperature and pressure effects on permeability, diffusivity and solubility in polymeric and mixed matrix membranes. Chem. Eng. Sci. 2019, 205, 58–73. [Google Scholar] [CrossRef]
- Shahid, S.; Baron, G.V.; Denayer, J.F.M.; Martens, J.A.; Wee, L.H.; Vankelecom, I.F.J. Hierarchical ZIF-8 composite membranes: Enhancing gas separation performance by exploiting molecular dynamics in hierarchical hybrid materials. J. Membr. Sci. 2021, 620, 118943. [Google Scholar] [CrossRef]
- Mozafari, M.; Rahimpour, A.; Abedini, R. Exploiting the effects of zirconium-based metal organic framework decorated carbon nanofibers to improve CO2/CH4 separation performance of thin film nanocomposite membranes. J. Ind. Eng. Chem. 2020, 85, 102–110. [Google Scholar] [CrossRef]
- Li, P.; Chung, T.S.; Paul, D.R. Gas sorption and permeation in PIM-1. J. Membr. Sci. 2013, 432, 50–57. [Google Scholar] [CrossRef]
- Salahshoori, I.; Seyfaee, A.; Babapoor, A.; Neville, F.; Moreno-Atanasio, R. Evaluation of the effect of silica nanoparticles, temperature and pressure on the performance of PSF/PEG/SiO2 mixed matrix membranes: A molecular dynamics simulation (MD) and design of experiments (DOE) study. J. Mol. Liq. 2021, 333, 115957. [Google Scholar] [CrossRef]
- Molki, B.; Aframehr, W.M.; Bagheri, R.; Salimi, J. Mixed matrix membranes of polyurethane with nickel oxide nanoparticles for CO2 gas separation. J. Membr. Sci. 2018, 549, 588–601. [Google Scholar] [CrossRef]
- Cheng, Y.; Ying, Y.; Zhai, L.; Liu, G.; Dong, J.; Wang, Y.; Christopher, M.P.; Long, S.; Wang, Y.; Zhao, D. Mixed matrix membranes containing MOF@COF hybrid fillers for efficient CO2/CH4 separation. J. Membr. Sci. 2019, 573, 97–106. [Google Scholar] [CrossRef]
- Soltani, B.; Asghari, M. Effects of ZnO Nanoparticle on the Gas Separation Performance of Polyurethane Mixed Matrix Membrane. Membrane 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.Z.; Peters, T.A.; Konnertz, N.M.; Visser, T.; Téllez, C.; Coronas, J.; Fila, V.; de Vos, W.M.; Benes, N.E. High-pressure CO2/CH4 separation of Zr-MOFs based mixed matrix membranes. Sep. Purif. Technol. 2020, 230, 115858. [Google Scholar] [CrossRef]
- Kida, K.; Maeta, Y.; Yogo, K. Pure silica CHA-type zeolite membranes for dry and humidified CO2/CH4 mixtures separation. Sep. Purif. Technol. 2018, 197, 116–121. [Google Scholar] [CrossRef]
- Ricci, E.; Gemeda, A.E.; Du, N.; Li, N.; De Angelis, M.G.; Guiver, M.D.; Sarti, G.C. Sorption of CO2/CH4 mixtures in TZ-PIM, PIM-1 and PTMSP: Experimental data and NELF-model analysis of competitive sorption and selectivity in mixed gases. J. Membr. Sci. 2019, 585, 136–149. [Google Scholar] [CrossRef]
- Genduso, G.; Wang, Y.; Ghanem, B.S.; Pinnau, I. Permeation, sorption, and diffusion of CO2-CH4mixtures in polymers of intrinsic microporosity: The effect of intrachain rigidity on plasticization resistance. J. Membr. Sci. 2019, 584, 100–109. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, L.; Xiang, D.; Cao, B.; Hosseini, S.; Li, P. Approaches to Suppress CO2-Induced Plasticization of Polyimide Membranes in Gas Separation Applications. Processes 2019, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Karimnezhad, H.; Navarchian, A.H.; Tavakoli Gheinani, T.; Zinadini, S. Amoxicillin removal by Fe-based nanoparticles immobilized on polyacrylonitrile membrane: Effects of input parameters and optimization by response surface methodology. Chem. Eng. Processing Process Intensif. 2020, 147, 107785. [Google Scholar] [CrossRef]






| Operational Parameters | Units | Coded Measurement Levels | ||
|---|---|---|---|---|
| −1 (Low) | 0 (Center) | 1 (High) | ||
| Feed Pressure (A) | Bar | 3.5 | 8.0 | 12.5 |
| Temperature (B) | °C | 30.0 | 40.0 | 50.0 |
| CO2 Feed Concentration (C) | (mol%) | 15.0 | 42.5 | 70.0 |
| Run | Operational Parameters | Significant Responses | ||||
|---|---|---|---|---|---|---|
| A: Pressure (Bar) | B: Temperature (°C) | C: CO2 Concentration (mol%) | CO2 Permeability (Barrer) | CH4 Permeability (Barrer) | CO2/CH4 Separation Factor | |
| 1 | 3.5 | 40.0 | 42.5 | 569.6 | 58.3 | 9.9 |
| 2 | 8.0 | 40.0 | 42.5 | 569.6 | 58.3 | 9.9 |
| 3 | 3.5 | 50.0 | 70.0 | 326.2 | 52.3 | 5.5 |
| 4 | 12.5 | 30.0 | 70.0 | 609.1 | 81.9 | 7.3 |
| 5 | 8.0 | 40.0 | 70.0 | 450.2 | 92.7 | 6.0 |
| 6 | 8.0 | 50.0 | 42.5 | 504.0 | 39.9 | 12.8 |
| 7 | 3.5 | 30.0 | 70.0 | 448.2 | 33.9 | 13.0 |
| 8 | 12.5 | 50.0 | 70.0 | 293.2 | 36.2 | 7.3 |
| 9 | 12.5 | 30.0 | 15.0 | 506.6 | 83.2 | 6.1 |
| 10 | 8.0 | 40.0 | 15.0 | 419.7 | 39.7 | 8.6 |
| 11 | 12.5 | 40.0 | 42.5 | 794.4 | 147.5 | 5.3 |
| 12 | 8.0 | 40.0 | 42.5 | 442.9 | 39.5 | 8.6 |
| 13 | 3.5 | 50.0 | 15.0 | 502.3 | 47.4 | 10.8 |
| 14 | 8.0 | 40.0 | 42.5 | 321.5 | 28.7 | 11.4 |
| 15 | 3.5 | 30.0 | 15.0 | 464.4 | 58.4 | 8.1 |
| 16 | 8.0 | 30.0 | 42.5 | 650.2 | 97.0 | 8.4 |
| 17 | 8.0 | 40.0 | 42.5 | 520.3 | 55.5 | 9.3 |
| 18 | 12.5 | 50.0 | 15.0 | 567.5 | 74.3 | 7.5 |
| 19 | 8.0 | 40.0 | 42.5 | 520.3 | 55.5 | 9.3 |
| 20 | 8.0 | 40.0 | 42.5 | 510.1 | 91.0 | 6.1 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| Model | 2.471 × 105 | 9 | 27,456.76 | 27.11 | <0.0001 a |
| A-Pressure | 45,922.31 | 1 | 45,922.31 | 45.34 | <0.0001 a |
| B-Temperature | 13,418.30 | 1 | 13,418.30 | 13.25 | 0.0045 a |
| C-Concentration | 96,228.25 | 1 | 96,228.25 | 95.00 | <0.0001 a |
| AB | 41,584.40 | 1 | 41,584.40 | 41.05 | <0.0001 a |
| AC | 9123.30 | 1 | 9123.30 | 9.01 | 0.0133 a |
| BC | 2111.85 | 1 | 2111.85 | 2.08 | 0.1794 b |
| A2 | 2307.53 | 1 | 2307.53 | 2.28 | 0.1621 b |
| B2 | 37,959.26 | 1 | 37,959.26 | 37.47 | 0.0001 a |
| C2 | 7009.65 | 1 | 7009.65 | 6.92 | 0.0251 a |
| Residual | 10,129.32 | 10 | 1012.93 | ||
| Lack of Fit | 5282.07 | 5 | 1056.41 | 1.09 | 0.4636 b |
| R2 | 0.96 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| Model | 15,005.47 | 9 | 1667.27 | 29.95 | <0.0001 a |
| A-Pressure | 5708.28 | 1 | 5708.28 | 102.54 | <0.0001 a |
| B-Temperature | 1892.55 | 1 | 1892.55 | 34.00 | 0.0002 a |
| C-Concentration | 1106.91 | 1 | 1106.91 | 19.88 | 0.0012 a |
| AB | 1171.28 | 1 | 1171.28 | 21.04 | 0.0010 a |
| AC | 393.68 | 1 | 393.68 | 7.07 | 0.0239 a |
| BC | 400.73 | 1 | 400.73 | 7.20 | 0.0230 a |
| A2 | 0.066 | 1 | 0.066 | 1.180 × 10−3 | 0.9733 b |
| B2 | 1587.90 | 1 | 1587.90 | 28.52 | 0.0003 a |
| C2 | 3716.16 | 1 | 3716.16 | 66.75 | <0.0001 a |
| Residual | 556.69 | 10 | 55.67 | ||
| Lack of Fit | 429.72 | 5 | 85.94 | 3.38 | 0.1035 b |
| R2 | 0.96 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| Model | 91.12 | 9 | 10.12 | 18.04 | <0.0001 a |
| A-Pressure | 33.27 | 1 | 33.27 | 59.29 | <0.0001 a |
| B-Temperature | 10.96 | 1 | 10.96 | 19.54 | 0.0013 a |
| C-Concentration | 7.12 | 1 | 7.12 | 12.69 | 0.0052 a |
| AB | 2.38 | 1 | 2.38 | 4.23 | 0.0666 b |
| AC | 1.71 | 1 | 1.71 | 3.05 | 0.1113 b |
| BC | 5.18 | 1 | 5.18 | 9.24 | 0.0125 a |
| A2 | 0.25 | 1 | 0.25 | 0.44 | 0.5229 b |
| B2 | 0.036 | 1 | 0.036 | 0.064 | 0.8057 b |
| C2 | 20.06 | 1 | 20.06 | 35.75 | 0.0001 a |
| Residual | 5.61 | 10 | 0.56 | ||
| Lack of Fit | 3.43 | 5 | 0.69 | 1.57 | 0.3168 b |
| R2 | 0.94 |
| Pressure (bar) | Temperature (°C) | Concentration (mol%) | CO2 Permeability (Barrer) | CH4 Permeability (Barrer) | CO2/CH4 Separation Factor | Desirability |
|---|---|---|---|---|---|---|
| 12.5 | 34.7 | 70.0 | 571.9 | 60.4 | 11.9 | 0.8 |
| Run | CO2 Permeability | Separation Factor | ||||
|---|---|---|---|---|---|---|
| Actual (Barrer) | Predicted (Barrer) | Error (%) | Actual | Predicted | Error (%) | |
| 1 | 609.3 | 571.9 | 6.5 | 11.6 | 11.9 | 2.5 |
| 2 | 592.7 | 571.9 | 3.6 | 11.8 | 11.9 | 0.8 |
| 3 | 605.3 | 571.9 | 5.8 | 11.3 | 11.9 | 5.0 |
| Average (%) | 5.3 | Average (%) | 2.8 | |||
| Standard deviation | 1.5 | Standard deviation | 2.1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhaimi, N.H.; Yeong, Y.F.; Jusoh, N.; Chew, T.L.; Bustam, M.A.; Mubashir, M. RSM Modeling and Optimization of CO2 Separation from High CO2 Feed Concentration over Functionalized Membrane. Polymers 2022, 14, 1371. https://doi.org/10.3390/polym14071371
Suhaimi NH, Yeong YF, Jusoh N, Chew TL, Bustam MA, Mubashir M. RSM Modeling and Optimization of CO2 Separation from High CO2 Feed Concentration over Functionalized Membrane. Polymers. 2022; 14(7):1371. https://doi.org/10.3390/polym14071371
Chicago/Turabian StyleSuhaimi, Nadia Hartini, Yin Fong Yeong, Norwahyu Jusoh, Thiam Leng Chew, Mohammad Azmi Bustam, and Muhammad Mubashir. 2022. "RSM Modeling and Optimization of CO2 Separation from High CO2 Feed Concentration over Functionalized Membrane" Polymers 14, no. 7: 1371. https://doi.org/10.3390/polym14071371
APA StyleSuhaimi, N. H., Yeong, Y. F., Jusoh, N., Chew, T. L., Bustam, M. A., & Mubashir, M. (2022). RSM Modeling and Optimization of CO2 Separation from High CO2 Feed Concentration over Functionalized Membrane. Polymers, 14(7), 1371. https://doi.org/10.3390/polym14071371