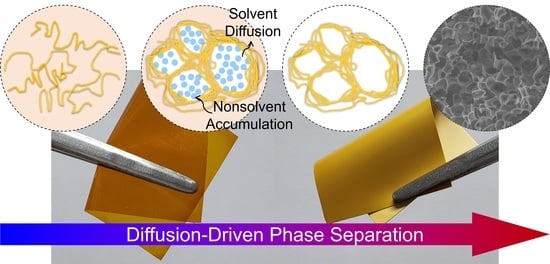
Polymer Concentration and Liquid—Liquid Demixing Time Correlation with Porous Structure of Low Dielectric Polyimide in Diffusion-Driven Phase Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hierarchically Porous Sponge-like Polyimide Film
2.3. Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hecht, J. The bandwidth bottleneck. Nature 2016, 536, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.G.; Buzzi, S.; Choi, W.; Hanly, S.V.; Lozano, A.; Soong, A.C.K.; Zhang, J.C. What will 5G be? IEEE J. Sel. Areas Commun. 2014, 32, 1065–1082. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, D.; Tong, F.; Lu, X.; Lu, Q. Low Dielectric Constant Polyimide Hybrid Films Prepared by in Situ Blow-Balloon Method. ACS Appl. Polym. Mater. 2019, 1, 2189–2196. [Google Scholar] [CrossRef]
- Bei, R.; Qian, C.; Zhang, Y.; Chi, Z.; Liu, S.; Chen, X.; Xu, J.; Aldred, M.P. Intrinsic low dielectric constant polyimides: Relationship between molecular structure and dielectric properties. J. Mater. Chem. C 2017, 5, 12807–12815. [Google Scholar] [CrossRef]
- Zhao, G.; Ishizaka, T.; Kasai, H.; Oikawa, H.; Nakanishi, H. Fabrication of unique porous polyimide nanoparticles using a reprecipitation method. Chem. Mater. 2007, 19, 1901–1905. [Google Scholar] [CrossRef]
- Kim, Y.; Nam, K.H.; Jung, Y.C.; Han, H. Interfacial adhesion and self-healing kinetics of multi-stimuli responsive colorless polymer bilayers. Compos. Part B Eng. 2020, 203, 108451. [Google Scholar] [CrossRef]
- Simpson, J.O.; St.Clair, A.K. Fundamental insight on developing low dielectric constant polyimides. Thin Solid Films 1997, 308–309, 480–485. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Lam, D.C.C. Properties and microstructures of low-temperature-processable ultralow-dielectric porous polyimide films. J. Electron. Mater. 2008, 37, 955–961. [Google Scholar] [CrossRef]
- Wu, H.W.; Su, Y.K.; Yang, R.Y.; Weng, M.H.; Lin, Y. Der Fabrication of low-loss thin film microstrip line on low-resistivity silicon for RF applications. Microelectron. J. 2007, 38, 304–309. [Google Scholar] [CrossRef]
- Han, S.; Li, Y.; Hao, F.; Zhou, H.; Qi, S.; Tian, G.; Wu, D. Ultra-low dielectric constant polyimides: Combined efforts of fluorination and micro-branched crosslink structure. Eur. Polym. J. 2021, 143, 110206. [Google Scholar] [CrossRef]
- Unterlass, M.M.; Kopetzki, D.; Antonietti, M.; Weber, J. Mechanistic study of hydrothermal synthesis of aromatic polyimides. Polym. Chem. 2011, 2, 1744–1753. [Google Scholar] [CrossRef]
- Taki, K.; Hosokawa, K.; Takagi, S.; Mabuchi, H.; Ohshima, M. Rapid production of ultralow dielectric constant porous polyimide films via CO2-tert -amine zwitterion-induced phase separation and subsequent photopolymerization. Macromolecules 2013, 46, 2275–2281. [Google Scholar] [CrossRef]
- Krause, B.; Koops, G.H.; van der Vegt, N.F.A.; Wessling, M.; Wübbenhorst, M.; van Turnhout, J. Ultralow-k dielectrics made by supercritical foaming of thin polymer films. Adv. Mater. 2002, 14, 1041–1046. [Google Scholar] [CrossRef]
- Kourakata, Y.; Onodera, T.; Kasai, H.; Jinnai, H.; Oikawa, H. Ultra-low dielectric properties of porous polyimide thin films fabricated by using the two kinds of templates with different particle sizes. Polymer 2021, 212, 123115. [Google Scholar] [CrossRef]
- Seo, K.; Nam, K.H.; Lee, S.; Han, H. Low stress polyimide/silica nanocomposites as dielectrics for wafer level chip scale packaging. Mater. Lett. 2020, 263, 127204. [Google Scholar] [CrossRef]
- Nam, K.H.; Seo, K.; Lee, S.; Han, H. Sulfur-doped hierarchically porous open cellular polymer/acid complex electrolyte membranes for efficient water-free proton transport. ACS Sustain. Chem. Eng. 2020, 8, 16156–16163. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, Y.M. Effects of Molecular Weight of Polyvinylpyrrolidone on Precipitation Kinetics During the Formation of Asymmetric Polyacrylonitrile Membrane. J. Appl. Polym. Sci. 2002, 85, 57–68. [Google Scholar] [CrossRef]
- Nishino, T.; Kotera, M.; Inayoshi, N.; Miki, N.; Nakamae, K. Residual stress and microstructures of aromatic polyimide with different imidization processes. Polymer 2000, 41, 6913–6918. [Google Scholar] [CrossRef]
- Pryde, C.A. IR studies of polyimides. I. Effects of chemical and physical changes during cure. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 711–724. [Google Scholar] [CrossRef]
- Guillen, G.R.; Ramon, G.Z.; Kavehpour, H.P.; Kaner, R.B.; Hoek, E.M.V. Direct microscopic observation of membrane formation by nonsolvent induced phase separation. J. Membr. Sci. 2013, 431, 212–220. [Google Scholar] [CrossRef]
- Lee, S.; Nam, K.H.; Seo, K.; Kim, G.; Han, H. Phase inversion-induced porous polybenzimidazole fuel cell membranes: An efficient architecture for high-temperature water-free proton transport. Polymers 2020, 12, 1604. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Phanthong, P.; Wakabayashi, T.; Yao, S. Fabrication of Porous Polyimide Membrane with Through-Hole via Multiple Solvent Displacement Method. ChemistryOpen 2021, 10, 352–359. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, L.; He, Z.; Xie, J.; Shi, L.; Zhang, M.; Zhang, W.; Cui, W. Tunable dielectric and other properties in high-performance sandwich-type polyimide films achieved by adjusting the porous structure. J. Mater. Chem. C 2019, 7, 7360–7370. [Google Scholar] [CrossRef]
- Lee, Y.J.; Huang, J.M.; Kuo, S.W.; Chang, F.C. Low-dielectric, nanoporous polyimide films prepared from PEO-POSS nanoparticles. Polymer 2005, 46, 10056–10065. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, J.; Kim, S.I.; Kim, M.; Lee, D.; Lee, S.; Kim, G.; Lee, J.; Han, H. One-step synthesis of nano-porous monolithic polyimide aerogel. Microporous Mesoporous Mater. 2016, 234, 35–42. [Google Scholar] [CrossRef]






| Sample | Tg (°C) | Td5% (°C) | Td10% (°C) | Char Residue at 800 °C (%) | Dielectric Constant at 1 MHz | Dielectric Loss at 1 MHz |
|---|---|---|---|---|---|---|
| Nonporous PI film | 381 | 564 | 589 | 57.47 | 3.29 | 5.60 |
| PI Sponge film | 392 | 577 | 596 | 53.56 | 2.01 | 3.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Son, J.; Park, H.; Jeong, E.; Nam, K.-H.; Bae, J.-S. Polymer Concentration and Liquid—Liquid Demixing Time Correlation with Porous Structure of Low Dielectric Polyimide in Diffusion-Driven Phase Separation. Polymers 2022, 14, 1425. https://doi.org/10.3390/polym14071425
Kim S, Son J, Park H, Jeong E, Nam K-H, Bae J-S. Polymer Concentration and Liquid—Liquid Demixing Time Correlation with Porous Structure of Low Dielectric Polyimide in Diffusion-Driven Phase Separation. Polymers. 2022; 14(7):1425. https://doi.org/10.3390/polym14071425
Chicago/Turabian StyleKim, Subin, Jaemin Son, Hwon Park, Euigyung Jeong, Ki-Ho Nam, and Jin-Seok Bae. 2022. "Polymer Concentration and Liquid—Liquid Demixing Time Correlation with Porous Structure of Low Dielectric Polyimide in Diffusion-Driven Phase Separation" Polymers 14, no. 7: 1425. https://doi.org/10.3390/polym14071425