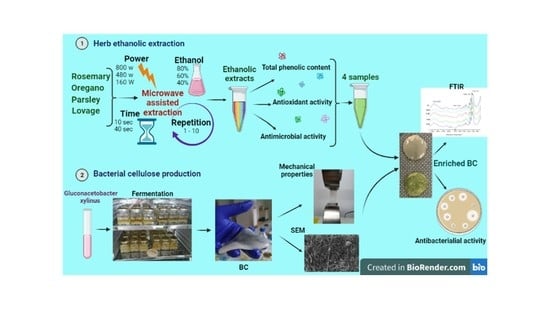
Antimicrobial Properties of Bacterial Cellulose Films Enriched with Bioactive Herbal Extracts Obtained by Microwave-Assisted Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strain and Chemicals
2.2. Herb Samples and Ethanolic Extraction
2.3. Total Phenolic Content (TPC)
2.4. Antioxidant Activity, DPPH Assay
2.5. Antimicrobial Activity of Herbal Extracts
2.5.1. Preparation of Microbial Strains
2.5.2. Disc-Diffusion Method
2.6. Obtaining of Bacterial Cellulose (BC)
Purification of Bacterial Cellulose (BC)
2.7. Mechanical Properties
2.8. Scanning Electron Microscopy (SEM)
2.9. Preparation of the Enriched BC Films
2.10. Fourier Transform Infrared Spectroscopy (FT-IR)
2.11. Antimicrobial Activity of Herbal Extracts of Bacterial Cellulose (BC) Enriched with Herbal Extracts
2.12. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Content (TPC) of Ethanolic Extracts
3.2. Antioxidant Activity (AA) of Ethanolic Herbal Extracts
3.3. Antimicrobial Activity of Herbal Extracts
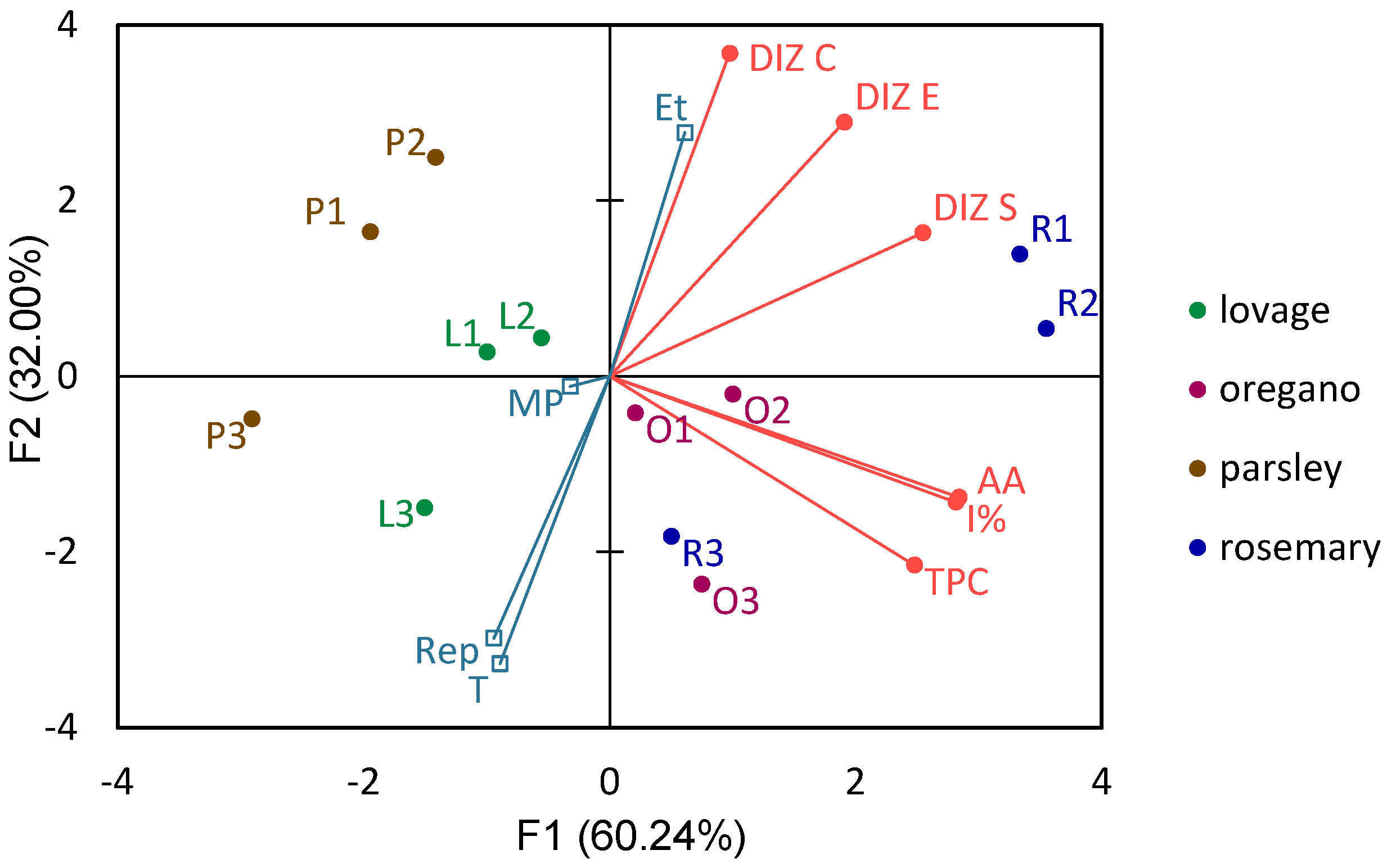
3.4. Principal Component Analysis of the Effect of Rosemary, Oregano, Lovage, and Parsley Extraction Parameters and Chemical Constituents on Selected Bacterial Strains
3.5. Characterization of the Physical Aspect of Bacterial-Cellulose (BC) Pellicles
3.6. Mechanical Properties of Bacterial-Cellulose (BC) Pellicles
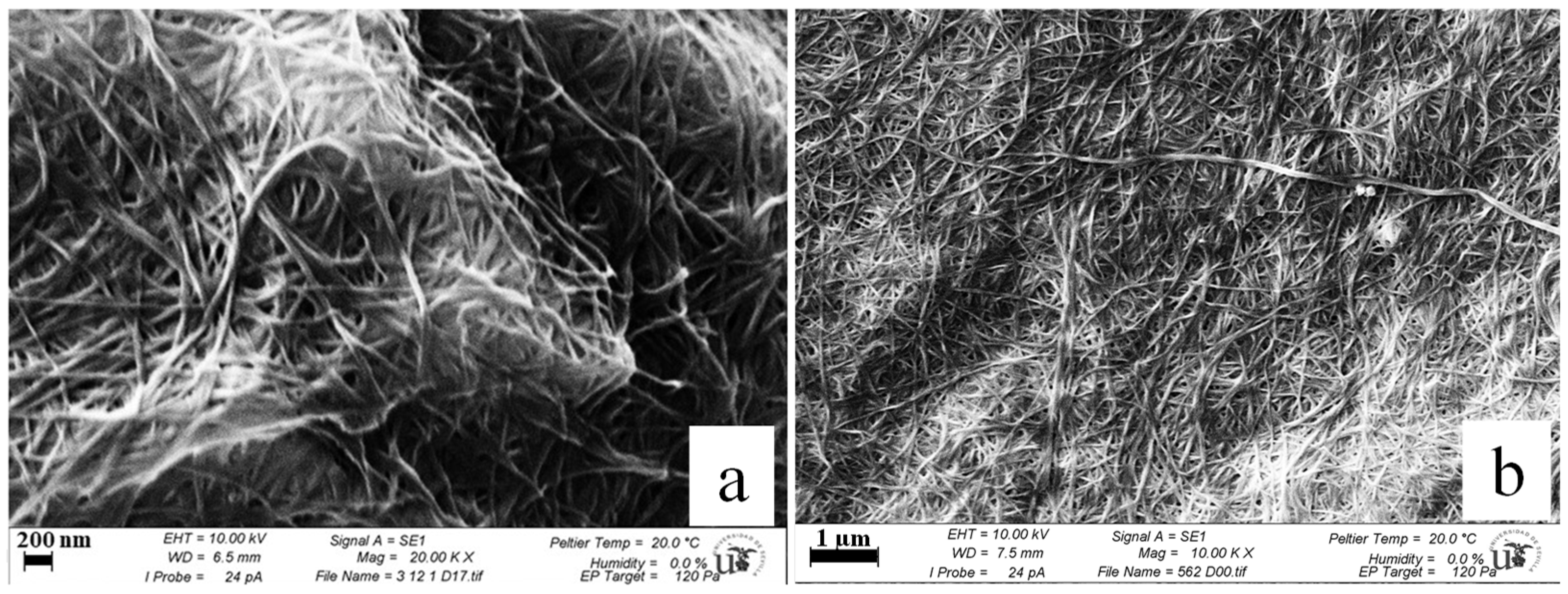
3.7. Scanning Electron Microscopy (SEM) of Bacterial Cellulose (BC) Pellicles
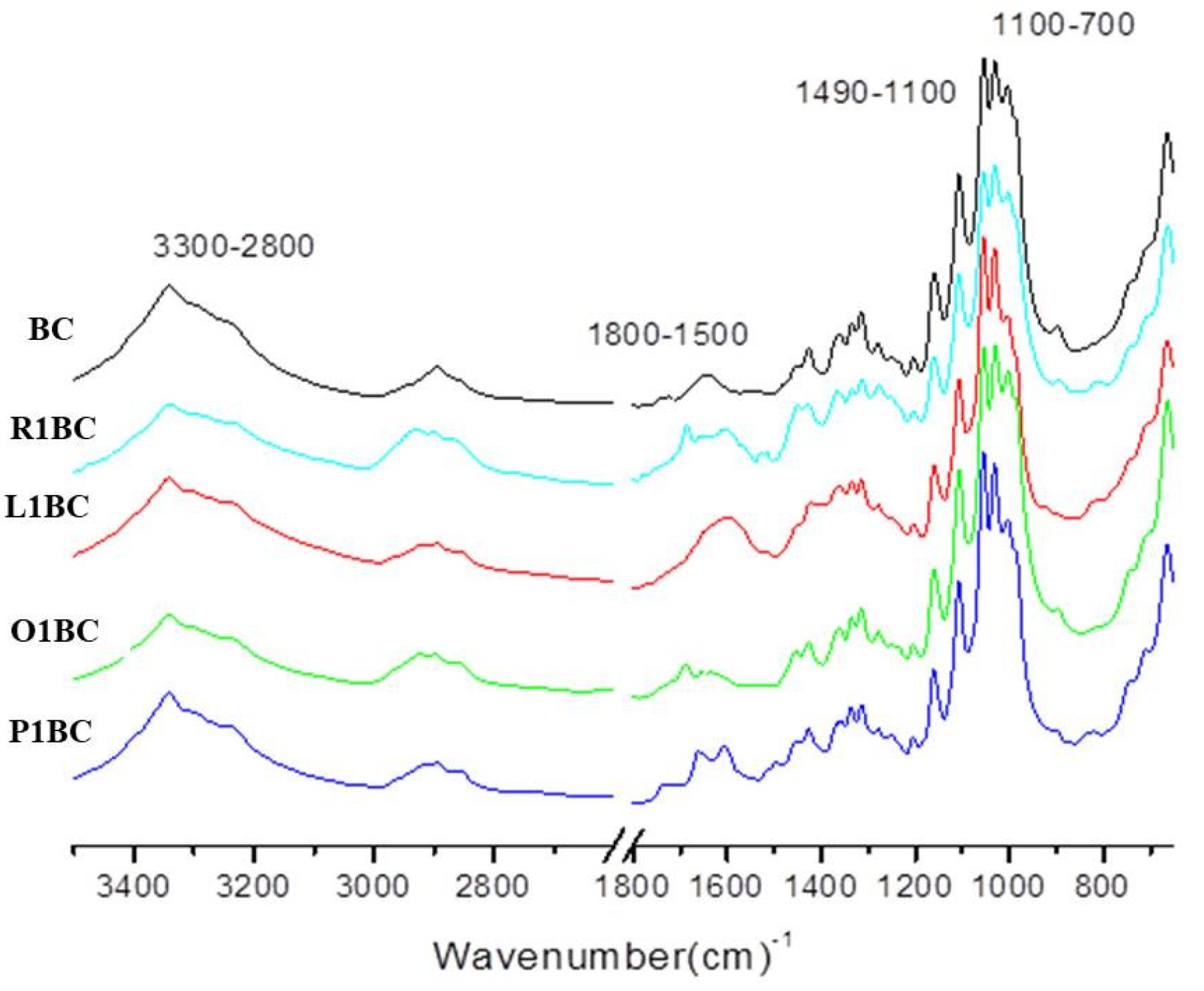
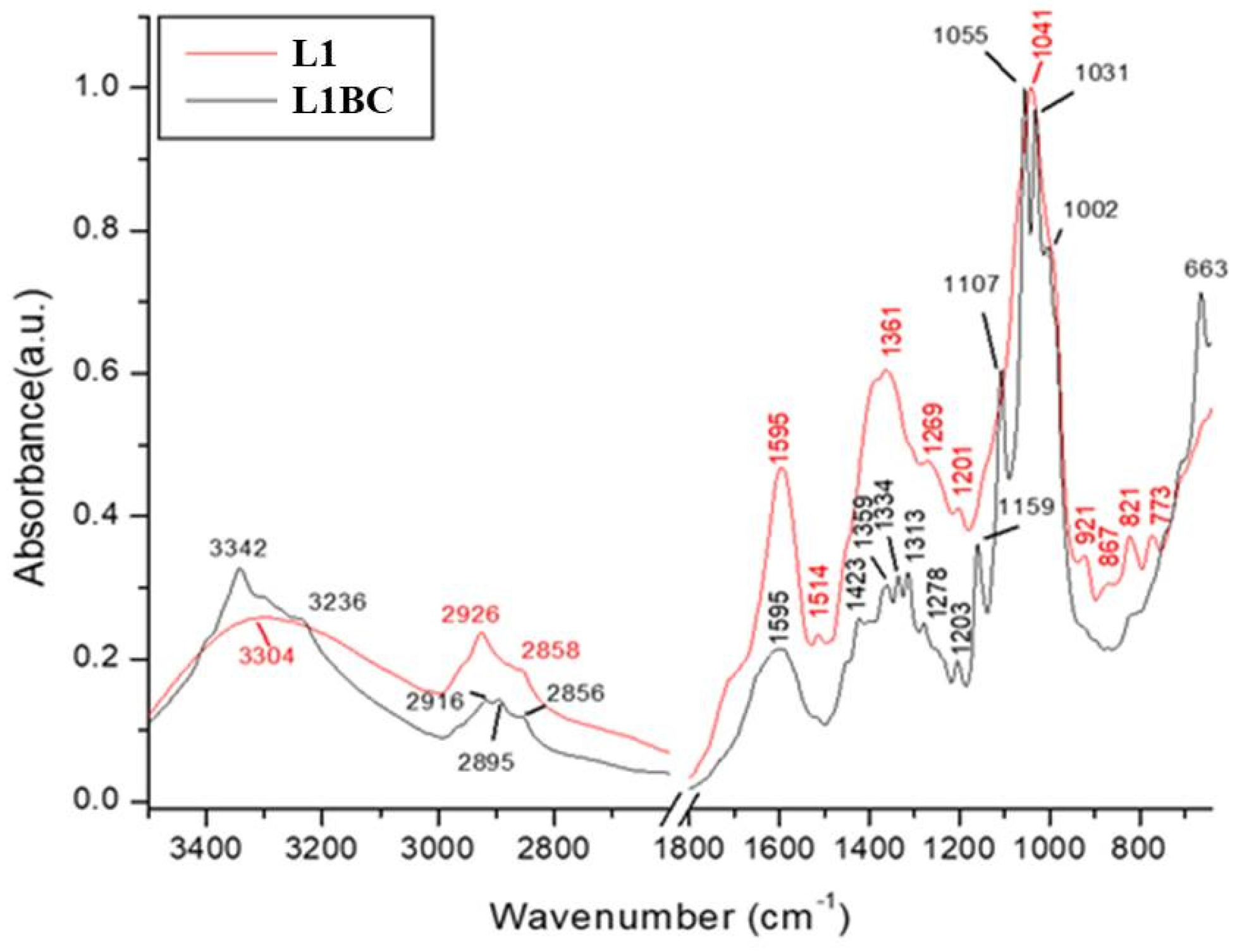
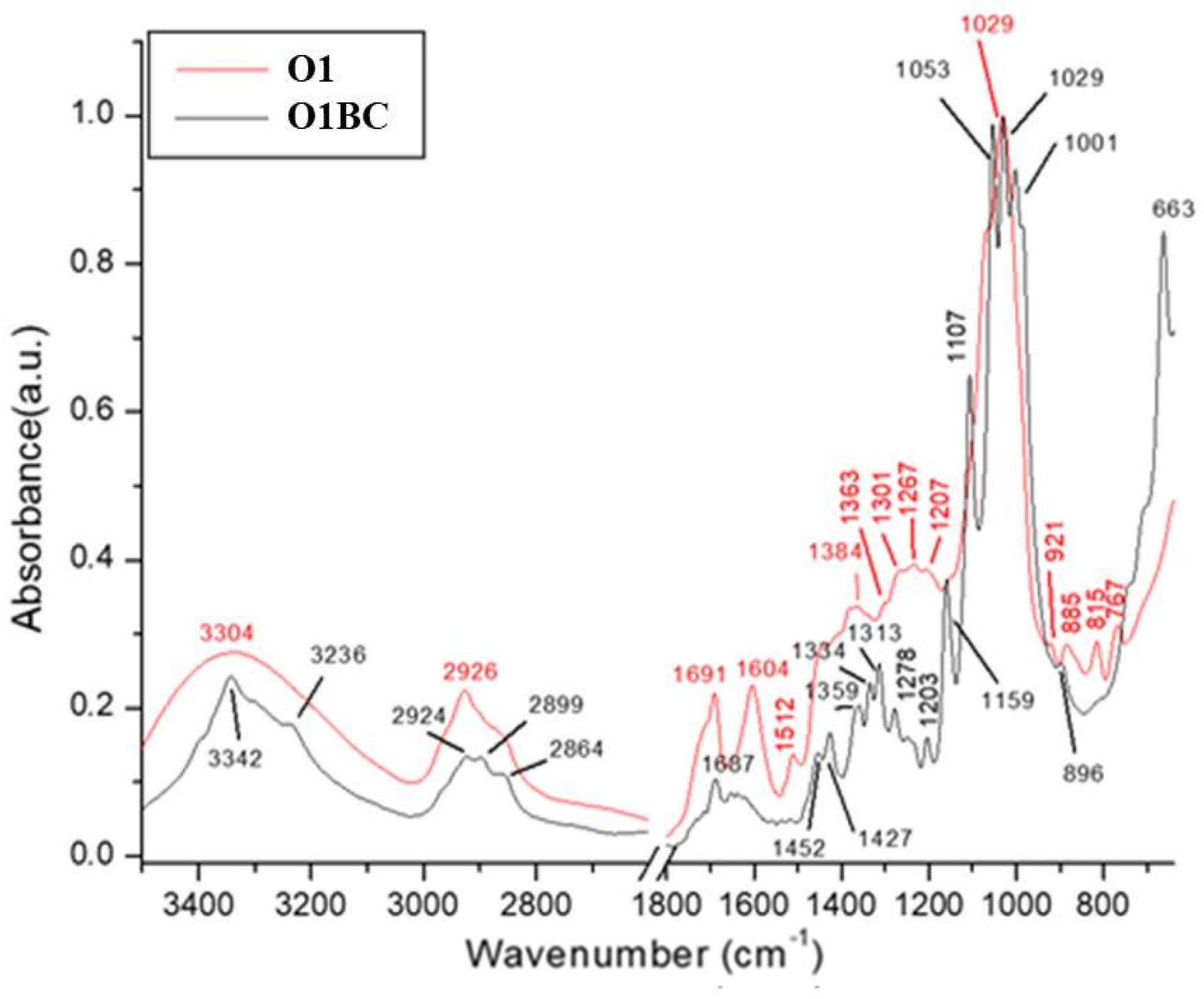
3.8. Fourier Transform Infrared Spectroscopy (FT-IR) of Bacterial-Cellulose (BC) Pellicles
3.9. Antimicrobial Activity of Bacterial Cellulose (BC) Enriched with Herbal Extracts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santos, D.C.; Ribeiro-Santos, R.; Ventura, L.A.F.; Melo, N.R.; Costa, B.; Rojas, E.E.G.; Salgado, N.L. Antimicrobial activity studies and characterization of cellulose acetate films containing essential oils. Ital. J. Food Sci. 2016, 28, 249–257. [Google Scholar] [CrossRef]
- Socaciu, M.I.; Fogarasi, M.; Semeniuc, C.A.; Socaci, S.A.; Rotar, M.A.; Muresan, V.; Pop, O.L.; Vodnar, D.C. Formulation and Characterization of Antimicrobial Edible Films Based on Whey Protein Isolate and Tarragon Essential Oil. Polymers 2020, 12, 1748. [Google Scholar] [CrossRef]
- Moradian, S.; Almasi, H.; Moini, S. Development of bacterial cellulose-based active membranes containing herbal extracts for shelf life extension of button mushrooms (Agaricus bisporus). J. Food Process. Preserv. 2018, 42, e13537. [Google Scholar] [CrossRef]
- Bodea, I.M.; Cătunescu, G.M.; Stroe, T.F.; Dîrlea, S.A.; Beteg, F.I. Applications of bacterial-synthesized cellulose in veterinary medicine—A review. Acta Vet. Brno 2019, 88, 451–471. [Google Scholar] [CrossRef]
- Mohite, B.V.; Koli, S.H.; Patil, S.V. Bacterial Cellulose-Based Hydrogels: Synthesis, Properties, and Applications. Polym. Polym. Compos. A Ref. Ser. 2019, 1255–1276. [Google Scholar] [CrossRef]
- Taokaew, S.; Nunkaew, N.; Siripong, P.; Phisalaphong, M. Characteristics and anticancer properties of bacterial cellulose films containing ethanolic extract of mangosteen peel. J. Biomater. Sci. Polym. Ed. 2014, 25, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Ossowicz-Rupniewska, P.; Rakoczy, R.; Konopacki, M.; Peruzynska, M.; Drozdzik, M.; Makuch, E.; Duchnik, W.; Kucharski, L.; Wenelska, K.; et al. Bacterial Cellulose Membrane Containing Epilobium angustifolium L. Extract as a Promising Material for the Topical Delivery of Antioxidants to the Skin. Int. J. Mol. Sci. 2021, 22, 6269. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Khan, M.S.; Manan, S.; Ullah, M.W.; Ul-Islam, M. Plant extract-loaded bacterial cellulose composite membrane for potential biomedical applications. J. Bioresour. Bioprod. 2021, 6, 26–32. [Google Scholar] [CrossRef]
- Garvey, M.I.; Rahman, M.M.; Gibbons, S.; Piddock, L.J. Medicinal plant extracts with efflux inhibitory activity against Gram-negative bacteria. Int. J. Antimicrob. Agents 2011, 37, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witkowska, A.M.; Hickey, D.K.; Alonso-Gomez, M.; Wilkinson, M. Evaluation of antimicrobial activities of commercial herb and spice extracts against selected food-borne bacteria. J. Food Res. 2013, 2, 37. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Eshraghi, A.; Mahzoonieh, M.R.; Lotfalian, S. Antibacterial and Antibiotic-Potentiation Activities of Levisticum officinale L. Extracts on Pathogenic Bacteria. Int. J. Infect. 2016, 4, e38768. [Google Scholar] [CrossRef]
- Bouyahya, A. Determination of Phenol Content and Antibacterial Activity of Five Medicinal Plants Ethanolic Extracts from North-West of Morocco. J. Plant Pathol. Microbiol. 2016, 7, 2. [Google Scholar] [CrossRef]
- Sukanya, S.L.; Sudisha, J.; Hariprasad, P.; Niranjana, S.R.; Prakash, H.S.; Fathima, S.K. Antimicrobial activity of leaf extracts of Indian medicinal plants against clinical and phytopathogenic bacteria. Afr. J. Biotechnol. 2009, 8, 6677–6682. [Google Scholar]
- Spiridon, I.; Colceru, S.; Anghel, N.; Teaca, C.A.; Bodirlau, R.; Armatu, A. Antioxidant capacity and total phenolic contents of oregano (Origanum vulgare), lavender (Lavandula angustifolia) and lemon balm (Melissa officinalis) from Romania. Nat. Prod. Res. 2011, 25, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Kitts, D. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006, 97, 505–515. [Google Scholar] [CrossRef]
- Heilerová, Ľ.; Bučková, M.; Tarapčík, P.; Šilhár, S.; Labuda, J. Comparison of antioxidative activity data for aqueous extracts of lemon balm (Melissa officinalis L.), oregano (Origanum vulgare L.), thyme (Thymus vulgaris L.), and agrimony (Agrimonia eupatoria L.) obtained by conventional methods and the DNA-based biosensor. Czech J. Food Sci. 2003, 21, 78. [Google Scholar]
- Ciolacu, D.; Oprea, A.M.; Anghel, N.; Cazacu, G.; Cazacu, M. New cellulose–lignin hydrogels and their application in controlled release of polyphenols. Mater. Sci. Eng. C 2012, 32, 452–463. [Google Scholar] [CrossRef]
- Althobaiti, F. Antibacterial Activity And Genotoxicity Effect of Ethanolic Leaves Extract of Rosmarinus Officinalis. J. Pure Appl. Microbiol. 2020, 14, 2165–2171. [Google Scholar] [CrossRef]
- Saǧdıç, O.; Özcan, M. Antibacterial activity of Turkish spice hydrosols. Food Control 2003, 14, 141–143. [Google Scholar] [CrossRef]
- Bunghez, F.; Rotar, A.; Vodnar, D.C.; Catunescu, G.M.; Socaciu, C. Comparative evaluation of phenolics’ profile and recovery in spray dried powders obtained from rosemary and oregano extracts in relation to their antibacterial activity in vitro. Rom. Biotechnol. Lett. 2016, 21, 11992–12004. [Google Scholar]
- Aljabri, M. Composition and antioxidant activities of rosemary (Rosmarinus officinalis) extracts. Eur. Asian J. BioSci. 2020, 14, 2179–2185. [Google Scholar]
- Pérez, M.B.; Calderón, N.L.; Croci, C.A. Radiation-induced enhancement of antioxidant activity in extracts of rosemary (Rosmarinus officinalis L.). Food Chem. 2007, 104, 585–592. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutierrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, A.; Camean, A.M. In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal Caco-2 cell line. Toxicol. In Vitro 2015, 29, 647–656. [Google Scholar] [CrossRef]
- Alakomi, H.L.; Maukonen, J.; Honkapaa, K.; Storgards, E.; Quirin, K.W.; Yang, B.; Saarela, M. Effect of Plant Antimicrobial Agents Containing Marinades on Storage Stability and Microbiological Quality of Broiler Chicken Cuts Packed with Modified Atmosphere Packaging. J. Food Prot. 2017, 80, 1689–1696. [Google Scholar] [CrossRef]
- Catunescu, G.M.; Rotar, I.; Vidican, R.; Bunghez, F.; Rotar, A.M. Gamma radiation enhances the bioactivity of fresh parsley (Petroselinum crispum (Mill.) Fuss Var. Neapolitanum). Radiat. Phys. Chem. 2017, 132, 22–29. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Zlotek, U.; Szymanowska, U.; Rybczynska-Tkaczyk, K.; Jederka, K.; Lewicki, S. In vitro Antioxidant, Anti-inflammatory, Anti-metabolic Syndrome, Antimicrobial, and Anticancer Effect of Phenolic Acids Isolated from Fresh Lovage Leaves [Levisticum officinale Koch] Elicited with Jasmonic Acid and Yeast Extract. Antioxidants 2020, 9, 554. [Google Scholar] [CrossRef]
- Brđanin, S.; Bogdanović, N.; Kolundžić, M.; Milenković, M.; Golić, N.; Kojić, M.; Kundaković, T. Antimicrobial activity of oregano (Origanum vulgare L.): And basil (Ocimum basilicum L.): Extracts. Adv. Technol. 2015, 4, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Miran, M.; Monsef Esfahani, H.; Jung, J.H.; Aliahmadi, A.; Skropeta, D.; Abbas-Mohammadi, M.; Nejad Ebrahimi, S.; Moridi Farimani, M. Characterization and Antibacterial Activity of Phthalides from the Roots of the Medicinal Herb Levisticum officinale W.D.J. Koch. Iran J. Pharm. Res. 2020, 19, 182–186. [Google Scholar] [CrossRef]
- Quiroz, J.Q.; Torres, A.C.; Ramirez, L.M.; Garcia, M.S.; Gomez, G.C.; Rojas, J. Optimization of the Microwave-Assisted Extraction Process of Bioactive Compounds from Annatto Seeds (Bixa orellana L.). Antioxidants 2019, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Celiktas, O.; Bedir, E.; Sukan, F. In vitro antioxidant activities of Rosmarinus officinalis extracts treated with supercritical carbon dioxide. Food Chem. 2007, 101, 1457–1464. [Google Scholar] [CrossRef]
- Zlotek, U.; Szymanowska, U.; Pecio, L.; Kozachok, S.; Jakubczyk, A. Antioxidative and Potentially Anti-inflammatory Activity of Phenolics from Lovage Leaves Levisticum officinale Koch Elicited with Jasmonic Acid and Yeast Extract. Molecules 2019, 24, 1441. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, J.; Langrish, T.A.G. Optimisation of total phenolic acids extraction from mandarin peels using microwave energy: The importance of the Maillard reaction. J. Food Eng. 2012, 109, 162–174. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Kabbashi, N.A. Extraction and characterization of bioactive compounds in Vernonia amygdalina leaf ethanolic extract comparing Soxhlet and microwave-assisted extraction techniques. J. Taibah Univ. Sci. 2019, 13, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.L.; Yue, T.L.; Yuan, Y.H.; Zhang, H.W. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef] [PubMed]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction—An innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Zhang, H.-F.; Yang, X.-H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Spigno, G.; De Faveri, D.M. Microwave-assisted extraction of tea phenols: A phenomenological study. J. Food Eng. 2009, 93, 210–217. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Olalere, O.A. Ethanolic extraction of bioactive compounds from Vernonia amygdalina leaf using response surface methodology as an optimization tool. J. Food Meas. Charact. 2018, 12, 1107–1122. [Google Scholar] [CrossRef]
- Simić, V.M.; Rajković, K.M.; Stojičević, S.S.; Veličković, D.T.; Nikolić, N.Č.; Lazić, M.L.; Karabegović, I.T. Optimization of microwave-assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep. Purif. Technol. 2016, 160, 89–97. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Biosci. 2019, 28, 66–73. [Google Scholar] [CrossRef]
- Kosakowska, O.; Weglarz, Z.; Pioro-Jabrucka, E.; Przybyl, J.L.; Krasniewska, K.; Gniewosz, M.; Baczek, K. Antioxidant and Antibacterial Activity of Essential Oils and Hydroethanolic Extracts of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare). Molecules 2021, 26, 988. [Google Scholar] [CrossRef] [PubMed]
- Farah, H.; Elbadrawy, E.; Al-Atoom, A.A. Evaluation of antioxidant and antimicrobial activities of ethanolic extracts of Parsley (Petroselinum erispum) and Coriander (Coriandrum sativum) plants grown in Saudi Arabia. Int. J. 2015, 3, 1244–1255. [Google Scholar]
- Nisca, A.; Stefanescu, R.; Moldovan, C.; Mocan, A.; Mare, A.D.; Ciurea, C.N.; Man, A.; Muntean, D.L.; Tanase, C. Optimization of Microwave Assisted Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant and Anti-Microbial Activity in Quercus cerris Bark Extracts. Plants 2022, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Purbowati, I.S.M.; Syamsu, K.; Warsiki, E. Optimization of phenols extraction from roselle (hibiscus sabdariffa) by microwave assisted extraction as antibacterial and antioxidant agents. J. Teknol. Ind. Pertan. 2016, 26, 23–30. [Google Scholar]
- Ertürk, Ö. Antibacterial and antifungal activity of ethanolic extracts from eleven spice plants. Biologia 2006, 61, 275–278. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, B.; Li, L.; Zhu, X. Microwave-assisted extraction and antioxidant activity of total phenolic compounds from pomegranate peel. J. Med. Plants Res. 2011, 5, 1004–1011. [Google Scholar]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Olalere, O.A. Ethanolic extraction of flavonoids, phenolics and antioxidants from Vernonia amygdalina leaf using two-level factorial design. J. King Saud Univ.-Sci. 2020, 32, 7–16. [Google Scholar] [CrossRef]
- Pavlović, M.D.; Buntić, A.V.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Ethanol influenced fast microwave-assisted extraction for natural antioxidants obtaining from spent filter coffee. Sep. Purif. Technol. 2013, 118, 503–510. [Google Scholar] [CrossRef]
- Xiao, W.; Han, L.; Shi, B. Microwave-assisted extraction of flavonoids from Radix Astragali. Sep. Purif. Technol. 2008, 62, 614–618. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, J.G.; Li, W.F.; Chen, J.; Wang, D.Y.; Zhu, L. Optimum extraction process of polyphenols from the bark of Phyllanthus emblica L. based on the response surface methodology. J. Sep. Sci. 2009, 32, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Gomes, C.L.; Danda, L.J.A.; Roberto, A.E.M.; Carvalho, A.M.R.; Ximenes, E.; Silva, R.; Angelos, M.A.; Rolim, L.A.; Rolim Neto, P.J. Optimized microwave-assisted extraction of polyphenols and tannins from Syzygium cumini (L.) Skeels leaves through an experimental design coupled to a desirability approach. An. Acad. Bras. Cienc. 2021, 93, e20190632. [Google Scholar] [CrossRef] [PubMed]
- Roman, I.; Stănilă, A.; Stănilă, S. Bioactive compounds and antioxidant activity of Rosa canina L. biotypes from spontaneous flora of Transylvania. Chem. Cent. J. 2013, 7, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol 2004, 26, 211–219. [Google Scholar]
- Akter, M.S.; Ahmed, M.; Eun, J.B. Solvent effects on antioxidant properties of persimmon (Diospyros kaki L. cv. Daebong) seeds. Int. J. Food Sci. Technol. 2010, 45, 2258–2264. [Google Scholar] [CrossRef]
- Marghitas, L.A.; MIHAI, C.M.; Chirila, F.; Dezmirean, D.S.; FIT, N.I. The study of the antimicrobial activity of Transylvanian (Romanian) propolis. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 40–44. [Google Scholar]
- Jiji, S.; Udhayakumar, S.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Thymol enriched bacterial cellulose hydrogel as effective material for third degree burn wound repair. Int. J. Biol. Macromol. 2019, 122, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Huang, T.R.; Lim, Z.H.; Luo, R.; Pasula, R.R.; Liao, L.D.; Lim, S.; Chen, C.H. Production of Hollow Bacterial Cellulose Microspheres Using Microfluidics to Form an Injectable Porous Scaffold for Wound Healing. Adv. Healthc. Mater. 2016, 5, 2983–2992. [Google Scholar] [CrossRef]
- Bodea, I.M.; Beteg, F.I.; Pop, C.R.; David, A.P.; Dudescu, M.C.; Vilau, C.; Stanila, A.; Rotar, A.M.; Catunescu, G.M. Optimization of Moist and Oven-Dried Bacterial Cellulose Production for Functional Properties. Polymers 2021, 13, 2088. [Google Scholar] [CrossRef]
- Dahman, Y.; Jayasuriya, K.E.; Kalis, M. Potential of biocellulose nanofibers production from agricultural renewable resources: Preliminary study. Appl. Biochem. Biotechnol. 2010, 162, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Gea, S.; Reynolds, C.T.; Roohpour, N.; Wirjosentono, B.; Soykeabkaew, N.; Bilotti, E.; Peijs, T. Investigation into the structural, morphological, mechanical and thermal behaviour of bacterial cellulose after a two-step purification process. Bioresour. Technol. 2011, 102, 9105–9110. [Google Scholar] [CrossRef] [PubMed]
- Paşcalău, V.; Popescu, V.; Popescu, G.; Dudescu, M.; Borodi, G.; Dinescu, A.; Perhaiţa, I.; Paul, M. The alginate/k-carrageenan ratio’s influence on the properties of the cross-linked composite films. J. Alloy. Compd. 2012, 536, S418–S423. [Google Scholar] [CrossRef]
- Santoso, S.P.; Chou, C.-C.; Lin, S.-P.; Soetaredjo, F.E.; Ismadji, S.; Hsieh, C.-W.; Cheng, K.C. Enhanced production of bacterial cellulose by Komactobacter intermedius using statistical modeling. Cellulose 2020, 27, 2497–2509. [Google Scholar] [CrossRef]
- Alonso, E.; Faria, M.; Mohammadkazemi, F.; Resnik, M.; Ferreira, A.; Cordeiro, N. Conductive bacterial cellulose-polyaniline blends: Influence of the matrix and synthesis conditions. Carbohydr. Polym. 2018, 183, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Kirdponpattara, S.; Khamkeaw, A.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Structural modification and characterization of bacterial cellulose-alginate composite scaffolds for tissue engineering. Carbohydr. Polym. 2015, 132, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Wang, L.; Zhao, J.C.; Zhu, P. Effect of Drying Methods on Structure and Mechanical Properties of Bacterial Cellulose Films. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2011; Volume 239, pp. 2667–2670. [Google Scholar] [CrossRef]
- Tercjak, A.; Gutierrez, J.; Barud, H.S.; Domeneguetti, R.R.; Ribeiro, S.J. Nano-and macroscale structural and mechanical properties of in situ synthesized bacterial cellulose/PEO-b-PPO-b-PEO biocomposites. ACS Appl. Mater. Interfaces 2015, 7, 4142–4150. [Google Scholar] [CrossRef] [PubMed]
- Cătunescu, G.M.; Rotar, A.M.; Pop, C.R.; Diaconeasa, Z.; Bunghez, F.; Socaciu, M.-I.; Semeniuc, C.A. Influence of extraction pre-treatments on some phytochemicals and biological activity of Transylvanian cranberries (Vaccinium vitis-idea L.). LWT 2019, 102, 385–392. [Google Scholar] [CrossRef]
- Ramamurthy, N.; Kannan, S. Fourier transform infrared spectroscopic analysis of a plant (Calotropis gigantea Linn) from an industrial village, Cuddalore dt, Tamilnadu, India. Rom. J. Biophys. 2007, 17, 269–276. [Google Scholar]
- Hosseini, H.; Bolourian, S.; Yaghoubi Hamgini, E.; Ghanuni Mahababadi, E. Optimization of heat- and ultrasound-assisted extraction of polyphenols from dried rosemary leaves using response surface methodology. J. Food Process. Preserv. 2018, 42, e13778. [Google Scholar] [CrossRef]
- Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitsuhashi, S.; Nishi, Y.; Uryu, M. The structure and mechanical properties of sheets prepared from bacterial cellulose. J. Mater. Sci. 1989, 24, 3141–3145. [Google Scholar] [CrossRef]
- Dorman, H.D.; Bachmayer, O.; Kosar, M.; Hiltunen, R. Antioxidant properties of aqueous extracts from selected Lamiaceae species grown in Turkey. J. Agric. Food Chem. 2004, 52, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Zhang, Y.; Seeram, N.P.; Lee, R.P.; Wang, P.; Bowerman, S.; Heber, D. Antioxidant capacity and phytochemical content of herbs and spices in dry, fresh and blended herb paste form. Int. J. Food Sci. Nutr. 2011, 62, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Słowianek, M.; Leszczyńska, J. Antioxidant properties of selected culinary spices. Herba Pol. 2016, 62, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Yesil-Celiktas, O.; Girgin, G.; Orhan, H.; Wichers, H.; Bedir, E.; Vardar-Sukan, F. Screening of free radical scavenging capacity and antioxidant activities of Rosmarinus officinalis extracts with focus on location and harvesting times. Eur. Food Res. Technol. 2007, 224, 443–451. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Regueiro, J.; Martinez-Huelamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Rababah, T.M.; Banat, F.; Rababah, A.; Ereifej, K.; Yang, W. Optimization of extraction conditions of total phenolics, antioxidant activities, and anthocyanin of oregano, thyme, terebinth, and pomegranate. J. Food Sci. 2010, 75, C626–C632. [Google Scholar] [CrossRef] [PubMed]
- Alshwaikh, R.M.A.; Al-Sorchee, S.M.A.; Ali, K.A.; Al Beer, W. Antibacterial activity of parsley and celery aqueous extract on the isolated bacteria from children UTI in Erbil city. Int. J. Adv. Res 2014, 2, 895–903. [Google Scholar]
- Chun, S.-S.; Vattem, D.A.; Lin, Y.-T.; Shetty, K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
- Chrpova, D.; Kouřimská, L.; Gordon, M.H.; Heřmanová, V.; Roubíčková, I.; Panek, J. Antioxidant activity of selected phenols and herbs used in diets for medical conditions. Czech J. Food Sci. 2010, 28, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Nour, V.; Trandafir, I.; Cosmulescu, S. Bioactive Compounds, Antioxidant Activity and Nutritional Quality of Different Culinary Aromatic Herbs. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 179–184. [Google Scholar] [CrossRef] [Green Version]
- El-Zaeddi, H.; Calin-Sanchez, A.; Nowicka, P.; Martinez-Tome, J.; Noguera-Artiaga, L.; Burlo, F.; Wojdylo, A.; Carbonell-Barrachina, A.A. Preharvest treatments with malic, oxalic, and acetylsalicylic acids affect the phenolic composition and antioxidant capacity of coriander, dill and parsley. Food Chem. 2017, 226, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Jałoszyński, K.; Figiel, A.; Wojdyło, A. Drying kinetics and antioxidant activity of oregano. Acta Agrophysica 2008, 11, 81–90. [Google Scholar]
- Gómez-Estaca, J.; Bravo, L.; Gómez-Guillén, M.C.; Alemán, A.; Montero, P. Antioxidant properties of tuna-skin and bovine-hide gelatin films induced by the addition of oregano and rosemary extracts. Food Chem. 2009, 112, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Marin, I.; Sayas-Barbera, E.; Viuda-Martos, M.; Navarro, C.; Sendra, E. Chemical Composition, Antioxidant and Antimicrobial Activity of Essential Oils from Organic Fennel, Parsley, and Lavender from Spain. Foods 2016, 5, 18. [Google Scholar] [CrossRef]
- Martinez, L.; Castillo, J.; Ros, G.; Nieto, G. Antioxidant and Antimicrobial Activity of Rosemary, Pomegranate and Olive Extracts in Fish Patties. Antioxidants 2019, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Zazharskiy, V.; Davydenko, P.; Kulishenko, O.; Borovik, I.; Brygadyrenko, V.; Zazharska, N. Antibacterial activity of herbal infusions against Staphylococcus aureus, Staphylococcus epidermidis and Pseudomonas aeruginosa in vitro. Magyar Állatorvosok Lapja 2019, 141, 693–704. [Google Scholar]
- Pasca, C.; Marghitas, L.; Dezmirean, D.; Bobis, O.; Bonta, V.; Chirila, F.; Matei, I.; Fit, N. Medicinal Plants Based Products Tested on Pathogens Isolated from Mastitis Milk. Molecules 2017, 22, 1473. [Google Scholar] [CrossRef] [Green Version]
- Ojala, T.; Remes, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haahtela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 2000, 73, 299–305. [Google Scholar] [CrossRef]
- Seyyednejad, S.; Maleki, S.; Damabi, N.M.; Motamedi, H. Antibacterial activity of Prunus mahaleb and Parsley (Petroselinum crispum) against some pathogen. Asian J. Biol. Sci. 2008, 1, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Jalosinska, M.; Wilczak, J. Influence of plant extracts on the microbiological shelf life of meat products. Pol. J. Food Nutr. Sci. 2009, 59, 303–308. [Google Scholar]
- Wang, S.; Jiang, F.; Xu, X.; Kuang, Y.; Fu, K.; Hitz, E.; Hu, L. Super-Strong, Super-Stiff Macrofibers with Aligned, Long Bacterial Cellulose Nanofibers. Adv. Mater. 2017, 29, 1702498. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, H.; Zheng, W.; Gong, N.; Chen, L.; Jiang, X.; Yang, G. Bacterial cellulose–hyaluronan nanocomposite biomaterials as wound dressings for severe skin injury repair. J. Mater. Chem. B 2015, 3, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Lien, C.C.; Yeh, H.J.; Yu, C.M.; Hsu, S.H. Bacterial cellulose and bacterial cellulose-chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Shumilova, A.A.; Shidlovskiy, I.P.; Nikolaeva, E.D.; Sukovatiy, A.G.; Vasiliev, A.D.; Shishatskaya, E.I. Antibacterial properties of films of cellulose composites with silver nanoparticles and antibiotics. Polym. Test. 2018, 65, 54–68. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Zhang, Y.; Li, C.; Wu, Z.; Zhuo, Q.; Huang, X.; Qiu, G.; Zhou, P.; Yang, G. Skin tissue repair materials from bacterial cellulose by a multilayer fermentation method. J. Mater. Chem. 2012, 22, 12349. [Google Scholar] [CrossRef]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Recent developments in the production and applications of bacterial cellulose fibers and nanocrystals. Crit. Rev. Biotechnol. 2017, 37, 510–524. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods-A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose 1998, 5, 187–200. [Google Scholar] [CrossRef]
- Wu, C.N.; Fuh, S.C.; Lin, S.P.; Lin, Y.Y.; Chen, H.Y.; Liu, J.M.; Cheng, K.C. TEMPO-Oxidized Bacterial Cellulose Pellicle with Silver Nanoparticles for Wound Dressing. Biomacromolecules 2018, 19, 544–554. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a colorimetric pH indicator based on bacterial cellulose nanofibers and red cabbage (Brassica oleraceae) extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.C.I.M.; Ahmad, N.; Halib, N.; Ahmad, I. Synthesis and characterization of thermo-and pH-responsive bacterial cellulose/acrylic acid hydrogels for drug delivery. Carbohydr. Polym. 2012, 88, 465–473. [Google Scholar] [CrossRef]
- Franca, A.S.; Nollet, L.M. Spectroscopic Methods in Food Analysis; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Nishi, Y.; Uryu, M.; Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitsuhashi, S. The structure and mechanical properties of sheets prepared from bacterial cellulose. J. Mater. Sci. 1990, 25, 2997–3001. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7381. [Google Scholar] [CrossRef]
- Yassine, F.; Bassil, N.; Chokr, A.; El Samrani, A.; Serghei, A.; Boiteux, G.; El Tahchi, M. Two-step formation mechanism of Acetobacter cellulosic biofilm: Synthesis of sparse and compact cellulose. Cellulose 2016, 23, 1087–1100. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Schlenger, P.; Garcia-Valverde, M. Monitoring changes in the structure and properties of humic substances following ozonation using UV-Vis, FTIR and (1)H NMR techniques. Sci. Total Environ. 2016, 541, 623–637. [Google Scholar] [CrossRef]
- Sivakesava, S.; Irudayaraj, J. Analysis of potato chips using FTIR photoacoustic spectroscopy. J. Sci. Food Agric. 2000, 80, 1805–1810. [Google Scholar] [CrossRef]
- Bellamy, L. Alkanes. In The Infrared Spectra of Complex Molecules; Springer: Berlin/Heidelberg, Germany, 1980; pp. 1–23. [Google Scholar]
- Rubio-Senent, F.; Martos, S.; Lama-Munoz, A.; Fernandez-Bolanos, J.G.; Rodriguez-Gutierrez, G.; Fernandez-Bolanos, J. Isolation and identification of minor secoiridoids and phenolic components from thermally treated olive oil by-products. Food Chem. 2015, 187, 166–173. [Google Scholar] [CrossRef]
- Zghari, B.; Doumenq, P.; Romane, A.; Boukir, A. GC-MS, FTIR and 1H, 13C NMR structural analysis and identification of phenolic compounds in olive mill wastewater extracted from oued Oussefrou effluent (Beni Mellal-Morocco). J. Mater. Environ. Sci. 2017, 8, 4496–4509. [Google Scholar] [CrossRef]
- Edison, T.J.; Sethuraman, M.G. Biogenic robust synthesis of silver nanoparticles using Punica granatum peel and its application as a green catalyst for the reduction of an anthropogenic pollutant 4-nitrophenol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 104, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Dammström, S.; Salmén, L.; Gatenholm, P. The effect of moisture on the dynamical mechanical properties of bacterial cellulose/glucuronoxylan nanocomposites. Polymer 2005, 46, 10364–10371. [Google Scholar] [CrossRef]
- Ashori, A.; Sheykhnazari, S.; Tabarsa, T.; Shakeri, A.; Golalipour, M. Bacterial cellulose/silica nanocomposites: Preparation and characterization. Carbohydr. Polym. 2012, 90, 413–418. [Google Scholar] [CrossRef]
- Barud, H.S.; Souza, J.L.; Santos, D.B.; Crespi, M.S.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J.L. Bacterial cellulose/poly(3-hydroxybutyrate) composite membranes. Carbohydr. Polym. 2011, 83, 1279–1284. [Google Scholar] [CrossRef]
- Boukir, A.; Guiliano, M.; Doumenq, P.; El Hallaoui, A.; Mille, G. Structural characterization of crude oil asphaltenes by infrared spectroscopy (FTIR). Application to photo-oxidation. Comptes Rendus L’Academie Des Sci. Ser. IIC Chem. 1998, 10, 597–602. [Google Scholar]
- Castro, C.; Zuluaga, R.; Putaux, J.-L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- Ragupathi Raja Kannan, R.; Arumugam, R.; Anantharaman, P. Fourier transform infrared spectroscopy analysis of seagrass polyphenols. Curr. Bioact. Compd. 2011, 7, 118–125. [Google Scholar] [CrossRef]
- Guiliano, M.; Boukir, A.; Doumenq, P.; Mille, G.; Crampon, C.; Badens, E.; Charbit, G. Supercritical fluid extraction of bal 150 crude oil asphaltenes. Energy Fuels 2000, 14, 89–94. [Google Scholar] [CrossRef]
- Sun, D.; Yang, J.; Wang, X. Bacterial cellulose/TiO2 hybrid nanofibers prepared by the surface hydrolysis method with molecular precision. Nanoscale 2010, 2, 287–292. [Google Scholar] [CrossRef]
- Huang, C.; Yang, X.Y.; Xiong, L.; Guo, H.J.; Luo, J.; Wang, B.; Zhang, H.R.; Lin, X.Q.; Chen, X.D. Evaluating the possibility of using acetone-butanol-ethanol (ABE) fermentation wastewater for bacterial cellulose production by Gluconacetobacter xylinus. Lett. Appl. Microbiol. 2015, 60, 491–496. [Google Scholar] [CrossRef]
- Castro, C.; Vesterinen, A.; Zuluaga, R.; Caro, G.; Filpponen, I.; Rojas, O.J.; Kortaberria, G.; Gañán, P. In situ production of nanocomposites of poly(vinyl alcohol) and cellulose nanofibrils from Gluconacetobacter bacteria: Effect of chemical crosslinking. Cellulose 2014, 21, 1745–1756. [Google Scholar] [CrossRef]
- Yassine, F.; Bassil, N.; Flouty, R.; Chokr, A.; Samrani, A.E.; Boiteux, G.; Tahchi, M.E. Culture medium pH influence on Gluconacetobacter physiology: Cellulose production rate and yield enhancement in presence of multiple carbon sources. Carbohydr. Polym. 2016, 146, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Droussi, Z.; D’Orazio, V.; Provenzano, M.R.; Hafidi, M.; Ouatmane, A. Study of the biodegradation and transformation of olive-mill residues during composting using FTIR spectroscopy and differential scanning calorimetry. J. Hazard. Mater. 2009, 164, 1281–1285. [Google Scholar] [CrossRef]
- Hajji, L.; Boukir, A.; Assouik, J.; Pessanha, S.; Figueirinhas, J.L.; Carvalho, M.L. Artificial aging paper to assess long-term effects of conservative treatment. Monitoring by infrared spectroscopy (ATR-FTIR), X-ray diffraction (XRD), and energy dispersive X-ray fluorescence (EDXRF). Microchem. J. 2016, 124, 646–656. [Google Scholar] [CrossRef]
- Garside, P.; Wyeth, P. Identification of cellulosic fibres by FTIR spectroscopy-thread and single fibre analysis by attenuated total reflectance. Stud. Conserv. 2003, 48, 269–275. [Google Scholar] [CrossRef] [Green Version]
- El Hajjouji, H.; Bailly, J.R.; Winterton, P.; Merlina, G.; Revel, J.C.; Hafidi, M. Chemical and spectroscopic analysis of olive mill waste water during a biological treatment. Bioresour. Technol. 2008, 99, 4958–4965. [Google Scholar] [CrossRef] [Green Version]
- Kalisz, G.; Gieroba, B.; Chrobak, O.; Suchora, M.; Starosta, A.L.; Sroka-Bartnicka, A. Vibrational Spectroscopic Analyses and Imaging of the Early Middle Ages Hemp Bast Fibres Recovered from Lake Sediments. Molecules 2021, 26, 1314. [Google Scholar] [CrossRef]
- Dayal, M.S.; Goswami, N.; Sahai, A.; Jain, V.; Mathur, G.; Mathur, A. Effect of media components on cell growth and bacterial cellulose production from Acetobacter aceti MTCC 2623. Carbohydr. Polym. 2013, 94, 12–16. [Google Scholar] [CrossRef]
- Goh, W.; Rosma, A.; Kaur, B.; Fazilah, A.; Karim, A.; Bhat, R. Microstructure and physical properties of microbial cellulose produced during fermentation of black tea broth (Kombucha). II. Int. Food Res. J. 2012, 19, 153–158. [Google Scholar]
- Zeinali Kalkhoran, A.H.; Naghib, S.M.; Vahidi, O.; Rahmanian, M. Synthesis and characterization of graphene-grafted gelatin nanocomposite hydrogels as emerging drug delivery systems. Biomed. Phys. Eng. Express 2018, 4, 055017. [Google Scholar] [CrossRef]
- Nagmetova, G.; Berthold-Pluta, A.; Garbowska, M.; Kurmanbayev, A.; Stasiak-Rozanska, L. Antibacterial Activity of Biocellulose with Oregano Essential Oil against Cronobacter Strains. Polymers 2020, 12, 1647. [Google Scholar] [CrossRef]
- Rajwade, J.M.; Paknikar, K.M.; Kumbhar, J.V. Applications of bacterial cellulose and its composites in biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A.; Tabuchi, M.; Uo, M.; Tatsumi, H.; Hideshima, K.; Kondo, S.; Sekine, J. Applicability of bacterial cellulose as an alternative to paper points in endodontic treatment. Acta Biomater. 2013, 9, 6116–6122. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yang, G.; Hong, F. Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr. Polym. 2011, 84, 533–538. [Google Scholar] [CrossRef]



| Sample | Ethanol Concentration (% v/v) | Microwave Power (W) | Extraction Time (s) | Extraction Repetitions |
|---|---|---|---|---|
| 1 | 80 | 800 | 10 | 5 |
| 2 | 60 | 160 | 10 | 1 |
| 3 | 40 | 480 | 40 | 10 |
| Botanical Family | Herb | Sample | Ethanol (%) | Microwave Power (W) | Extraction Time (s) | Times Extracted | TPC (mg GAE/100 g DW) | AA (mM TE/100 g DW) | I (%) |
|---|---|---|---|---|---|---|---|---|---|
| Lamiaceae | Oregano Origanum vulgare | O1 | 80 | 800 | 10 | 5 | 3213.48 ± 55.13 c | 48.61 ± 1.66 e | 40.39 ± 2.02 d |
| O2 | 60 | 160 | 10 | 1 | 3960.82 ± 94.39 b | 58.54 ± 0.96 d | 46.01 ± 1.18 c | ||
| O3 | 40 | 480 | 40 | 10 | 4314.06 ± 81.33 a | 73.99 ± 1.66 c | 57.05 ± 2.08 b | ||
| rosemary Rosmarinus officinalis | R1 | 80 | 800 | 10 | 5 | 4230.19 ± 88.30 a | 82.81 ± 0.83 b | 62.04 ± 1.13 a | |
| R2 | 60 | 160 | 10 | 1 | 4240.28 ± 44.77 a | 87.23 ± 1.38 a | 65.01 ± 1.77 a | ||
| R3 | 40 | 480 | 40 | 10 | 4059.91 ± 11.20 b | 58.63 ± 1.57 d | 46.04 ± 1.94 c | ||
| Apiaceae | lovage Levisticum officinale | L1 | 80 | 800 | 10 | 5 | 2385.02 ± 21.69 e | 24.15 ± 1.52 g | 16.90 ± 1.89 f |
| L2 | 60 | 160 | 10 | 1 | 2949.17 ± 40.35 d | 25.53 ± 0.97 g | 18.09 ± 1.20 ef | ||
| L3 | 40 | 480 | 40 | 10 | 3015.25 ± 70.02 d | 32.06 ± 1.20 f | 22.08 ± 1.50 e | ||
| parsley Petroselinum crispum | P1 | 80 | 800 | 10 | 5 | 452.90 ± 81.89 h | 6.58 ± 0.57 h | 4.57 ± 0.70 g | |
| P2 | 60 | 160 | 10 | 1 | 600.35 ± 45.25 g | 7.78 ± 1.10 h | 5.77 ± 1.46 g | ||
| P3 | 40 | 480 | 40 | 10 | 747.73 ± 21.32 f | 8.42 ± 1.11 h | 6.20 ± 1.32 g |
| Botanical Family | Herb | Sample | Ethanol (%) | Microwave Power (W) | Extraction Time (s) | Extraction Repetition | DIZ S. aureus (mm) | DIZ E. coli (mm) | DIZ C. albicans (mm) |
|---|---|---|---|---|---|---|---|---|---|
| Lamiaceae | Oregano Origanum vulgare | O1 | 80 | 800 | 10 | 5 | 1.50 ± 0.71 cd | 1.00 ± 0.00 ef | 1.00 ± 0.00 d |
| O2 | 60 | 160 | 10 | 1 | 1.00 ± 0.00 cd | 3.00 ± 1.41 bcd | 1.00 ± 0.00 d | ||
| O3 | 40 | 480 | 40 | 10 | 1.00 ± 0.00 cd | R f | R e | ||
| rosemary Rosmarinus officinalis | R1 | 80 | 800 | 10 | 5 | 4.00 ± 0.00 b | 3.50 ± 0.71 bc | 2.50 ± 0.71 b | |
| R2 | 60 | 160 | 10 | 1 | 4.50 ± 2.12 b | 4.50 ± 0.71 b | 1.00 ± 0.00 d | ||
| R3 | 40 | 480 | 40 | 10 | 2.00 ± 0.00 c | R f | R e | ||
| Apiaceae | lovage Levisticum officinale | L1 | 80 | 800 | 10 | 5 | 1.50 ± 0.71 cd | 1.00 ± 0.00 ef | 1.00 ± 0.00 d |
| L2 | 60 | 160 | 10 | 1 | 1.50 ± 0.71 cd | 1.00 ± 0.00 cde | 1.00 ± 0.00 d | ||
| L3 | 40 | 480 | 40 | 10 | R d | R f | R e | ||
| parsley Petroselinum crispum | P1 | 80 | 800 | 10 | 5 | 1.50 ± 0.71 cd | 1.50 ± 0.71 def | 1.50 ± 0.71 cd | |
| P2 | 60 | 160 | 10 | 1 | 1.50 ± 0.71 cd | 3.00 ± 1.41 bcd | 2.00 ± 0.00 bc | ||
| P3 | 40 | 480 | 40 | 10 | 0.15 ± 0.07 d | R f | R e | ||
| amoxicillin/ clavulanic acid | 18.50 ± 0.24 a | 7.00 ± 0.47 a | NA | ||||||
| miconazole | NA | NA | 10.83 ± 0.24 a |
| PCA Variable | Correlations between Variables and PCA Factors | Contribution of the Variables (%) | Correlations between Variables and PCA Factors | |||
|---|---|---|---|---|---|---|
| F1 | F2 | F1 | F2 | F1 | F2 | |
| TPC | 0.82 | −0.52 | 18.50 | 13.94 | 0.67 | 0.27 |
| AA | 0.94 | −0.33 | 24.31 | 5.71 | 0.88 | 0.11 |
| I% | 0.93 | −0.34 | 23.87 | 6.19 | 0.86 | 0.12 |
| DIZ S | 0.84 | 0.39 | 19.51 | 8.06 | 0.71 | 0.15 |
| DIZ E | 0.63 | 0.70 | 10.95 | 25.28 | 0.40 | 0.49 |
| DIZ C | 0.32 | 0.89 | 2.87 | 40.83 | 0.10 | 0.78 |
| Et | 0.20 | 0.67 | 0.04 | 0.45 | ||
| MP | −0.11 | −0.03 | 0.01 | 0.00 | ||
| T | −0.29 | −0.79 | 0.09 | 0.62 | ||
| Rep | −0.31 | −0.72 | 0.10 | 0.52 | ||
| Samples Wavenumber (cm−1) | Tentative Assignments | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BC | R1BC | R1 | L1BC | L1 | O1BC | O1 | P1BC | P1 | Functional Group | |
| 665 | 663 | 663 | 661 | 663 | 661 | 665 | 663 | C–OH in alcohols of C–O–H bending | [119] | |
| 767 | 773 | 767 | 770 | γ C–Har (2C–Har adjacent) out-of-plane deformation of Aromatic groups | [112,128] | |||||
| 815 | 821 | 816 | 820 | |||||||
| 896 | 894 | 896 | 898 | 898 | ѵ s (C–O–C) in-plane | [129,130] | ||||
| 1002 | 1001 | 1002 | 1001 | 1001 | C–3…O–3 stretching | [127] | ||||
| 1029 | 1029 | 1031 | 1030 | 1031 | 1029 | 1029 | 1031 | 1033 | Bending of C–O–H bond of carbohydrates | [121] |
| 1043 | 1041 | 1045 | 1041 | ѵ C–O stretching of polysaccharides or polysaccharide-like substances | [125,126] | |||||
| 1055 | 1055 | 1055 | 1053 | 1055 | C–O stretching vibrations in primary alcohol; The bending of C–O–H bond of carbohydrates or C–O–C pyranose ring skeletal vibration | [116,121,122,123,124] | ||||
| 1109 | 1107 | 1107 | 1107 | 1107 | C–C bonds of the monomer units of polysaccharide or C–O bending vibration | [118] | ||||
| 1112 | 1114 | 1112 | 1114 | ѵ C–O stretching of aryl ethers and phenols | [120] | |||||
| 1157 | 1161 | 1161 | 1159 | 1160 | 1159 | 1163 | 1161 | 1166 | C–O–C antisymmetric bridge stretching of 1,4-b-D-glucoside | [103] |
| 1203 | 1203 | 1201 | 1207 | 1203 | Unidentified | |||||
| 1276 | 1280 | 1278 | 1269 | 1278 | 1267 | 1278 | 1279 | Ar–O in aryl ethers | [119] | |
| 1313 | 1313 | 1313 | 1313 | 1313 | Out-of-plane wagging of the CH2 groups | [118] | ||||
| 1334 | 1336 | 1334 | 1334 | 1334 | C–H deformation or O–H in-plane bending | [118] | ||||
| 1373 | 1371 | 1359 | 1361 | 1363 | 1359 | 1369 | 1370 | ѵ COO– antisymmetric stretching C–H bending of CH2 and CH3 groups | [117] | |
| 1427 | 1425 | 1423 | 1427 | 1425 | CH2 symmetric bending or O–H in plane bending | [114,115,116] | ||||
| 1450 | 1450 | 1452 | 1450 | Aromatic –C=C– bond | [113] | |||||
| 1514 | 1514 | 1512 | 1512 | ѵ Car=Car aromatic-stretching vibrations | [112] | |||||
| 1604 | 1600 | 1595 | 1595 | 1600 | 1604 | 1604 | 1606 | Stretching vibration of C–C | [17] | |
| - | 1686 | 1697 | 1687 | 1691 | 1660 | ѵ C=O stretching of amide groups (Amide I band), C=O of quinone and/or H–bonded conjugated ketones | [108] | |||
| - | 1732 | 1717 | ѵ C=O stretching vibrations in associated carboxyl COOH, ketone groups, and esters | [111] | ||||||
| 2895 | 2895 | 2895 | 2899 | 2894 | C–H stretching of CH2 and CH3 groups | [67] | ||||
| - | 2926 | 2927 | 2925 | 2926 | 2924 | 2926 | 2924 | 2926 | CH3 and CH2 in aliphatic compounds, CH anti sym and symmetric or CH3 attached to O or N | [69,109,110] |
| 3342 | 3342 | 3307 | 3342 | 3304 | 3342 | 3304 | 3342 | 3307 | O–H stretching vibration or ѵ (OH) hydroxyl groups in (phenols, alcohols, and organic acids) | [107,108] |
| Sample | DIZ S. aureus (mm) | DIZ E coli (mm) | DIZ C. albicans (mm) |
|---|---|---|---|
| O1 | 1.15 ± 0.5 b | R | R |
| R1 | 5.15 ± 0.5 a | R | R |
| L1 | 2.15 ± 0.5 b | R | R |
| P1 | R | R | R |
| BC | R | R | R |
| amoxicillin/ clavulanic acid | 19.5 ± 0.5 | R | NA |
| miconazole | NA | NA | 7.5 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodea, I.M.; Cătunescu, G.M.; Pop, C.R.; Fiț, N.I.; David, A.P.; Dudescu, M.C.; Stănilă, A.; Rotar, A.M.; Beteg, F.I. Antimicrobial Properties of Bacterial Cellulose Films Enriched with Bioactive Herbal Extracts Obtained by Microwave-Assisted Extraction. Polymers 2022, 14, 1435. https://doi.org/10.3390/polym14071435
Bodea IM, Cătunescu GM, Pop CR, Fiț NI, David AP, Dudescu MC, Stănilă A, Rotar AM, Beteg FI. Antimicrobial Properties of Bacterial Cellulose Films Enriched with Bioactive Herbal Extracts Obtained by Microwave-Assisted Extraction. Polymers. 2022; 14(7):1435. https://doi.org/10.3390/polym14071435
Chicago/Turabian StyleBodea, Ioana M., Giorgiana M. Cătunescu, Carmen R. Pop, Nicodim I. Fiț, Adriana P. David, Mircea C. Dudescu, Andreea Stănilă, Ancuța M. Rotar, and Florin I. Beteg. 2022. "Antimicrobial Properties of Bacterial Cellulose Films Enriched with Bioactive Herbal Extracts Obtained by Microwave-Assisted Extraction" Polymers 14, no. 7: 1435. https://doi.org/10.3390/polym14071435
APA StyleBodea, I. M., Cătunescu, G. M., Pop, C. R., Fiț, N. I., David, A. P., Dudescu, M. C., Stănilă, A., Rotar, A. M., & Beteg, F. I. (2022). Antimicrobial Properties of Bacterial Cellulose Films Enriched with Bioactive Herbal Extracts Obtained by Microwave-Assisted Extraction. Polymers, 14(7), 1435. https://doi.org/10.3390/polym14071435