In-Depth Sulfhydryl-Modified Cellulose Fibers for Efficient and Rapid Adsorption of Cr(VI)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
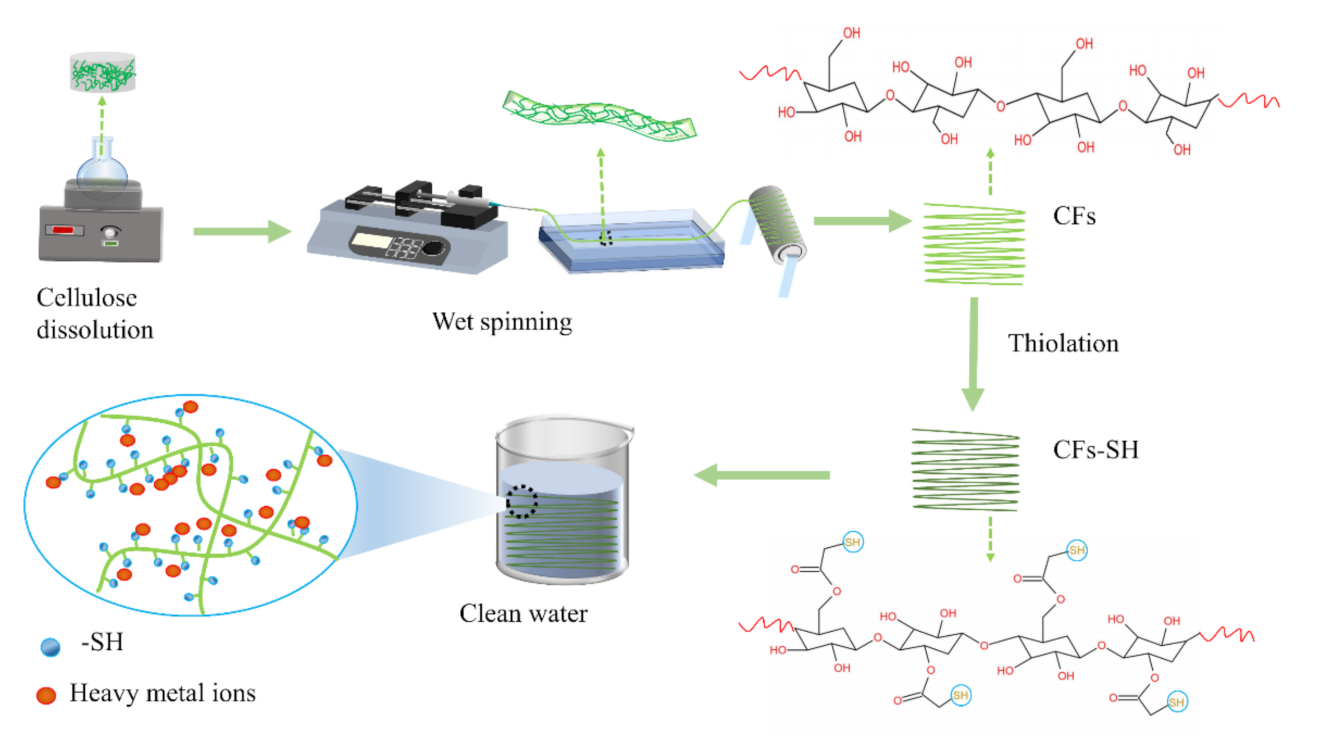
2.3. Preparation of Pure CFs and CFs–SH
2.4. Adsorption of Cr(VI)
3. Test Results and Discussion
3.1. Characterization of Pure CFs and CFs–SH
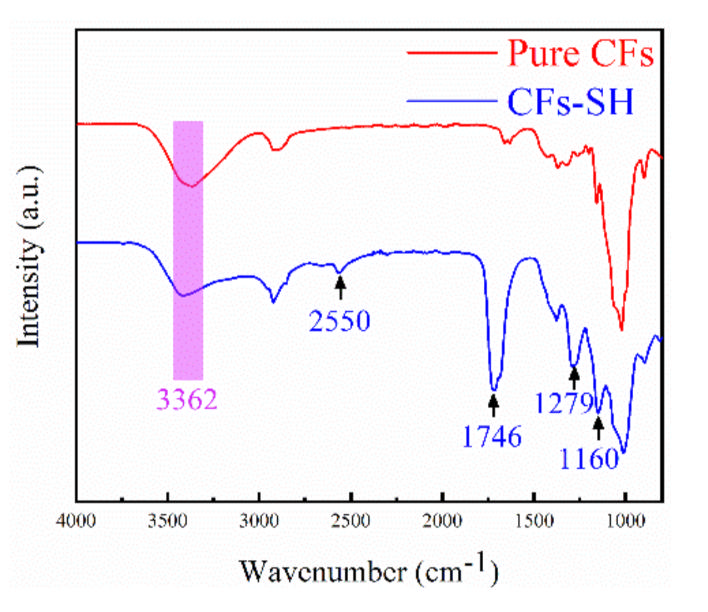
3.1.1. FTIR Analysis
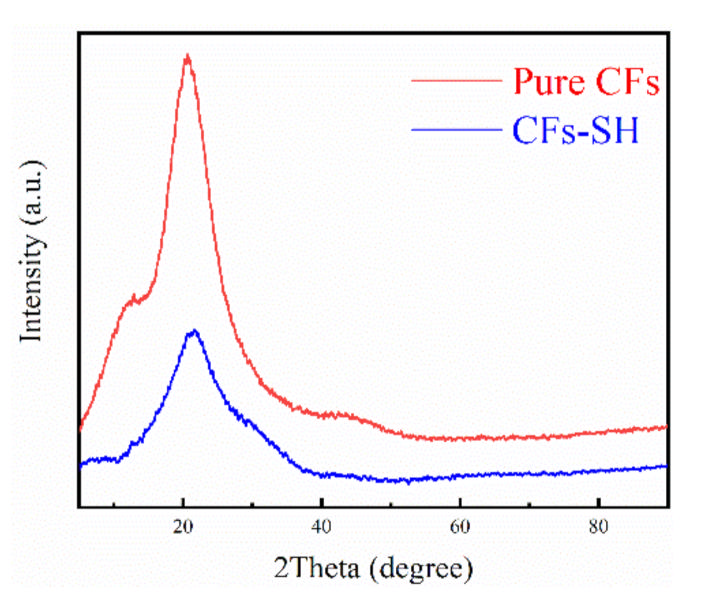
3.1.2. XRD Analysis
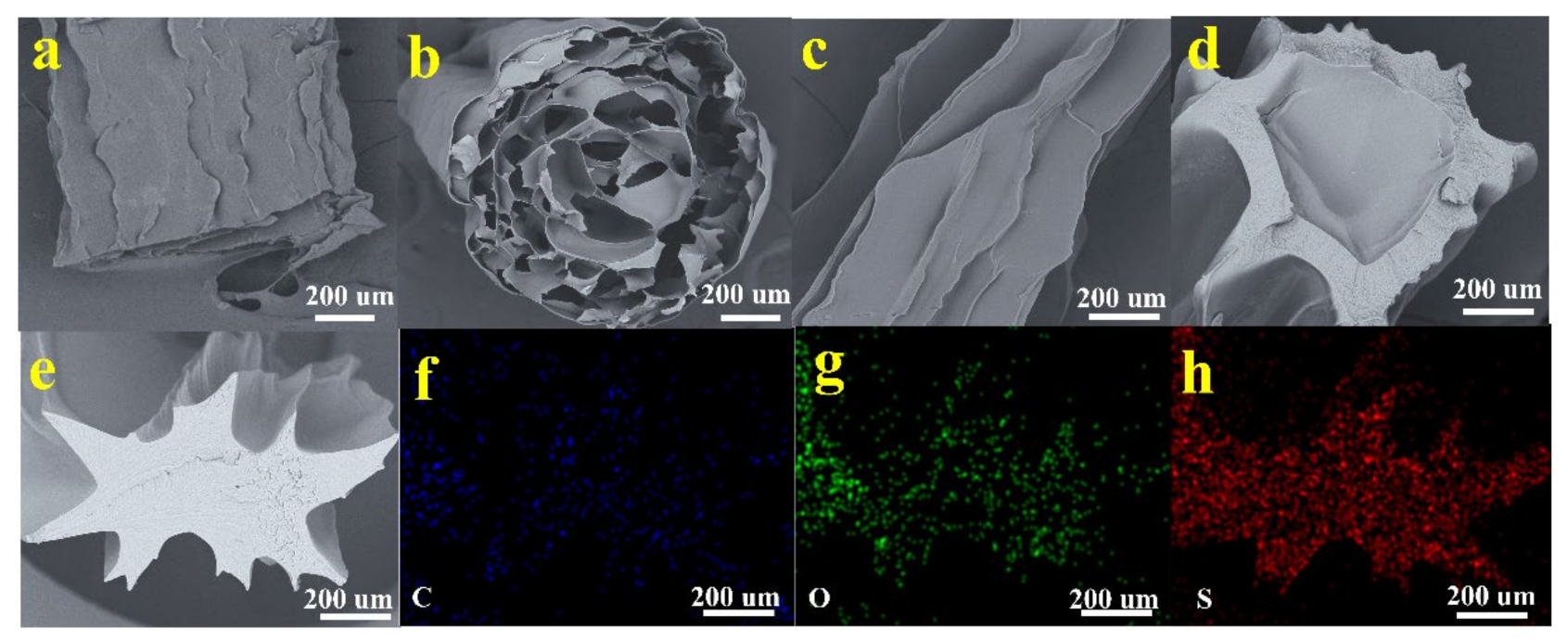
3.1.3. SEM-EDS Analysis
3.1.4. TG/DTG Analysis
3.2. Cr(VI) Adsorption
3.2.1. Effect of Contact Time and Adsorption Kinetics
3.2.2. Cr(VI) Adsorption Isotherm on CFs–SH
3.2.3. Adsorption Ability of CFs–SH
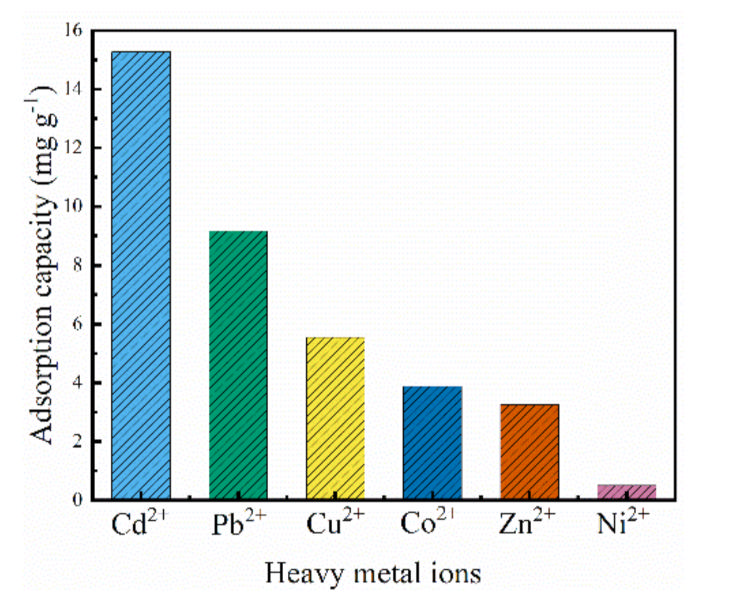
3.3. Other Heavy Metal Ions Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Ukhurebor, K.E.; Aigbe, U.O.; Onyancha, R.B.; Nwankwo, W.; Osibote, O.A.; Paumo, H.K.; Ama, O.M.; Adetunji, C.O.; Siloko, I.U. Effect of hexavalent chromium on the environment and removal techniques: A review. J. Environ. Manag. 2021, 280, 111809. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Gong, Y.; Gao, J.; Sun, T.; Liu, Y.; Oturan, N.; Oturan, M.A. The reduction of Cr(VI) to Cr(III) mediated by environmentally relevant carboxylic acids: State-of-the-art and perspectives. J. Hazard. Mater. 2019, 365, 205–226. [Google Scholar] [CrossRef]
- Murad, H.A.; Ahmad, M.; Bundschuh, J.; Hashimoto, Y.; Zhang, M.; Sarkar, B.; Ok, Y.S. A remediation approach to chromium-contaminated water and soil using engineered biochar derived from peanut shell. Environ. Res. 2021, 204, 112125. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Liu, Y.; Xu, Y.; Li, R.; Hong, H.; Shen, L.; Lin, H.; Liao, B.-Q. Enhanced permeability and antifouling performance of polyether sulfone (PES) membrane via elevating magnetic Ni@MXene nanoparticles to upper layer in phase inversion process. J. Membr. Sci. 2021, 623, 119080. [Google Scholar] [CrossRef]
- Chen, F.; Guo, S.; Wang, Y.; Ma, L.; Li, B.; Song, Z.; Huang, L.; Zhang, W. Concurrent adsorption and reduction of chromium(VI) to chromium(III) using nitrogen-doped porous carbon adsorbent derived from loofah sponge. Front. Environ. Sci. Eng. 2021, 16, 57. [Google Scholar] [CrossRef]
- Li, T.; Shen, J.; Huang, S.; Li, N.; Ye, M. Hydrothermal carbonization synthesis of a novel montmorillonite supported carbon nanosphere adsorbent for removal of Cr (VI) from waste water. Appl. Clay Sci. 2014, 93–94, 48–55. [Google Scholar] [CrossRef]
- Dong, X.; Ma, L.Q.; Li, Y. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 2011, 190, 909–915. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, Z.; Si, M.; Zhu, F.; Yang, W.; Zhao, F.; Shi, Y. Application of biochars in the remediation of chromium contamination: Fabrication, mechanisms, and interfering species. J. Hazard. Mater. 2020, 407, 124376. [Google Scholar] [CrossRef]
- Xia, S.; Song, Z.; Jeyakumar, P.; Shaheen, S.M.; Rinklebe, J.; Ok, Y.S.; Bolan, N.; Wang, H. A critical review on bioremediation technologies for Cr(VI)-contaminated soils and wastewater. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1027–1078. [Google Scholar] [CrossRef]
- Khan, A.R.; Awan, S.K.; Husnain, S.M.; Abbas, N.; Anjum, D.H.; Abbas, N.; Benaissa, M.; Mirza, C.R.; Mujtaba-ul-Hassan, S.; Shahzad, F. 3d flower like 8-Mno2/Mxene nano-hybrids for the removal of hexavalent Cr from wastewater. Ceram. Int. 2021, 47, 25951–25958. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Kousha, M.; Hashtjin, A.M.; Saratale, G.D.; Maiti, A.; Vithanage, M.; Bhatnagar, A. Hexavalent chromium removal from water by microalgal-based materials: Adsorption, desorption and recovery studies. Bioresour. Technol. 2019, 293, 122064. [Google Scholar] [CrossRef] [PubMed]
- Dilamian, M.; Noroozi, B. Rice straw agri-waste for water pollutant adsorption: Relevant mesoporous super hydrophobic cellulose aerogel. Carbohydr. Polym. 2021, 251, 117016. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhao, J.; Zhu, J.; Guo, X.; Chen, P.; Duan, G.; Liu, X.; Li, Y. A mussel-inspired polydopamine-filled cellulose aerogel for solar-enabled water remediation. ACS Appl. Mater. Interfaces 2021, 13, 7617–7624. [Google Scholar] [CrossRef]
- Ali, N.; Awais; Kamal, T.; Ul-Islam, M.; Khan, A.; Shah, S.J.; Zada, A. Chitosan-coated cotton cloth supported copper nanoparticles for toxic dye reduction. Int. J. Biol. Macromol. 2018, 111, 832–838. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, J.; Zhang, Z.; Liu, X.; Zhang, X. Trends and research progresses on the recycling of ionic liquids. Chem. Ind. Eng. Prog. 2019, 38, 100–110. [Google Scholar]
- Choi, H.Y.; Bae, J.H.; Hasegawa, Y.; An, S.; Kim, I.S.; Lee, H.; Kim, M. Thiol-functionalized cellulose nanofiber membranes for the effective adsorption of heavy metal ions in water. Carbohydr. Polym. 2020, 234, 115881. [Google Scholar] [CrossRef]
- Dickinson, E. Biopolymer-based particles as stabilizing agents for emulsions and foams. Food Hydrocoll. 2017, 68, 219–231. [Google Scholar] [CrossRef]
- Lin, X.; Tran, D.T.; Song, M.H.; Yun, Y.S. Development of quaternized polyethylenimine-cellulose fibers for fast recovery of Au(Cn)2(-) in alkaline wastewater: Kinetics, isotherm, and thermodynamic study. J. Hazard. Mater. 2022, 422, 126940. [Google Scholar]
- Orelma, H.; Virtanen, T.; Spoljaric, S.; Lehmonen, J.; Seppala, J.; Rojas, O.J.; Harlin, A. Cyclodextrin-functionalized fiber yarns spun from deep eutectic cellulose solutions for nonspecific hormone capture in aqueous matrices. Biomacromolecules 2018, 19, 652–661. [Google Scholar]
- Liu, F.; Wang, A.; Xiang, M.; Hu, Q.; Hu, B. Effective adsorption and immobilization of Cr(VI) and U(VI) from aqueous solution by magnetic amine-functionalized SBA-15. Sep. Purif. Technol. 2021, 282, 120042. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, X.; Wang, H.; Liang, D.; Zhang, J.; Li, J. Sulfhydryl-modified sodium alginate film for lead-ion adsorption. Mater. Lett. 2019, 254, 149–153. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Zhang, A.; Liu, C.; Sun, R. Direct preparation of green and renewable aerogel materials from crude bagasse. Cellulose 2016, 23, 1325–1334. [Google Scholar] [CrossRef] [Green Version]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Derradji, M.; Bessa, W. Ecofriendly isolation and characterization of microcrystalline cellulose from giant reed using various acidic media. Cellulose 2019, 26, 7635–7651. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, S.; Ma, L.; Yu, Y.; Dai, H.; Zhang, Y. Extraction and comparison of cellulose nanocrystals from lemon (Citrus limon) seeds using sulfuric acid hydrolysis and oxidation methods. Carbohydr. Polym. 2020, 238, 116180. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Zhang, Y.; Zhang, H.; Huang, H. Green and facile fabrication of pineapple peel cellulose/magnetic diatomite hydrogels in ionic liquid for methylene blue adsorption. Cellulose 2019, 26, 3825–3844. [Google Scholar] [CrossRef]
- Park, M.J.; Nisola, G.M.; Vivas, E.L.; Limjuco, L.; Lawagon, C.P.; Gil Seo, J.; Kim, H.; Shon, H.K.; Chung, W.-J. Mixed matrix nanofiber as a flow-through membrane adsorber for continuous Li+ recovery from seawater. J. Membr. Sci. 2016, 510, 141–154. [Google Scholar] [CrossRef]
- Shi, X.-C.; Zhang, Z.-B.; Zhou, D.-F.; Zhang, L.-F.; Chen, B.-Z.; Yu, L.-L. Synthesis of Li+ adsorbent (H2TiO3) and its adsorption properties. Trans. Nonferrous Met. Soc. China 2013, 23, 253–259. [Google Scholar] [CrossRef]
- Limjuco, L.; Nisola, G.M.; Lawagon, C.P.; Lee, S.-P.; Gil Seo, J.; Kim, H.; Chung, W.-J. H2TiO3 composite adsorbent foam for efficient and continuous recovery of Li+ from liquid resources. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 504, 267–279. [Google Scholar] [CrossRef]
- Ju, P.; Liu, Q.; Zhang, H.; Chen, R.; Liu, J.; Yu, J.; Liu, P.; Zhang, M.; Wang, J. Hyperbranched topological swollen-layer constructs of multi-active sites polyacrylonitrile (PAN) adsorbent for uranium(VI) extraction from seawater. Chem. Eng. J. 2019, 374, 1204–1213. [Google Scholar] [CrossRef]
- Xiao, G.; Tong, K.; Zhou, L.; Xiao, J.; Sun, S.; Li, P.; Yu, J. Adsorption and desorption behavior of lithium ion in spherical PVC-MnO2 ion sieve. Ind. Eng. Chem. Res. 2012, 51, 10921–10929. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, K.; Luo, J.; Luo, S.; Crittenden, J. Capturing lithium from wastewater using a fixed bed packed with 3-D MnO2 ion cages. Environ. Sci. Technol. 2016, 50, 13002–13012. [Google Scholar] [CrossRef] [PubMed]
- Santoso, S.P.; Kurniawan, A.; Soetaredjo, F.E.; Cheng, K.-C.; Putro, J.; Ismadji, S.; Ju, Y.-H. Eco-friendly cellulose–bentonite porous composite hydrogels for adsorptive removal of azo dye and soilless culture. Cellulose 2019, 26, 3339–3358. [Google Scholar] [CrossRef]
- Wang, N.; Feng, J.; Yan, W.; Zhang, L.; Liu, Y.; Mu, R. Dual-functional sites for synergistic adsorption of Cr(Vi) and Sb(V) by polyaniline-Tio2 hydrate: Adsorption behaviors, sites and mechanisms. Front. Environ. Sci. Eng. 2022, 16, 105. [Google Scholar] [CrossRef]
- Vaghetti, J.C.; Lima, E.C.; Royer, B.; Brasil, J.L.; da Cunha, B.M.; Simon, N.M.; Cardoso, N.F.; Noreña, C.P.Z. Application of Brazilian-pine fruit coat as a biosorbent to removal of Cr(VI) from aqueous solution—Kinetics and equilibrium study. Biochem. Eng. J. 2008, 42, 67–76. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Liang, D.; Xiao, Z.; Xie, Y.; Li, J. Sulfhydryl-modified chitosan aerogel for the adsorption of heavy metal ions and organic dyes. Ind. Eng. Chem. Res. 2020, 59, 14531–14536. [Google Scholar] [CrossRef]



| Material | Pseudo First Order | Pseudo Second Order | ||||
|---|---|---|---|---|---|---|
| qe (mg g−1) | k1 × 10−3 (min−1) | r2 | qe (mg g−1) | k1 × 10−3 (min−1) | r2 | |
| Pure CFs | 12.34 | 58.52 | 0.672 | 11.49 | 77.20 | 0.992 |
| CFs–SH | 79.73 | 11.55 | 0.906 | 78.32 | 5.86 | 0.954 |
| Temperature | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | k1 × 10−3 | r2 | k2 | n | r2 | |
| 20 °C | 105.08 | 9.42 | 0.999 | 0.185 | 3.77 | 0.471 |
| 30 °C | 113.57 | 8.80 | 0.999 | 0.198 | 3.78 | 0.491 |
| 40 °C | 115.58 | 8.61 | 0.999 | 0.205 | 3.81 | 0.470 |
| 50 °C | 122.51 | 8.15 | 0.999 | 0.203 | 3.88 | 0.393 |
| 60 °C | 120.60 | 8.24 | 0.999 | 0.200 | 3.89 | 0.376 |
| Composites | Adsorption Capacity (mg g−1) | Adsorption Rate (mg g−1 s−1) | Ref. |
|---|---|---|---|
| Peanut shell biochar | 22.93 | 0.013 | [4] |
| HI | 353.87 | 0.098 | [11] |
| SBA-15 | 66.50 | 0.004 | [21] |
| PAIN/TiO2 | 394.43 | 0.219 | [35] |
| Brazilian-pine fruit coat | 240.00 | 0.008 | [36] |
| CFs–SH | 120.60 | 0.319 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yu, F.; Ba, Z.; Qian, H.; Zhao, S.; Liu, J.; Jiang, W.; Li, J.; Liang, D. In-Depth Sulfhydryl-Modified Cellulose Fibers for Efficient and Rapid Adsorption of Cr(VI). Polymers 2022, 14, 1482. https://doi.org/10.3390/polym14071482
Wang W, Yu F, Ba Z, Qian H, Zhao S, Liu J, Jiang W, Li J, Liang D. In-Depth Sulfhydryl-Modified Cellulose Fibers for Efficient and Rapid Adsorption of Cr(VI). Polymers. 2022; 14(7):1482. https://doi.org/10.3390/polym14071482
Chicago/Turabian StyleWang, Wenxuan, Feihan Yu, Zhichen Ba, Hongbo Qian, Shuai Zhao, Jie Liu, Wei Jiang, Jian Li, and Daxin Liang. 2022. "In-Depth Sulfhydryl-Modified Cellulose Fibers for Efficient and Rapid Adsorption of Cr(VI)" Polymers 14, no. 7: 1482. https://doi.org/10.3390/polym14071482
APA StyleWang, W., Yu, F., Ba, Z., Qian, H., Zhao, S., Liu, J., Jiang, W., Li, J., & Liang, D. (2022). In-Depth Sulfhydryl-Modified Cellulose Fibers for Efficient and Rapid Adsorption of Cr(VI). Polymers, 14(7), 1482. https://doi.org/10.3390/polym14071482







