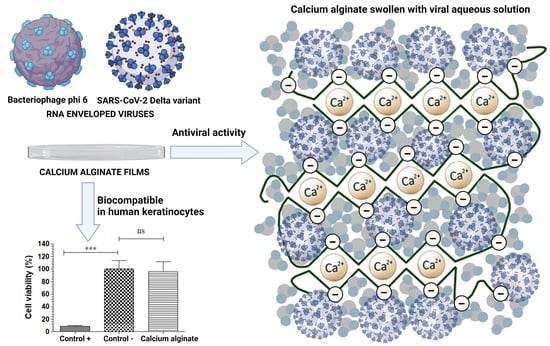
Biocompatible Films of Calcium Alginate Inactivate Enveloped Viruses Such as SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Toxicological Study
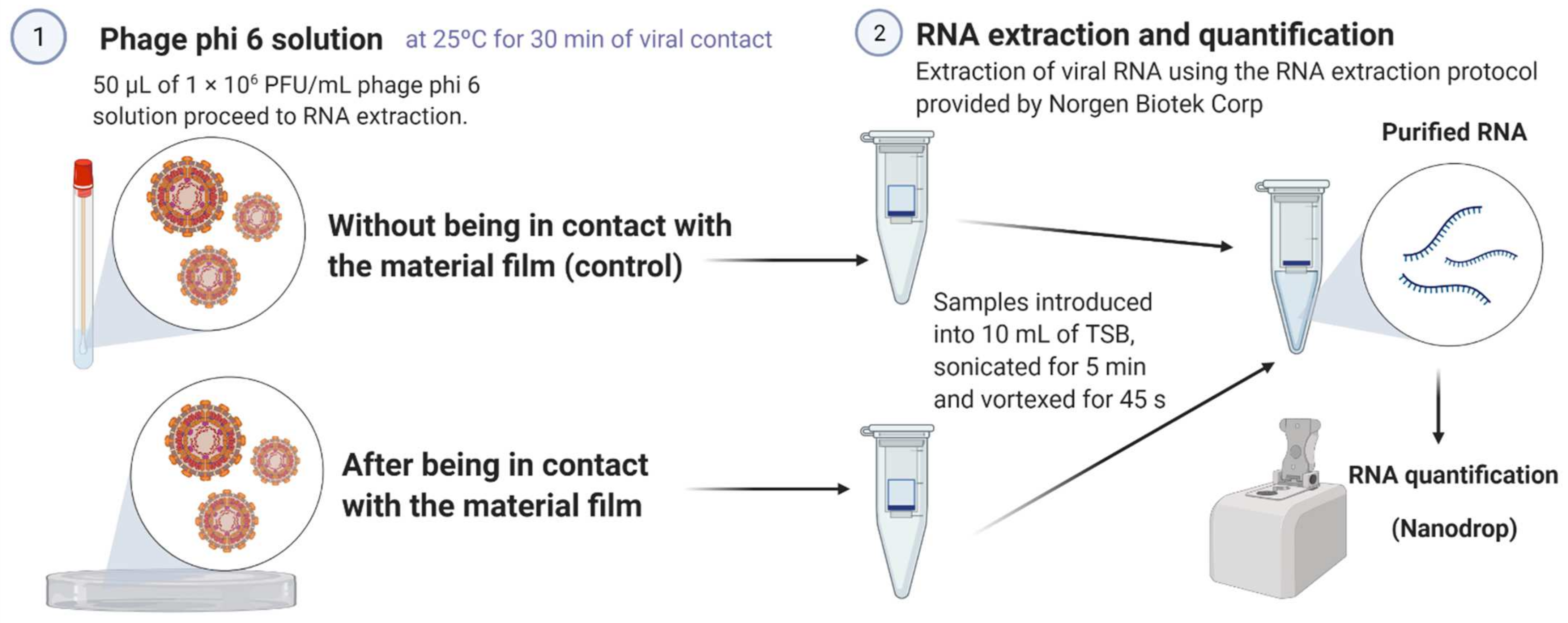
2.4. Antiviral Test with the Bacteriophage Phi 6
2.5. Bacteriophage RNA Extraction and Quantification
2.6. Antiviral Test with SARS-CoV-2 Delta Variant
2.7. Statistical Analysis
3. Results
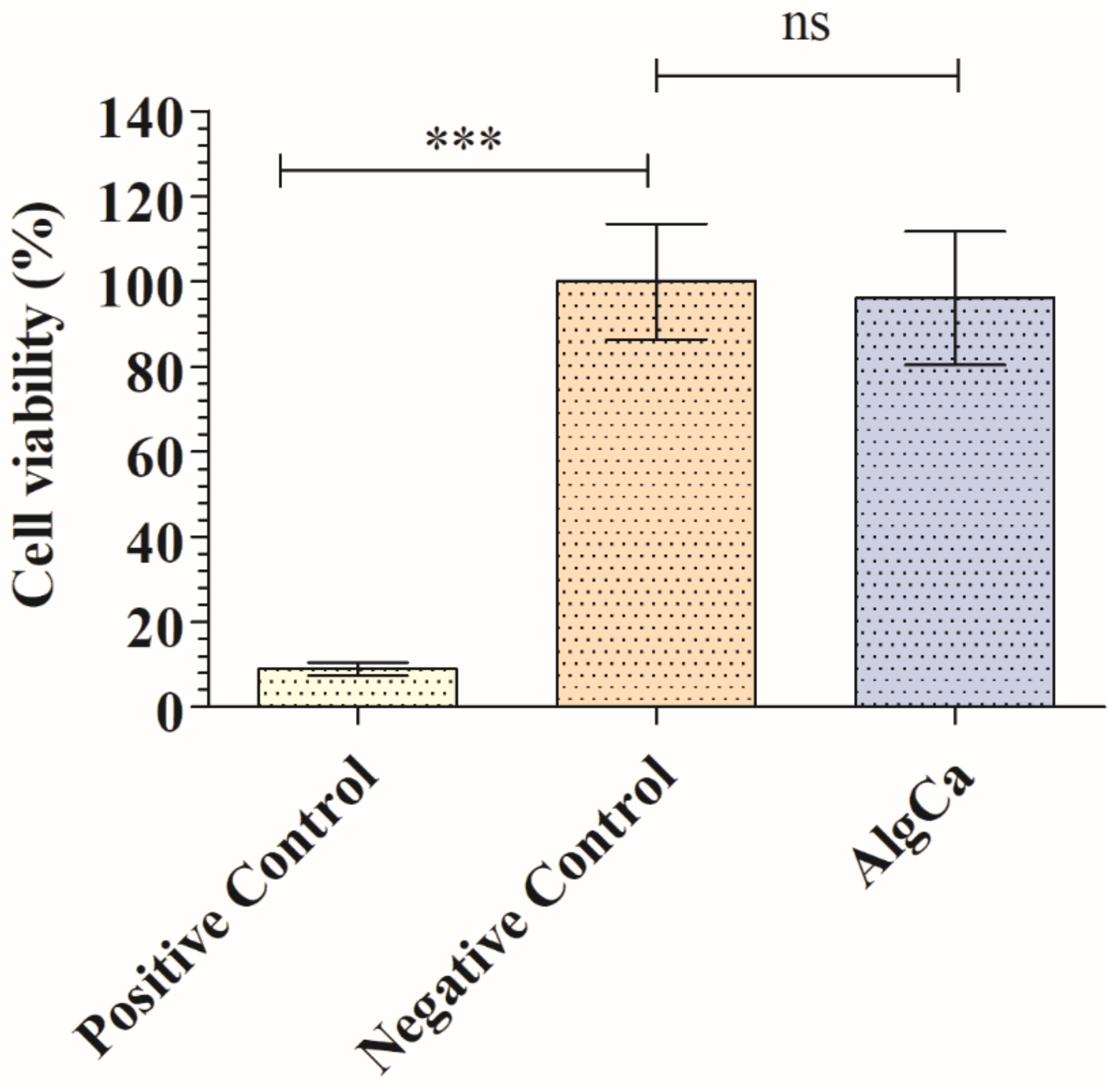
3.1. Toxicological Study
3.2. Antiviral Assays with the Enveloped Bacteriophage Phi 6
3.3. Bacteriophage RNA Extraction and Quantification
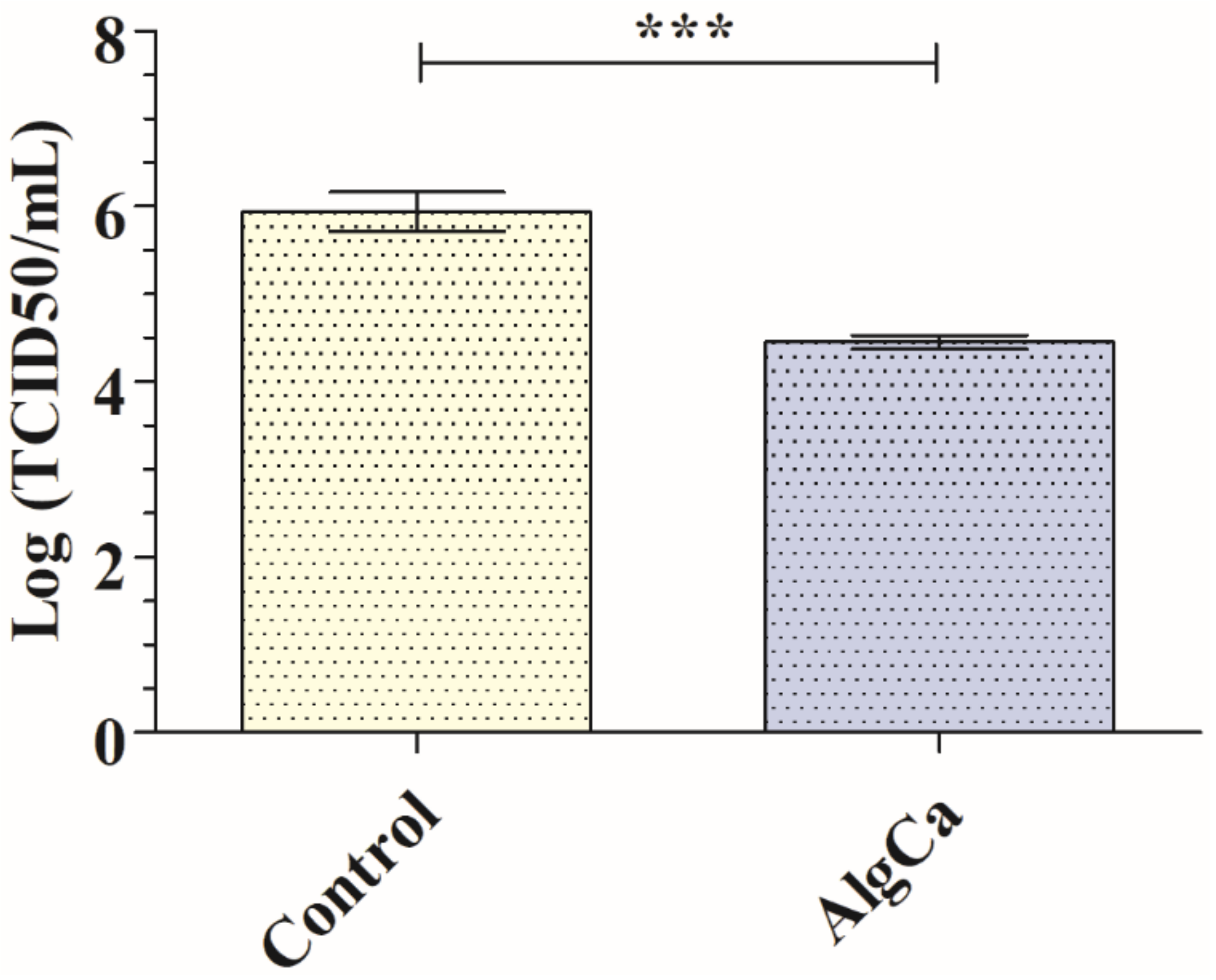
3.4. Antiviral Assays with the SARS-CoV-2 Delta Variant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, H.H.; Kim, H.; Lee, H.S.; Seo, E.U.; Kim, J.E.; Lee, J.H.; Mun, Y.H.; Yoo, S.Y.; An, J.; Yun, M.Y.; et al. PEGylated nanoparticle albumin-bound steroidal ginsenoside derivatives ameliorate SARS-CoV-2-mediated hyper-inflammatory responses. Biomaterials 2021, 273, 120827. [Google Scholar] [CrossRef]
- Gray, L.T.; Raczy, M.M.; Briquez, P.S.; Marchell, T.M.; Alpar, A.T.; Wallace, R.P.; Volpatti, L.R.; Sasso, M.S.; Cao, S.; Nguyen, M.; et al. Generation of potent cellular and humoral immunity against SARS-CoV-2 antigens via conjugation to a polymeric glyco-adjuvant. Biomaterials 2021, 278, 121159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jin, Y.; Chi, C.; Han, G.; Jiang, W.; Wang, Z.; Cheng, H.; Zhang, C.; Wang, G.; Sun, C.; et al. Sponge particulates for biomedical applications: Biofunctionalization, multi-drug shielding, and theranostic applications. Biomaterials 2021, 273, 120824. [Google Scholar] [CrossRef]
- Wibowo, D.; Jorritsma, S.H.T.; Gonzaga, Z.J.; Evert, B.; Chen, S.; Rehm, B.H.A. Polymeric nanoparticle vaccines to combat emerging and pandemic threats. Biomaterials 2021, 268, 120597. [Google Scholar] [CrossRef] [PubMed]
- Oladapo, B.I.; Ismail, S.O.; Afolalu, T.D.; Olawade, D.B.; Zahedi, M. Review on 3D printing: Fight against COVID-19. Mater. Chem. Phys. 2021, 258, 123943. [Google Scholar] [CrossRef] [PubMed]
- Reina, G.; Peng, S.; Jacquemin, L.; Andrade, A.F.; Bianco, A. Hard Nanomaterials in Time of Viral Pandemics. ACS Nano 2020, 14, 9364–9388. [Google Scholar] [CrossRef] [PubMed]
- Donskyi, I.S.; Nie, C.; Ludwig, K.; Trimpert, J.; Ahmed, R.; Quaas, E.; Achazi, K.; Radnik, J.; Adeli, M.; Haag, R.; et al. Graphene Sheets with Defined Dual Functionalities for the Strong SARS-CoV-2 Interactions. Small 2021, 17, 2007091. [Google Scholar] [CrossRef] [PubMed]
- Hutasoit, N.; Kennedy, B.; Hamilton, S.; Luttick, A.; Rahman Rashid, R.A.; Palanisamy, S. Sars-CoV-2 (COVID-19) inactivation capability of copper-coated touch surface fabricated by cold-spray technology. Manuf. Lett. 2020, 25, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, Á.; Ferrandis-Montesinos, M.; Wang, R. Antiviral Properties of Alginate-Based Biomaterials: Promising Antiviral Agents against SARS-CoV-2. ACS Appl. Bio Mater. 2021, 4, 5897–5907. [Google Scholar] [CrossRef] [PubMed]
- Salesa, B.; Llorens-Gámez, M.; Serrano-Aroca, Á. Study of 1D and 2D carbon nanomaterial in alginate films. Nanomaterials 2020, 10, 206. [Google Scholar] [CrossRef] [Green Version]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.A.; Kipke, D.R.; Brandon, T. Calcium alginate gel: A biocompatible and mechanically stable polymer for endovascular embolization. J. Biomed. Mater. Res. 2001, 54, 76–86. [Google Scholar] [CrossRef]
- Tai, C.; Bouissil, S.; Gantumur, E.; Carranza, M.S.; Yoshii, A.; Sakai, S.; Pierre, G.; Michaud, P.; Delattre, C. Use of anionic polysaccharides in the development of 3D bioprinting technology. Appl. Sci. 2019, 9, 2596. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Pujana, A.; Orive, G.; Pedraz, J.L.; Santos-Vizcaino, E.; Hernandez, R.M. Alginate Microcapsules for Drug Delivery. In Alginates and Their Biomedical Applications; Springer: Singapore, 2018; pp. 67–100. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, Y.; Turgeman, G.; Pelled, G.; Xu, N.; Moutsatsos, I.K.; Hortelano, G.; Gazit, D. Polymer-encapsulated engineered adult mesenchymal stem cells secrete exogenously regulated rhBMP-2, and induce osteogenic and angiogenic tissue formation. Polym. Adv. Technol. 2002, 13, 863–870. [Google Scholar] [CrossRef]
- Kaplan, D.L. (Ed.) Biopolymers from Renewable Resources; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Yugay, Y.A.; Usoltseva, R.V.; Silant’ev, V.E.; Egorova, A.E.; Karabtsov, A.A.; Kumeiko, V.V.; Ermakova, S.P.; Bulgakov, V.P.; Shkryl, Y.N. Synthesis of bioactive silver nanoparticles using alginate, fucoidan and laminaran from brown algae as a reducing and stabilizing agent. Carbohydr. Polym. 2020, 245, 116547. [Google Scholar] [CrossRef]
- Akoulina, E.; Dudun, A.; Bonartsev, A.; Bonartseva, G.; Voinova, V. Effect of bacterial alginate on growth of mesenchymal stem cells. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 115–118. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Ruiz-Pividal, J.F.; Llorens-Gámez, M. Enhancement of water diffusion and compression performance of crosslinked alginate with a minuscule amount of graphene oxide. Sci. Rep. 2017, 7, 11684. [Google Scholar] [CrossRef]
- Llorens-Gámez, M.; Salesa, B.; Serrano-Aroca, Á. Physical and biological properties of alginate/carbon nanofibers hydrogel films. Int. J. Biol. Macromol. 2020, 151, 499–507. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Sano, Y. Antiviral activity of alginate against infection by tobacco mosaic virus. Carbohydr. Polym. 1999, 38, 183–186. [Google Scholar] [CrossRef]
- Gong, Y.; Han, G.T.; Li, X.L.; Wu, Y.; Zhang, Y.M.; Xia, Y.Z.; Yue, C.Q.; Wu, D.W. Cytotoxicity and Antiviral Activity of Calcium Alginate Fibers and Zinc Alginate Fibers. Adv. Mater. Res. 2010, 152–153, 1475–1478. [Google Scholar] [CrossRef]
- Tran, N.M.; Dufresne, M.; Helle, F.; Hoffmann, T.W.; Francois, C.; Brochot, E.; Paullier, P.; Legallais, C.; Duverlie, G.; Castelain, S. Alginate hydrogel protects encapsulated hepatic HuH-7 cells against hepatitis C virus and other viral infections. PLoS ONE 2014, 9, e109969. [Google Scholar]
- Martí, M.; Tuñón-Molina, A.; Aachmann, F.L.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, Á. Protective Face Mask Filter Capable of Inactivating SARS-CoV-2, and Methicillin-Resistant Staphylococcus aureus and Staphylococcus epidermidis. Polymers 2021, 13, 207. [Google Scholar] [CrossRef]
- Prussin, A.J.; Schwake, D.O.; Lin, K.; Gallagher, D.L.; Buttling, L.; Marr, L.C. Survival of the enveloped virus Phi6 in droplets as a function of relative humidity, absolute humidity, and temperature. Appl. Environ. Microbiol. 2018, 84, e00551-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farinholt, T.; Doddapaneni, H.; Qin, X.; Menon, V.; Meng, Q.; Metcalf, G.; Chao, H.; Gingras, M.-C.; Farinholt, P.; Agrawal, C.; et al. Transmission event of SARS-CoV-2 Delta variant reveals multiple vaccine breakthrough infections. BMC Med. 2021, 19, 255. [Google Scholar] [CrossRef]
- Riemersma, K.K.; Grogan, B.E.; Kita-Yarbro, A.; Halfmann, P.; Kocharian, A.; Florek, K.R.; Westergaard, R.; Bateman, A.; Jeppson, G.E.; Kawaoka, Y.; et al. Shedding of Infectious SARS-CoV-2 Despite Vaccination when the Delta Variant is Prevalent—Wisconsin, July 2021. medRxiv 2021. [Google Scholar] [CrossRef]
- Shitrit, P.; Zuckerman, N.S.; Mor, O.; Gottesman, B.-S.; Chowers, M. Nosocomial outbreak caused by the SARS-CoV-2 Delta variant in a highly vaccinated population, Israel, July 2021. Eurosurveillance 2021, 26, 2100822. [Google Scholar] [CrossRef]
- Eyre, D.W.; Taylor, D.; Purver, M.; Chapman, D.; Fowler, T.; Pouwels, K.; Walker, A.S.; Peto, T.E.A. The impact of SARS-CoV-2 vaccination on Alpha and Delta variant transmission. medRxiv 2021. [Google Scholar] [CrossRef]
- ASTM F2259-10(2012)e1 Standard Test Method for Determining the Chemical Composition and Sequence in Alginate by Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy. Available online: https://www.astm.org/f2259-10r12e01.html (accessed on 26 March 2022).
- Liling, G.; Di, Z.; Jiachao, X.; Xin, G.; Xiaoting, F.; Qing, Z. Effects of ionic crosslinking on physical and mechanical properties of alginate mulching films. Carbohydr. Polym. 2016, 136, 259–265. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yetter, A.B. Correlation of visual in vitro cytotoxicity ratings of biomaterials with quantitative in vitro cell viability measurements. Cell Biol. Toxicol. 2008, 24, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Komeri, R.; Kasoju, N.; Anil Kumar, P.R. In vitro cytotoxicity and cytocompatibility assays for biomaterial testing under regulatory platform. In Biomedical Product and Materials Evaluation; Woodhead Publishing: Cambridge, UK, 2022; pp. 329–353. [Google Scholar]
- Martí, M.; Frígols, B.; Salesa, B.; Serrano-Aroca, Á. Calcium alginate/graphene oxide films: Reinforced composites able to prevent Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis infections with no cytotoxicity for human keratinocyte HaCaT cells. Eur. Polym. J. 2019, 110, 14–21. [Google Scholar] [CrossRef]
- Frígols, B.; Martí, M.; Salesa, B.; Hernández-Oliver, C.; Aarstad, O.; Ulset, A.-S.T.; Sætrom, G.I.; Aachmann, F.L.; Serrano-Aroca, Á. Graphene oxide in zinc alginate films: Antibacterial activity, cytotoxicity, zinc release, water sorption/diffusion, wettability and opacity. PLoS ONE 2019, 14, e0212819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 2009, 501, 69–76. [Google Scholar]
- Norgen Biotek Corp. Total RNA Purification Kit Product Insert. Available online: https://norgenbiotek.com/sites/default/files/resources/Total-RNA-Purification-Kit-Insert-PI17200-32-M14.pdf (accessed on 25 February 2022).
- Pietropaolo, V.; Seganti, L.; Marchetti, M.; Sinibaldi, L.; Orsi, N.; Nicoletti, R. Effect of natural and semisynthetic polymers on rabies virus infection in CER cells. Res. Virol. 1993, 144, 151–158. [Google Scholar] [CrossRef]
- Pardee, K.I.; Ellis, P.; Bouthillier, M.; Towers, G.H.N.; French, C.J. Plant virus inhibitors from marine algae. Can. J. Bot. 2004, 82, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Astani, A.; Ghosh, T.; Schnitzler, P.; Ray, B. Polysaccharides from Sargassum tenerrimum: Structural features, chemical modification and anti-viral activity. Phytochemistry 2010, 71, 235–242. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative Study on the Antiviral Activity of Selected Monoterpenes Derived from Essential Oils. Phyther. Res. 2008, 22, 544–549. [Google Scholar] [CrossRef]
- Schnitzler, P.; Schneider, S.; Stintzing, F.C.; Carle, R.; Reichling, J. Efficacy of an aqueous Pelargonium sidoides extract against herpesvirus. Phytomedicine 2008, 15, 1108–1116. [Google Scholar] [CrossRef]
- Mastromarino, P.; Petruzziello, R.; Macchia, S.; Rieti, S.; Nicoletti, R.; Orsi, N. Antiviral activity of natural and semisynthetic polysaccharides on the early steps of rubella virus infection. J. Antimicrob. Chemother. 1997, 39, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanmartín-Santos, I.; Gandía-Llop, S.; Salesa, B.; Martí, M.; Lillelund Aachmann, F.; Serrano-Aroca, Á. Enhancement of Antimicrobial Activity of Alginate Films with a Low Amount of Carbon Nanofibers (0.1% w/w). Appl. Sci. 2021, 11, 2311. [Google Scholar] [CrossRef]
- Blázquez, E.; Rodríguez, C.; Ródenas, J.; Navarro, N.; Riquelme, C.; Rosell, R.; Campbell, J.; Crenshaw, J.; Segalés, J.; Joan, P.; et al. Evaluation of the effectiveness of the surepure turbulator ultraviolet-C irradiation equipment on inactivation of different enveloped and non-enveloped viruses inoculated in commercially collected liquid animal plasma. PLoS ONE 2019, 14, e0212332. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Vicent, A.; Hashimoto, R.; Takayama, K.; Serrano-Aroca, Á. Biocompatible Films of Calcium Alginate Inactivate Enveloped Viruses Such as SARS-CoV-2. Polymers 2022, 14, 1483. https://doi.org/10.3390/polym14071483
Cano-Vicent A, Hashimoto R, Takayama K, Serrano-Aroca Á. Biocompatible Films of Calcium Alginate Inactivate Enveloped Viruses Such as SARS-CoV-2. Polymers. 2022; 14(7):1483. https://doi.org/10.3390/polym14071483
Chicago/Turabian StyleCano-Vicent, Alba, Rina Hashimoto, Kazuo Takayama, and Ángel Serrano-Aroca. 2022. "Biocompatible Films of Calcium Alginate Inactivate Enveloped Viruses Such as SARS-CoV-2" Polymers 14, no. 7: 1483. https://doi.org/10.3390/polym14071483
APA StyleCano-Vicent, A., Hashimoto, R., Takayama, K., & Serrano-Aroca, Á. (2022). Biocompatible Films of Calcium Alginate Inactivate Enveloped Viruses Such as SARS-CoV-2. Polymers, 14(7), 1483. https://doi.org/10.3390/polym14071483