Polydopamine-Coated Natural Rubber Sponge for Highly Efficient Vapor Generation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
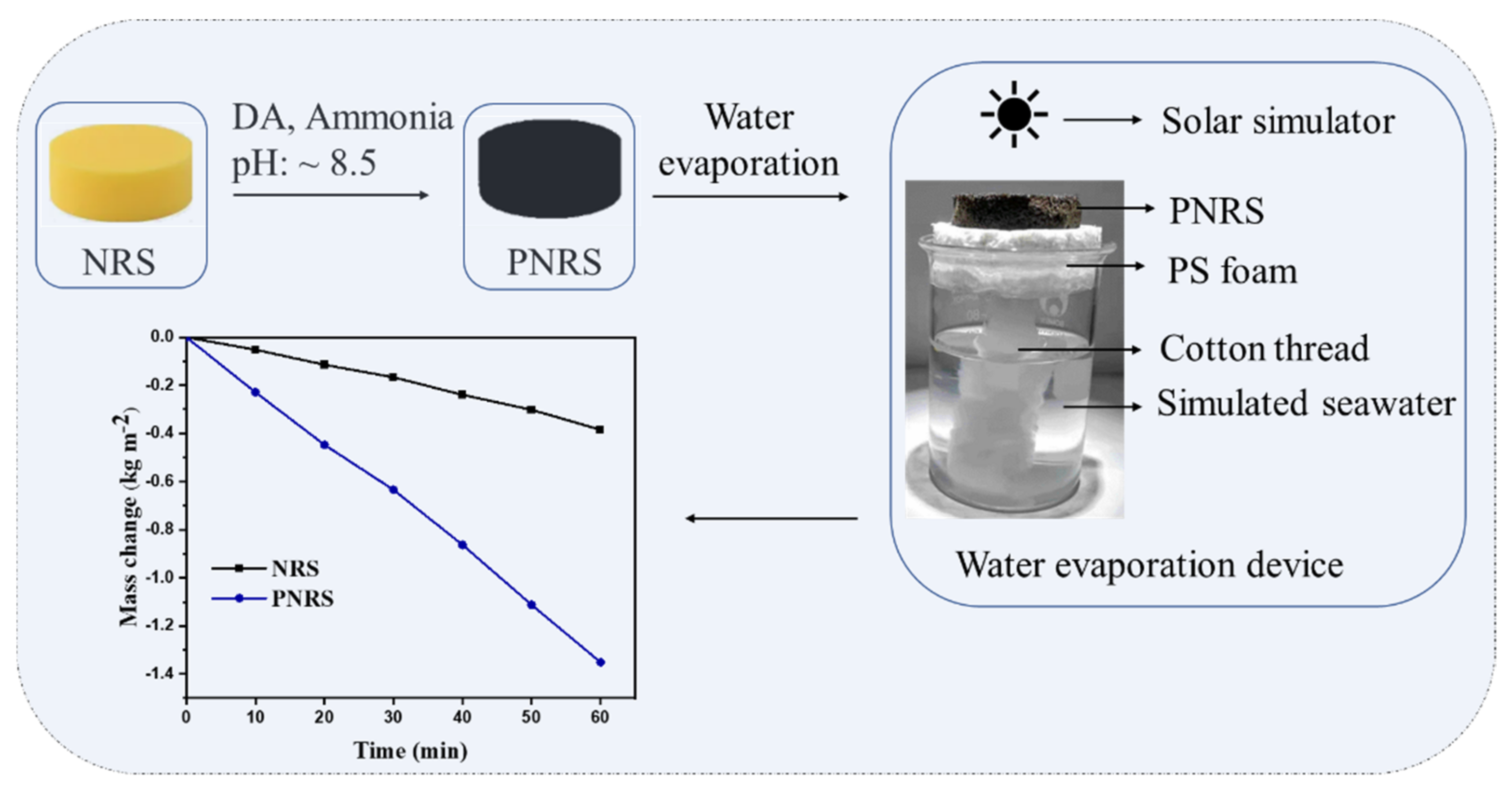
2.2. Preparation Process of PNRS
2.3. Solar Evaporation Tests
2.4. Characterization
3. Results and Discussion
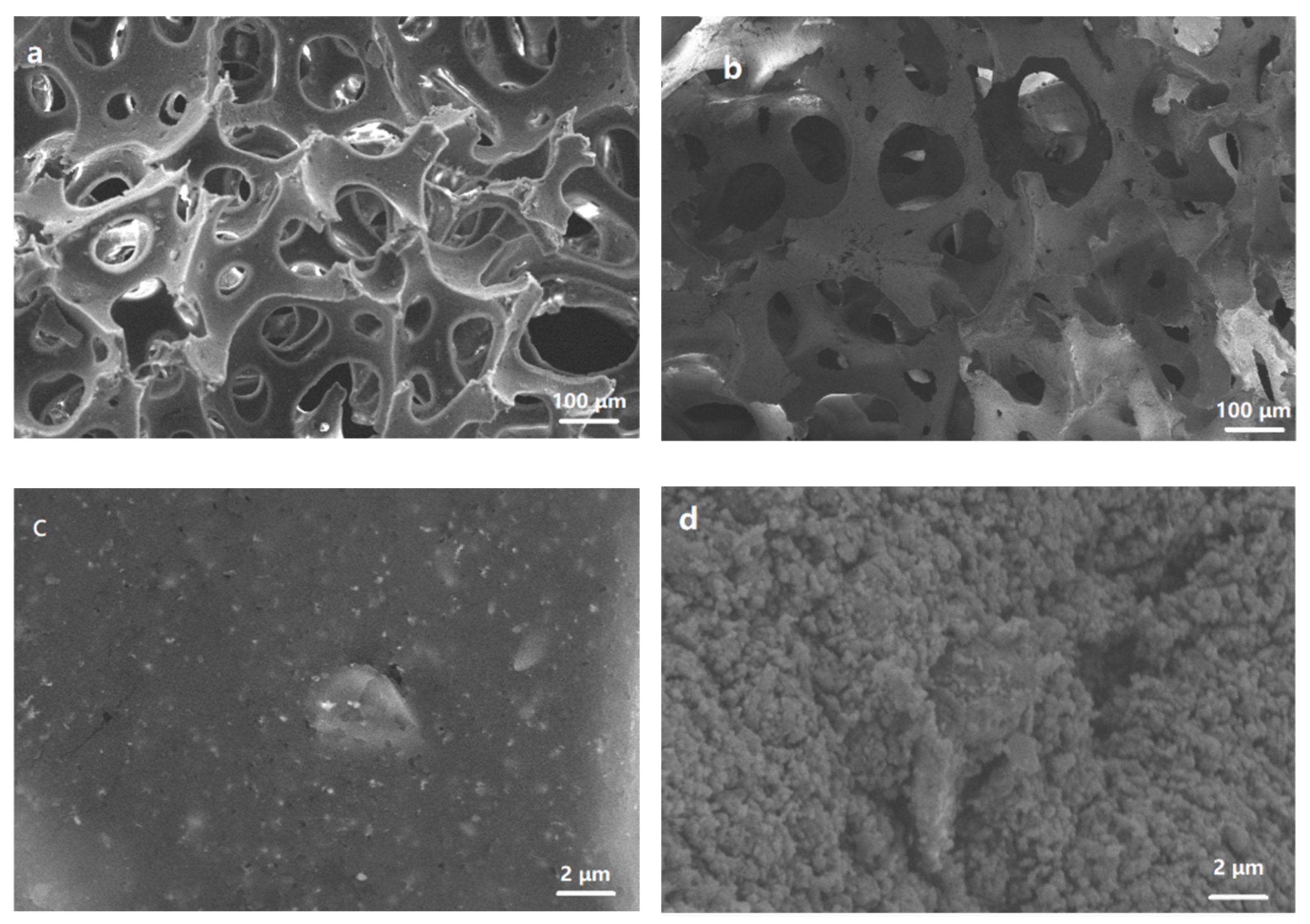
3.1. SEM Analysis
3.2. XPS Analysis
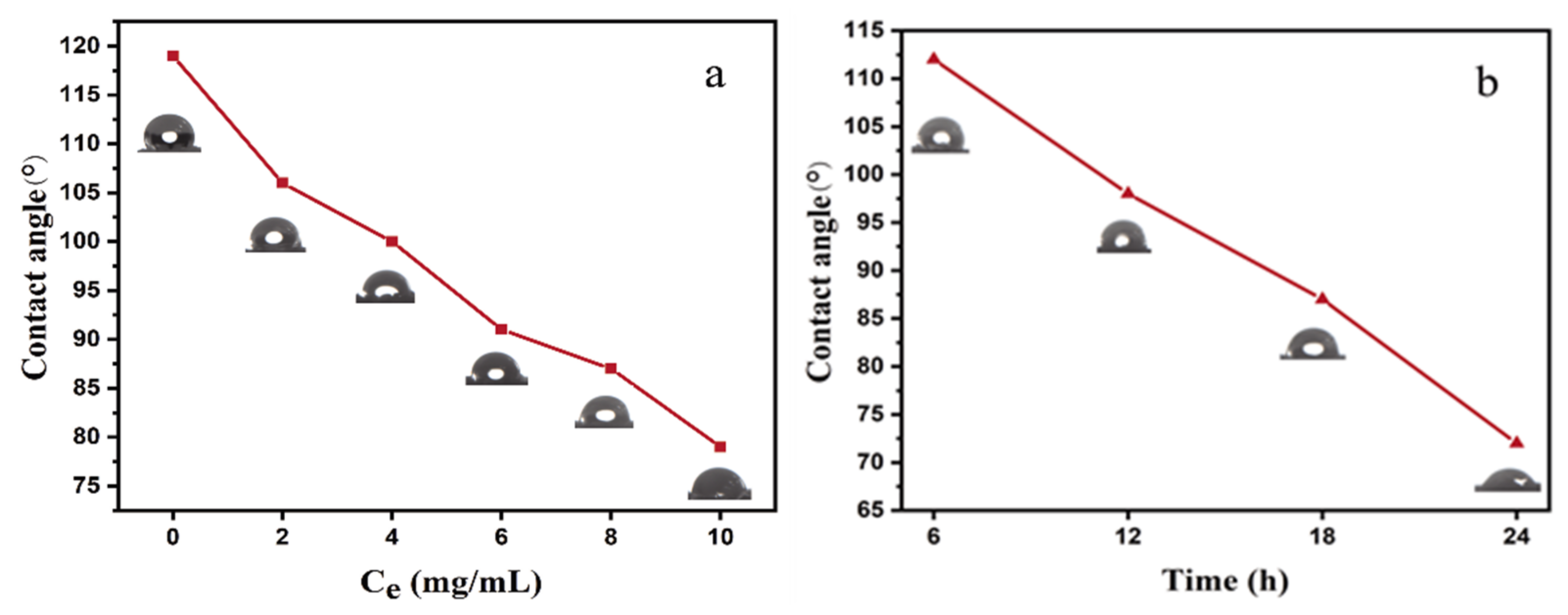
3.3. Water Contact Angle (CA) of NRS and PNRS
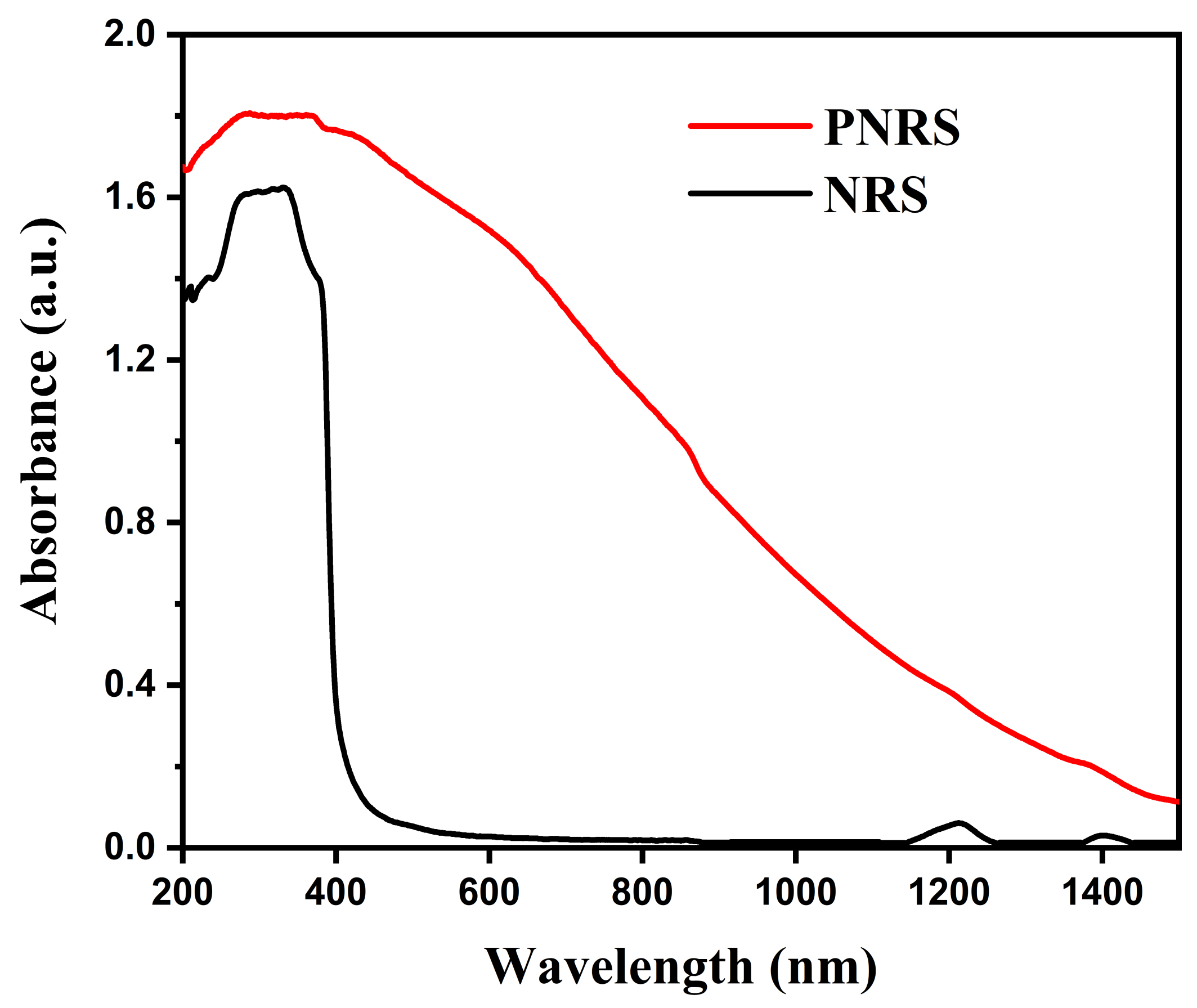
3.4. Optical Absorption Performance of NRS and PNRS
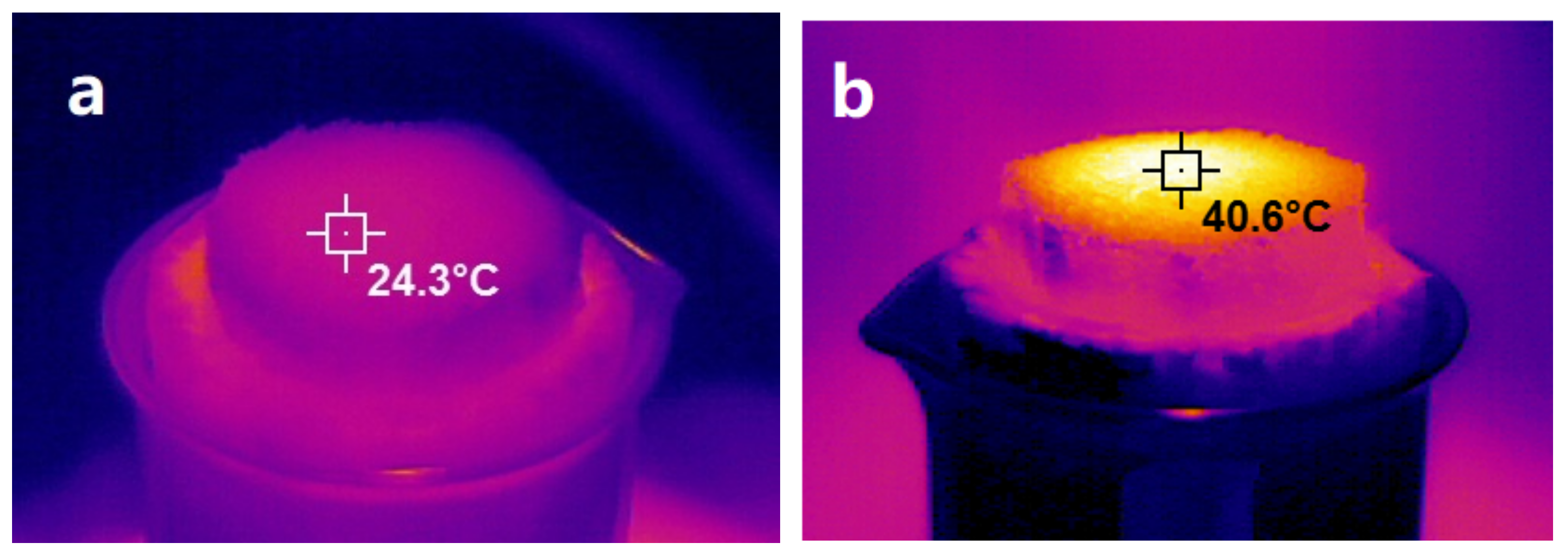
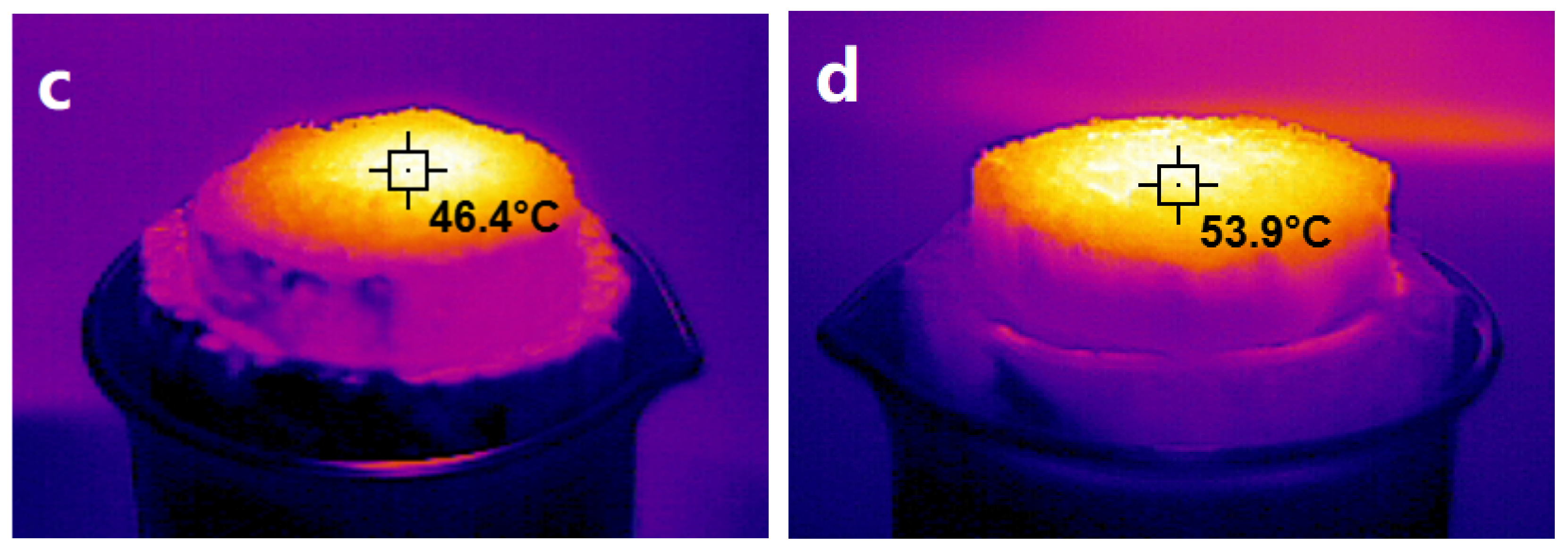
3.5. Evaluation of Photothermal Performance of NRS and PNRS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vörösmarty, C.J.; Green, P.; Salisbury, J.; Lammers, R.B. Global Water Resources: Vulnerability from Climate Change and Population Growth. Science 2000, 289, 284–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodell, M.; Famiglietti, J.S.; Wiese, D.N.; Reager, J.T.; Beaudoing, H.K.; Landerer, F.W.; Lo, M.-H. Emerging trends in global freshwater availability. Nature 2018, 557, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, X.; Yu, B.; Wang, X.; Guo, Q.; Yang, J. Recyclable polydopamine-functionalized sponge for high efficiency clean water generation with dual-purpose solar evaporation and contaminant adsorption. ACS Appl. Mater. Interfaces 2019, 11, 32559–32568. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Yin, P.; Zhao, M.; Luo, Z.; Huang, Y.; He, Q.; Yu, Y.; Liu, Z.; Hu, Z.; Chen, B.; et al. MOF-Based Hierarchical Structures for Solar-Thermal Clean Water Production. Adv. Mater. 2019, 31, e1808249. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Liang, H.W.; Cao, X.; Zhang, W.J.; Lin, H.T.; Zhou, F.; Chen, L.F.; Yu, S.H. Robust and highly efficient free-standing carbonaceous nanofiber membranes for water purification. Adv. Funct. Mater. 2011, 21, 3851–3858. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.; Peng, X.; Zhang, L.; Gao, C. Polyamide membranes with nanoscale Turing structures for water purification. Science 2018, 360, 518–521. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Wang, L.; Bradley, R.; Zhao, B.; Wu, W. Highly efficient solar seawater desalination with environmentally friendly hierarchical porous carbons derived from halogen-containing polymers. RSC Adv. 2019, 9, 29414–29423. [Google Scholar] [CrossRef] [Green Version]
- Neumann, O.; Neumann, A.D.; Silva, E.; Ayala-Orozco, C.; Tian, S.; Nordlander, P.; Halas, N.J. Nanoparticle-Mediated, Light-Induced Phase Separations. Nano Lett. 2015, 15, 7880–7885. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Kang, G.; Cho, S.K.; Park, W.; Kim, K.; Padilla, W.J. Flexible thin-film black gold membranes with ultrabroadband plasmonic nanofocusing for efficient solar vapour generation. Nat. Commun. 2015, 6, 10103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Liu, Y.; Tao, P.; Shen, Q.; Yi, N.; Zhang, F.; Liu, Q.; Song, C.; Zhang, D.; Shang, W.; et al. Bio-inspired evaporation through plasmonic film of nanoparticles at the air–water interface. Small 2014, 10, 3234–3239. [Google Scholar] [CrossRef] [PubMed]
- El-Agouz, S.; El-Aziz, G.A.; Awad, A. Solar desalination system using spray evaporation. Energy 2014, 76, 276–283. [Google Scholar] [CrossRef]
- Neumann, O.; Urban, A.S.; Day, J.; Lal, S.; Nordlander, P.; Halas, N.J. Solar Vapor Generation Enabled by Nanoparticles. ACS Nano 2013, 7, 42–49. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Peh, C.K.; Ho, G.W. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energ. Environ. Sci. 2019, 12, 841–864. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Ni, G.W.; Zhu, S.; Zhu, J. The revival of thermal utilization from the Sun: Interfacial solar vapor generation. Natl. Sci. Rev. 2019, 6, 562–578. [Google Scholar] [CrossRef] [Green Version]
- Sajadi, S.M.; Farokhnia, N.; Irajizad, P.; Hasnain, M.; Ghasemi, H. Flexible artificially networked structure for ambient/high pressure solar steam generation. J. Mater. Chem. A 2016, 6, 4700–4705. [Google Scholar] [CrossRef]
- Ghasemi, H.; Ni, G.; Marconnet, A.M.; Loomis, J.; Yerci, S.; Miljkovic, N.; Chen, G. Solar steam generation by heat localization. Nat. Commun. 2014, 5, 4449. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Tan, Y.L.; Wang, J.Y.; Xu, W.C.; Yuan, Y.; Cai, W.S.; Zhu, S.N.; Zhu, J. 3D self-assembly of aluminium nano-particles for plasmon-enhanced solar desalination. Nat. Photonics 2016, 10, 393–400. [Google Scholar] [CrossRef]
- Sui, Y.; Hao, D.; Guo, Y.; Cai, Z.; Xu, B. A flowerlike sponge coated with carbon black nanoparticles for enhanced solar vapor generation. J. Mater. Sci. 2019, 55, 298–308. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Tang, M.; Zhou, L.; Zhu, B.; Zhu, S.; Zhu, J. Graphene oxide-based efficient and scalable solar desalination under one sun with a confined 2D water path. Proc. Natl. Acad. Sci. USA 2016, 113, 13953–13958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, A.; Ming, X.; Fu, Y.; Wang, G.; Wang, X. Fiber-Based, Double-Sided, Reduced Graphene Oxide Films for Efficient Solar Vapor Generation. ACS Appl. Mater. Interfaces 2017, 9, 29958–29964. [Google Scholar] [CrossRef] [PubMed]
- Dao, V.-D.; Choi, H.-S. Carbon-Based Sunlight Absorbers in Solar-Driven Steam Generation Devices. Glob. Chall. 2018, 2, 1700094. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tan, Y.; Ji, D.; Zhu, B.; Zhang, P.; Xu, J.; Gan, Q.; Yu, Z.; Zhu, J. Self-assembly of highly efficient, broadband plasmonic absorbers for solar steam generation. Sci. Adv. 2016, 2, e1501227. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Tang, B.; Wu, J.; Li, R.; Wang, P. Hydrophobic Light-to-Heat Conversion Membranes with Self-Healing Ability for Interfacial Solar Heating. Adv. Mater. 2015, 27, 4889–4894. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Li, J.; Lv, L.; Zhao, Y.; Qu, L. Vertically Aligned Graphene Sheets Membrane for Highly Efficient Solar Thermal Generation of Clean Water. ACS Nano 2017, 11, 5087–5093. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, M.; Peh, C.K.N.; Ho, G.W. Recent progress in solar-driven interfacial water evaporation: Advanced designs and applications. Nano Energy 2018, 57, 507–518. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, F.; Huang, A.; Qin, Y.; Song, S.; Li, Y.; Arroyo, M.A.C. MoS2@sponge with double layer structure for high-efficiency solar desalination. Desalination 2020, 481, 114359. [Google Scholar] [CrossRef]
- Hao, D.; Yang, Y.; Xu, B.; Cai, Z. Efficient solar water vapor generation enabled by water-absorbing polypyrrole coated cotton fabric with enhanced heat localization. Appl. Therm. Eng. 2018, 141, 406–412. [Google Scholar] [CrossRef]
- Mu, P.; Bai, W.; Zhang, Z.; He, J.; Sun, H.; Zhu, Z.; Liang, W.; Li, A. Robust aerogels based on conjugated microporous polymer nanotubes with exceptional mechanical strength for efficient solar steam generation. J. Mater. Chem. A 2018, 6, 18183–18190. [Google Scholar] [CrossRef]
- Huang, H.; Hao, H.; Yu, Q.; Lin, P.; Xu, J.; Yin, X.; Chen, S.; Wang, H.; Wang, L. Flexible and Highly Efficient Bilayer Photothermal Paper for Water Desalination and Purification: Self-Floating, Rapid Water Transport, and Localized Heat. ACS Appl. Mater. Interfaces 2020, 12, 11204–11213. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Du, Z. A Flexible and Highly Sensitive Pressure Sensor Based on AgNWs/NRLF for Hand Motion Monitoring. Nanomaterials 2019, 9, 945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, J.; Yao, H.-B.; Wang, X.; Ye, Y.-D.; Wang, J.-L.; Wu, Z.-Y.; Liu, J.-W.; Fan, F.-J.; Gao, H.-L.; Zhang, C.-L.; et al. Stretchable Conductors Based on Silver Nanowires: Improved Performance through a Binary Network Design. Angew. Chem. Int. Ed. 2013, 52, 1654–1659. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, B.; Wang, J.; Mohamed, A.; Jia, H. Ultrasensitive and wearable strain sensors based on natural rubber/graphene foam. J. Alloy. Compd. 2019, 785, 1001–1008. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Yu, F.; Guo, Z.; Cheng, H.; Yin, J.; Yan, L.; Wang, X. Flexible and Efficient Solar Thermal Generators Based on Polypyrrole Coated Natural Latex Foam for Multimedia Purification. ACS Sustain. Chem. Eng. 2020, 8, 12053–12062. [Google Scholar] [CrossRef]
- Wu, X.; Wu, L.; Tan, J.; Chen, G.Y.; Owens, G.; Xu, H. Evaporation above a bulk water surface using an oil lamp in-spired highly efficient solar steam generation strategy. J. Mater. Chem. A 2018, 6, 12267–12274. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Liu, Z.; Singer, M.; Li, C.; Cheney, A.R.; Ji, D.; Zhou, L.; Zhang, N.; Zeng, X.; et al. Cold Vapor Generation beyond the Input Solar Energy Limit. Adv. Sci. 2018, 5, 1800222. [Google Scholar] [CrossRef]
- Bashir, A.S.M.; Manusamy, Y.; Chew, T.L.; Ismail, H.; Ramasamy, S. Mechanical, thermal, and morphological properties of (eggshell powder)-filled natural rubber latex foam. J. Vinyl. Addit. Techn. 2017, 23, 3–12. [Google Scholar] [CrossRef]
- Phomrak, S.; Nimpaiboon, A.; Newby, B.-M.Z.; Phisalaphong, M. Natural Rubber Latex Foam Reinforced with Micro- and Nanofibrillated Cellulose via Dunlop Method. Polymers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Derami, H.G.; Ghim, D.; Cao, S.; Jun, Y.-S.; Singamaneni, S. Polydopamine-filled bacterial nanocellulose as biodegradable interfacial photothermal evaporator for highly efficient solar steam generation. J. Mater. Chem. A 2017, 5, 18397–18402. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Wang, S.; Yang, Y.; Qin, B.; Wang, K.; Xie, T.; Wei, Y.; Ji, Y. Polydopamine coated shape memory polymer: Enabling light triggered shape recovery, light controlled shape reprogramming and surface functionalization. Chem. Sci. 2016, 7, 4741–4747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.; Ku, S.H.; Lee, H.; Park, C.B. Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Wu, X.; Chen, G.Y.; Zhang, W.; Liu, X.; Xu, H. A plant-transpiration-process-inspired strategy for highly efficient solar evaporation. Adv. Sustain. Syst. 2017, 1, 1700046. [Google Scholar] [CrossRef]
- Lee, M.; Kim, J.U.; Lee, J.S.; Lee, B.I.; Shin, J.; Park, C.B. Mussel-inspired plasmonic nanohybrids for light harvesting. Adv. Mater. 2014, 26, 4463–4468. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Yang, J.; Pang, Y.; Huang, W.; Shaw, S.K.; Schiffbauer, J.; Pillers, M.A.; Mu, X.; Luo, S.; Zhang, T.; Huang, Y.; et al. Functionalized Graphene Enables Highly Efficient Solar Thermal Steam Generation. ACS Nano 2017, 11, 5510–5518. [Google Scholar] [CrossRef]
- Li, K.; Gao, M.; Li, Z.; Yang, H.; Jing, L.; Tian, X.; Li, Y.; Li, S.; Li, H.; Wang, Q.; et al. Multi-interface engineering of solar evaporation devices via scalable, synchronous thermal shrinkage and foaming. Nano Energy 2020, 74, 104875. [Google Scholar] [CrossRef]
- Fan, Y.; Bai, W.; Mu, P.; Su, Y.; Zhu, Z.; Sun, H.; Liang, W.; Li, A. Conductively monolithic polypyrrole 3-D porous architecture with micron-sized channels as superior salt-resistant solar steam generators. Sol. Energ. Mater. Sol. C 2020, 206, 110347. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Feng, R.; Bernard, A.; Liu, Y.; Zhang, Y.; Duan, H.; Shang, W.; Tao, P.; Song, C.; et al. A Bioinspired, Reusable, Paper-Based System for High-Performance Large-Scale Evaporation. Adv. Mater. 2015, 27, 2768–2774. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, H.; Li, J.; Huang, J.; Wang, D.; Tang, B.Z. Doping AIE Photothermal Molecule into All-Fiber Aerogel with Self-Pumping Water Function for Efficiency Solar Steam Generation. ACS Appl. Mater. Interfaces 2020, 12, 26033–26040. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Xu, Y.; Ding, T.; Li, J.; Yin, J.; Fei, W.; Cao, Y.; Yu, J.; Yuan, L.; Gong, L.; et al. Water-evaporation-induced electricity with nanostructured carbon materials. Nat. Nanotechnol. 2017, 12, 317–321. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Shi, Y.; Ding, A.; Liao, J.; Gui, H.; Chen, Y. Polydopamine-Coated Natural Rubber Sponge for Highly Efficient Vapor Generation. Polymers 2022, 14, 1486. https://doi.org/10.3390/polym14071486
Yu H, Shi Y, Ding A, Liao J, Gui H, Chen Y. Polydopamine-Coated Natural Rubber Sponge for Highly Efficient Vapor Generation. Polymers. 2022; 14(7):1486. https://doi.org/10.3390/polym14071486
Chicago/Turabian StyleYu, Han, Yuqi Shi, Aiwu Ding, Jianhe Liao, Hongxing Gui, and Yongping Chen. 2022. "Polydopamine-Coated Natural Rubber Sponge for Highly Efficient Vapor Generation" Polymers 14, no. 7: 1486. https://doi.org/10.3390/polym14071486





