Synthesis of 3D Hollow Layered Double Hydroxide-Molybdenum Disulfide Hybrid Materials and Their Application in Flame Retardant Thermoplastic Polyurethane
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of 3D Hollow NiFeCe-LDH and NiFeTb-LDH
2.3. Synthesis of 3D Hollow NiFeCe-LDH/MoS2 and NiFeTb-LDH/MoS2 Hybrid Materials
2.4. Synthesis of TPU Composites
2.5. Characterization
3. Results and Discussion
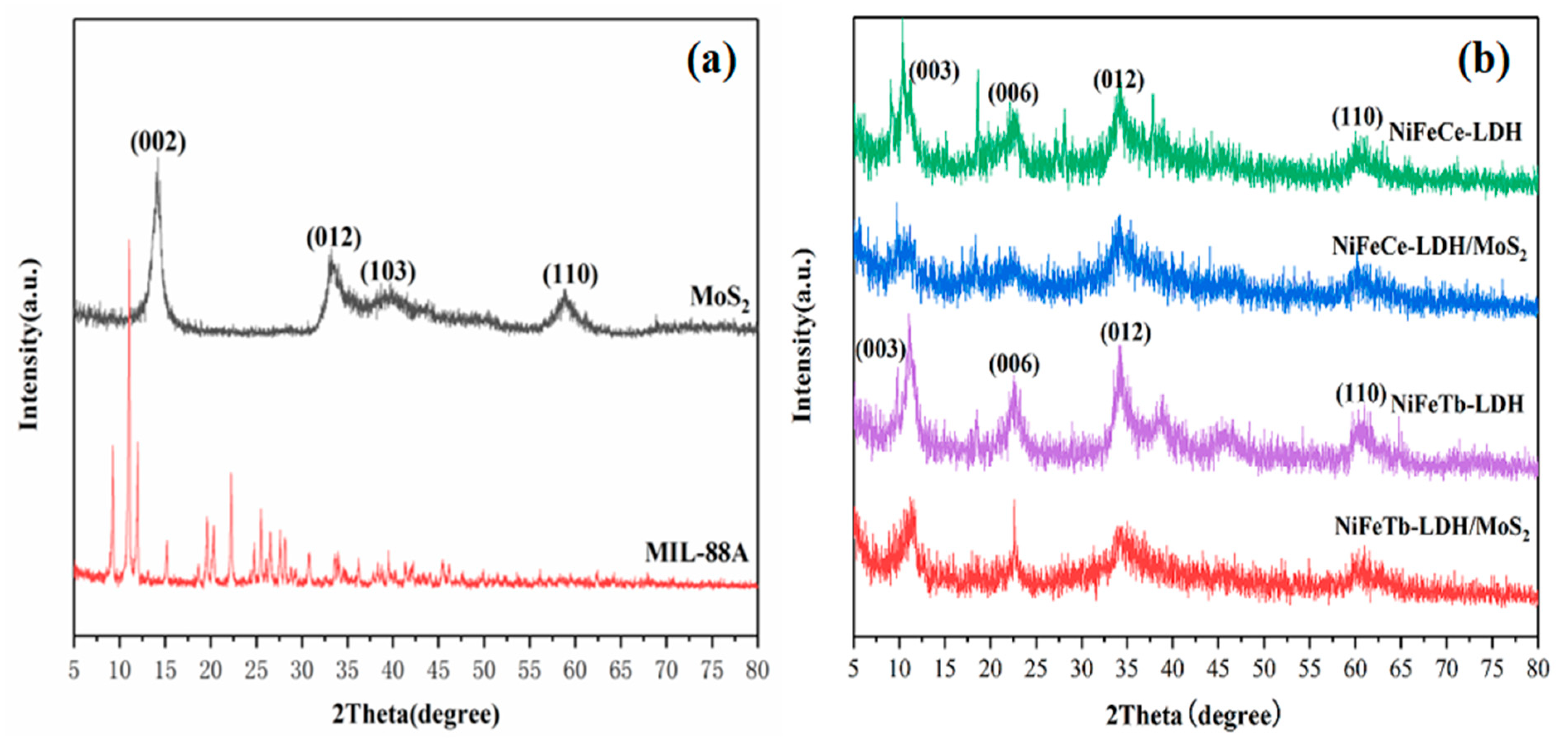
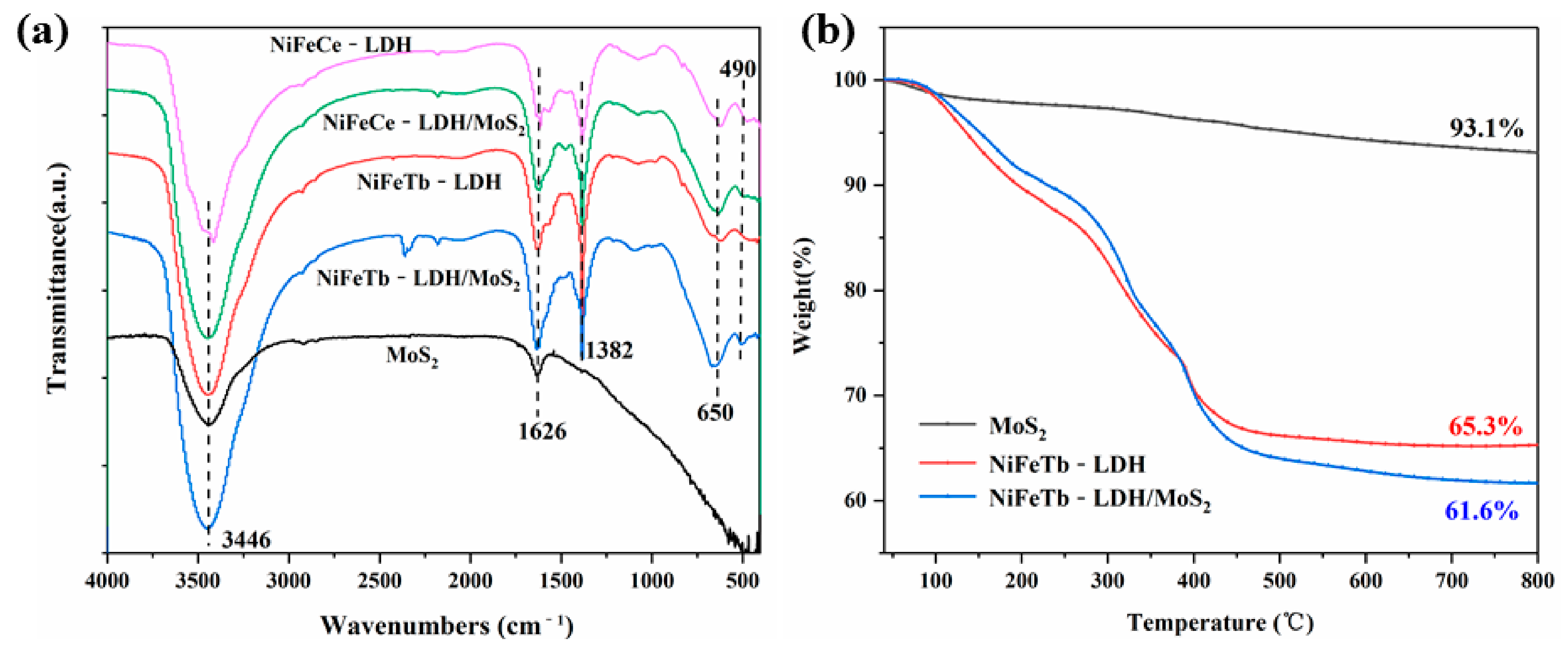
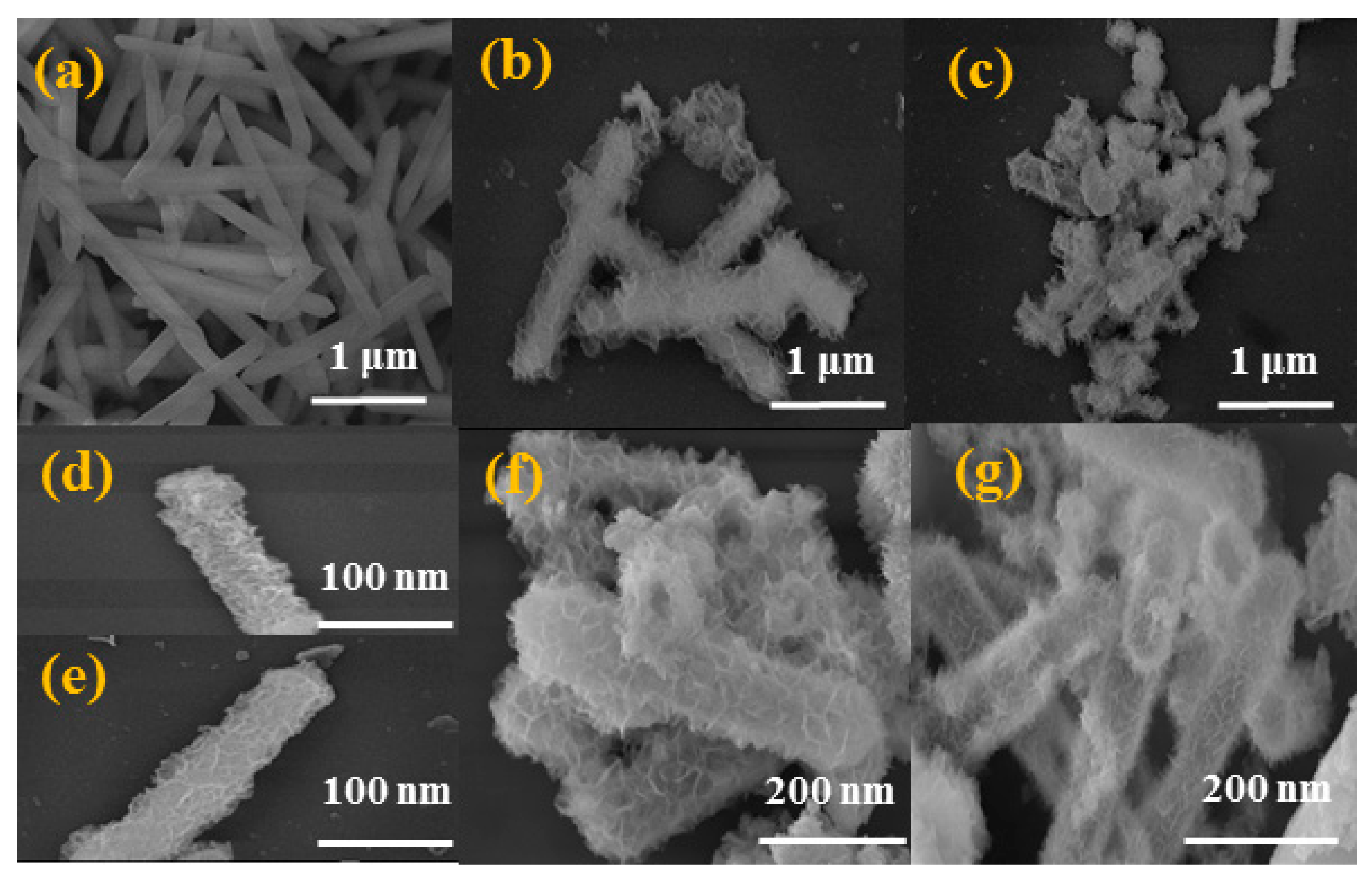
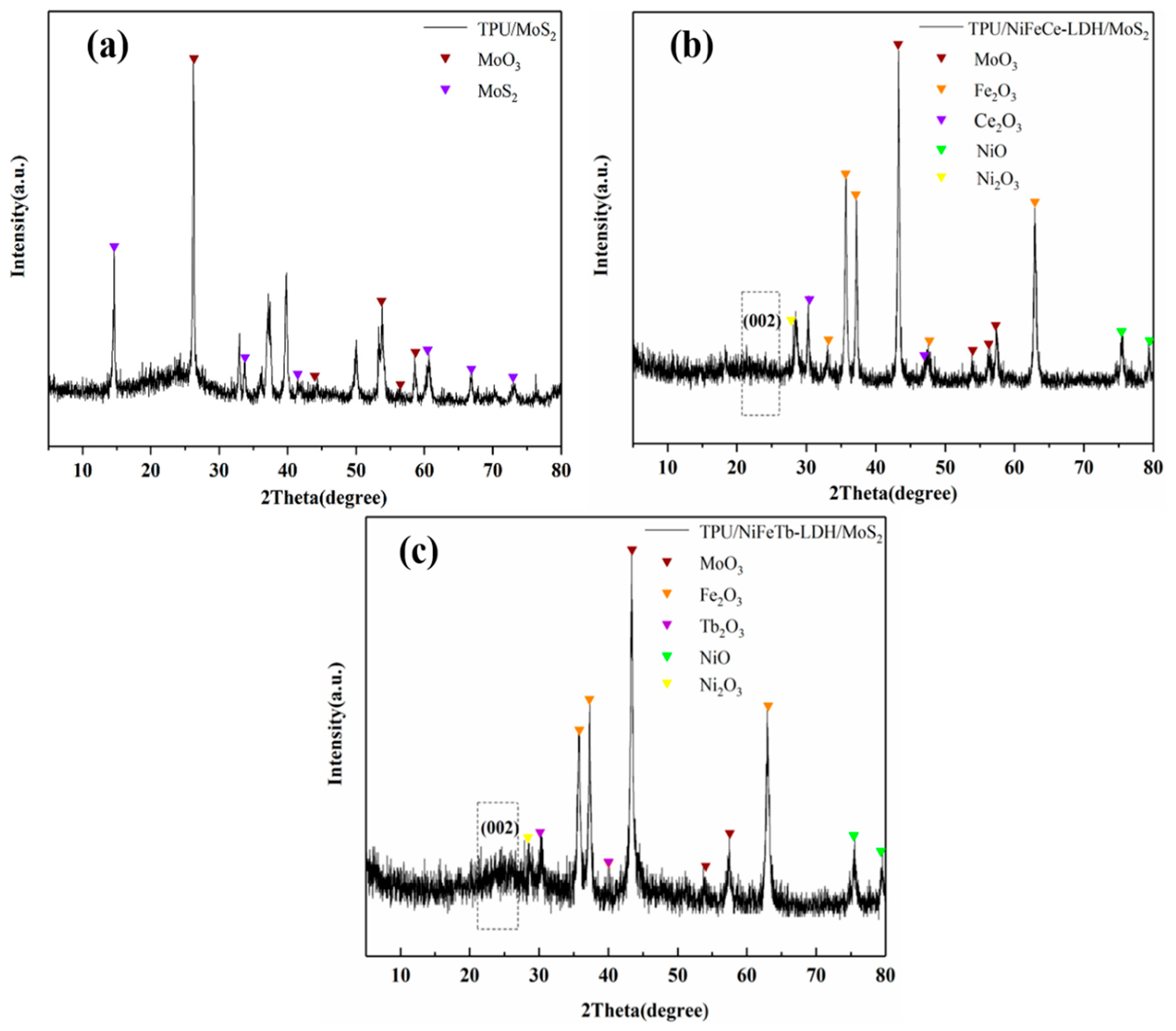
3.1. Characterization of 3D Hollow LDH and Its Hybrid Materials
3.2. CCT Tests of TPU Composites
3.3. Thermal Stability of TPU Composites
3.4. Char Residues Analysis of TPU Composites
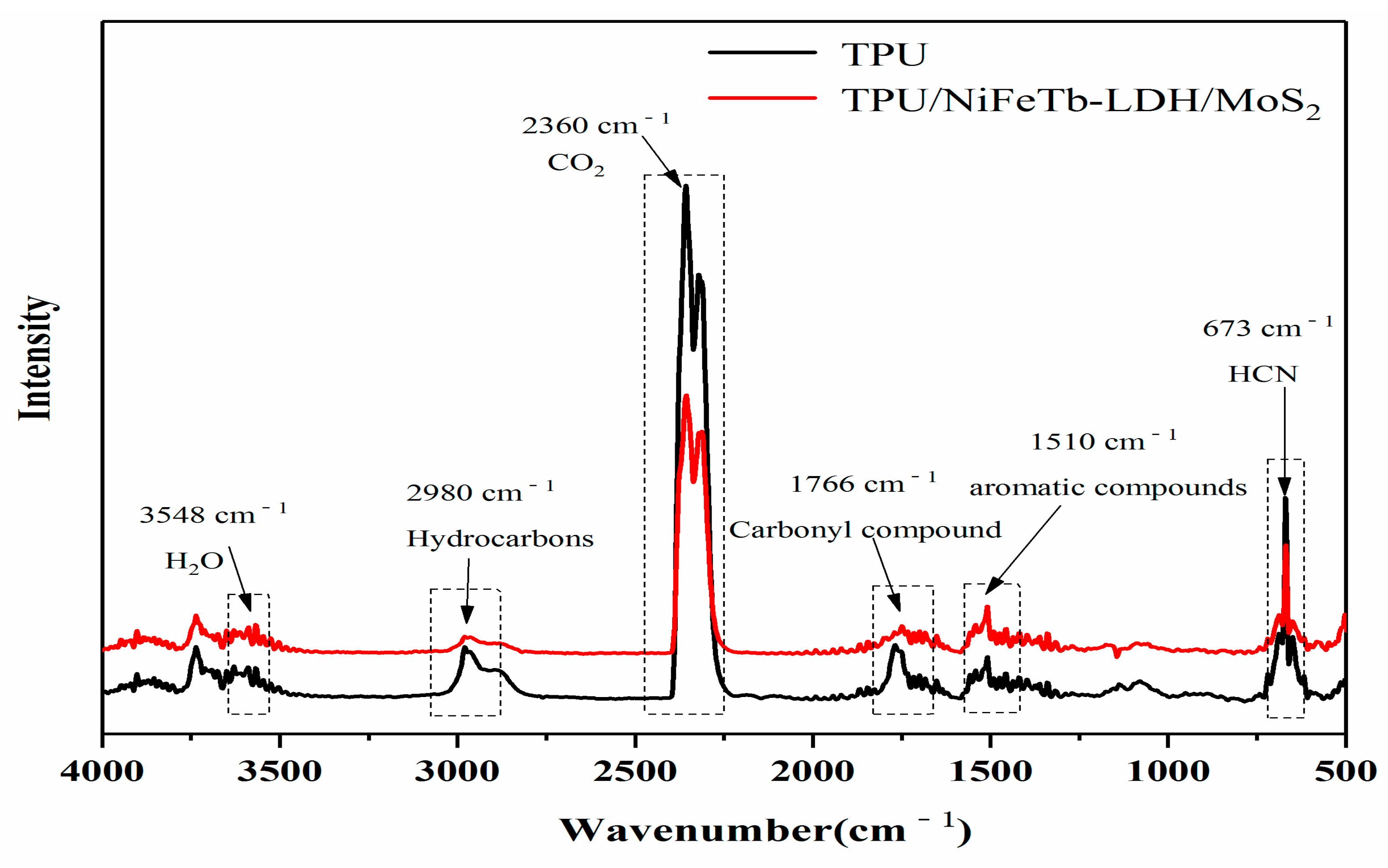
3.5. Gas Analysis
3.6. Flame Retardant Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Toldy, A.; Harakály, G.; Szolnoki, B.; Zimonyi, E.; Marosi, G. Flame retardancy of thermoplastics polyurethanes. Polym. Degrad. Stab. 2012, 97, 2524–2530. [Google Scholar] [CrossRef]
- Lei, J.; Yao, G.; Sun, Z.; Wang, B.; Yu, C.; Zheng, S. Fabrication of a novel antibacterial TPU nanofiber membrane containing Cu-loaded zeolite and its antibacterial activity toward Escherichia coli. Mater. Sci. 2019, 54, 11682–11693. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, W.; Gong, X.; Sun, Q.; Cao, X.; Su, Y.; Yu, B.; Li, R.K.; Vellaisamy, R.A. Surface decoration of halloysite nanotubes with POSS for fire-safe thermoplastic polyurethane nanocomposites. J. Mater. Sci. Technol. 2022, 101, 107–117. [Google Scholar] [CrossRef]
- Hirschler, M.M. Flame retardants and heat release: Review of data on individual polymers. Fire Mater. 2015, 39, 232–258. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Peng, C.; Yuan, S.; Zhang, M. Thermal and flame retardant properties of novel intumescent flame retardant polypropylene composites. J. Therm. Anal. Calorim. 2013, 113, 865–871. [Google Scholar] [CrossRef]
- Elbasuney, S. Surface engineering of layered double hydroxide (LDH) nanoparticles for polymer flame retardancy. Powder Technol. 2015, 277, 63–73. [Google Scholar] [CrossRef]
- Wenelska, K.; Mijowska, E. Preparation, thermal conductivity, and thermal stability of flame retardant polyethylene with exfoliated MoS2/MxOy. New J. Chem. 2017, 41, 13287–13292. [Google Scholar] [CrossRef]
- Huang, W.; Huang, J.; Yu, B.; Meng, Y.; Cao, X.; Zhang, Q.; Wu, W.; Shi, D.; Jiang, T.; Li, R.K. Facile preparation of phosphorus containing hyperbranched polysiloxane grafted graphene oxide hybrid toward simultaneously enhanced flame retardancy and smoke suppression of thermoplastic polyurethane nanocomposites. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106614. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, D.; El Hankari, S.; Zou, Y.; Wang, S. Recent progress on layered double hydroxides and their derivatives for electrocatalytic water splitting. Adv. Sci. 2018, 5, 1800064. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, P.; Wu, D.; Frost, R.L. Novel approach to fabricate organo-LDH hybrid by the intercalation of sodium hexadecyl sulfate into tricalcium aluminate. Appl. Clay Sci. 2017, 140, 25–30. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Z.; Qin, Z.; Sun, H.; Jiao, X.; Chen, D. LDH nanocages synthesized with MOF templates and their high performance as supercapacitors. Nanoscale 2013, 5, 11770–11775. [Google Scholar] [CrossRef]
- Huo, J.; Wang, Y.; Yan, L.; Xue, Y.; Li, S.; Hu, M.; Jiang, Y.; Zhai, Q.-G. In situ semi-transformation from heterometallic MOFs to Fe-Ni LDH/MOF hierarchical architectures for boosted oxygen evolution reaction. Nanoscale 2020, 12, 14514–14523. [Google Scholar] [CrossRef]
- Zhou, X.; Mu, X.; Cai, W.; Wang, J.; Chu, F.; Xu, Z.; Song, L.; Xing, W.; Hu, Y. Design of hierarchical NiCo-LDH@PZS hollow dodecahedron architecture and application in high-performance epoxy resin with excellent fire safety. ACS Appl. Mater. Interfaces 2019, 11, 41736–41749. [Google Scholar] [CrossRef]
- Yue, X.; Li, C.; Ni, Y.; Xu, Y.; Wang, J. Flame retardant nanocomposites based on 2D layered nanomaterials: A review. J. Mater. Sci. 2019, 54, 13070–13105. [Google Scholar] [CrossRef]
- Yang, L.; Mukhopadhyay, A.; Jiao, Y.; Yong, Q.; Chen, L.; Xing, Y.; Hamel, J.; Zhu, H. Ultralight, highly thermally insulating and fire resistant aerogel by encapsulating cellulose nanofibers with two-dimensional MoS2. Nanoscale 2017, 9, 11452–11462. [Google Scholar] [CrossRef]
- Jiang, J.W. Graphene versus MoS2: A short review. Front. Phys. 2015, 10, 287–302. [Google Scholar] [CrossRef] [Green Version]
- Campbell, P.M.; Perini, C.J.; Chiu, J.; Gupta, A.; Ray, H.S.; Chen, H.; Wenzel, K.; Snyder, E.; Wagner, B.K.; Ready, J.; et al. Plasma-assisted synthesis of MoS2. 2D Mater. 2017, 5, 015005. [Google Scholar] [CrossRef]
- Xu, H.; Shan, C.; Wu, X.; Sun, M.; Huang, B.; Tang, Y.; Yan, C.-H. Fabrication of layered double hydroxide microcapsules mediated by cerium doping in metal-organic frameworks for boosting water splitting. Energy Environ. Sci. 2020, 13, 2949–2956. [Google Scholar] [CrossRef]
- Sanikop, R.; Budumuru, A.K.; Gautam, S.; Chae, K.H.; Sudakar, C. Robust ferromagnetism in Li-intercalated and-deintercalated MoS2 nanosheets: Implications for 2D spintronics. ACS Appl. Nano Mater. 2020, 3, 11825–11837. [Google Scholar] [CrossRef]
- Wu, H.; Ma, M.D.; Gai, W.Z.; Yang, H.; Zhou, J.G.; Cheng, Z.; Xu, P.; Deng, Z.Y. Arsenic removal from water by metal-organic framework MIL-88A microrods. Environ. Sci. Pollut. Res. 2018, 25, 27196–27202. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Valdes, S.; Ramírez-Vargas, E.; Rodriguez-Gonzalez, J.A.; Uribe-Calderón, J.A.; De-Valle, L.F.R.; Zuluaga-Parra, J.D.; Martínez-Colunga, J.G.; Solís-Rosales, S.G.; Sánchez-Martínez, A.C.; Flores-Flores, R.; et al. Organopalygorskite and molybdenum sulfide combinations to produce mechanical and processing enhanced flame-retardant PE/EVA blend composites with low magnesium hydroxide loading. J. Vinyl. Addit. Technol. 2020, 26, 434–442. [Google Scholar] [CrossRef]
- Wang, J.; Pan, Q.; Liu, Q.; He, Y.; Mann, T.; Li, R.; Zhang, M.; Liu, L. Synthesis and photoluminescence properties of europium doped Mg-Al layered double hydroxides intercalated with MoO42− anions. Solid State Sci. 2012, 14, 562–566. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Wang, D.Y. Hierarchical layered double hydroxide nanosheets/phosphorus-containing organosilane functionalized hollow glass microsphere towards high performance epoxy composite: Enhanced interfacial adhesion and bottom-up charring behavior. Polymer 2020, 210, 123018. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Menzel, R.; Wang, Y.; Garcia-Gallastegui, A.; Bawaked, S.M.; Obaid, A.Y.; Basahel, S.N.; Mokhtar, M. Graphene-oxide-supported CuAl and CoAl layered double hydroxides as enhanced catalysts for carbon-carbon coupling via Ullmann reaction. J. Solid State Chem. 2017, 246, 130–137. [Google Scholar] [CrossRef]
- Mourit, A.P.; Mathys, Z.; Gibson, A.G. Heat release of polymer composites in fire. Compos. Part A 2006, 37, 1040–1054. [Google Scholar] [CrossRef]
- Wang, D.; Song, L.; Zhou, K.; Yu, X.; Hu, Y.; Wang, J. Anomalous nano-barrier effects of ultrathin molybdenum disulfide nanosheets for improving the flame retardance of polymer nanocomposites. J. Mater. Chem. A 2015, 3, 14307–14317. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Song, L.; Yu, B.; Yuan, Y.; Hu, Y.; Yuen, R.K.K. Aluminum hypophosphite in combination with expandable graphite as a novel flame retardant system for rigid polyurethane foams. Polym. Adv. Technol. 2015, 25, 1034–1043. [Google Scholar] [CrossRef]
- Nyambo, C.; Kandare, E.; Wang, D.; Wilkie, C.A. Flame-retarded polystyrene: Investigating chemical interactions between ammonium polyphosphate and MgAl layered double hydroxide. Polym. Degrad. Stab. 2008, 93, 1656–1663. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Xu, B.; Li, A.; Wang, X.; Wang, G. Flame retardancy and smoke suppression of MgAl layered double hydroxides containing P and Si in polyurethane elastomer. Ind. Eng. Chem. Res. 2016, 55, 11175–11185. [Google Scholar] [CrossRef]
- Kalali, E.N.; Wang, X.; Wang, D.Y. Functionalized layered double hydroxide-based epoxy nanocomposites with improved flame retardancy and mechanical properties. J. Mater. Chem. A 2015, 3, 6819–6826. [Google Scholar] [CrossRef] [Green Version]
- Gürü, M.; Güngör, G.; Yılmaz Aydın, D.; Çakanyıldırım, Ç. The investigation of synthesis parameters, kinetic and flame retardant properties of magnesium fluoroborate. Chem. Pap. 2022, 76, 1313–1320. [Google Scholar] [CrossRef]
- Tang, Q.; Song, Y.; He, J.; Yang, R. Synthesis and characterization of inherently flame-retardant and anti-dripping thermoplastic poly (imides-urethane)s. J. Appl. Polym. Sci. 2014, 131, 776–781. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhan, J.; Zhou, K.; Lu, H.; Zeng, W.; Stec, A.A.; Hull, R.; Hu, Y.; Gui, Z. The influence of carbon nanotubes on the combustion toxicity of PP/intumescent flame retardant composites. Polym. Degrad. Stab. 2015, 115, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Gui, Z.; Hu, Y. The influence of graphene based smoke suppression agents on reduced fire hazards of polystyrene composites. Compos. Part A Appl. Sci. Manuf. 2016, 80, 217–227. [Google Scholar] [CrossRef]
- Liu, X.; Ge, X.; Liu, M.; Zhou, K.; Zhu, Q.; Chen, D.; Liu, C.; Wang, C.; Liu, X.; Tang, G. Facile fabrication of NiAl-LDH and its application in TPU nanocomposites targets for reducing fire hazards. Plast. Rubber Compos. 2021, 50, 1–14. [Google Scholar] [CrossRef]
- Dong, Y.; Gui, Z.; Hu, Y.; Wu, Y.; Jiang, S. The influence of titanate nanotube on the improved thermal properties and the smoke suppression in poly(methyl methacrylate). J. Hazard. Mater. 2012, 209, 34–39. [Google Scholar] [CrossRef]
- Ma, H.; Tong, L.; Xu, Z.; Fang, Z. Synergistic effect of carbon nanotube and clay for improving the flame retardancy of ABS resin. Nanotechnology 2007, 18, 375602. [Google Scholar] [CrossRef]



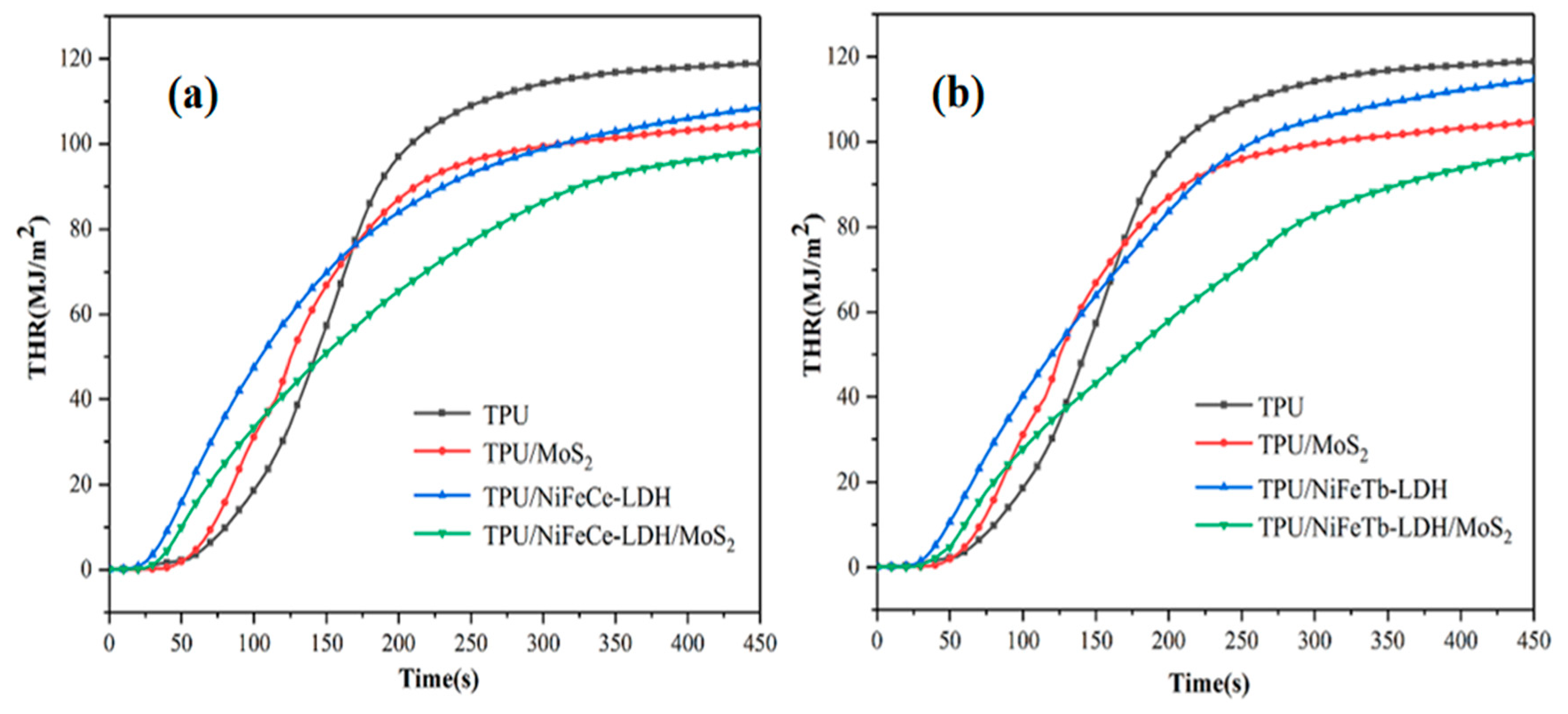
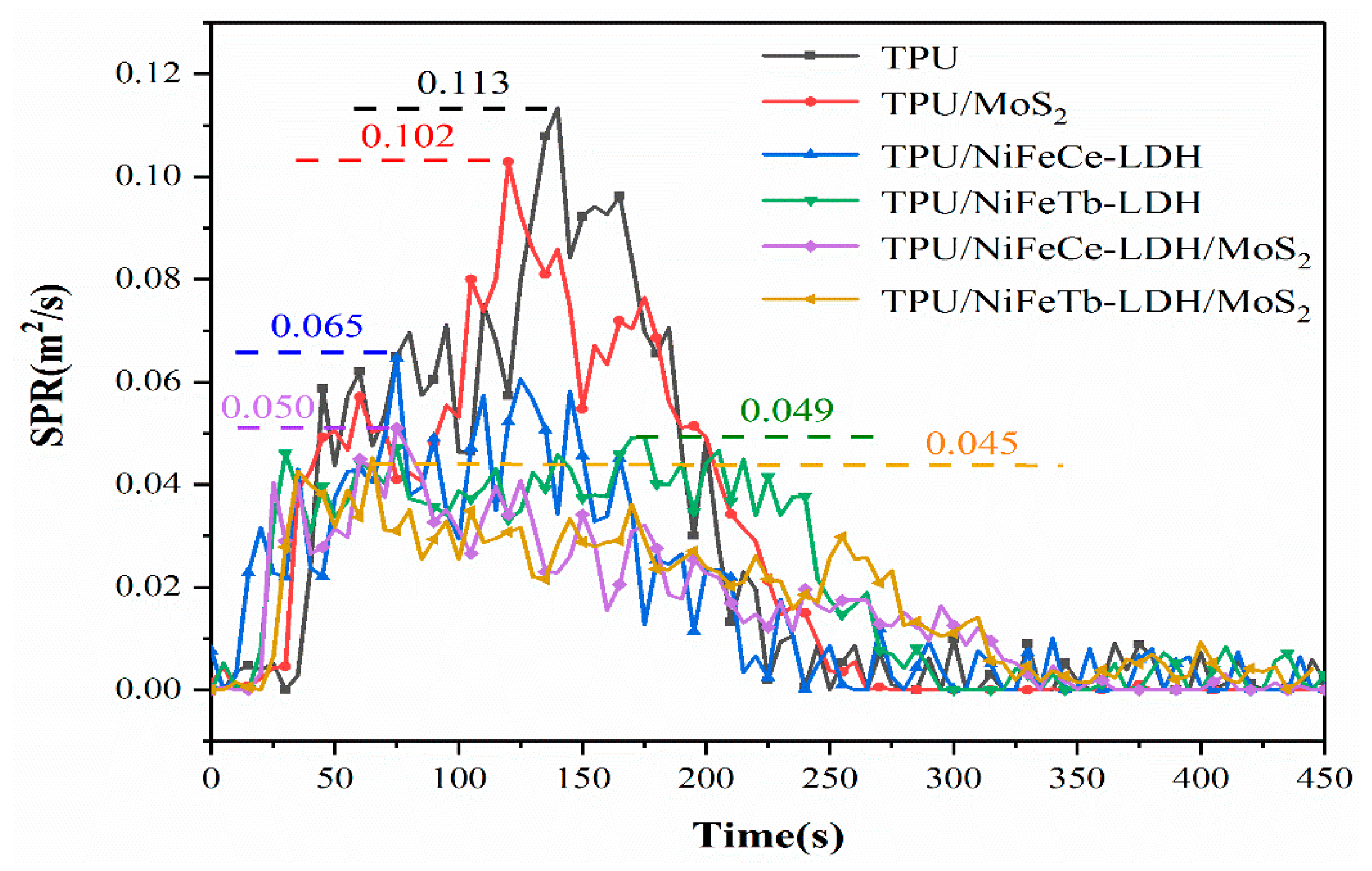
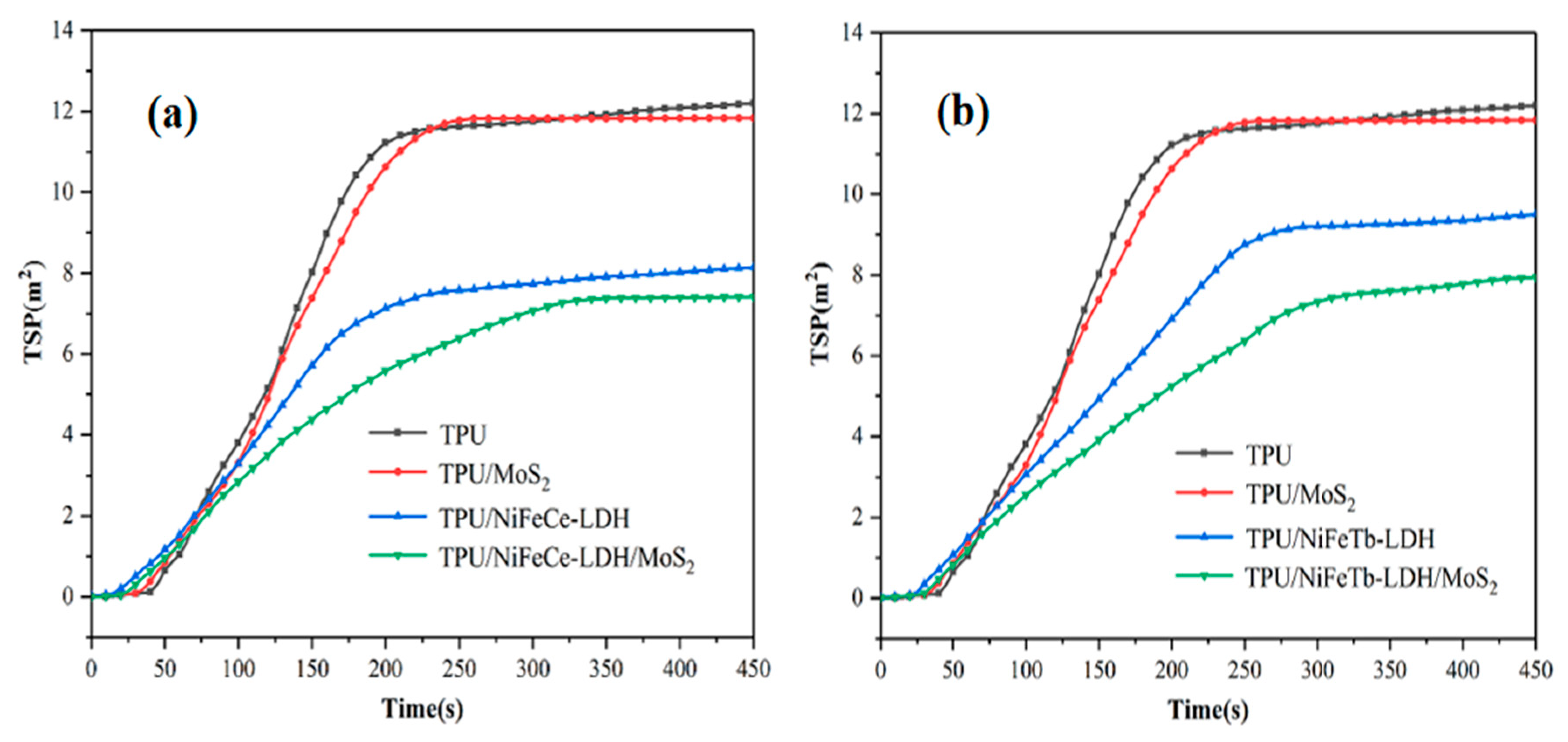


| Sample Code | PHRR kW/m2 | THR MJ/m2 | PSPR m2/s | TSP m2 |
|---|---|---|---|---|
| TPU | 1135 | 118.8 | 0.113 | 12.3 |
| TPU/MoS2 | 804 | 104.6 | 0.103 | 11.9 |
| TPU/NiFeCe-LDH | 710 | 108.5 | 0.065 | 8.1 |
| TPU/NiFeTb-LDH | 652 | 114.6 | 0.049 | 9.5 |
| TPU/NiFeCe-LDH/MoS2 | 581 | 98.4 | 0.050 | 7.4 |
| TPU/NiFeTb-LDH/MoS2 | 557 | 97.2 | 0.045 | 7.9 |
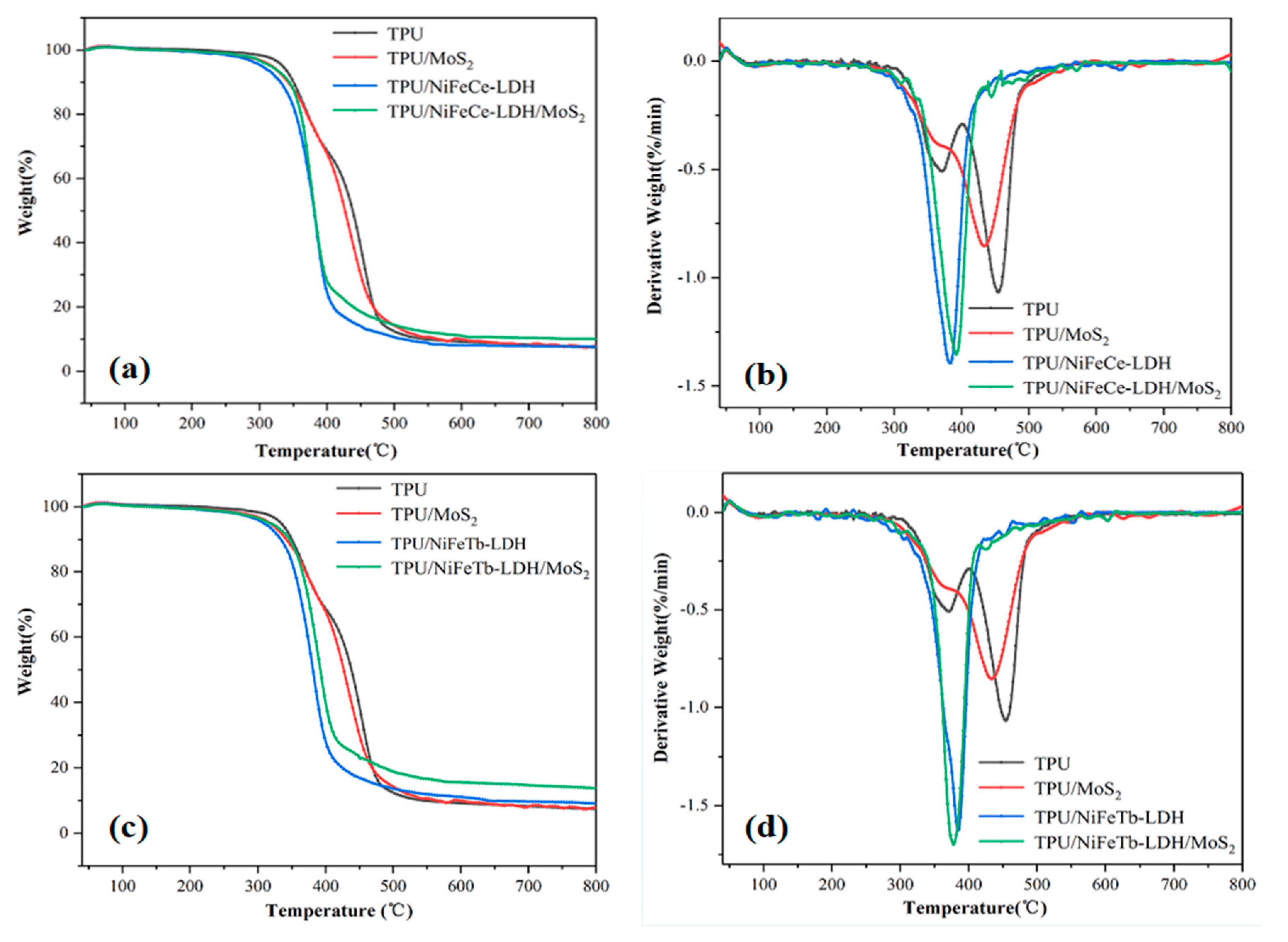
| Sample Code | T-5% (°C) | Tmax (°C) | Char Yield (%) |
|---|---|---|---|
| TPU | 333 | 454 | 5.85 |
| TPU/MoS2 | 317 | 432 | 7.93 |
| TPU/NiFeCe-LDH | 302 | 385 | 7.58 |
| TPU/NiFeTb-LDH | 308 | 382 | 9.05 |
| TPU/NiFeCe-LDH/MoS2 | 316 | 376 | 10.11 |
| TPU/NiFeTb-LDH/MoS2 | 316 | 394 | 13.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Y.; Su, W.; Li, L.; Fu, H.; Li, J.; Zhang, Y. Synthesis of 3D Hollow Layered Double Hydroxide-Molybdenum Disulfide Hybrid Materials and Their Application in Flame Retardant Thermoplastic Polyurethane. Polymers 2022, 14, 1506. https://doi.org/10.3390/polym14081506
Qian Y, Su W, Li L, Fu H, Li J, Zhang Y. Synthesis of 3D Hollow Layered Double Hydroxide-Molybdenum Disulfide Hybrid Materials and Their Application in Flame Retardant Thermoplastic Polyurethane. Polymers. 2022; 14(8):1506. https://doi.org/10.3390/polym14081506
Chicago/Turabian StyleQian, Yi, Wenyuan Su, Long Li, Haoyan Fu, Jiayin Li, and Yihao Zhang. 2022. "Synthesis of 3D Hollow Layered Double Hydroxide-Molybdenum Disulfide Hybrid Materials and Their Application in Flame Retardant Thermoplastic Polyurethane" Polymers 14, no. 8: 1506. https://doi.org/10.3390/polym14081506
APA StyleQian, Y., Su, W., Li, L., Fu, H., Li, J., & Zhang, Y. (2022). Synthesis of 3D Hollow Layered Double Hydroxide-Molybdenum Disulfide Hybrid Materials and Their Application in Flame Retardant Thermoplastic Polyurethane. Polymers, 14(8), 1506. https://doi.org/10.3390/polym14081506





