Unfunctionalized and Functionalized Multiwalled Carbon Nanotubes/Polyamide Nanocomposites as Selective-Layer Polysulfone Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
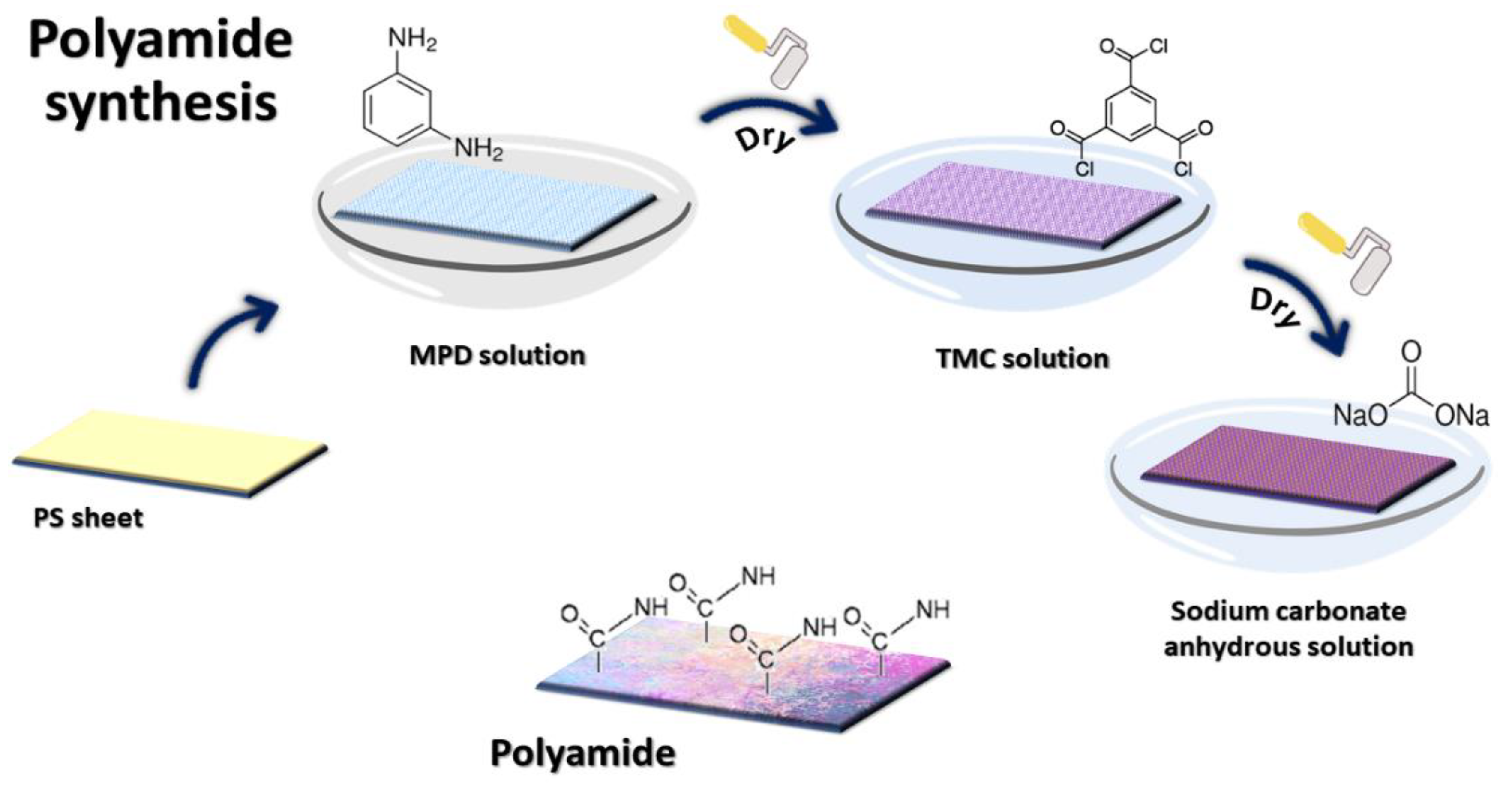
2.2. Preparation of Membrane
2.2.1. Polyamide Membrane
2.2.2. PA-MWCNT Membranes
2.2.3. PA-EtOH
2.3. Characterization and Evaluation
2.3.1. Sonication Time
2.3.2. Thermal Stability
2.3.3. Zeta Potential (ZP)
2.3.4. Scanning Electron Microscope (SEM)
2.3.5. Salt Rejection of Water
3. Results
3.1. Optimization of Sonication Time
3.2. Thermogravimetric Analysis (TGA)
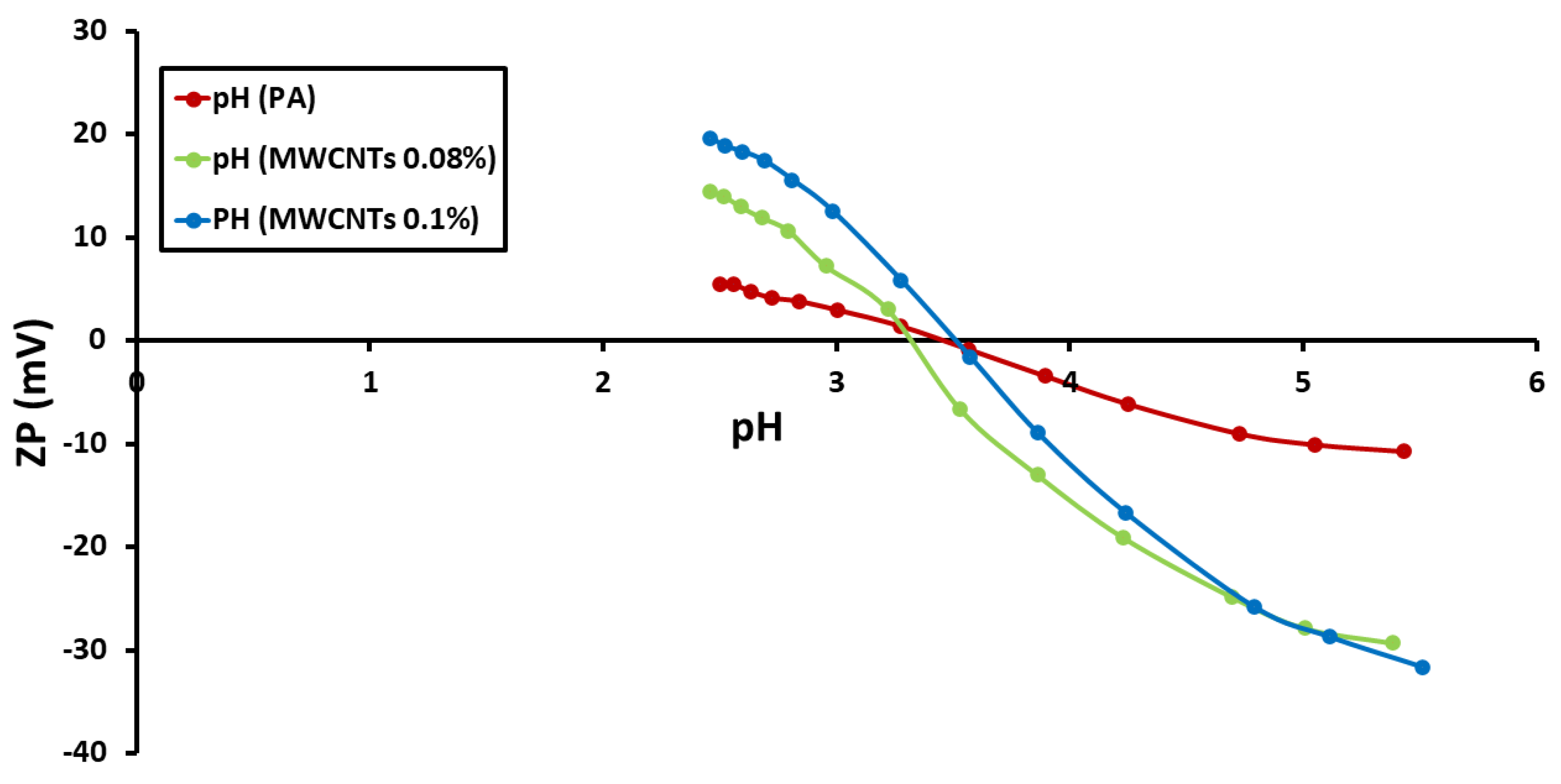
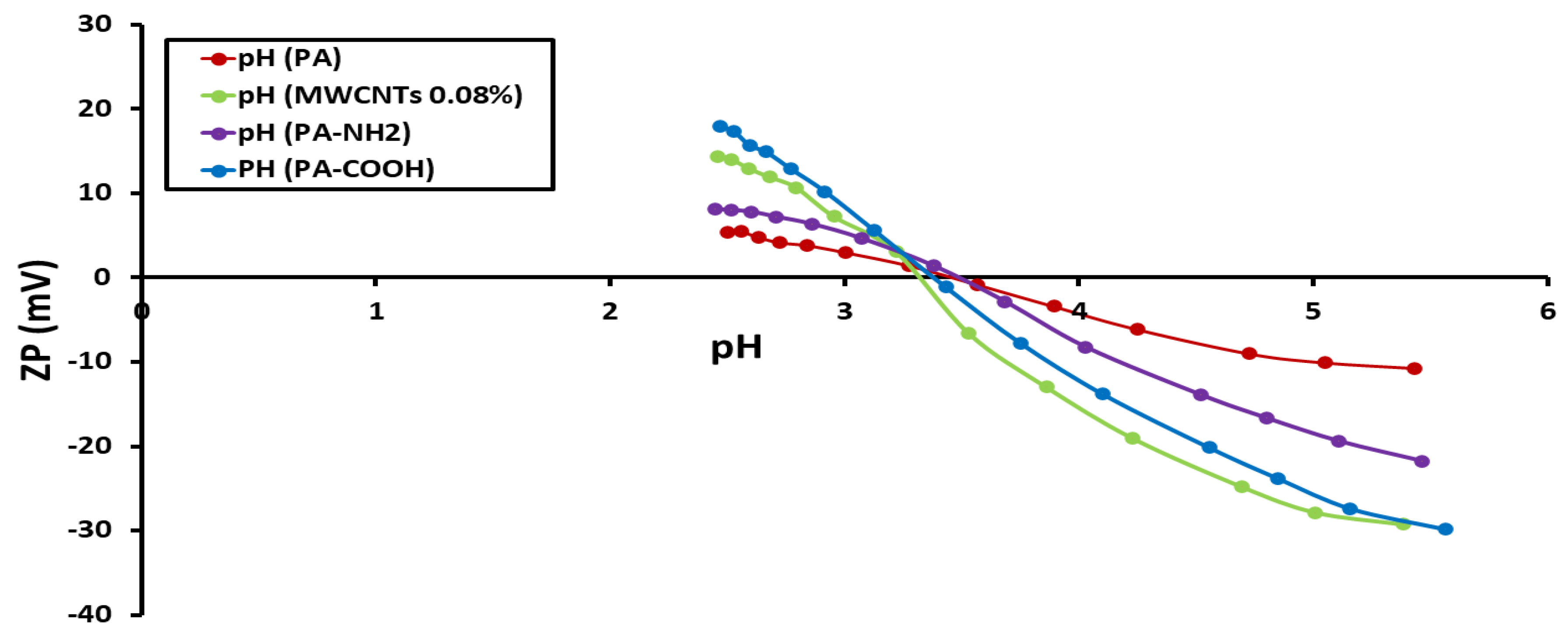
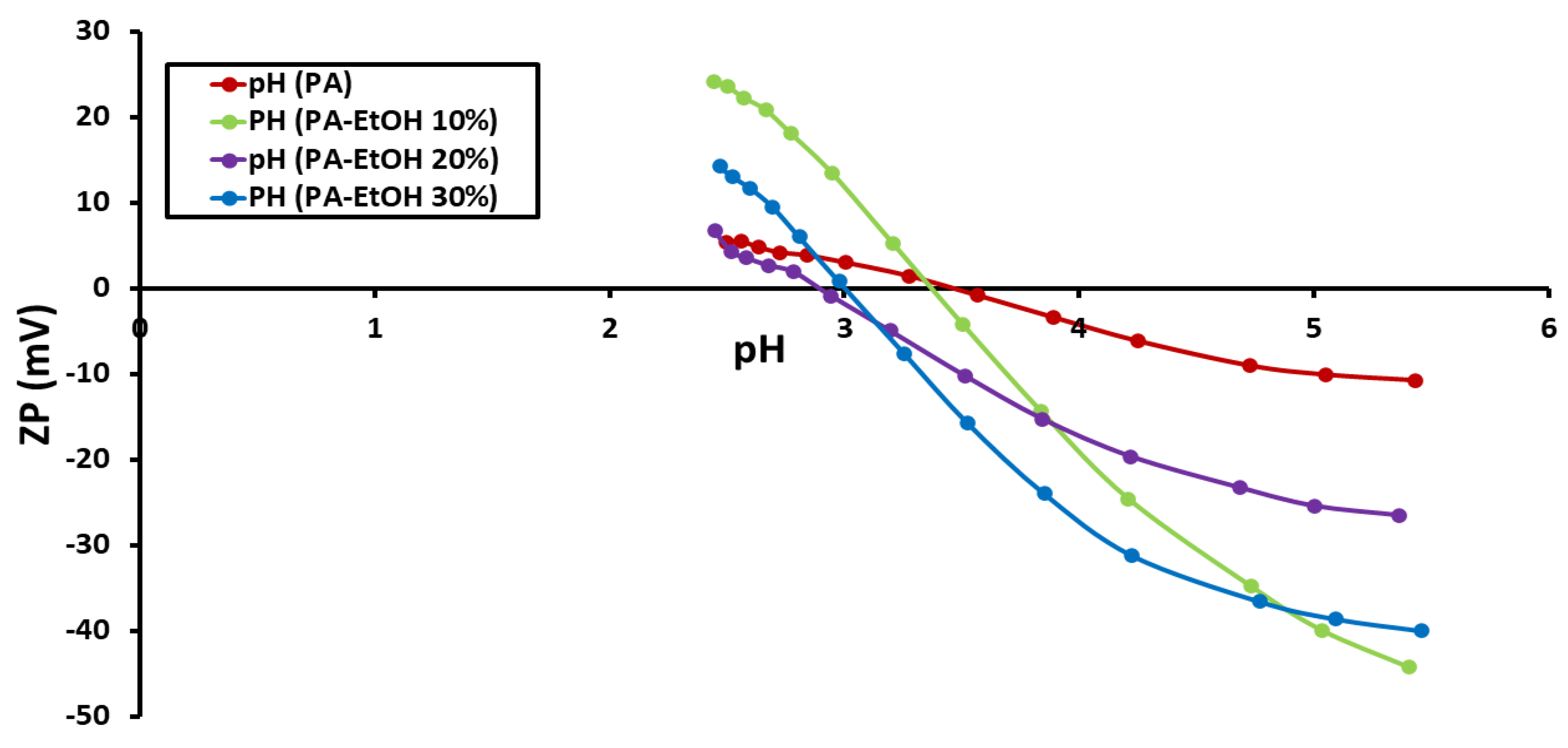
3.3. Determination of Zeta Potential (ZP)
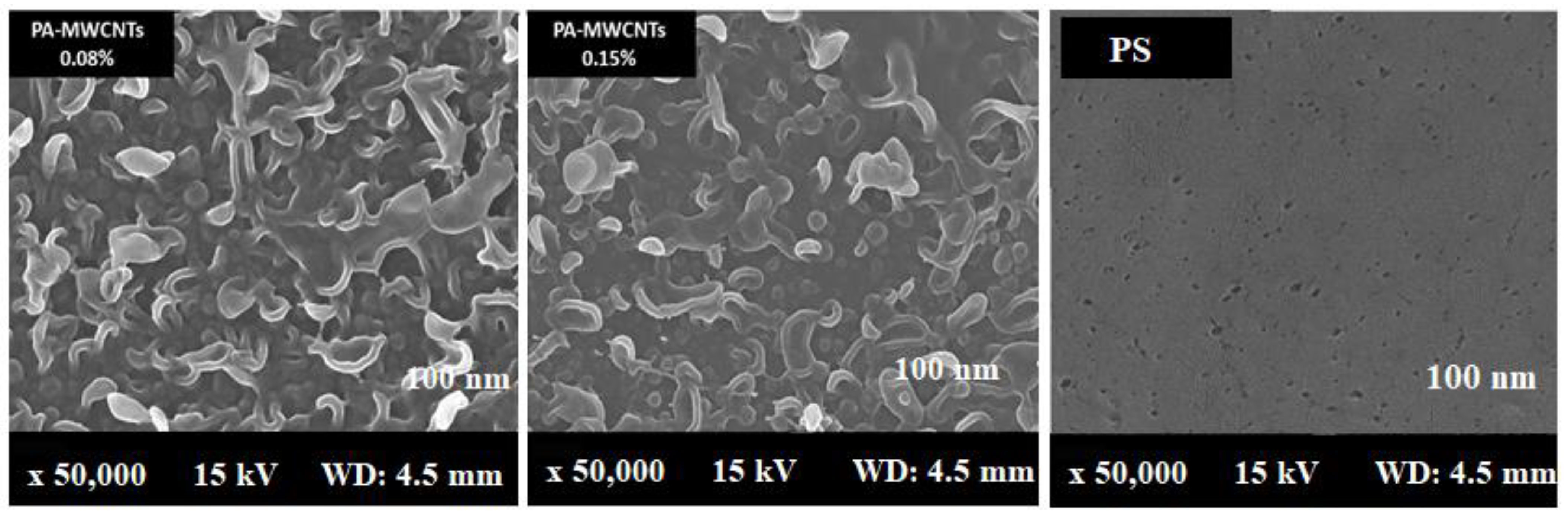
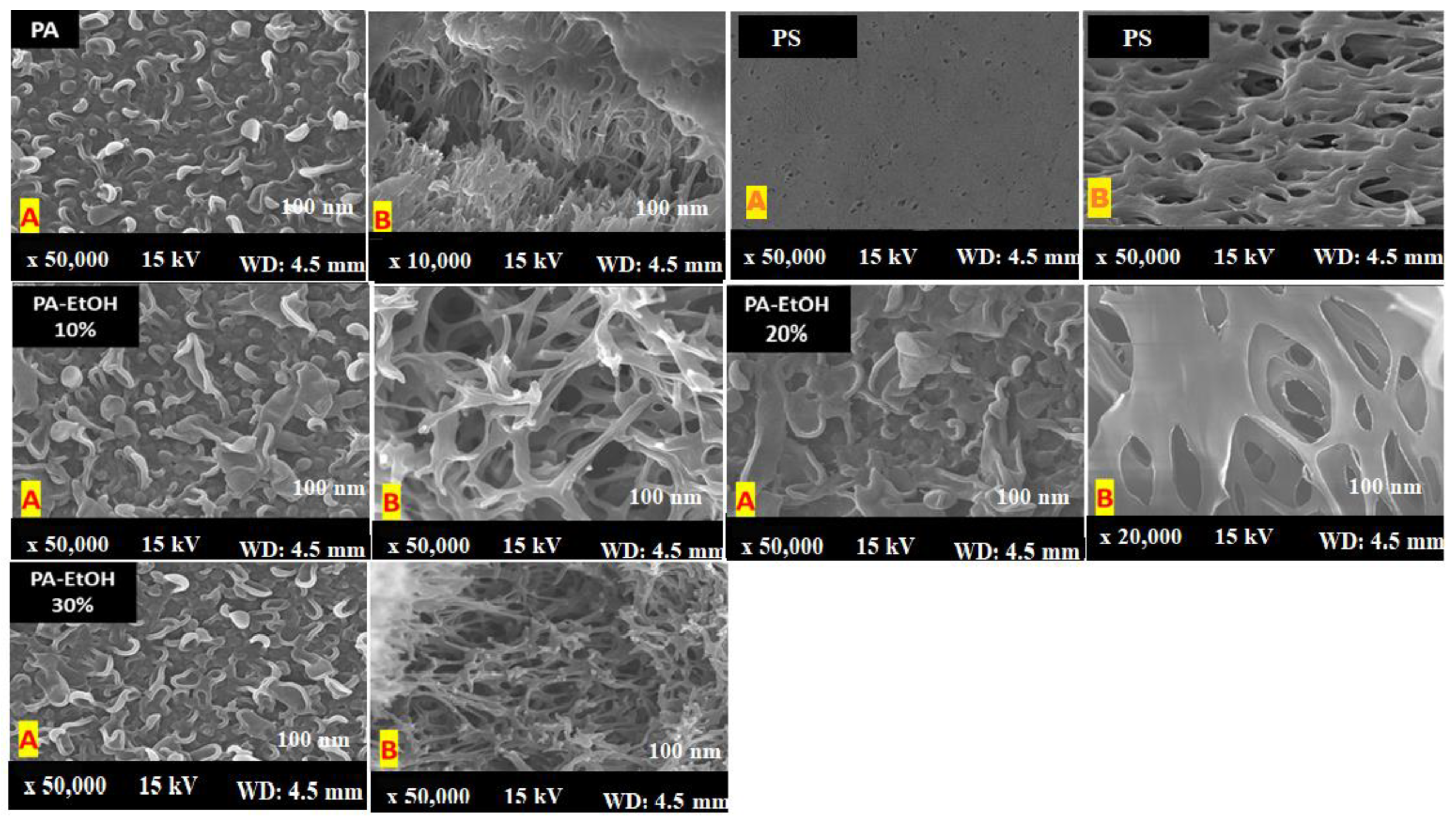
3.4. Scanning Electron Microscope Analysis (SEM)
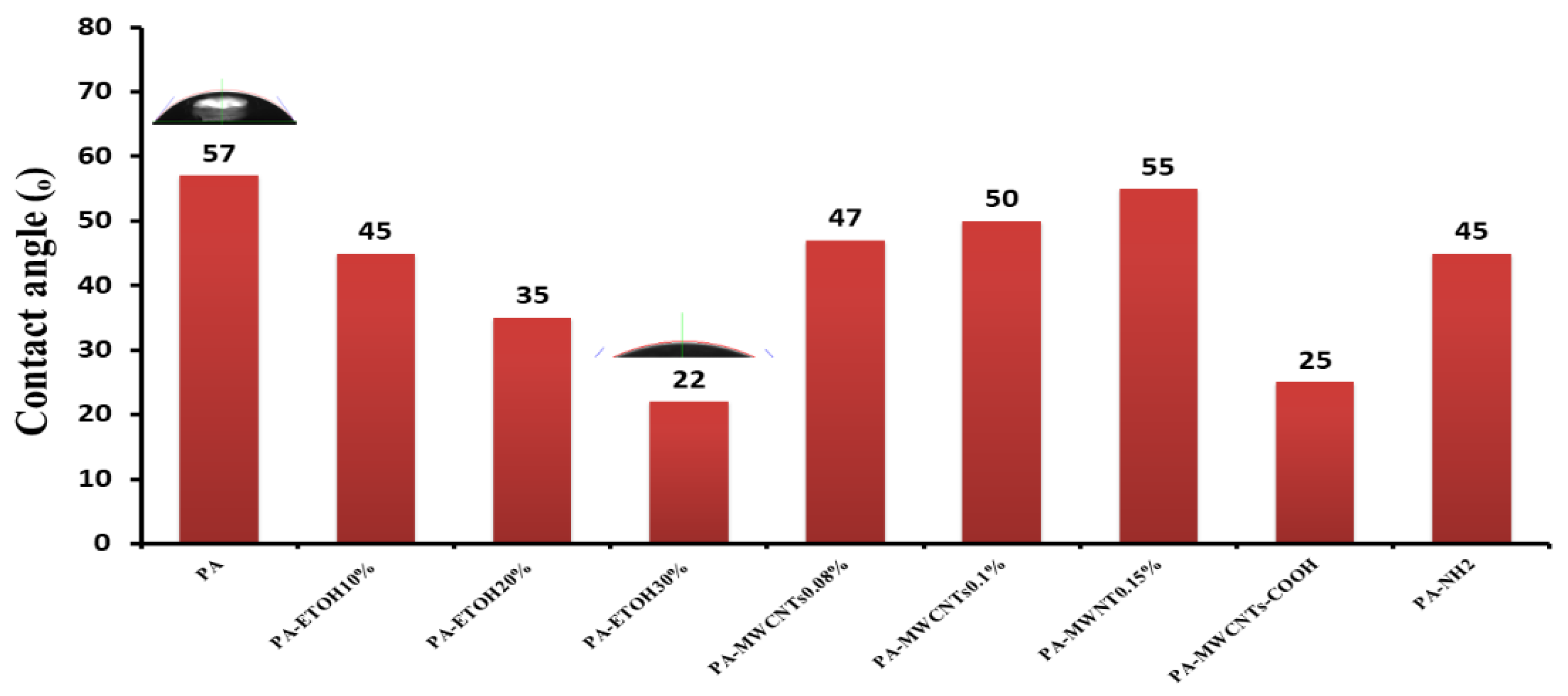
3.5. Contact Angle Measurement
4. Membrane Performance Evaluation
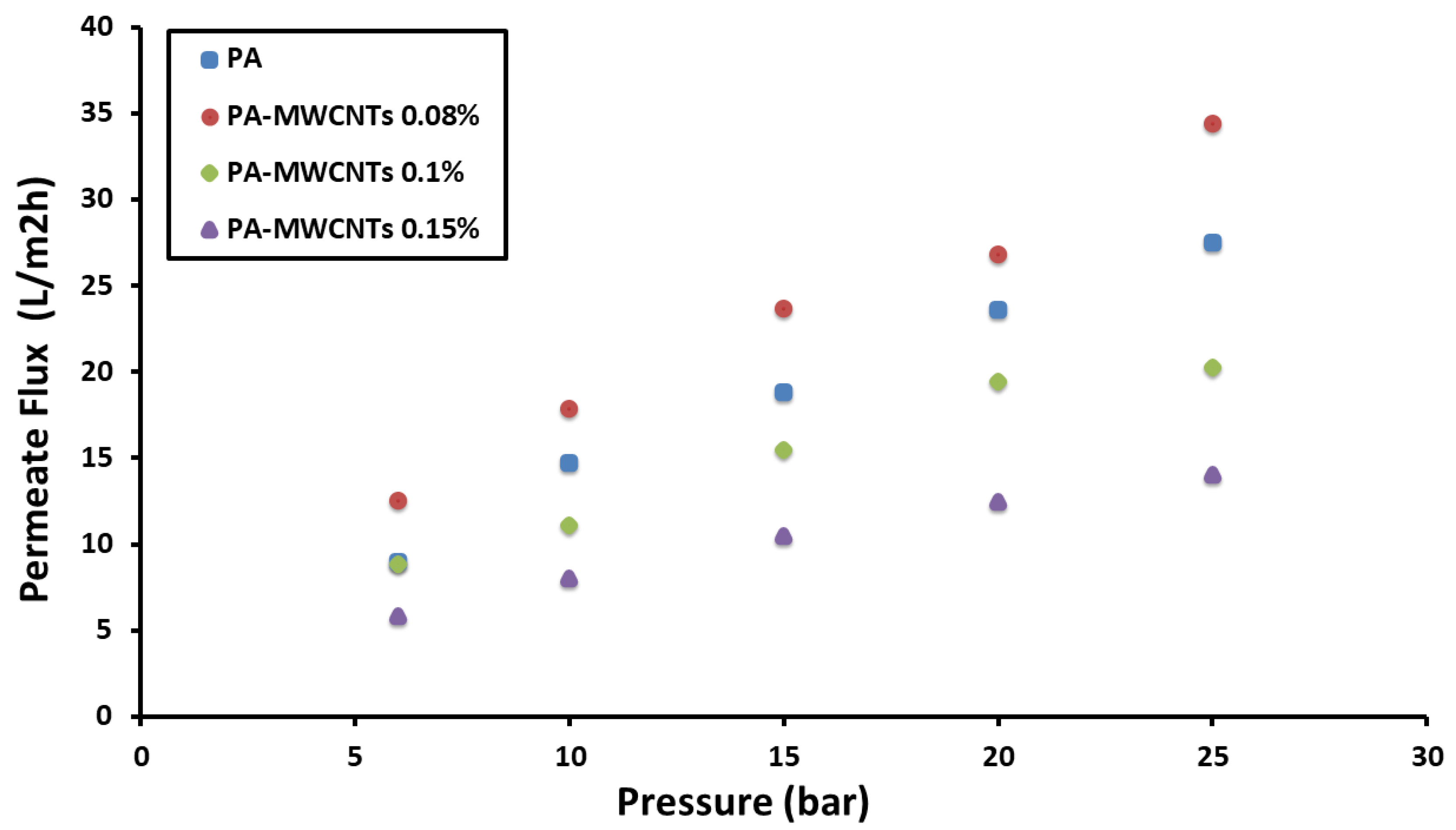
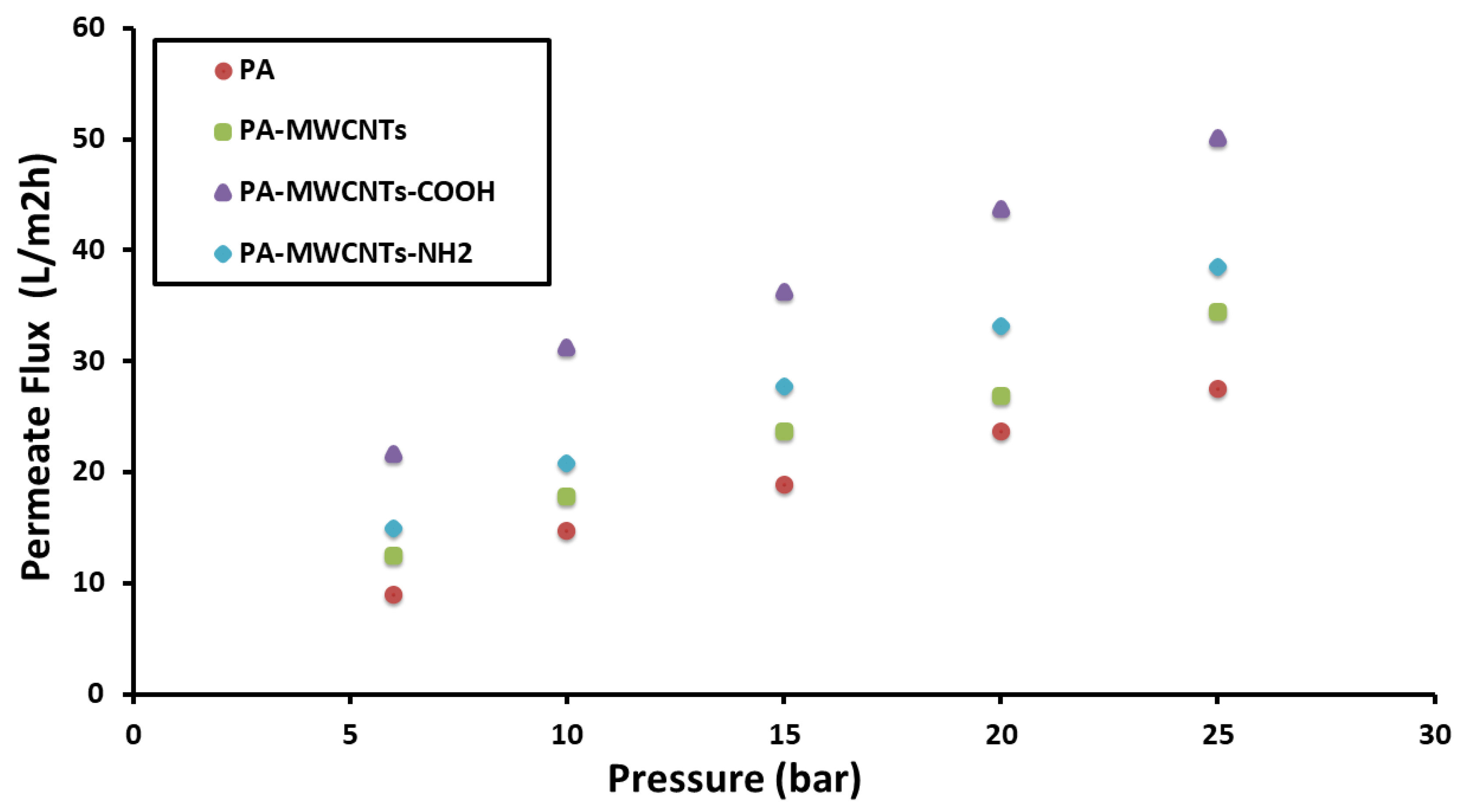
4.1. Water Permeability
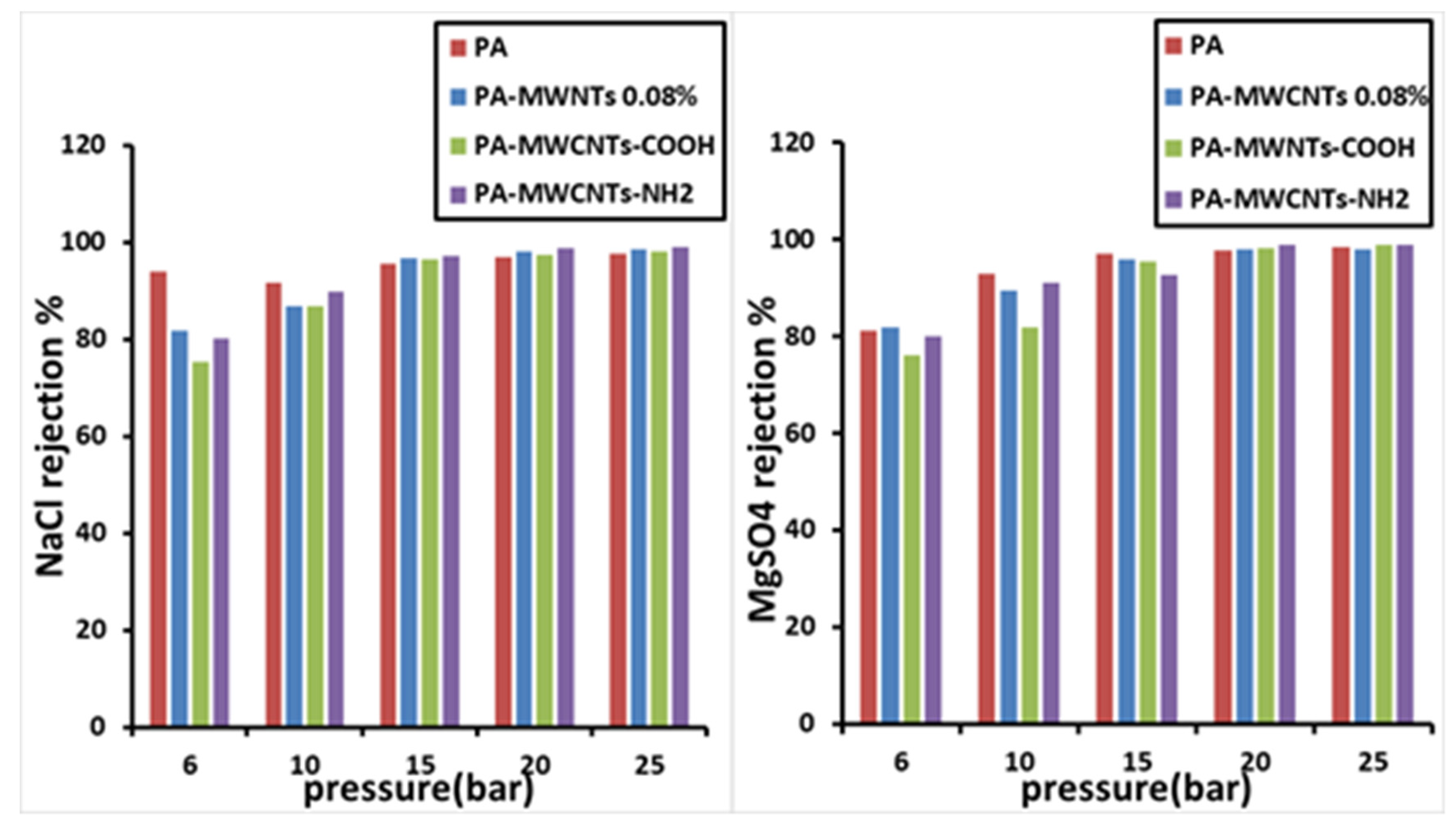
4.2. Salt Rejection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.; Azam, T. Water purification technologies. In Bottled and Packaged Water; Woodhead Pulishing: Cambridge, UK, 2019; pp. 83–120. [Google Scholar] [CrossRef]
- Prisciandaro, M.; di Celso, G.M. Back-flush effects on superficial water ultrafiltration. Desalination 2010, 256, 22–26. [Google Scholar] [CrossRef]
- Nabeela Nasreen, S.A.A.; Sundarrajan, S.; Syed Nizar, S.A.; Ramakrishna, S. Nanomaterials: Solutions to water-concomitant challenges. Membranes 2019, 9, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gohil, J.M.; Choudhury, R.R. Introduction to nanostructured and nano-enhanced polymeric membranes: Preparation, function, and application for water purification. Nanoscale Mater. Water Pur. 2019, 25–57. [Google Scholar] [CrossRef]
- Liu, F.; Wang, L.; Li, D.; Liu, Q.; Deng, B. A review: The effect of the microporous support during interfacial polymerization on the morphology and performances of a thin film composite membrane for liquid purification. RSC Adv. 2019, 9, 35417–35428. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xiao, K.; Liu, Z.; Gao, T.; Liang, S.; Huang, X. Large-scale membrane bioreactors for industrial wastewater treatment in China: Technical and economic features, driving forces, and perspectives. Engineering 2021, 7, 868–880. [Google Scholar] [CrossRef]
- Teella, A.; Huber, G.W.; Ford, D.M. Separation of acetic acid from the aqueous fraction of fast pyrolysis bio-oils using nanofiltration and reverse osmosis membranes. J. Memb. Sci. 2011, 378, 495–502. [Google Scholar] [CrossRef]
- Shatat, M.; Riffat, S.B. Water desalination technologies utilizing conventional and renewable energy sources. Int. J. Low-Carbon Technol. 2014, 9, 1–19. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Ang, W.L.; Leo, C.P.; Mohammad, A.W.; Hilal, N. Current advances in membrane technologies for saline wastewater treatment: A comprehensive review. Desalination 2021, 517, 115170. [Google Scholar] [CrossRef]
- Ounifi, I.; Guesmi, Y.; Ursino, C.; Castro-Munoz, R.; Agougui, H.; Jabli, M.; Hafiane, A.; Figoli, A.; Ferjani, E. Synthesis and Characterization of a Thin-Film Composite Nanofiltration Membrane Based on Polyamide-Cellulose Acetate: Application for Water Purification. J. Polym. Environ. 2022, 30, 707–718. [Google Scholar] [CrossRef]
- Habib, S.; Weinman, S.T. A review on the synthesis of fully aromatic polyamide reverse osmosis membranes. Desalination 2021, 502, 114939. [Google Scholar] [CrossRef]
- Abdellah Ali, S.F.; Gad, E.S.; Elnaggar, E.M.; Ali, M.E.; Sammak, A.A. Performance of Nanocomposite Polysulfone-Polyaniline Substrates for Enhanced Thin Film Membranes. Egypt. J. Chem. 2022, 65, 3. [Google Scholar] [CrossRef]
- El-Aassar, A.H.M.A. Polyamide thin film composite membranes using interfacial polymerization: Synthesis, characterization and reverse osmosis performance for water desalination. Aust. J. Basic Appl. Sci. 2012, 6, 382–391. [Google Scholar]
- Tiraferri, A.; Yip, N.Y.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. Relating performance of thin-film composite forward osmosis membranes to support layer formation and structure. J. Memb. Sci. 2011, 367, 340–352. [Google Scholar] [CrossRef] [Green Version]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Dong, X.; Chen, M.; Zhu, L.; Wang, C.; Yang, F.; Dong, Y. Fabrication and water treatment application of carbon nanotubes (CNTs)-based composite membranes: A review. Membranes 2017, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.S.; Deng, B. Fabrication of polyamide thin-film nano-composite (PA-TFN) membrane with hydrophilized ordered mesoporous carbon (H-OMC) for water purifications. J. Memb. Sci. 2011, 375, 46–54. [Google Scholar] [CrossRef]
- Alshahrani, A.A.; Alsohaimi, I.H.; Alshehri, S.; Alawady, A.R.; El-Aassar, M.R.; Nghiem, L.D.; Panhuis, M. Nanofiltration membranes prepared from pristine and functionalised multiwall carbon nanotubes/biopolymer composites for water treatment applications. J. Mater. Res. Technol. 2020, 9, 9080–9092. [Google Scholar] [CrossRef]
- Alawady, A.R.; Alshahrani, A.A.; Aouak, T.A.; Alandis, N.M. Polysulfone membranes with CNTs/Chitosan biopolymer nanocomposite as selective layer for remarkable heavy metal ions rejection capacity. Chem. Eng. J. 2020, 388, 124267. [Google Scholar] [CrossRef]
- Fahmey, M.S.; El-Aassar, A.H.M.; Abo-Elfadel, M.M.; Orabi, A.S.; Das, R. Comparative performance evaluations of nanomaterials mixed polysulfone: A scale-up approach through vacuum enhanced direct contact membrane distillation for water desalination. Desalination 2019, 451, 111–116. [Google Scholar] [CrossRef]
- Das, R.; Leo, B.F.; Murphy, F. The toxic truth about carbon nanotubes in water purification: A perspective view. Nanoscale Res. Lett. 2018, 13, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, R.; Hamid, S.B.A.; Ali, M.; Annuar, M.S.M.; Samsudin, E.M.B.; Bagheri, S. Covalent functionalization schemes for tailoring solubility of multi-walled carbon nanotubes in water and acetone solvents. Sci. Adv. Mater. 2015, 7, 2726–2737. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes. J. Memb. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Bilici, Z.; Ozay, Y.; Yuzer, A.; Ince, M.; Ocakoglu, K.; Dizge, N. Fabrication and characterization of polyethersulfone membranes functionalized with zinc phthalocyanines embedding different substitute groups. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126288. [Google Scholar] [CrossRef]
- Wilson, R.; George, G.; Jose, A.J. Polymer membranes reinforced with carbon-based nanomaterials for water purification. New Polym. Nanocom. Environ. Remed. 2018, 457–468. [Google Scholar] [CrossRef]
- Mamah, S.C.; Goh, P.S.; Ismail, A.F.; Suzaimi, N.D.; Yogarathinam, L.T.; Raji, Y.O.; El-badawy, T.H. Recent development in modification of polysulfone membrane for water treatment application. J. Water Proc. Eng. 2021, 40, 101835. [Google Scholar] [CrossRef]
- Baatiyyah, H. The impact of chemical cleaning on separation efficiency and properties of reverse osmosis membrane. King Abd. Univ. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Hurwitz, G.; Guillen, G.R.; Hoek, E.M. Probing polyamide membrane surface charge, zeta potential, wettability, and hydrophilicity with contact angle measurements. J. Memb. Sci. 2010, 349, 349–357. [Google Scholar] [CrossRef]
- Yakavalangi, E.M.; Rimaz, S.; Vatanpour, V. Effect of surface properties of polysulfone support on the performance of thin film composite polyamide reverse osmosis membranes. J. Appl. Polym. Sci. 2016, 134. [Google Scholar] [CrossRef]
- Vatanpour, V.; Dehqan, A.; Harifi-Mood, A.R. Ethaline deep eutectic solvent as a hydrophilic additive in modification of polyethersulfone membrane for antifouling and separation improvement. J. Memb. Sci. 2020, 614, 118528. [Google Scholar] [CrossRef]
- Wang, W.Y.; Shi, J.Y.; Wang, J.L.; Li, Y.L.; Gao, N.N.; Liu, Z.X.; Lian, W.T. Preparation and characterization of PEG-g-MWCNTs/PSf nano-hybrid membranes with hydrophilicity and antifouling properties. RSC Adv. 2015, 5, 84746–84753. [Google Scholar] [CrossRef]
- Sianipar, M.; Kim, S.H.; Iskandar, I.G.; Wenten, F. Functionalized carbon nanotube (CNT) membrane: Progress and challenges. RSC Adv. 2017, 7, 51175–51198. [Google Scholar] [CrossRef] [Green Version]
- Pillai, S.; Santana, A.; Das, R.; Shrestha, B.R.; Manalastas, E.; Mishra, H. A molecular to macro level assessment of direct contact membrane distillation for separating organics from water. J. Memb. Sci. 2020, 608, 118140. [Google Scholar] [CrossRef]
- Idarraga-Mora, J.I.; Lemelin, M.A.; Weinman, S.T.; Husson, S.M. Effect of short-term contact with C1–C4 monohydric alcohols on the water permeance of MPD-TMC thin-film composite reverse osmosis membranes. Membranes 2019, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Wang, Z.; Wu, J.; Zhao, S.; Wang, J.; Wang, S. Enhancing the flux of brackish water TFC RO membrane by improving support surface porosity via a secondary pore-forming method. J. Memb. Sci. 2016, 498, 227–241. [Google Scholar] [CrossRef]
- Wang, J.; Xu, R.; Yang, F.; Kang, J.; Cao, Y.; Xiang, M. Probing influences of support layer on the morphology of polyamide selective layer of thin film composite membrane. J. Memb. Sci. 2018, 556, 374–383. [Google Scholar] [CrossRef] [Green Version]






| Salt Rejection at 15 bar (%) | Water Permeability at 15 bar (L m−2 h−1 bar−1) | Contact Angle (°) | Membrane | |
|---|---|---|---|---|
| MgSO4 | NaCl | |||
| 97.12 | 95.55 | 18.85 | 57 ± 3 | PA |
| 99.35 | 98.46 | 30.9 | 45 ± 5 | PA-EtOH 10% |
| 99.62 | 99.09 | 33.75 | 35 ± 4 | PA-EtOH 20% |
| 98.27 | 99.29 | 44.21 | 22 ± 4 | PA-EtOH 30% |
| 96.03 | 96.79 | 23.662 | 47 ± 4 | PA-MWCNTs 0.08% |
| 96.89 | 95.98 | 15.45 | 50 ± 3 | PA-MWCNTs 0.1% |
| 91.5 | 93.42 | 10.5 | 55 ± 5 | PA-MWCNTs 0.15% |
| 95.43 | 96.43 | 36.225 | 25 ± 2 | PA-MWCNTs-COOH |
| 92.77 | 97.11 | 27.75 | 45 ± 2 | PA-MWNHs-NH2 |
| Membrane | Test Conditions | Flux (L/m2 h) | NaCl Salt Rejection % | Ref. |
|---|---|---|---|---|
| PA-SiO2 1% | 15 bar, 2000 ppm NaCl solutions | 47.9 | 98.9% | [36] |
| PA-DMF 12% | 16 bar, 2000 ppm NaCl solutions | 3.98 | 98.8% | [37] |
| PA-NMP 82% | 15 bar, 2000 ppm NaCl solutions | 36 | 95% | [29] |
| PA-EtOH 30% | 15 bar, 2000 ppm NaCl solutions | 44.21 | 99.29% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alterary, S.S.; Alyabes, R.M.; Alshahrani, A.A.; Al-Alshaikh, M.A. Unfunctionalized and Functionalized Multiwalled Carbon Nanotubes/Polyamide Nanocomposites as Selective-Layer Polysulfone Membranes. Polymers 2022, 14, 1544. https://doi.org/10.3390/polym14081544
Alterary SS, Alyabes RM, Alshahrani AA, Al-Alshaikh MA. Unfunctionalized and Functionalized Multiwalled Carbon Nanotubes/Polyamide Nanocomposites as Selective-Layer Polysulfone Membranes. Polymers. 2022; 14(8):1544. https://doi.org/10.3390/polym14081544
Chicago/Turabian StyleAlterary, Seham S., Raya M. Alyabes, Ahmed A. Alshahrani, and Monirah A. Al-Alshaikh. 2022. "Unfunctionalized and Functionalized Multiwalled Carbon Nanotubes/Polyamide Nanocomposites as Selective-Layer Polysulfone Membranes" Polymers 14, no. 8: 1544. https://doi.org/10.3390/polym14081544





