Electrospun Biodegradable α-Amino Acid-Substituted Poly(organophosphazene) Fiber Mats for Stem Cell Differentiation towards Vascular Smooth Muscle Cells
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PαAPz
2.3. Macromolecular Substitution
2.4. PDCP and PαAPz Characterization with 31P-NMR and 1H-NMR
2.5. Preparation of PαAPz Thin Films
2.6. Electrospinning of PαAPz
2.7. Cell Culture Studies on PαAPz and Smooth Muscle Cell Differentiation
2.8. Quantitative Real-Time qPCR and Western Blot Analysis
2.9. Immunofluorescence Microscopy
2.10. Statistical Analysis
3. Results and Discussion
3.1. PαAPz Synthesis and Characterization
3.2. Electrospinning of PαAPz
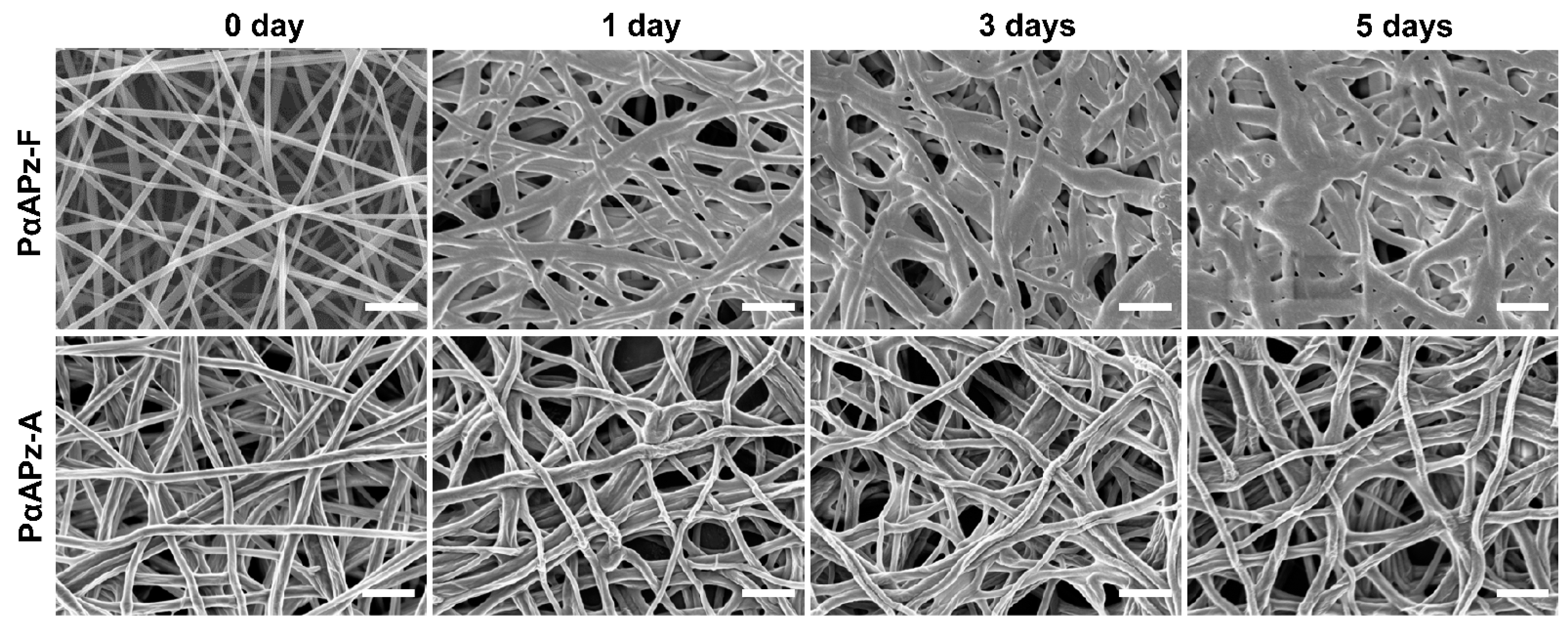
3.3. Short-Term In Vitro Degradation Study
3.4. Cell Adhesion and Morphology on PαAPz-A Films
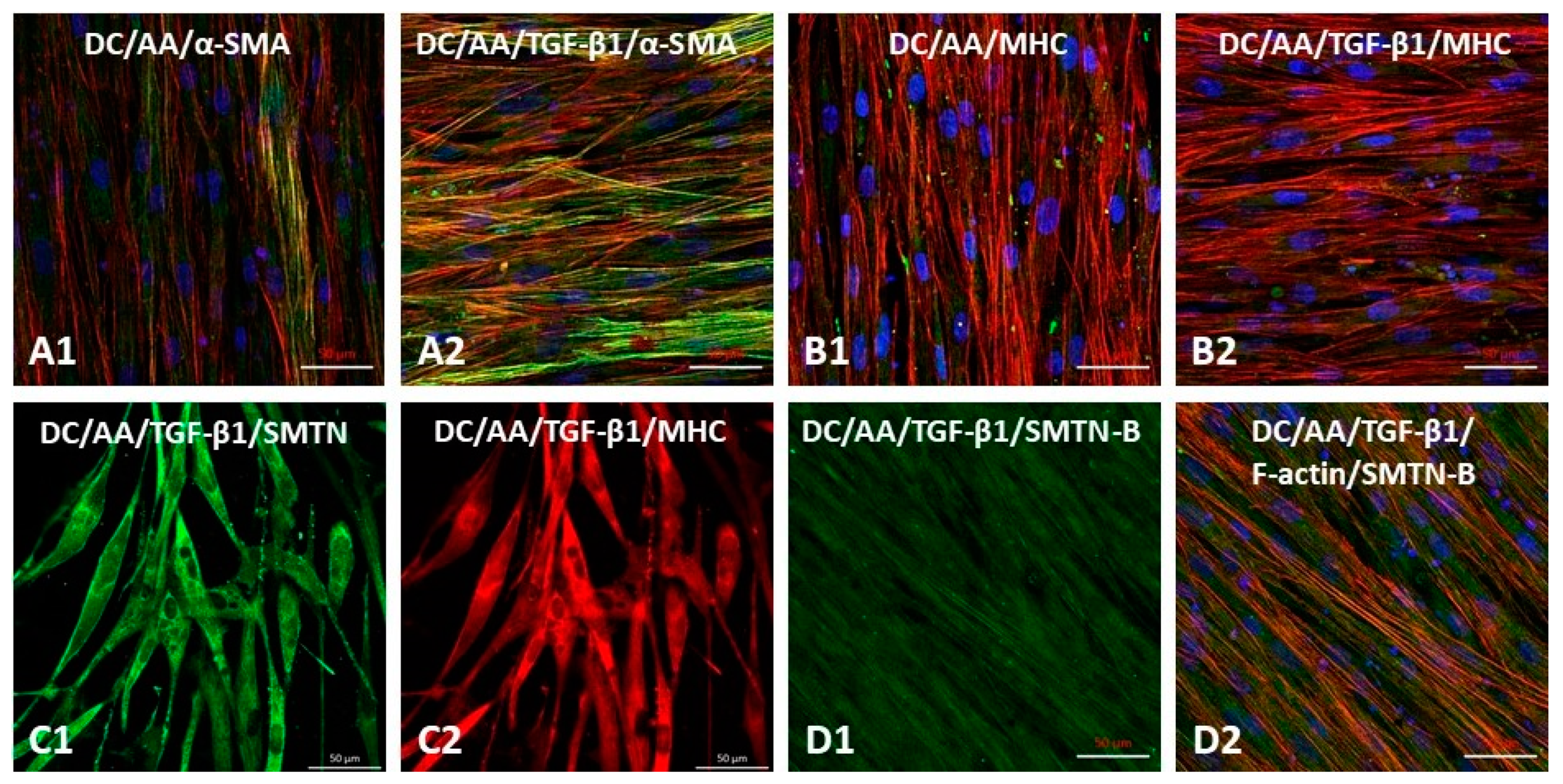
3.5. Differentiation Potential of iMSCs towards Smooth Muscle Phenotype
3.6. Differentiation of iMSCs towards Smooth Muscle Phenotype on Electrospun PαAPz-A Fibrous Mats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogueri, K.S.; Allcock, H.R.; Laurencin, C.T. Polyphosphazene polymers: The next generation of biomaterials for regenerative engineering and therapeutic drug delivery. J. Vac. Sci. Technol. B. Nanotechnol. Microelectron. 2020, 38, 030801. [Google Scholar] [CrossRef] [PubMed]
- Ogueri, K.S.; Ogueri, K.S.; Ude, C.C.; Allcock, H.R.; Laurencin, C.T. Biomedical applications of polyphosphazenes. Med. Devices Sens. 2020, 3, 10113. [Google Scholar] [CrossRef] [PubMed]
- Ogueri, K.S.; Escobar Ivirico, J.L.; Nair, L.S.; Allcock, H.R.; Laurencin, C.T. Biodegradable polyphosphazene-based blends for regenerative engineering. Regen. Eng. Transl. Med. 2017, 3, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Yu, L.; Ding, J. PEG-based thermosensitive and biodegradable hydrogels. Acta Biomater. 2021, 128, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Decollibus, D.P.; Marin, A.; Andrianov, A.K. Effect of environmental factors on hydrolytic degradation of water-soluble polyphosphazene polyelectrolyte in aqueous solutions. Biomacromolecules 2010, 11, 2033–2038. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.K.; Marin, A. Degradation of polyaminophosphazenes: Effects of hydrolytic environment and polymer processing. Biomacromolecules 2006, 7, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Ogueri, K.S.; Jafari, T.; Escobar Ivirico, J.L.; Laurencin, C.T. Polymeric biomaterials for scaffold-based bone regenerative engineering. Regen. Eng. Transl. Med. 2019, 5, 128–154. [Google Scholar] [CrossRef] [PubMed]
- Peach, M.S.; Ramos, D.M.; James, R.; Morozowich, N.L.; Mazzocca, A.D.; Doty, S.B.; Allcock, H.R.; Kumbar, S.G.; Laurencin, C.T. Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering. PLoS ONE 2017, 12, e0174789. [Google Scholar] [CrossRef]
- Baillargeon, A.L.; Mequanint, K. Biodegradable polyphosphazene biomaterials for tissue engineering and delivery of therapeutics. Biomed. Res. Int. 2014, 2014, 761373. [Google Scholar] [CrossRef]
- Brown, J.L.; Nair, L.S.; Laurencin, C.T. Solvent/non-solvent sintering: A novel route to create porous microsphere scaffolds for tissue regeneration. J. Biomed. Mater. Res. B 2008, 86, 396–406. [Google Scholar] [CrossRef] [Green Version]
- Nukavarapu, S.P.; Kumbar, S.G.; Brown, J.L.; Krogman, N.R.; Weikel, A.L.; Hindenlang, M.D.; Nair, L.S.; Allcock, H.R.; Laurencin, C.T. Polyphosphazene/nano-hydroxyapatite composite microsphere scaffolds for bone tissue engineering. Biomacromolecules 2008, 9, 1818–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, S.; Kumbar, S.G.; Khan, Y.M.; Nair, L.S.; Singh, A.; Krogman, N.R.; Brown, P.W.; Allcock, H.R.; Laurencin, C.T. Biodegradable polyphosphazene-nanohydroxyapatite composite nanofibers: Scaffolds for bone tissue engineering. J. Biomed. Nanotechnol. 2009, 5, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Conconi, M.T.; Lora, S.; Menti, A.M.; Carampin, P.; Parnigotto, P.P. In vitro evaluation of poly[bis(ethyl alanato)phosphazene] as a scaffold for bone tissue engineering. Tissue Eng. 2006, 12, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Conconi, M.T.; Lora, S.; Baiguera, S.; Boscolo, E.; Folin, M.; Scienza, R.; Rebuffat, P.; Parnigotto, P.P.; Nussdorfer, G.G. In vitro culture of rat neuromicrovascular endothelial cells on polymeric scaffolds. J. Biomed. Mater. Res. A 2004, 71, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Carampin, P.; Conconi, M.T.; Lora, S.; Menti, A.M.; Baiguera, S.; Bellini, S.; Grandi, C.; Parnigotto, P.P. Electrospun polyphosphazene nanofibers for in vitro rat endothelial cells proliferation. J. Biomed. Mater. Res. A 2007, 80, 661–668. [Google Scholar] [CrossRef]
- Srinath, D.; Lin, S.; Knight, D.K.; Rizkalla, A.S.; Mequanint, K. Fibrous biodegradable l-alanine-based scaffolds for vascular tissue engineering. J. Tissue Eng. Regen. Med. 2014, 8, 578–588. [Google Scholar] [CrossRef]
- Allo, B.A.; Lin, S.; Mequanint, K.; Rizkalla, A.S. Role of bioactive 3D hybrid fibrous scaffolds on mechanical behavior and spatiotemporal osteoblast gene expression. ACS Appl. Mater. Interfaces 2013, 5, 7574–7583. [Google Scholar] [CrossRef]
- Afra, S.; Matin, M.M. Potential of mesenchymal stem cells for bioengineered blood vessels in comparison with other eligible cell sources. Cell Tissue Res. 2020, 380, 1–13. [Google Scholar] [CrossRef]
- Gong, Z.; Niklason, L.E. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J. 2008, 22, 1635–1648. [Google Scholar] [CrossRef] [Green Version]
- Gu, W.; Hong, X.; Le Bras, A.; Nowak, W.N.; Issa Bhaloo, S.; Deng, J.; Xie, Y.; Hu, Y.; Ruan, X.Z.; Xu, Q. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J. Biol. Chem. 2018, 293, 8089–8102. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Tsai, A.L.; Li, P.C.; Huang, C.W.; Wu, C.C. Endothelial differentiation of bone marrow mesenchyme stem cells applicable to hypoxia and increased migration through Akt and NFκB signals. Stem Cell Res. Ther. 2017, 8, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittenger, M.F.; Martin, B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004, 95, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhu, L.; Fu, S.; Qian, Y.; Wang, D.; Wang, C. Small diameter blood vessels bioengineered from human adipose-derived stem cells. Sci. Rep. 2016, 6, 35422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beeravolu, N.; McKee, C.; Alamri, A.; Mikhael, S.; Brown, C.; Perez-Cruet, M.; Chaudhry, G.R. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J. Vis. Exp. 2017, 122, e55224. [Google Scholar] [CrossRef] [Green Version]
- Bieback, K.; Kern, S.; Kocaömer, A.; Ferlik, K.; Bugert, P. Comparing mesenchymal stromal cells from different human tissues: Bone marrow, adipose tissue and umbilical cord blood. Biomed. Mater. Eng. 2008, 18, S71–S76. [Google Scholar]
- Sensebé, L.; Gadelorge, M.; Fleury-Cappellesso, S. Production of mesenchymal stromal/stem cells according to good manufacturing practices: A review. Stem Cell Res. Ther. 2013, 4, 66. [Google Scholar] [CrossRef] [Green Version]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; et al. Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS ONE 2008, 3, e2213. [Google Scholar] [CrossRef] [Green Version]
- Kang, L.; Wang, J.; Zhang, Y.; Kou, Z.; Gao, S. iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell 2009, 5, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Park, I.H.; Zhao, R.; West, J.A.; Yabuuchi, A.; Huo, H.; Ince, T.A.; Lerou, P.H.; Lensch, M.W.; Daley, G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008, 451, 141–146. [Google Scholar] [CrossRef]
- Hsia, L.T.; Ashley, N.; Ouaret, D.; Wang, L.M.; Wilding, J.; Bodmer, W.F. Myofibroblasts are distinguished from activated skin fibroblasts by the expression of AOC3 and other associated markers. Proc. Natl. Acad. Sci. USA 2016, 113, E2162–E2171. [Google Scholar] [CrossRef] [Green Version]
- Von Kleeck, R.; Castagnino, P.; Roberts, E.; Talwar, S.; Ferrari, G.; Assoian, R.K. Decreased vascular smooth muscle contractility in Hutchinson-Gilford Progeria Syndrome linked to defective smooth muscle myosin heavy chain expression. Sci. Rep. 2021, 11, 10625. [Google Scholar] [CrossRef] [PubMed]
- Rothdiener, M.; Hegemann, M.; Uynuk-Ool, T.; Walters, B.; Papugy, P.; Nguyen, P.; Claus, V.; Seeger, T.; Stoeckle, U.; Boehme, K.A.; et al. Stretching human mesenchymal stromal cells on stiffness-customized collagen type I generates a smooth muscle marker profile without growth factor addition. Sci. Rep. 2016, 6, 35840. [Google Scholar] [CrossRef] [PubMed]
- Fakhrieh, M.; Darvish, M.; Ardeshirylajimi, A.; Taheri, M.; Omrani, M.D. Improved bladder smooth muscle cell differentiation of the mesenchymal stem cells when grown on electrospun polyacrylonitrile/polyethylene oxide nanofibrous scaffold. J. Cell Biochem. 2019, 120, 15814–15822. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasu, H.; Sadiq, A.; Ratheesh, G.; Sridhar, S.; Ramakrishna, S.; Ab Rahim, M.H.; Yusoff, M.M.; Jose, R.; Reddy, V.J. Functionalized core/shell nanofibers for the differentiation of mesenchymal stem cells for vascular tissue engineering. Nanomedicine 2019, 14, 201–214. [Google Scholar] [CrossRef]
- Baillargeon, A.L.; Penev, K.I.; Mequanint, K. One-pot substitution approach for the syntheses of nonfunctional and functional poly [(amino acid ester) phosphazene] Biomaterials. Macromol. Mater. Eng. 2017, 302, 1600318. [Google Scholar] [CrossRef]
- Luo, J.; Qin, L.; Kural, M.H.; Schwan, J.; Li, X.; Bartulos, O.; Cong, X.Q.; Ren, Y.; Gui, L.; Li, G.; et al. Vascular smooth muscle cells derived from inbred swine induced pluripotent stem cells for vascular tissue engineering. Biomaterials 2017, 147, 116–132. [Google Scholar] [CrossRef]
- Atchison, L.; Zhang, H.; Cao, K.; Truskey, G.A. A Tissue engineered blood vessel model of Hutchinson-Gilford Progeria Syndrome using human iPSC-derived smooth muscle cells. Sci. Rep. 2017, 7, 8168. [Google Scholar] [CrossRef]
- Glenn, G.; Shogren, R.; Jin, X.; Orts, W.; Hart-Cooper, W.; Olson, L. Per- and polyfluoroalkyl substances and their alternatives in paper food packaging. Compr. Rev. Food Sci. F 2021, 20, 2596–2625. [Google Scholar] [CrossRef]
- Lemmouchi, Y.; Schacht, E.; Dejardin, S. Biodegradable poly[(amino acid ester)phosphazenes] for biomedical applications. J. Bioact. Compat. Pol. 1998, 13, 4–18. [Google Scholar] [CrossRef]
- Nichol, J.L.; Morozowich, N.L.; Allcock, H.R. Biodegradable alanine and phenylalanine alkyl ester polyphosphazenes as potential ligament and tendon tissue scaffolds. Polym. Chem. 2013, 4, 600–606. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Nair, L.S.; Singh, A.; Krogman, N.R.; Greish, Y.E.; Brown, P.W.; Allcock, H.R.; Laurencin, C.I. Electrospinning of poly[bis(ethyl alanato) phosphazene] nanofibers. J. Biomed. Nanotechnol. 2006, 2, 36–45. [Google Scholar] [CrossRef]
- Härdelin, L.; Perzon, E.; Hagström, B.; Walkenström, P.; Gatenholm, P. Influence of molecular weight and rheological behavior on electrospinning cellulose nanofibers from ionic liquids. J. Appl. Polym. Sci. 2013, 130, 2303–2310. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Sanchez, G.; López-Rubio, A. Impact of molecular weight on the formation of electrosprayed chitosan microcapsules as delivery vehicles for bioactive compounds. Carbohydr. Polym. 2016, 150, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colmenares-Roldán, G.J.; Quintero-Martínez, Y.; Agudelo-Gómez, L.M.; Rodríguez-Vinasco, L.F.; Hoyos-Palacio, L.M. Influence of the molecular weight of polymer, solvents and operational condition in the electrospinning of polycaprolactone. Rev. Fac. Ing. Univ. Antioq. 2017, 84, 35–45. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng. Part C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolo, A.; Mehr, M.; Janner-Jametti, T.; Graumann, U.; Aebli, N.; Baur, M.; Ferguson, S.J.; Stoyanov, J.V. An in vitro expansion score for tissue-engineering applications with human bone marrow-derived mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2016, 10, 149–161. [Google Scholar] [CrossRef]
- Wang, C.; Meng, H.; Wang, X.; Zhao, C.; Peng, J.; Wang, Y. Differentiation of bone marrow mesenchymal stem cells in osteoblasts and adipocytes and its role in treatment of osteoporosis. Med. Sci. Monit. 2016, 22, 226–233. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Li, G. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Chen, M.; Su, W.; Tao, X.; Sun, M.; Zou, X.; Ying, R.; Wei, W.; Wang, B. The differentiation of mesenchymal stem cells to vascular cells regulated by the HMGB1/RAGE axis: Its application in cell therapy for transplant arteriosclerosis. Stem Cell Res. Ther. 2018, 9, 85. [Google Scholar] [CrossRef]
- Maguire, E.M.; Xiao, Q.; Xu, Q. Differentiation and application of induced pluripotent stem cell-derived vascular smooth muscle cells. Arter. Thromb. Vasc. Biol. 2017, 37, 2026–2037. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, V.K.; Mistriotis, P.; Loh, Y.H.; Daley, G.Q.; Andreadis, S.T. Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc. Res. 2012, 96, 391–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaram, S.; One, J.; Siewert, J.; Teodosescu, S.; Zhao, L.; Dimitrievska, S.; Qian, H.; Huang, A.H.; Niklason, L. Tissue-engineered vascular grafts created from human induced pluripotent stem cells. Stem Cells Transl. Med. 2014, 3, 1535–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eoh, J.H.; Shen, N.; Burke, J.A.; Hinderer, S.; Xia, Z.; Schenke-Layland, K.; Gerecht, S. Enhanced elastin synthesis and maturation in human vascular smooth muscle tissue derived from induced-pluripotent stem cells. Acta Biomater. 2017, 52, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.L.; Gabbiani, G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- Yoshida, T.; Owens, G.K. Molecular determinants of vascular smooth muscle cell diversity. Circ. Res. 2005, 96, 280–291. [Google Scholar] [CrossRef]
- Peach, M.S.; Kumbar, S.G.; James, R.; Toti, U.S.; Balasubramaniam, D.; Deng, M.; Ulery, B.; Mazzocca, A.D.; McCarthy, M.B.; Morozowich, N.L.; et al. Design and optimization of polyphosphazene functionalized fiber matrices for soft tissue regeneration. J. Biomed. Nanotechnol. 2012, 8, 107–124. [Google Scholar] [CrossRef]
- Peach, M.S.; James, R.; Toti, U.S.; Deng, M.; Morozowich, N.L.; Allcock, H.R.; Laurencin, C.T.; Kumbar, S.G. Polyphosphazene functionalized polyester fiber matrices for tendon tissue engineering: In vitro evaluation with human mesenchymal stem cells. Biomed. Mater. 2012, 7, 045016. [Google Scholar] [CrossRef]




| Gene | Forward Primer (5′→3′) | Reverse Primer (3′→5′) |
|---|---|---|
| Human Acta2 | CAA GTG ATC ACC ATC GGA AAT G | GAC TCC ATC CCG ATG AAG GA |
| Human Cnn1 | TGA AGC CCC ACG ACA TTT TT | GGG TGG ACT GCA CCT GTG TA |
| Human Myh11 | GTC CAG GAG ATG AGG CAG AAA C | GTC TGC GTT CTC TTT CTC CAG C |
| Human SMTN | CAG GAC AAC AAG GAG AAC TGG | CAG TCA ATT CCT CCA CAT CGT |
| Human 18S | GCG GTT CTA TTT TGT TGG TTT | CTC CGA CTT TCG TTC TTG ATT |
| Method | Reaction Time at 230 °C | Integration of +20 ppm Peak | Integration of −17 ppm Peak | % Conversion |
|---|---|---|---|---|
| GS | 25 h | 1 | 1.19 | 54 |
| GS-Ar | 54 h | 1 | 6.51 | 86 |
| FS | 58 h | 1 | 5.70 | 85 |
| FS-Ar | 93 h | 1 | 5.32 | 84 |
| FS-Vacuum | 92 h | 1 | 18.91 | 95 |
| R-FS-Vacuum | 92 h | 1 | 15.38 | 94 |
| Sample Abbreviations | Solvent Ratio | Distance (cm) | Voltage (kV) | Concentration (wt%) | Flow Rate (mL/h) |
|---|---|---|---|---|---|
| PDCP from FS-vacuum method | |||||
| PαAPz-A | CF:DMSO (3:1) | 12–15(*12) | 15–20(*20) | 7.5–12.5(*10) | 0.2–0.6(*0.2) |
| PαAPz-F | THF:CF (9:1) | 9–15(*12) | 12–20(*12) | 10–15(*10) | 0.2–0.6(*0.2) |
| PDCP from R-FS-vacuum method | |||||
| PαAPz-A | CF:DMSO (3:1) | 12–15(*12) | 15–20(*15) | 7.5–12.5(*10) | 0.2–0.6(*0.2) |
| PαAPz-A | THF:CF (9:1) | 12–15(*12) | 15–20(*15) | 7.5–12.5(*10) | 0.2–0.6(*0.2) |
| PαAPz-F | THF:CF (9:1) | 9–15(*12) | 12–20(*12) | 10–15(*10) | 0.2–0.6(*0.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Lin, S.; Mequanint, K. Electrospun Biodegradable α-Amino Acid-Substituted Poly(organophosphazene) Fiber Mats for Stem Cell Differentiation towards Vascular Smooth Muscle Cells. Polymers 2022, 14, 1555. https://doi.org/10.3390/polym14081555
Wang M, Lin S, Mequanint K. Electrospun Biodegradable α-Amino Acid-Substituted Poly(organophosphazene) Fiber Mats for Stem Cell Differentiation towards Vascular Smooth Muscle Cells. Polymers. 2022; 14(8):1555. https://doi.org/10.3390/polym14081555
Chicago/Turabian StyleWang, Meng, Shigang Lin, and Kibret Mequanint. 2022. "Electrospun Biodegradable α-Amino Acid-Substituted Poly(organophosphazene) Fiber Mats for Stem Cell Differentiation towards Vascular Smooth Muscle Cells" Polymers 14, no. 8: 1555. https://doi.org/10.3390/polym14081555
APA StyleWang, M., Lin, S., & Mequanint, K. (2022). Electrospun Biodegradable α-Amino Acid-Substituted Poly(organophosphazene) Fiber Mats for Stem Cell Differentiation towards Vascular Smooth Muscle Cells. Polymers, 14(8), 1555. https://doi.org/10.3390/polym14081555






