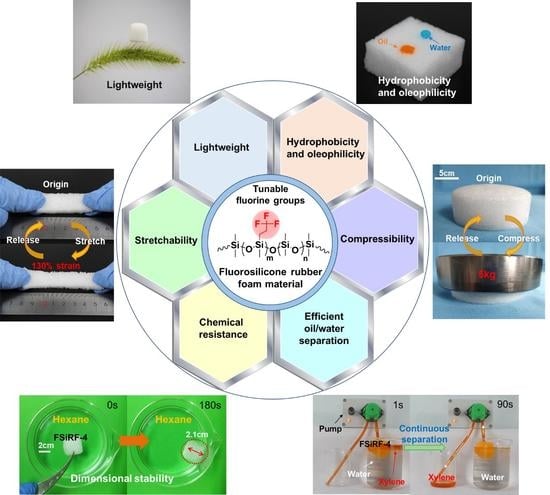
Green and Rapid Preparation of Fluorosilicone Rubber Foam Materials with Tunable Chemical Resistance for Efficient Oil–Water Separation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PDFS-Vi-X
2.3. Preparation of FSiRF Materials
2.4. Characterizations
3. Results and Discussion
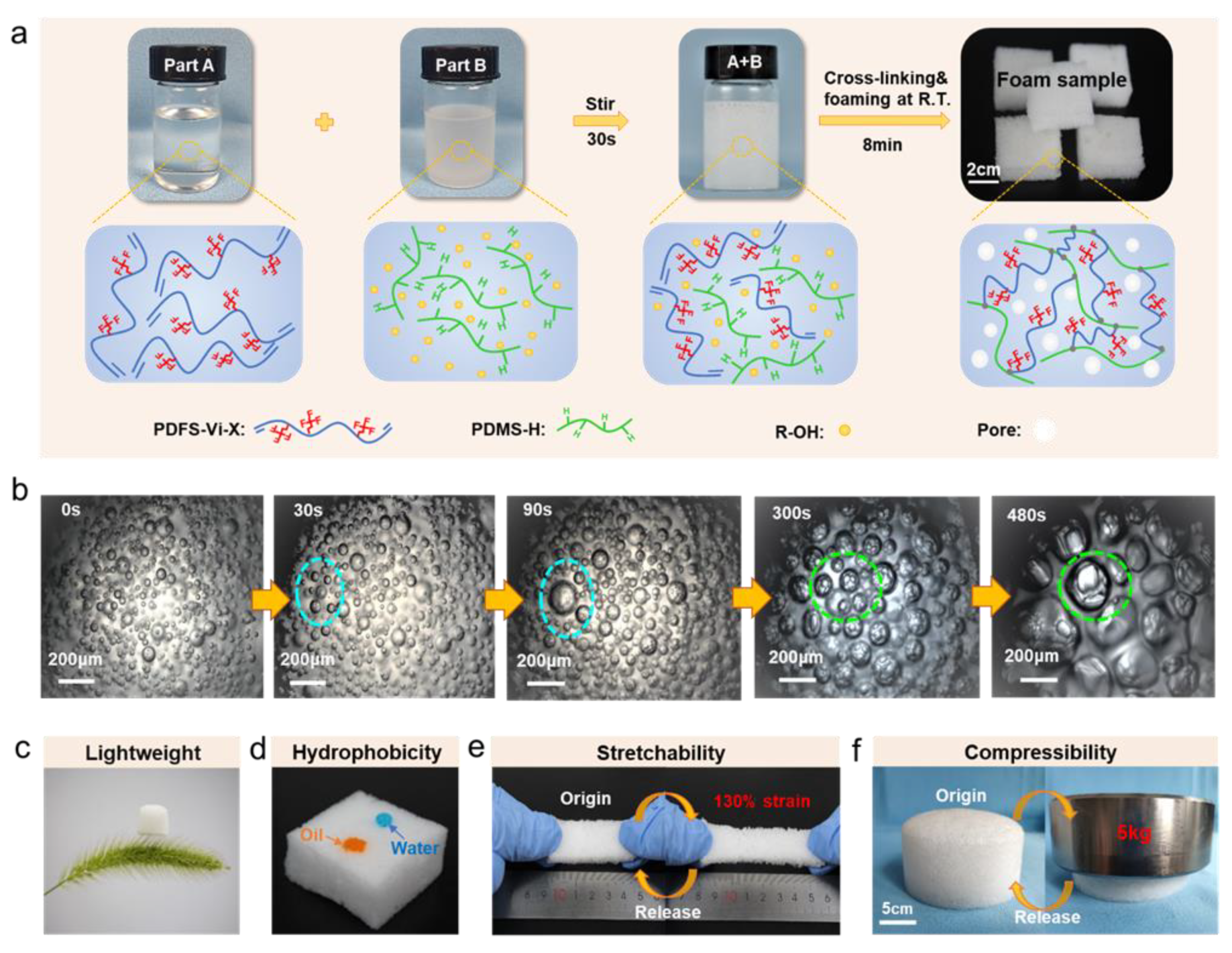
3.1. Preparation and Performance of FSiRF Materials
3.2. Molecular Design and Synthesis of PDFS-Vi-X
3.3. Pore Microstructures of FSiRF Materials
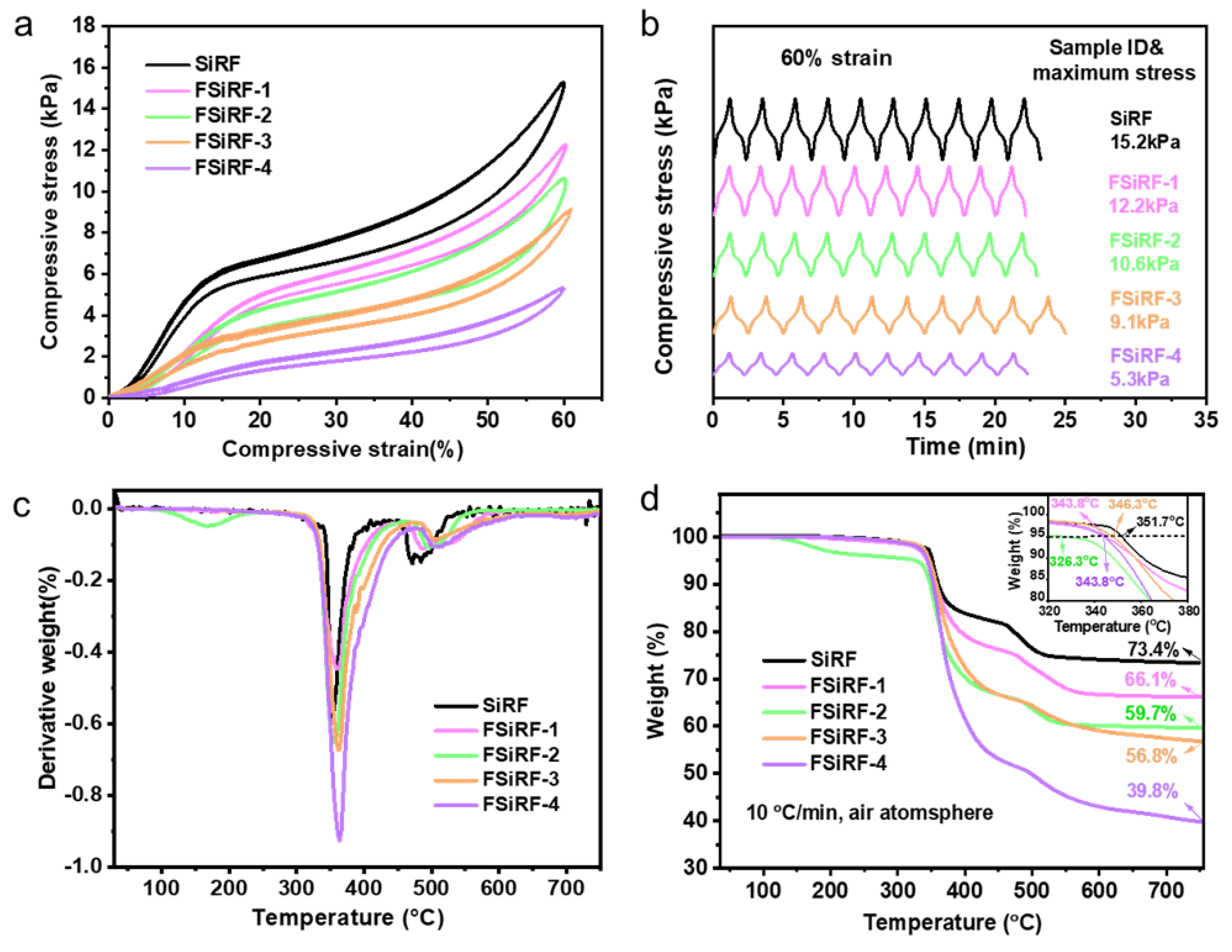
3.4. Mechanical and Thermal Properties of FSiRF Materials
3.5. Chemical Resistance of FSiRF Materials
3.6. Oil–Water Separation of the FSiRF Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rostami-Tapeh-Esmaeil, E.; Vahidifar, A.; Esmizadeh, E.; Rodrigue, D. Chemistry, processing, properties, and applications of rubber foams. Polymers 2021, 13, 1565. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, J.; Ren, J.; Rong, L.; Cao, P.F.; Advincula, R.C. 3D printed multifunctional, hyperelastic silicone rubber foam. Adv. Funct. Mater. 2019, 29, 1900469. [Google Scholar] [CrossRef]
- Dai, S.-W.; Gu, Y.-L.; Zhao, L.; Zhang, W.; Gao, C.-H.; Wu, Y.-X.; Shen, S.-C.; Zhang, C.; Kong, T.-T.; Li, Y.-T.; et al. Bamboo-inspired mechanically flexible and electrically conductive polydimethylsiloxane foam materials with designed hierarchical pore structures for ultra-sensitive and reliable piezoresistive pressure sensor. Compos. Part. B-Eng. 2021, 225, 109243. [Google Scholar] [CrossRef]
- Yang, J.; Liao, X.; Wang, G.; Chen, J.; Song, P.; Tang, W.; Guo, F.; Liu, F.; Li, G. Heterogeneous silicon rubber composite foam with gradient porous structure for highly absorbed ultra-efficient electromagnetic interference shielding. Compos. Sci. Technol. 2021, 206, 108663. [Google Scholar] [CrossRef]
- Yan, H.; Wang, K.; Zhao, Y. Fabrication of silicone rubber foam with tailored porous structures by supercritical CO2. Macromol. Mater. Eng. 2017, 302, 1600377. [Google Scholar] [CrossRef]
- Zhang, G.-D.; Wu, Z.-H.; Xia, Q.-Q.; Qu, Y.-X.; Pan, H.-T.; Hu, W.-J.; Zhao, L.; Cao, K.; Chen, E.-Y.; Yuan, Z.; et al. Ultrafast Flame-Induced Pyrolysis of Poly(dimethylsiloxane) Foam Materials toward Exceptional Superhydrophobic Surfaces and Reliable Mechanical Robustness. ACS Appl. Mater. Interfaces 2021, 13, 23161–23172. [Google Scholar] [CrossRef]
- Mao, M.; Xu, H.; Guo, K.-Y.; Zhang, J.-W.; Xia, Q.-Q.; Zhang, G.-D.; Zhao, L.; Gao, J.-F.; Tang, L.-C. Mechanically flexible, super-hydrophobic and flame-retardant hybrid nano-silica/graphene oxide wide ribbon decorated sponges for efficient oil/water separation and fire warning response. Compos. Part. A 2021, 140, 106191. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, L.; Wang, Y.; Xu, T.; Zhang, C. Thermal insulation and stability of polysiloxane foams containing hydroxyl-terminated polydimethylsiloxanes. RSC Adv. 2018, 8, 9901–9909. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Zhao, X.; Jiang, X.; Zhang, L.; Zhang, H.; Liu, Y.; Zhu, H. A porous graphene/polydimethylsiloxane composite by chemical foaming for simultaneous tensile and compressive strain sensing. FlatChem 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Zhu, D.; Handschuh-Wang, S.; Zhou, X. Recent progress in fabrication and application of polydimethylsiloxane sponges. J. Mater. Chem. A 2017, 5, 16467–16497. [Google Scholar] [CrossRef]
- Cao, C.-F.; Wang, P.-H.; Zhang, J.-W.; Guo, K.-Y.; Li, Y.; Xia, Q.-Q.; Zhang, G.-D.; Zhao, L.; Chen, H.; Wang, L.; et al. One-step and green synthesis of lightweight, mechanically flexible and flame-retardant polydimethylsiloxane foam nanocomposites via surface-assembling ultralow content of graphene derivative. Chem. Eng. J. 2020, 393, 124724. [Google Scholar] [CrossRef]
- Xiang, B.; Jia, Y.; Lei, Y.; Zhang, F.; He, J.; Liu, T.; Luo, S. Mechanical properties of microcellular and nanocellular silicone rubber foams obtained by supercritical carbon dioxide. Polym. J. 2019, 51, 559–568. [Google Scholar] [CrossRef]
- Cai, J.-H.; Huang, M.-L.; Chen, X.-D.; Wang, M. Thermo-expandable microspheres strengthened polydimethylsiloxane foam with unique softening behavior and high-efficient energy absorption. Appl. Surf. Sci. 2021, 540, 148364. [Google Scholar] [CrossRef]
- Li, Y.; Cao, C.-F.; Li, S.-N.; Huang, N.-J.; Mao, M.; Zhang, J.-W.; Wang, P.-H.; Guo, K.-Y.; Gong, L.-X.; Zhang, G.-D.; et al. In situ reactive self-assembly of a graphene oxide nano-coating in polymer foam materials with synergistic fire shielding properties. J. Mater. Chem. A 2019, 7, 27032–27040. [Google Scholar] [CrossRef]
- Turco, A.; Primiceri, E.; Frigione, M.; Maruccio, G.; Malitesta, C. An innovative, fast and facile soft-template approach for the fabrication of porous PDMS for oil–water separation. J. Mater. Chem. A 2017, 5, 23785–23793. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Mavrona, E.; Sacré, D.; Kummer, N.; Cao, J.; Müller, L.A.E.; Hack, E.; Zolliker, P.; Nyström, G. Terahertz Birefringent Biomimetic Aerogels Based on Cellulose Nanofibers and Conductive Nanomaterials. ACS. Nano 2021, 15, 7451–7462. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Wu, T.; Han, D.; Ren, Q.; Siqueira, G.; Nyström, G. Ultralight, Flexible, and Biomimetic Nanocellulose/Silver Nanowire Aerogels for Electromagnetic Interference Shielding. ACS. Nano 2020, 14, 2927–2938. [Google Scholar] [CrossRef]
- Hailan, S.M.; Ponnamma, D.; Krupa, I. The Separation of Oil/Water Mixtures by Modified Melamine and Polyurethane Foams: A Review. Polymers 2021, 13, 4142. [Google Scholar] [CrossRef]
- Cao, C.-F.; Liu, W.-J.; Xu, H.; Yu, K.-X.; Gong, L.-X.; Guo, B.-F.; Li, Y.-T.; Feng, X.-L.; Lv, L.-Y.; Pan, H.-T.; et al. Temperature-induced resistance transition behaviors of melamine sponge composites wrapped with different graphene oxide derivatives. J. Mater. Sci. Technol. 2021, 85, 194–204. [Google Scholar] [CrossRef]
- Guo, K.-Y.; Wu, Q.; Mao, M.; Chen, H.; Zhang, G.-D.; Zhao, L.; Gao, J.-F.; Song, P.; Tang, L.-C. Water-based hybrid coatings toward mechanically flexible, super-hydrophobic and flame-retardant polyurethane foam nanocomposites with high-efficiency and reliable fire alarm response. Compos. Part. B-Eng. 2020, 193, 108017. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, J.; Wang, S.; Chen, B.; Feng, Y.; Pei, Y.; Yan, Y.; Tang, L.; Qiu, H.; Wu, L. Exceptionally flame-retardant flexible polyurethane foam composites: Synergistic effect of the silicone resin/graphene oxide coating. Front. Chem. Sci. Eng. 2021, 15, 969–983. [Google Scholar] [CrossRef]
- Yu, Z.-R.; Mao, M.; Li, S.-N.; Xia, Q.-Q.; Cao, C.-F.; Zhao, L.; Zhang, G.-D.; Zheng, Z.-J.; Gao, J.-F.; Tang, L.-C. Facile and green synthesis of mechanically flexible and flame-retardant clay/graphene oxide nanoribbon interconnected networks for fire safety and prevention. Chem. Eng. J. 2021, 405, 126620. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Hong, L.-Y.; Chung, Y.-M.; Kim, D.-P.; Lee, C.-S. Solvent-Resistant PDMS Microfluidic Devices with Hybrid Inorganic/Organic Polymer Coatings. Adv. Funct. Mater. 2009, 19, 3796–3803. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Kwon, T.-H.; Im, H.; Moon, D.-I.; Baek, D.J.; Seol, M.-L.; Duarte, J.P.; Choi, Y.-K. A Polydimethylsiloxane (PDMS) Sponge for the Selective Absorption of Oil from Water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef]
- Shin, J.H.; Heo, J.-H.; Jeon, S.; Park, J.H.; Kim, S.; Kang, H.-W. Bio-inspired hollow PDMS sponge for enhanced oil–water separation. J. Hazard. Mater. 2019, 365, 494–501. [Google Scholar] [CrossRef]
- Guan, Y.; Cheng, F.; Pan, Z. Superwetting Polymeric Three Dimensional (3D) Porous Materials for Oil/Water Separation: A Review. Polymers 2019, 11, 806. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.V.; Nguyen, C.H.; Lin, W.-C.; Juang, R.-S. Improved stability of a supported liquid membrane process via hydrophobic modification of PVDF support by plasma activation and chemical vapor deposition. Sep. Purif. Technol. 2021, 277, 119615. [Google Scholar] [CrossRef]
- Evsyukova, N.V.; Myshkovskii, A.M.; Polukhina, L.M.; Serenko, O.A.; Nikitin, L.N.; Muzafarov, A.M. Making fabrics water-repellent with fluorine-containing silane in supercritical carbon dioxide medium. Fibre Chem. 2009, 41, 46–52. [Google Scholar] [CrossRef]
- Peng, Z.; Song, J.; Gao, Y.; Liu, J.; Lee, C.; Chen, G.; Wang, Z.; Chen, J.; Leung, M.K.H. A fluorinated polymer sponge with superhydrophobicity for high-performance biomechanical energy harvesting. Nano Energy 2021, 85, 106021. [Google Scholar] [CrossRef]
- Indulekha, K.; Mathew, A.; Rajeev, R.S.; Ninan, K.N.; Gouri, C. A facile route to fluoroalkylsiloxane polymers having resistance to corrosive acidic conditions: Synthesis and characterization. Eur. Polym. J. 2017, 97, 94–103. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kotera, M. Synthesis of fluorosilicone having highly fluorinated alkyl side chains based on the hydrosilylation of fluorinated olefins with polyhydromethylsiloxane. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3120–3128. [Google Scholar] [CrossRef]
- Cui, Y.; Jiang, W.; Li, D.; Niu, C.; Jiezhang; Feng, S. Preparation and properties of fluorosilicone and fluorosilicone elastomer with various contents of trifluoropropyl groups. e-Polymers 2011, 11. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Y.; Zhao, C.; Diao, S.; Duan, B. Preparation of fluorosilicone rubber containing perfluorocyclobutyl aryl ether. Polym. Adv. Technol. 2021, 32, 538–543. [Google Scholar] [CrossRef]
- Guan, Y.; Yang, R.; Huang, Y.; Yu, C.; Li, X.; Wei, D.; Xu, X. Multi-walled carbon nanotubes acting as antioxidant for fluorosilicone rubber. Polym. Degrad. Stab. 2018, 156, 161–169. [Google Scholar] [CrossRef]
- Métivier, T.; Cassagnau, P. Foaming behavior of silicone/fluorosilicone blends. Polymer 2018, 146, 21–30. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, Y.; Meng, L.; Wang, Y.; Pang, A.; Guo, X.; Xiao, J.; Li, W. A review of poly[(3,3,3-trifluoropropyl)methylsiloxane]: Synthesis, properties and applications. Eur. Polym. J. 2022, 163, 110903. [Google Scholar] [CrossRef]
- Indulekha, K.; Behera, P.K.; Rajeev, R.S.; Gouri, C.; Ninan, K.N. Polyfluoroalkyl siloxanes with varying trifluoropropyl content: Synthesis, characterization and solvent resistance studies. J. Fluor. Chem. 2017, 200, 24–32. [Google Scholar] [CrossRef]
- Kählig, H.; Zöllner, P.; Mayer-Helm, B.X. Characterization of degradation products of poly[(3,3,3-trifluoropropyl)methylsiloxane] by nuclear magnetic resonance spectroscopy, mass spectrometry and gas chromatography. Polym. Degrad. Stab. 2009, 94, 1254–1260. [Google Scholar] [CrossRef]
- Shmeleva, O.A.; Mileshkevich, V.P. Study of the equilibrium copolymerization of dimethyl- and methyl(3,3,3-trifluoropropyl)cyclosiloxanes. Polym. Sci. USSR. 1990, 32, 2112–2117. [Google Scholar] [CrossRef]
- Li, B.; Chen, S.; Zhang, J. Synthesis and characterization of vinyl-terminated copolysiloxanes containing 3,3,3-trifluoropropyl groups. Polym. Chem. 2012, 3, 2366–2376. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, J.; Qu, L.; Dai, X.; Li, P.; Sui, Y.; Zhang, C. Fabrication of polysiloxane foam with a pendent phenyl group for improved thermal insulation capacity and thermal stability. New J. Chem. 2019, 43, 6136–6145. [Google Scholar] [CrossRef]
- Fei, H.-F.; Gao, X.; Han, X.; Wang, Q.; Hu, T.; Zhang, Z.; Xie, Z. Synthesis, characterization, and properties of vinyl-terminated copolysiloxanes containing trifluoropropyl and 4-trifluoromethylphenyl groups. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1023–1031. [Google Scholar] [CrossRef]
- Tang, W.; Liao, X.; Zhang, Y.; Li, J.; Wang, G.; Li, G. Mechanical–Microstructure Relationship and Cellular Failure Mechanism of Silicone Rubber Foam by the Cell Microstructure Designed in Supercritical CO2. J. Phys. Chem. C. 2019, 123, 26947–26956. [Google Scholar] [CrossRef]
- Li, B.; Chen, S.; Zhang, J. Effects of CH2CH2CF3 on properties of RTV polysiloxane rubber: Processability, thermal stability, and oil/solvent resistance. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- You, Y.; Chen, J.; Zheng, A.; Wei, D.; Xu, X.; Guan, Y. Effect of silanol on the thermal stability of poly[methyl(trifluoropropyl)siloxane]. J. Appl. Polym. Sci. 2020, 137, 49347. [Google Scholar] [CrossRef]
- González-Rivera, J.; Iglio, R.; Barillaro, G.; Duce, C.; Tinè, M. Structural and Thermoanalytical Characterization of 3D Porous PDMS Foam Materials: The Effect of Impurities Derived from a Sugar Templating Process. Polymers 2018, 10, 616. [Google Scholar] [CrossRef] [Green Version]
- Dawir, M. Sealing in the automotive industry with liquid fluoro-silicone elastomers. Seal. Technol. 2008, 2008, 10–14. [Google Scholar] [CrossRef]
- Liao, S.; He, Y.; Chu, Y.; Liao, H.; Wang, Y. Solvent-resistant and fully recyclable perfluoropolyether-based elastomer for microfluidic chip fabrication. J. Mater. Chem. A 2019, 7, 16249–16256. [Google Scholar] [CrossRef]
- Ma, Q.; Liao, S.; Ma, Y.; Chu, Y.; Wang, Y. An Ultra-Low-Temperature Elastomer with Excellent Mechanical Performance and Solvent Resistance. Adv. Mater. 2021, 33, 2102096. [Google Scholar] [CrossRef]
- Ge, J.; Ye, Y.D.; Yao, H.B.; Zhu, X.; Wang, X.; Wu, L.; Wang, J.L.; Ding, H.; Yong, N.; He, L.H.; et al. Pumping through Porous Hydrophobic/Oleophilic Materials: An Alternative Technology for Oil Spill Remediation. Angew. Chem. 2014, 53, 3612–3616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Chen, Z.Y.; Tang, Y.H.; Li, Y.T.; Ma, D.; Zhang, G.D.; Rabah, B.; Cao, C.F.; Gong, L.X.; Song, P.; et al. Silicone/graphene oxide co-cross-linked aerogels with wide-temperature mechanical flexibility, super-hydrophobicity and flame resistance for exceptional thermal insulation and oil/water separation. J. Mater. Sci. Technol. 2022, 114, 131–142. [Google Scholar] [CrossRef]




| Sample Code | Fluorine Content (mol%) | Mn (g/mol) | Mw (g/mol) | PDI (Mw/Mn) |
|---|---|---|---|---|
| PDFS-Vi-0 | 0.0% | 116833 | 153094 | 1.36 |
| PDFS-Vi-12.5 | 12.5% | 83139 | 116431 | 1.40 |
| PDFS-Vi-25.0 | 25.0% | 73677 | 103899 | 1.41 |
| PDFS-Vi-37.5 | 37.5% | 78373 | 105495 | 1.35 |
| PDFS-Vi-50.0 | 50.0% | 87643 | 122486 | 1.39 |
| Sample Code | Swelling Ratio (100%) | ||||
|---|---|---|---|---|---|
| Hexane | Xylene | N-Octane | Acetone | Isopropanol | |
| SiRF | 4.90 | 4.98 | 4.87 | 4.79 | 4.15 |
| FSiRF-1 | 4.25 | 4.41 | 4.01 | 4.21 | 3.90 |
| FSiRF-2 | 3.40 | 3.57 | 3.37 | 3.28 | 3.47 |
| FSiRF-3 | 2.32 | 2.47 | 2.39 | 2.54 | 2.77 |
| FSiRF-4 | 1.48 | 1.08 | 1.24 | 1.18 | 1.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.-J.; Xia, Q.-Q.; Pan, H.-T.; Chen, H.-Y.; Qu, Y.-X.; Chen, Z.-Y.; Zhang, G.-D.; Zhao, L.; Gong, L.-X.; Xue, C.-G.; et al. Green and Rapid Preparation of Fluorosilicone Rubber Foam Materials with Tunable Chemical Resistance for Efficient Oil–Water Separation. Polymers 2022, 14, 1628. https://doi.org/10.3390/polym14081628
Hu W-J, Xia Q-Q, Pan H-T, Chen H-Y, Qu Y-X, Chen Z-Y, Zhang G-D, Zhao L, Gong L-X, Xue C-G, et al. Green and Rapid Preparation of Fluorosilicone Rubber Foam Materials with Tunable Chemical Resistance for Efficient Oil–Water Separation. Polymers. 2022; 14(8):1628. https://doi.org/10.3390/polym14081628
Chicago/Turabian StyleHu, Wan-Jun, Qiao-Qi Xia, Hong-Tao Pan, Hai-Yang Chen, Yong-Xiang Qu, Zuan-Yu Chen, Guo-Dong Zhang, Li Zhao, Li-Xiu Gong, Chang-Guo Xue, and et al. 2022. "Green and Rapid Preparation of Fluorosilicone Rubber Foam Materials with Tunable Chemical Resistance for Efficient Oil–Water Separation" Polymers 14, no. 8: 1628. https://doi.org/10.3390/polym14081628
APA StyleHu, W.-J., Xia, Q.-Q., Pan, H.-T., Chen, H.-Y., Qu, Y.-X., Chen, Z.-Y., Zhang, G.-D., Zhao, L., Gong, L.-X., Xue, C.-G., & Tang, L.-C. (2022). Green and Rapid Preparation of Fluorosilicone Rubber Foam Materials with Tunable Chemical Resistance for Efficient Oil–Water Separation. Polymers, 14(8), 1628. https://doi.org/10.3390/polym14081628