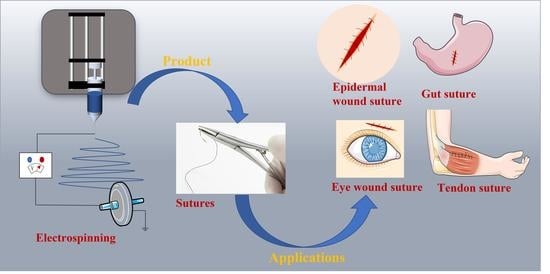
Electrospun Medical Sutures for Wound Healing: A Review
Abstract
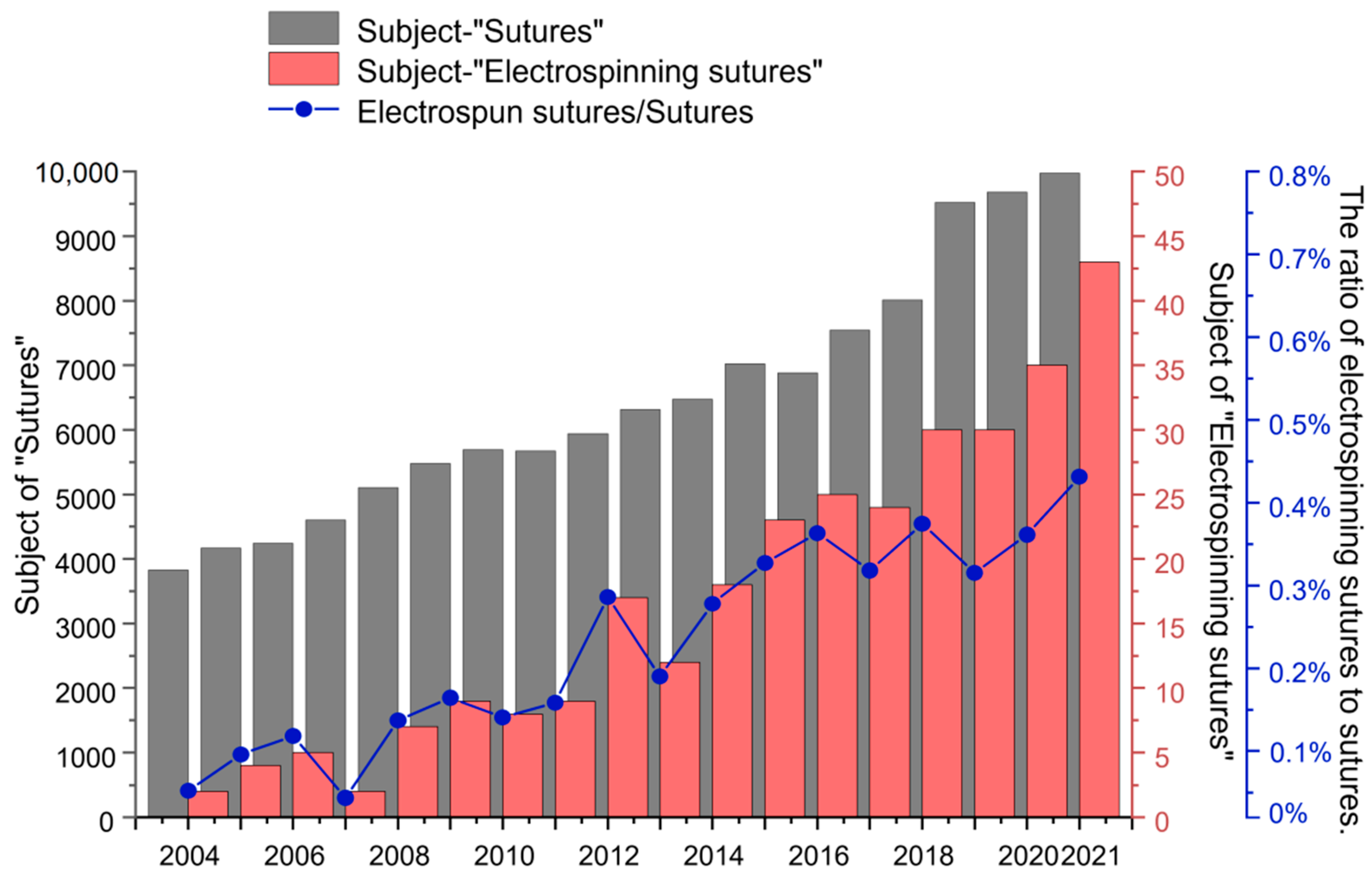
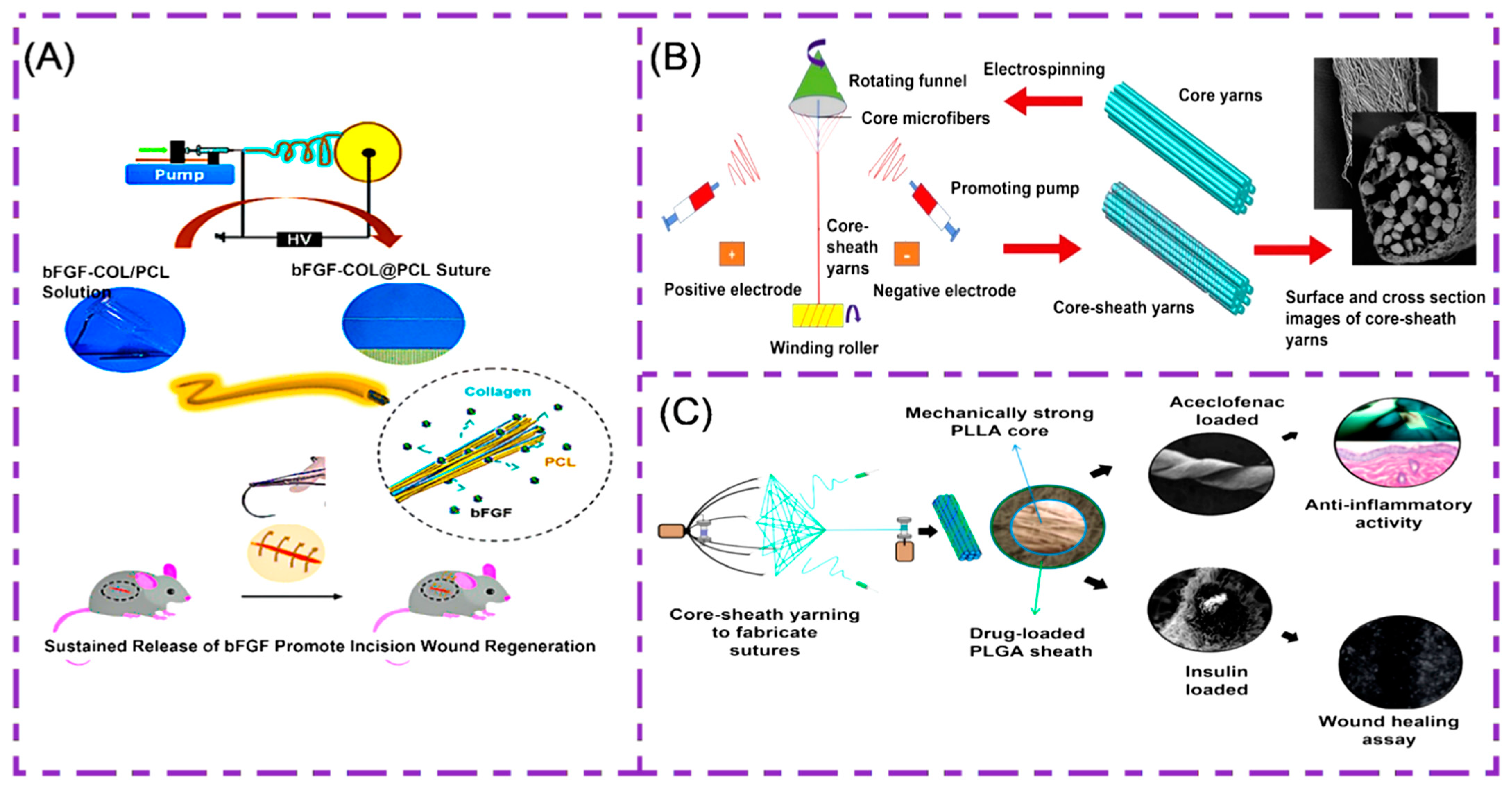
:1. Introduction
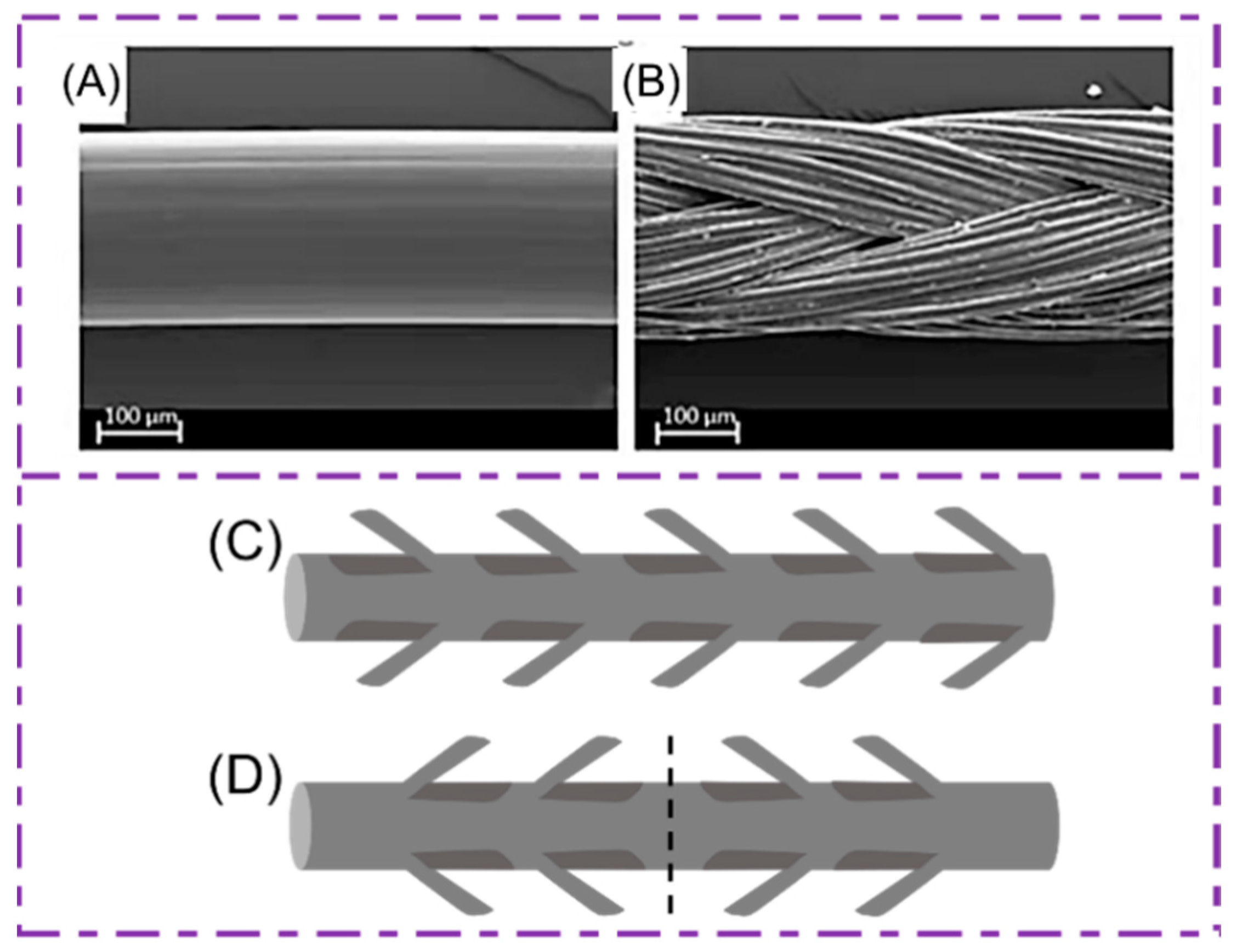
2. Structure of Sutures
2.1. Monofilament Sutures
2.2. Multifilament Sutures
2.3. Barb Sutures
3. Performance Analysis of Sutures
3.1. Physical Performance Analysis
3.2. Operational Performance Analysis
3.3. Biological Performance Analysis
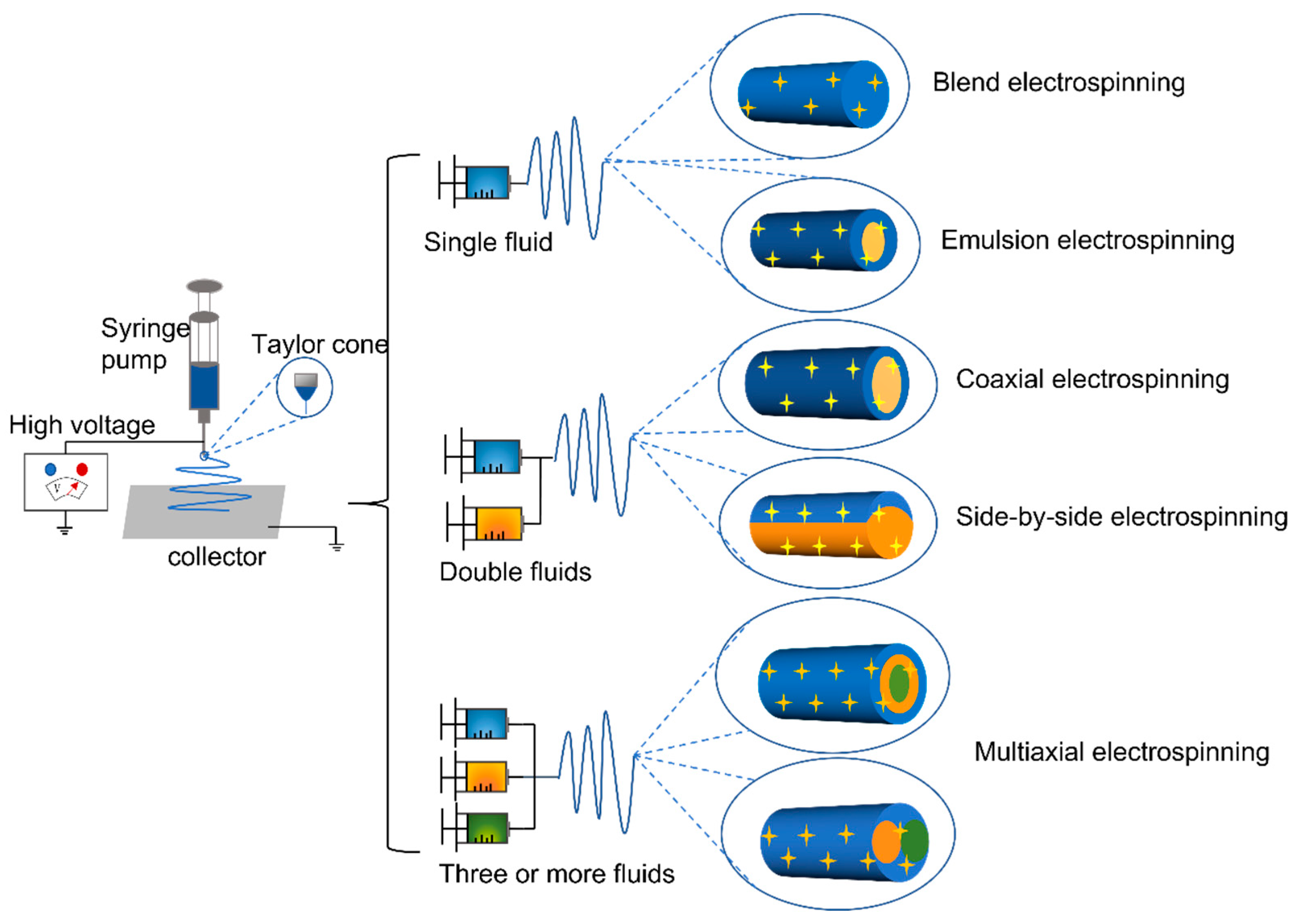
4. Electrospinning Processes
4.1. Single-Fluid Electrospinning
4.2. Double-Fluid Electrospinning
4.3. Multi-Fluid Electrospinning
4.4. In Situ Electrospinning
4.5. Improved Receiver Used to Prepare Nanowires
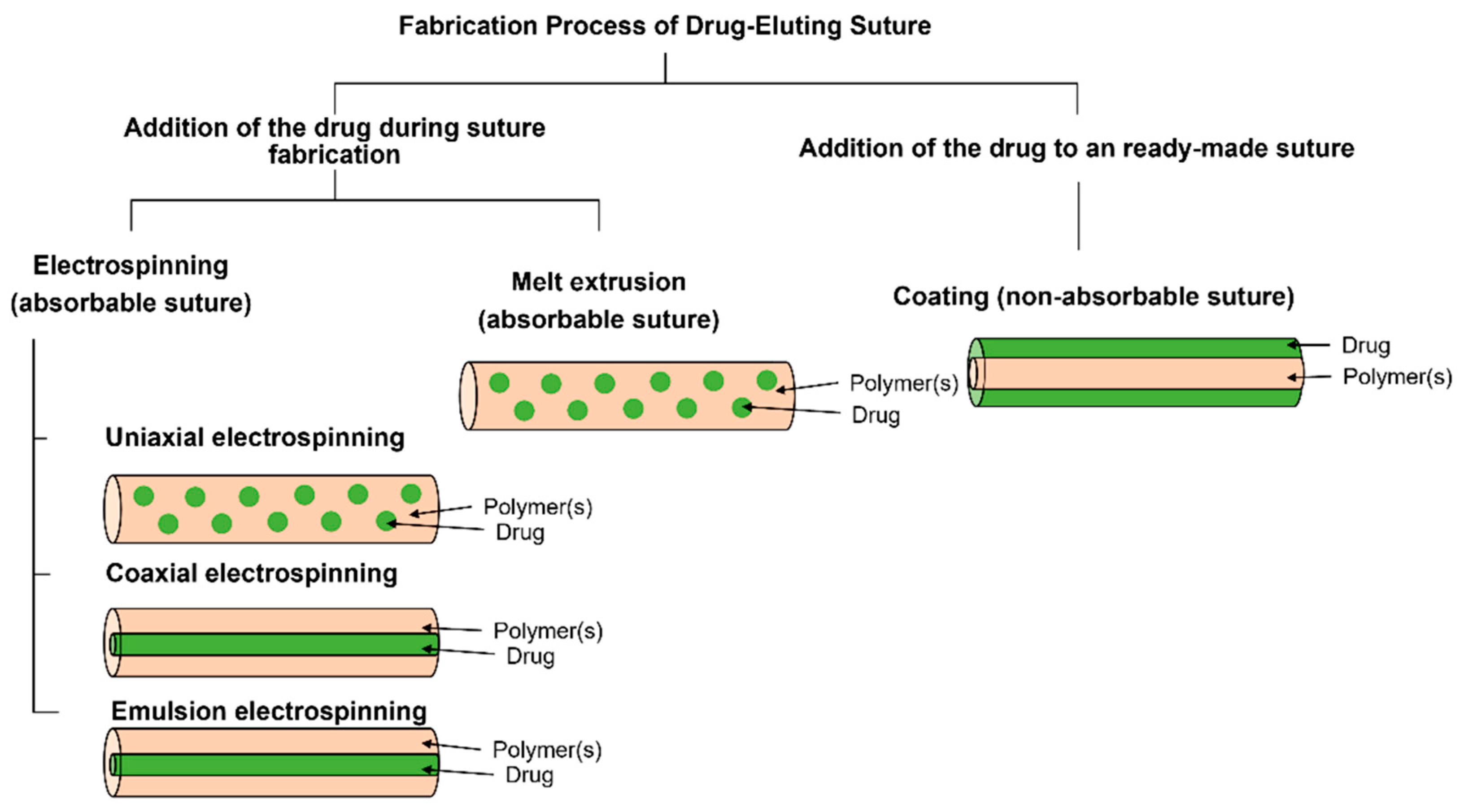
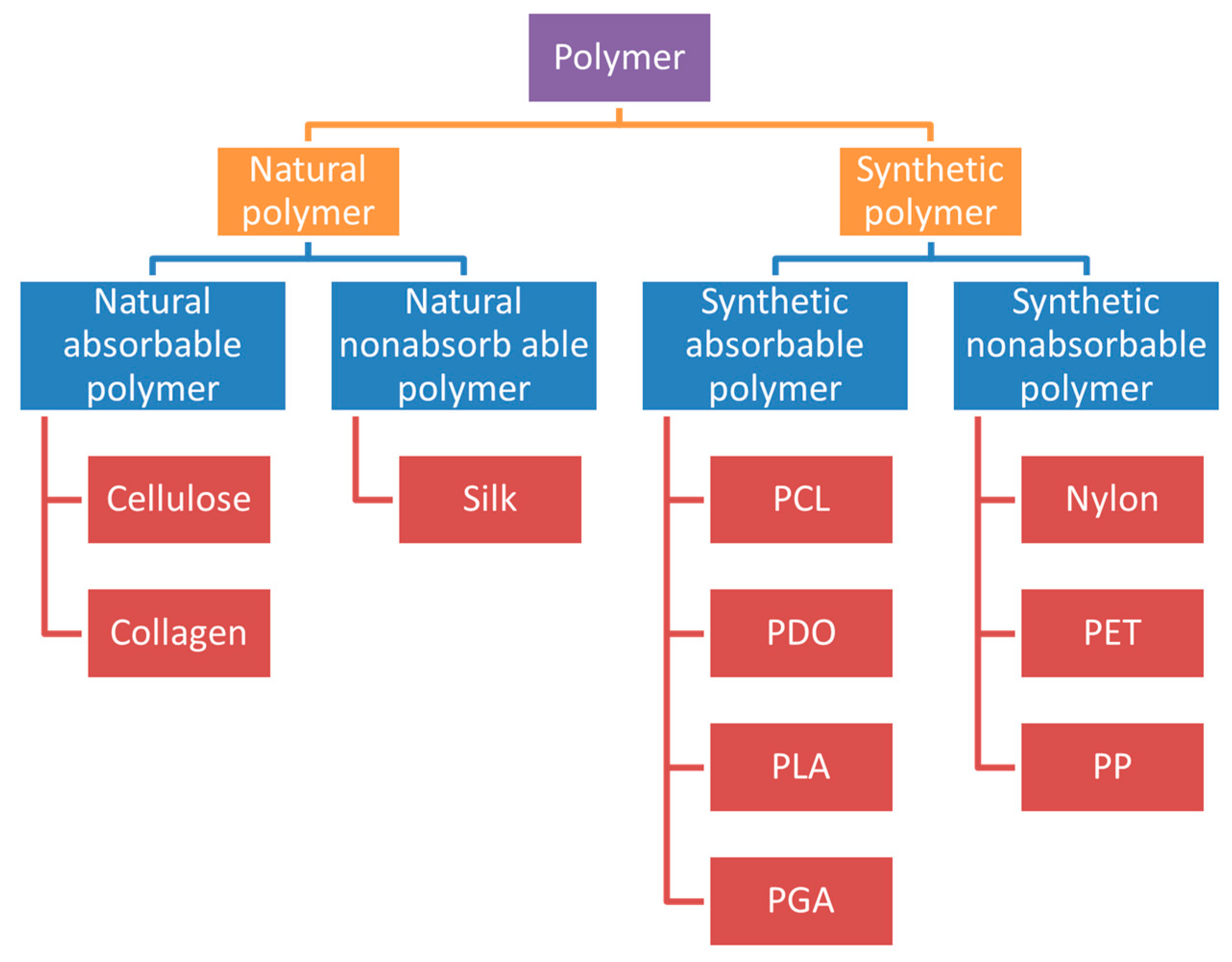
5. The Type of Sutures and the Polymer Materials of Sutures
5.1. Absorbable Sutures
| Name | Structure | Characteristic | Preparation Method | Ref. | |
|---|---|---|---|---|---|
| Natural absorbable suture material | Catgut/chrome gut | Monofilament | Monofilament, easy to degrade, low tensile strength, susceptible to bacterial infection | Washing and drying | [102] |
| Regenerated cellulose | Monofilament | Ideal biodegradability and mechanical properties | Wet spinning | [104] | |
| Synthetic absorbable suture material | P3BV-co-HB | Monofilament | Strong toughness, biodegradable, non-toxic, promote cell proliferation | Blend electrospinning | [106,108] |
| PCL | Monofilament or woven | Good biocompatibility, degradability, mechanical properties and shape memory properties are excellent | Blend electrospinning | [110] | |
| PDO | Monofilament | Colorless, biodegradable, mechanically flexible | Blend electrospinning | [116] | |
| PLA | Monofilament | Biocompatible, good degradability | Blend electrospinning | [117] | |
| PLGA | Monofilament | Degradable, non-toxic and harmless, good tensile properties | Blend electrospinning | [118] | |
| PU | Monofilament | Biocompatible, degradable | Blend electrospinning | [120] |
5.2. Non-Absorbable Sutures
| Name | Structure | Characteristic | Preparation Method | Ref. | |
|---|---|---|---|---|---|
| Natural non-absorbable suture material | silk | monofilament | Good biocompatibility, low tensile strength, easy bacterial infection, | Wet spinning | [125] |
| Synthetic non-absorbable suture material | nylon | monofilament | A certain tensile strength can reduce tissue infection and fight thrombosis | Melting, forming, cooling | [132] |
| PP | monofilament | Low tissue reactivity, high tensile strength, high plasticity, adapted to wound edema | Chemical synthesis, usually surface coating to prepare sutures | [135] | |
| PET | multifilament | Extremely high tensile strength, good operability, not easy to degrade | Chemical synthesis, usually surface coating to prepare sutures | [137] |
5.3. Bioactive Substances for Medical Sutures
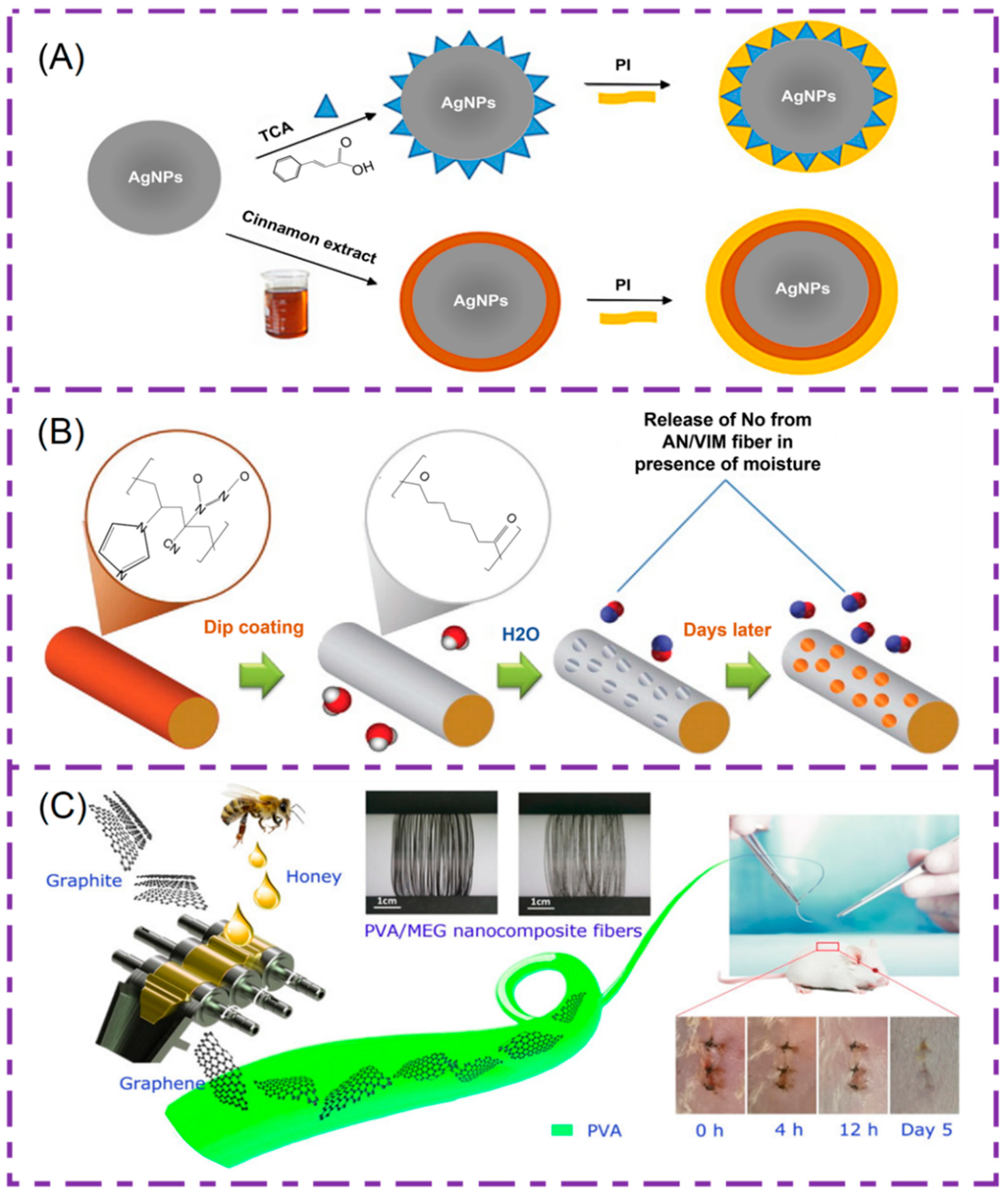
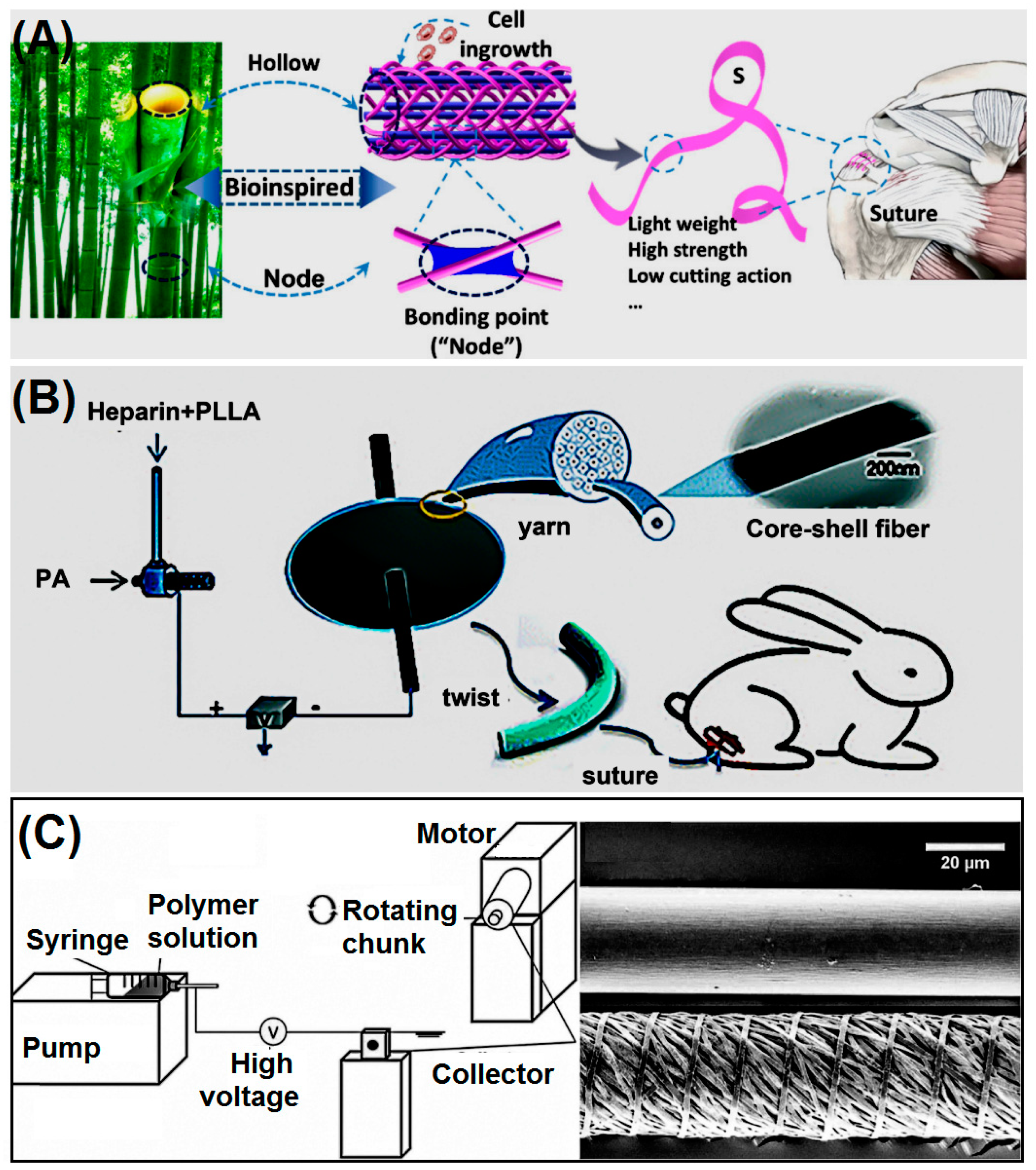
6. Applications
6.1. Tendon Rupture Repair
6.2. Oral and Periodontal Surgery
6.3. Prevention of Corneal Repair Infection
7. Summary and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSI | Surgical Site Infections |
| S. epidermidis | Staphylococcus epidermidis |
| S. aureus | Staphylococcus aureus |
| P. aeruginosa | Pseudomonas aeruginosa |
| E. coli | Escherichia coli |
| API | Active Pharmaceutical Ingredient |
| USP | United States Pharmacopeia |
| PBS | phosphate buffer saline |
| PCL | Polycaprolactone |
| SEM | Scanning electron microscope |
| PLLA | Poly(l-lactic acid) |
| PGA | Polyglycolic acid |
| PP | Polypropylene |
| PE | Polyethylene |
| PET | Polyester |
| PDO | Poly-p-dioxanone |
| TCH | Tetracycline hydrochloride |
| ORC | Oxidative regenerated cellulose |
| BCNCCs | Bacterial cellulose nanocrystals |
| RC | Regenerated chitin |
| P3BV-co-HB | Poly (3-hydroxybutyrate-co-4-hydroxybutyrate) |
| EC | Ethyl cellulose |
| PLACL | Poly(l-lactide-co-ε-caprolactone) |
| PLGA | Poly (lactic-co-glycolic acid) |
| FDA | Food and Drug Administration |
| NO | Nitric oxide |
| GO | Graphene oxide |
| VEGF | Vascular endothelial growth factor |
| BFGF | Fibroblast growth factor |
| PEO | Poly(ethylene oxide) |
| COL-HA | Collagen-hyaluronic acid |
References
- Lekic, N.; Dodds, S.D. Suture materials, needles, and methods of skin closure: What every hand surgeon should know. J. Hand Surg. 2022, 47, 160–171.e1. [Google Scholar] [CrossRef] [PubMed]
- Gierek, M.; Kuśnierz, K.; Lampe, P.; Ochała, G.; Kurek, J.; Hekner, B.; Merkel, K.; Majewski, J. Absorbable sutures in general surgery–review, available materials, and optimum choices. Pol. J. Surg. 2018, 90, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Pacer, E.; Griffin, D.W.; Anderson, A.B.; Tintle, S.M.; Potter, B.K. Suture and needle characteristics in orthopaedic surgery. JBJS Rev. 2020, 8, e19.00133. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.L.; Paladini, F.; Romano, A.; Verri, T.; Quattrini, A.; Sannino, A.; Pollini, M. Efficacy of silver coated surgical sutures on bacterial contamination, cellular response and wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Baygar, T.; Sarac, N.; Ugur, A.; Karaca, I.R. Antimicrobial characteristics and biocompatibility of the surgical sutures coated with biosynthesized silver nanoparticles. Bioorg. Chem. 2019, 86, 254–258. [Google Scholar] [CrossRef]
- Joseph, B.; George, A.; Gopi, S.; Kalarikkal, N.; Thomas, S. Polymer sutures for simultaneous wound healing and drug delivery—A review. Int. J. Pharm. 2017, 524, 454–466. [Google Scholar] [CrossRef]
- Namivandi-Zangeneh, R.; Wong, E.H.H.; Boyer, C. Synthetic antimicrobial polymers in combination therapy: Tackling antibiotic resistance. ACS Infect. Dis. 2021, 7, 215–253. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Jiang, D.; Pei, Y.; Wang, Z.; Zhou, X.; Wu, J.; Mo, X.; Wang, H. Delivery of mRNA vaccines and anti-PDL1 siRNA through non-invasive transcutaneous route effectively inhibits tumor growth. Compos. Part B Eng. 2022, 233, 109648. [Google Scholar] [CrossRef]
- Balasubramaniam, B.; Prateek; Ranjan, S.; Saraf, M.; Kar, P.; Singh, S.P.; Thakur, V.K.; Singh, A.; Gupta, R.K. Antibacterial and antiviral functional materials: Chemistry and biological activity toward tackling COVID-19-like pandemics. ACS Pharmacol. Transl. Sci. 2021, 4, 8–54. [Google Scholar] [CrossRef]
- Konai, M.M.; Bhattacharjee, B.; Ghosh, S.; Haldar, J. Recent progress in polymer research to tackle infections and antimicrobial resistance. Biomacromolecules 2018, 19, 1888–1917. [Google Scholar] [CrossRef]
- Kourtis, A.P. Vital Signs: Epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections-United states. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, M.; Ahmed, N.; Permana, A.D.; Rodgers, A.M.; Donnelly, R.F.; Rehman, A.U. Enhancement in site-Specific delivery of carvacrol against methicillin resistant Staphylococcus aureus induced skin infections using enzyme responsive nanoparticles: A proof of concept study. Pharmaceutics 2019, 11, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Li, J.; Tu, J.; Yang, H.; Chen, Q.; Liu, H. Fabrication of porous chitosan membranes composed of nanofibers by low temperature thermally induced phase separation, and their adsorption behavior for Cu2+. Carbohydr. Polym. 2017, 178, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Menk, F.; Kim, Y.; Lim, J.; Char, K.; Zentel, R.; Choi, T.-L. Living light-induced crystallization-driven self-assembly for rapid preparation of semiconducting nanofibers. J. Am. Chem. Soc. 2018, 140, 6088–6094. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, G.R. Energy-saving electrospinning with a concentric teflon-core rod spinneret to create medicated nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- He, H.; Wu, M.; Zhu, J.; Yang, Y.; Ge, R.; Yu, D.-G. Engineered spindles of little molecules around electrospun nanofibers for biphasic drug release. Adv. Fiber Mater. 2021, 3, 305–317. [Google Scholar] [CrossRef]
- Lan, X.; Liu, Y.; Wang, Y.; Tian, F.; Miao, X.; Wang, H.; Tang, Y. Coaxial electrospun PVA/PCL nanofibers with dual release of tea polyphenols and ε-poly (l-lysine) as antioxidant and antibacterial wound dressing materials. Int. J. Pharm. 2021, 601, 120525. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Caramella, C.; Del Fante, C.; Perotti, C.; Miele, D.; Vigani, B.; Ferrari, F. Bioactive medications for the delivery of platelet derivatives to skin wounds. Curr. Drug Deliv. 2019, 16, 472–483. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Qin, Y.; Li, C. Functional electrospun nanocomposites for efficient oxygen reduction reaction. Chem. Res. Chin. Univ. 2021, 37, 379–393. [Google Scholar] [CrossRef]
- Jiang, S.; Schmalz, H.G.; Agarwal, S.; Greiner, A. Electrospinning of ABS nanofibers and their high filtration performance. Adv. Fiber Mater. 2020, 2, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, G.; Zhao, Y.; Zhou, M.; Zhong, A.; Sun, J. Promotion of skin regeneration through co-axial electrospun fibers loaded with basic fibroblast growth factor. Adv. Compos. Hybrid Mater. 2022, 5, 1–15. [Google Scholar] [CrossRef]
- Khalid, G.M.; Selmin, F.; Musazzi, U.M.; Gennari, C.G.M.; Minghetti, P.; Cilurzo, F. Trends in the characterization methods of orodispersible films. Curr. Drug Deliv. 2021, 18, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, L.; Yang, D.; Nie, J.; Ma, G. Electrospun core–shell fibrous 2D scaffold with biocompatible poly (glycerol sebacate) and poly-l-lactic acid for wound healing. Adv. Fiber Mater. 2020, 2, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Ortega, C.A.; Favier, L.S.; Cianchino, V.A.; Cifuente, D.A. New orodispersible mini tablets of enalapril maleate by direct compression for pediatric patients. Curr. Drug. Deliv. 2020, 17, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Hou, J.S.; Yu, D.G.; Li, S.Y.; Zhu, J.W.; Chen, Z.Z. Electrospun tri-layer nanodepots for sustained release of acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Bukhary, H.; Williams, G.R.; Orlu, M. Fabrication of electrospun levodopa-carbidopa fixed-dose combinations. Adv. Fiber Mater. 2020, 2, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.R.; Hou, L.L.; Yue, G.C.; Li, H.K.; Zhang, J.S.; Liu, J.; Miao, B.B.; Wang, N.; Bai, J.; Cui, Z.M.; et al. Progress of fabrication and applications of electrospun hierarchically porous nanofibers. Adv. Fiber Mater. 2022, 4, 1–27. [Google Scholar] [CrossRef]
- Ziyadi, H.; Baghali, M.; Bagherianfar, M.; Mehrali, F.; Faridi-Majidi, R. An investigation of factors affecting the electrospinning of poly (vinyl alcohol)/kefiran composite nanofibers. Adv. Compos. Hybrid Mater. 2021, 4, 768–779. [Google Scholar] [CrossRef]
- Ghazalian, M.; Afshar, S.; Rostami, A.; Rashedi, S.; Bahrami, S.H. Fabrication and characterization of chitosan-polycaprolactone core-shell nanofibers containing tetracycline hydrochloride. Colloids Surface. A 2022, 636, 128163. [Google Scholar] [CrossRef]
- Huang, C.; Dong, J.; Zhang, Y.; Chai, S.; Wang, X.; Kang, S.; Yu, D.; Wang, P.; Jiang, Q. Gold nanoparticles-loaded polyvinylpyrrolidone/ethylcellulose coaxial electrospun nanofibers with enhanced osteogenic capability for bone tissue regeneration. Mater. Des. 2021, 212, 110240. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R.; et al. Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial Activities. Nano Res. 2022, 1–13. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.G.; Wang, K. Electrospun nanofiber membranes for air filtration: A review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Y.; Li, D.; Xu, G.; Yin, D.; Xiao, Y.; Xu, T.; Chen, X.; Zhu, X.; Shi, X. Negative isolation of circulating tumor cells using a microfluidic platform integrated with streptavidin-functionalized PLGA nanofibers. Adv. Fiber Mater. 2021, 3, 192–202. [Google Scholar] [CrossRef]
- Zhan, L.; Deng, J.; Ke, Q.; Li, X.; Ouyang, Y.; Huang, C.; Liu, X.; Qian, Y. Grooved fibers: Preparation principles through electrospinning and potential applications. Adv. Fiber Mater. 2021, 3, 203–213. [Google Scholar] [CrossRef]
- Guo, S.; Jiang, W.; Shen, L.; Zhang, G.; Gao, Y.; Yang, Y.Y.; Yu, D.G. Electrospun hybrid films for fast and convenient delivery of active herbs extracts. Membranes 2022, 12, 398. [Google Scholar] [CrossRef]
- Bigogno, E.R.; Soares, L.; Mews, M.H.R.; Zétola, M.; Bazzo, G.C.; Stulzer, H.K.; Pezzini, B.R. It is possible to achieve tablets with good tabletability from solid dispersions—The case of the high dose drug gemfibrozil. Curr. Drug Deliv. 2021, 18, 460–470. [Google Scholar] [CrossRef]
- Ejeta, F.; Gabriel, T.; Joseph, N.M.; Belete, A. Formulation, optimization and in vitro evaluation of fast disintegrating tablets of salbutamol sulphate using a combination of superdisintegrant and subliming agent. Curr. Drug Deliv. 2021, 19, 129–141. [Google Scholar] [CrossRef]
- Reinbold, J.; Uhde, A.-K.; Müller, I.; Weindl, T.; Geis-Gerstorfer, J.; Schlensak, C.; Wendel, H.-P.; Krajewski, S. Preventing surgical site infections using a natural, biodegradable, antibacterial coating on surgical sutures. Molecules 2017, 22, 1570. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Qasim, M.; Ali, A. Engineering and polymeric composition of drug-eluting suture: A review. J. Biomed. Mater. Res. A 2021, 109, 2065–2081. [Google Scholar] [CrossRef]
- Serrano, C.; García-Fernández, L.; Fernández-Blázquez, J.P.; Barbeck, M.; Ghanaati, S.; Unger, R.; Kirkpatrick, J.; Arzt, E.; Funk, L.; Turón, P.; et al. Nanostructured medical sutures with antibacterial properties. Biomaterials 2015, 52, 291–300. [Google Scholar] [CrossRef]
- Yu, D.G. Preface—Bettering drug delivery knowledge from pharmaceutical techniques and excipients. Curr. Drug Deliv. 2021, 18, 2–3. [Google Scholar] [CrossRef]
- Dennis, C.; Sethu, S.; Nayak, S.; Mohan, L.; Morsi, Y.; Manivasagam, G. Suture materials—Current and emerging trends. J. Biomed. Mater. Res. A 2016, 104, 1544–1559. [Google Scholar] [CrossRef] [PubMed]
- Umranikar, S.A.; Ubee, S.S.; Selvan, M.; Cooke, P. Barbed suture tissue closure device in urological surgery—A comprehensive review. J. Clin. Urol. 2017, 10, 476–484. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Boyce, M.; Ortiz, C.; Baur, J.; Song, J.; Li, Y. The effects of morphological irregularity on the mechanical be-havior of interdigitated biological sutures under tension. J. Biomech. 2017, 58, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kanchanathepsak, T.; Wairojanakul, W.; Suppaphol, S.; Watcharananan, I.; Tuntiyatorn, P.; Tawonsawatruk, T. Evaluation of biomechanical properties on partial and complete epitendinous suture in human cadaver flexor tendon repair. J. Orthop. Surg. 2021, 16, 489. [Google Scholar] [CrossRef]
- Chen, X.; Hou, D.; Tang, X.; Wang, L. Quantitative physical and handling characteristics of novel antibacterial braided silk suture materials. J. Mech. Behav. Biomed. Mater. 2015, 50, 160–170. [Google Scholar] [CrossRef]
- Hashemi, N.; Asayesh, A.; Jeddi, A.A.A.; Tehrani, M.A. The role of geometry on the viscoelastic materials properties: Tensile and stress relaxation of the yarn. J. Text. Inst. 2019, 110, 1733–1739. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, J.; Ren, T.; Zhu, P. Microstructural and tribological properties of a dopamine hydrochloride and graphene oxide coating applied to multifilament surgical sutures. Polymers 2020, 12, 1630. [Google Scholar] [CrossRef]
- Lipatov, V.A.; Severinov, D.A.; Denisov, A.A.; Lazarenko, S.V.; Grigor’yev, N.N. Research of physical and mechanical characteristics of suture material in experiment in operations on liver. IP Pavlov Russ. Med. Biol. Her. 2020, 28, 193–199. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, J.; Cheng, J.; Wu, G.; Zhang, Y.; Ni, Z.; Zhao, G. A Poly(L-Lactic Acid) monofilament with high mechanical properties for application in biodegradable biliary stents. J. Appl. Polym. Sci. 2021, 138, 49656. [Google Scholar] [CrossRef]
- Byrne, M.; Aly, A. The surgical suture. Aesthet. Surg. J. 2019, 39, S67–S72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.-Z. Study on standard midline diameter of surgical suture. Chin. Med. J. 2019, 43, 369–371. (In Chinese) [Google Scholar] [CrossRef]
- Nout, E.; Lange, J.F.; Salu, N.E.; Wijsmuller, A.R.; Hop, W.C.J.; Goossens, R.H.M.; Snijders, C.J.; Jeekel, J.; Kleinrensink, G.-J. Creep behavior of commonly used suture materials in abdominal wall surgery. J. Surg. Res. 2007, 138, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Tobias, K.M.; Kidd, C.E.; Mulon, P.-Y.; Zhu, X. Tensile properties of synthetic, absorbable monofilament suture materials before and after incubation in phosphate-buffered saline. Vet. Surg. 2020, 49, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ge, L.; Mueller, A.; Carlson, M.A.; Teusink, M.J.; Shuler, F.D.; Xie, J. Twisting electrospun nanofiber fine strips into functional sutures for sustained co-delivery of gentamicin and silver. Nanomedicine 2017, 13, 1435–1445. [Google Scholar] [CrossRef] [Green Version]
- Asvar, Z.; Mirzaei, E.; Azarpira, N.; Geramizadeh, B.; Fadaie, M. Evaluation of electrospinning parameters on the tensile strength and suture retention strength of polycaprolactone nanofibrous scaffolds through surface response methodology. J. Mech. Behav. Biomed. Mater. 2017, 75, 369–378. [Google Scholar] [CrossRef]
- Tomita, N.; Tamai, S.; Morihara, T.; Ikeuchi, K.; Ikada, Y. Handling characteristics of braided suture materials for tight tying. J. Appl. Biomater. 1993, 4, 61–65. [Google Scholar] [CrossRef]
- Zhang, L.; Li, W.; Lin, C.; Zhou, Z. Friction behavior at the interface between surgical sutures and tissues. Tribol. Lett. 2017, 65, 127. [Google Scholar] [CrossRef]
- Bezwada, R.S.; Jamiolkowski, D.D.; Lee, I.-Y.; Agarwal, V.; Persivale, J.; Trenka-Benthin, S.; Erneta, M.; Suryadevara, J.; Yang, A.; Liu, S. Monocryl® suture, a new ultra-pliable absorbable monofilament suture. Biomaterials 1995, 16, 1141–1148. [Google Scholar] [CrossRef]
- Viju, S.; Thilagavathi, G. Effect of chitosan coating on properties of silk braided sutures. J. Ind. Text. 2013, 42, 256–268. [Google Scholar] [CrossRef]
- Griesser, H.J.; Chatelier, R.C.; Martin, C.; Vasic, Z.R.; Gengenbach, T.R.; Jessup, G. Elimination of stick-slip of elastomeric sutures by radiofrequency glow discharge deposited coatings. J. Biomed. Mater. Res. 2000, 53, 235–243. [Google Scholar] [CrossRef]
- Richard, A.S.; Verma, R.S. Bioactive nano yarns as surgical sutures for wound healing. Mater. Sci. Eng. C 2021, 128, 112334. [Google Scholar] [CrossRef]
- James, B.; Ramakrishnan, R.; Aprem, A.S. Development of environmentally safe biodegradable, antibacterial surgical sutures using nanosilver particles. J. Polym. Environ. 2021, 29, 2282–2288. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A. Electrospun nanofibers as carriers of microorganisms, stem cells, proteins, and nucleic acids in therapeutic and other applications. Front. Bioeng. Biotechnol. 2020, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Sa’adon, S.; Ansari, M.N.M.; Razak, S.I.A.; Anand, J.S.; Nayan, N.H.M.; Ismail, A.E.; Khan, M.U.A.; Haider, A. Preparation and physicochemical characterization of a diclofenac sodium-dual layer polyvinyl alcohol patch. Polymers 2021, 13, 2459. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating current electrospinning: The impacts of various high-voltage signal shapes and frequencies on the spinnability and productivity of polycaprolactone nanofifibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Wang, M.; Yu, D.G.; Li, X.; Williams, G.R. The development and bio-applications of multifluid electrospinning. Mater. Highlight. 2020, 1, 1–13. [Google Scholar] [CrossRef]
- Yu, D.-G.; Lv, H. Preface-striding into nano drug delivery. Curr. Drug Deliv. 2022, 19, 1–3. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, F.; Wang, M.; Lv, H.; Yu, D.G.; Liu, X.; Shen, H. Electrospun hierarchical structural films for effective wound healing. Biomater. Adv. 2022, 212795. [Google Scholar] [CrossRef]
- Aidana, Y.; Wang, Y.; Li, J.; Chang, S.; Wang, K.; Yu, D.-G. Fast dissolution electrospun medicated nanofibers for effective delivery of poorly water-soluble drug. Curr. Drug Deliv. 2022, 19, 422–435. [Google Scholar] [CrossRef]
- Shepa, I.; Mudra, E.; Dusza, J. Electrospinning through the prism of time. Mater. Today Chem. 2021, 21, 100543. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Lv, H.; Zhou, Y.; Yang, Y.; Yu, D.-G. Electrospun polyacrylonitrile-based lace nanostructures and their Cu(II) adsorption. Sep. Purif. Technol. 2022, 288, 120643. [Google Scholar] [CrossRef]
- Yu, D.-G.; Wang, M.; Ge, R. Strategies for sustained drug release from electrospun multi-layer nanostructures. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Polymer-based nanofiber-nanoparticle hybrids and their medical applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; He, Y.; Yu, D.-G.; Li, W.; Wang, K. Drug–zein@lipid hybrid nanoparticles: Electrospraying preparation and drug extended release application. Colloids Surf. B Biointerfaces 2021, 201, 111629. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Hao, J. Identifying new pathways and targets for wound healing and therapeutics from natural sources. Curr. Drug Deliv. 2021, 18, 1064–1084. [Google Scholar] [CrossRef]
- Pizarro, G.d.C.; Alavia, W.; González, K.; Díaz, H.; Marambio, O.G.; Martin-Trasanco, R.; Sánchez, J.; Oyarzún, D.P.; Neira-Carrillo, A. Design and study of a photo-switchable polymeric system in the presence of ZnS nanoparticles under the influence of UV light irradiation. Polymers 2022, 14, 945. [Google Scholar] [CrossRef]
- Monfared, M.; Taghizadeh, S.; Zare-Hoseinabadi, A.; Mousavi, S.M.; Hashemi, S.A.; Ranjbar, S.; Amani, A.M. Emerging frontiers in drug release control by core–shell nanofibers: A review. Drug Metab. Rev. 2019, 51, 589–611. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Wu, M.; Yu, D.-G. Nanofibers-based food packaging. ES Food Agrofor. 2021, 7, 186B. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, K.; Yu, D.G.; Zheng, X.; Huang, C. Advances in biosensing and environmental monitoring based on electrospun nanofibers. Adv. Fiber Mater. 2022, 9. [Google Scholar] [CrossRef]
- Song, Y.; Huang, H.; He, D.; Yang, M.; Wang, H.; Zhang, H.; Li, J.; Li, Y.; Wang, C. Gallic acid/2-hydroxypropyl-β-cyclodextrin inclusion complexes electrospun nanofibrous webs: Fast dissolution, improved aqueous solubility and antioxidant property of gallic acid. Chem. Res. Chin. Univ. 2021, 37, 450–455. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Liu, Y.; Gao, Y.; Liu, P. Electrospun coaxial fibers to optimize the release of poorly water-soluble drug. Polymers 2022, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Ning, T.; Zhou, Y.; Xu, H.; Guo, S.; Wang, K.; Yu, D.-G. Orodispersible membranes from a modified coaxial electrospinning for fast dissolution of diclofenac sodium. Membranes 2021, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Rasekh, A.; Raisi, A. Electrospun nanofibrous polyether-block-amide membrane containing silica nanoparticles for water desalination by vacuum membrane distillation. Sep. Purif. Technol. 2021, 275, 119149. [Google Scholar] [CrossRef]
- Kesici Güler, H.; Cengiz Çallıoğlu, F.; Sesli Çetin, E. Antibacterial PVP/cinnamon essential oil nanofibers by emulsion electrospinning. J. Text. Inst. 2019, 110, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Jiang, Y.; Zhang, W.; Elfawal, G.; Wang, K.; Jiang, D.; Hong, H.; Wu, J.; He, C.; Mo, X.; et al. Transcutaneous tumor vaccination combined with anti-programmed death-1 monoclonal antibody treatment produces a synergistic antitumor effect. Acta Biomater. 2022, 140, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, X.; Li, S.; Song, W.-L.; Yu, D.-G.; Annie Bligh, S.W. The effect of drug heterogeneous distributions within core-sheath nanostructures on its sustained release profiles. Biomolecules 2021, 11, 1330. [Google Scholar] [CrossRef]
- Zhao, K.; Lu, Z.-H.; Zhao, P.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Modified tri–axial electrospun functional core–shell nanofibrous membranes for natural photodegradation of antibiotics. Chem. Eng. J. 2021, 425, 131455. [Google Scholar] [CrossRef]
- Rathore, P.; Schiffman, J.D. Beyond the single-nozzle: Coaxial electrospinning enables innovative nanofiber chemistries, geometries, and applications. ACS Appl. Mater. Interfaces 2021, 13, 48–66. [Google Scholar] [CrossRef]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.-G. Electrospun structural hybrids of acyclovir-polyacrylonitrile at acyclovir for modifying drug release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef]
- He, C.-L.; Huang, Z.-M.; Han, X.-J. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. J. Biomed. Mater. Res. A 2009, 89, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Branford-White, C.J.; Chatterton, N.P.; White, K.; Zhu, L.-M.; Shen, X.-X.; Nie, W. Electrospinning of concentrated polymer solutions. Macromolecules 2010, 43, 10743–10746. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, M.; Zhou, Y.; Sun, M.; Xie, Y.; Yu, D.-G. Comparisons of antibacterial performances between electrospun polymer@drug nanohybrids with drug-polymer nanocomposites. Adv. Compos. Hybrid Mater. 2022, 5, 1–13. [Google Scholar] [CrossRef]
- Zheng, G.; Peng, H.; Jiang, J.; Kang, G.; Liu, J.; Zheng, J.; Liu, Y. Surface functionalization of PEO nanofibers using a TiO2 suspension as sheath fluid in a modified coaxial electrospinning process. Chem. Res. Chin. Univ. 2021, 37, 571–577. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.-T.; Hu, P.-Y.; Liu, J.-J.; Liu, X.-F.; Hu, M.; Cui, Z.; Wang, N.; Niu, Z.; Xiang, H.-F.; et al. Laparoscopic electrospinning for in situ hemostasis in minimally invasive operation. Chem. Eng. J. 2020, 395, 125089. [Google Scholar] [CrossRef]
- Padmakumar, S.; Joseph, J.; Neppalli, M.H.; Mathew, S.E.; Nair, S.V.; Shankarappa, S.A.; Menon, D. Electrospun polymeric core–sheath yarns as drug eluting surgical sutures. ACS Appl. Mater. Interfaces 2016, 8, 6925–6934. [Google Scholar] [CrossRef]
- Weldon, C.B.; Tsui, J.H.; Shankarappa, S.A.; Nguyen, V.T.; Ma, M.; Anderson, D.G.; Kohane, D.S. Electrospun drug-eluting sutures for local anesthesia. J. Control. Release 2012, 161, 903–909. [Google Scholar] [CrossRef]
- Wu, Z.; Kong, B.; Liu, R.; Sun, W.; Mi, S. Engineering of corneal tissue through an aligned PVA/Collagen composite nanofibrous electrospun scaffold. Nanomaterials 2018, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Song, J.Y.; Ryu, H.I.; Lee, J.M.; Bae, S.H.; Lee, J.W.; Yi, C.C.; Park, S.M. Conformal fabrication of an electrospun nanofiber mat on a 3D ear cartilage-shaped hydrogel collector based on hydrogel-assisted electrospinning. Nanoscale Res. Lett. 2021, 16, 116. [Google Scholar] [CrossRef]
- Erçin, E.; Karahan, M. Literature review of suture materials. In Knots in Orthopedic Surgery; Springer: Berlin/Heidelberg, Germany, 2018; pp. 177–180. [Google Scholar] [CrossRef]
- Regula, C.G.; Yag-Howard, C. Suture products and techniques: What to use, where, and why. Dermatol. Surg. 2015, 41, S187. [Google Scholar] [CrossRef]
- Hosseini, R.; Mansoorli, S.; Pirjani, R.; Eslamian, L.; Rabiee, M. A comparison of the effects of two suture materials on isthmocele formation: A cohort study. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101933. [Google Scholar] [CrossRef] [PubMed]
- Bichon, D.; Borloz, W.; Cassano-Zoppi, A.L. In vivo evaluation of a new polyurethane-coated catgut suture. Biomaterials 1984, 5, 255–263. [Google Scholar] [CrossRef]
- Li, H.; Cheng, F.; Chávez-Madero, C.; Choi, J.; Wei, X.; Yi, X.; Zheng, T.; He, J. Manufacturing and physical characterization of absorbable oxidized regenerated cellulose braided surgical sutures. Int. J. Biol. Macromol. 2019, 134, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Williams, G.R.; Wu, J.; Wu, J.; Niu, S.; Li, H.; Wang, H.; Zhu, L. Regenerated chitin fibers reinforced with bacterial cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018, 180, 304–313. [Google Scholar] [CrossRef]
- Chen, X.; Yang, X.; Pan, J.; Wang, L.; Xu, K. Degradation behaviors of bioabsorbable P3/4HB monofilament suture in vitro and in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 447–455. [Google Scholar] [CrossRef]
- El-Shanshory, A.A.; Agwa, M.M.; Abd-Elhamid, A.I.; Soliman, H.M.A.; Mo, X.; Kenawy, E.-R. Metronidazole topically immobilized electrospun nanofibrous scaffold: Novel secondary intention wound healing accelerator. Polymers 2022, 14, 454. [Google Scholar] [CrossRef]
- Keridou, I.; Franco, L.; Martínez, J.C.; Turon, P.; Del Valle, L.J.; Puiggalí, J. Electrospun scaffolds for wound healing applications from poly(4-hydroxybutyrate): A biobased and biodegradable linear polymer with high elastomeric properties. J. Appl. Polym. Sci. 2022, 139, 51447. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Sanfilippo, M.; Gallo, G.; Palazzolo, G.; Puglia, A.M. Combining in the melt physical and biological properties of poly(caprolactone) and chlorhexidine to obtain antimicrobial surgical monofilaments. Appl. Microbiol. Biotechnol. 2013, 97, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Reddy, B.S.; In, K.H.; Panigrahi, B.B.; Paturi, U.M.R.; Cho, K.K.; Reddy, N.S. Modeling tensile strength and suture retention of polycaprolactone electrospun nanofibrous scaffolds by artificial neural networks. Mater. Today Commun. 2021, 26, 102115. [Google Scholar] [CrossRef]
- Bai, Y.; Jiang, C.; Wang, Q.; Wang, T. A novel high mechanical strength shape memory polymer based on ethyl cellulose and polycaprolactone. Carbohydr. Polym. 2013, 96, 522–527. [Google Scholar] [CrossRef]
- Goonoo, N.; Jeetah, R.; Bhaw-Luximon, A.; Jhurry, D. Polydioxanone-based bio-materials for tissue engineering and drug/gene delivery applications. Eur. J. Pharm. Biopharm. 2015, 97, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Boland, E.D.; Coleman, B.D.; Barnes, C.P.; Simpson, D.G.; Wnek, G.E.; Bowlin, G.L. Electrospinning polydioxanone for biomedical applications. Acta Biomater. 2005, 1, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.S.; Chen, P.; Ruan, R.; Davachi, S.M.; Al-Salami, H.; Pardo, E.D.J.; Zheng, M.; Doyle, B. A novel biocompatible polymeric blend for applications requiring high toughness and tailored degradation rate. J. Mater. Chem. B 2021, 9, 2532–2546. [Google Scholar] [CrossRef] [PubMed]
- Im, J.N.; Kim, J.K.; Kim, H.-K.; In, C.H.; Lee, K.Y.; Park, W.H. In vitro and in vivo degradation behaviors of synthetic absorbable bicomponent monofilament suture prepared with poly(p-dioxanone) and its copolymer. Polym. Degrad. Stab. 2007, 92, 667–674. [Google Scholar] [CrossRef]
- Zhu, L.; Liang, K.; Ji, Y. Prominent reinforcing effect of chitin nanocrystals on electrospun polydioxanone nanocomposite fiber mats. J. Mech. Behav. Biomed. Mater. 2015, 44, 35–42. [Google Scholar] [CrossRef]
- Liu, S.; Wu, G.; Chen, X.; Zhang, X.; Yu, J.; Liu, M.; Zhang, Y.; Wang, P. Degradation behavior in vitro of carbon nanotubes (CNTs)/poly(lactic acid) (PLA) composite suture. Polymers 2019, 11, 1015. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.Y.; Poh, C.K.; Wang, W. Poly (lactic-co-glycolic acid) as a controlled release delivery device. J. Mater. Sci. Mater. Med. 2009, 20, 1669–1675. [Google Scholar] [CrossRef]
- Ott, L.M.; Zabel, T.A.; Walker, N.K.; Farris, A.L.; Chakroff, J.T.; Ohst, D.G.; Johnson, J.K.; Gehrke, S.H.; Weatherly, R.A.; Detamore, M.S. Mechanical evaluation of gradient electrospun scaffolds with 3D printed ring reinforcements for tracheal defect repair. Biomed. Mater. 2016, 11, 025020. [Google Scholar] [CrossRef] [Green Version]
- Lendlein, A.; Langer, R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 2002, 296, 1673–1676. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Liu, K.; Zhang, G.; Mao, Y.; Chen, L.; Peng, Y.; Tao, T.H. Biomimicking antibacterial opto-electro sensing sutures made of regenerated silk proteins. Adv. Mater. 2021, 33, 2004733. [Google Scholar] [CrossRef]
- Joo, Y.-S.; Cha, J.-R.; Gong, M.-S. Biodegradable shape-memory polymers using polycaprolactone and isosorbide based polyurethane blends. Mater. Sci. Eng. C 2018, 91, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Houshyar, S.; Bhattacharyya, A.; Khalid, A.; Rifai, A.; Dekiwadia, C.; Kumar, G.S.; Tran, P.A.; Fox, K. Multifunctional sutures with temperature sensing and infection control. Macromol. Biosci. 2021, 21, 2000364. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guan, S.; Su, J.; Liang, J.; Cui, L.; Zhang, K. The development of hyaluronic acids used for skin tissue regeneration. Curr. Drug Deliv. 2021, 18, 836–846. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, J.; Meyer, K.M.; Marion, M.D. Suture choice and other methods of skin closure. Surg. Clin. N. Am. 2009, 89, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Swanson, N.A.; Tromovitch, T.A. Suture materials, 1980s: Properties, uses, and abuses. Int. J. Dermatol. 1982, 21, 373–378. [Google Scholar] [CrossRef]
- Wu, D.-Q.; Cui, H.-C.; Zhu, J.; Qin, X.-H.; Xie, T. Novel amino acid based nanogel conjugated suture for antibacterial application. J. Mater. Chem. B 2016, 4, 2606–2613. [Google Scholar] [CrossRef]
- Rodeheaver, G.T.; Thacker, J.G.; Owen, J.; Strauss, M.; Masterson, T.; Edlich, R.F. Knotting and handling characteristics of coated synthetic absorbable sutures. J. Surg. Res. 1983, 35, 525–530. [Google Scholar] [CrossRef]
- Huang, W.; Ling, S.; Li, C.; Omenetto, F.G.; Kaplan, D.L. Silkworm silk-based materials and devices generated using bionanotechnology. Chem. Soc. Rev. 2018, 47, 6486–6504. [Google Scholar] [CrossRef]
- Mohammadi, H.; Alihosseini, F.; Hosseini, S.A. Improving physical and biological properties of nylon monofilament as suture by chitosan/hyaluronic acid. Int. J. Biol. Macromol. 2020, 164, 3394–3402. [Google Scholar] [CrossRef]
- Mahesh, L.; Kumar, V.R.; Jain, A.; Shukla, S.; Aragoneses, J.M.; Martínez González, J.M.; Fernández-Domínguez, M.; Calvo-Guirado, J.L. Bacterial adherence around sutures of different material at grafted site: A microbiological analysis. Materials 2019, 12, 2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asher, R.; Chacartchi, T.; Tandlich, M.; Shapira, L.; Polak, D. Microbial accumulation on different suture materials following oral surgery: A randomized controlled study. Clin. Oral Investig. 2019, 23, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Veleirinho, B.; Coelho, D.S.; Dias, P.F.; Maraschin, M.; Pinto, R.; Cargnin-Ferreira, E.; Peixoto, A.; Souza, J.A.; Ribeiro-do-Valle, R.M.; Lopes-da-Silva, J.A. Foreign body reaction associated with PET and PET/Chitosan electrospun nanofibrous abdominal meshes. PLoS ONE 2014, 9, e95293. [Google Scholar] [CrossRef]
- France, L.A.; Fancey, K.S. Viscoelastically active sutures—A stitch in time? Mater. Sci. Eng. C 2021, 121, 111695. [Google Scholar] [CrossRef] [PubMed]
- East, B.; Plencner, M.; Kralovic, M.; Rampichova, M.; Sovkova, V.; Vocetkova, K.; Otahal, M.; Tonar, Z.; Kolinko, Y.; Amler, E.; et al. A polypropylene mesh modified with poly-ε-caprolactone nanofibers in hernia repair: Large animal experiment. Int. J. Nanomed. 2018, 13, 3129–3143. [Google Scholar] [CrossRef] [Green Version]
- Swar, S.; Zajícová, V.; Rysová, M.; Lovětinská-Šlamborová, I.; Voleský, L.; Stibor, I. Biocompatible surface modification of poly(ethylene terephthalate) focused on pathogenic bacteria: Promising prospects in biomedical applications. J. Appl. Polym. Sci. 2017, 134, 44990. [Google Scholar] [CrossRef]
- Hoshino, S.; Yoshida, Y.; Tanimura, S.; Yamauchi, Y.; Noritomi, T.; Yamashita, Y. A study of the efficacy of antibacterial sutures for surgical site infection: A retrospective controlled trial. Int. Surg. 2013, 98, 129–132. [Google Scholar] [CrossRef]
- Rouhollahi, F.; Hosseini, S.A.; Alihosseini, F.; Allafchian, A.; Haghighat, F. Investigation on the biodegradability and antibacterial properties of nanohybrid suture based on silver incorporated PGA-PLGA nanofibers. Fibers Polym. 2018, 19, 2056–2065. [Google Scholar] [CrossRef]
- Edis, Z.; Haj Bloukh, S.; Ibrahim, M.R.; Abu Sara, H. “Smart” antimicrobial nanocomplexes with potential to decrease surgical site infections (SSI). Pharmaceutics 2020, 12, 361. [Google Scholar] [CrossRef] [Green Version]
- Tummalapalli, M.; Anjum, S.; Kumari, S.; Gupta, B. Antimicrobial surgical sutures: Recent developments and strategies. Polym. Rev. 2016, 56, 607–630. [Google Scholar] [CrossRef]
- Lowe, A.; Deng, W.; Smith, D.W.; Balkus, K.J. Acrylonitrile-based nitric oxide releasing melt-spun fibers for enhanced wound healing. Macromolecules 2012, 45, 5894–5900. [Google Scholar] [CrossRef]
- Ma, Y.; Bai, D.; Hu, X.; Ren, N.; Gao, W.; Chen, S.; Chen, H.; Lu, Y.; Li, J.; Bai, Y. Robust and antibacterial polymer/mechanically exfoliated graphene nanocomposite fibers for biomedical applications. ACS Appl. Mater. Interfaces 2018, 10, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, L.; Huang, X.; Wang, H.; Shao, H.; Xie, M.; Xu, Y.; Zhang, Y. Tough and VEGF-Releasing scaffolds composed of artificial silk fibroin mats and a natural acellular matrix. RSC Adv. 2015, 5, 16748–16758. [Google Scholar] [CrossRef]
- Hu, J.; Song, Y.; Zhang, C.; Huang, W.; Chen, A.; He, H.; Zhang, S.; Chen, Y.; Tu, C.; Liu, J.; et al. Highly aligned electrospun collagen/polycaprolactone surgical sutures with sustained release of growth factors for wound regeneration. Acs Appl. Bio. Mater. 2020, 3, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Yin, H.; Wang, J.; Ma, L.; Morsi, Y.; Mo, X. Fabrication and characterization of TGF-Β1-loaded electrospun poly (lactic-co-glycolic acid) core-sheath sutures. Colloids Surf. B Biointerfaces 2018, 161, 331–338. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, J.J.; Atala, A.; Lee, S.J. The effect of controlled release of PDGF-BB from heparin-conjugated electrospun PCL/gelatin scaffolds on cellular bioactivity and infiltration. Biomaterials 2012, 33, 6709–6720. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.; DiBalsi, M.J.; Meilinger, N.; Zhang, C.; Beal, E.; Korneva, G.; Brown, R.O.; Kornev, K.G.; Lee, J.S. Heparin-eluting electrospun nanofiber yarns for antithrombotic vascular sutures. ACS Appl. Mater. Interfaces 2018, 10, 8426–8435. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Min, T. Curcumin, curcumin nanoparticles and curcumin nanospheres: A review on their pharmacodynamics based on monogastric farm animal, poultry and fish nutrition. Pharmaceutics 2020, 12, 447. [Google Scholar] [CrossRef]
- Sridhar, R.; Ravanan, S.; Venugopal, J.R.; Sundarrajan, S.; Pliszka, D.; Sivasubramanian, S.; Gunasekaran, P.; Prabhakaran, M.; Madhaiyan, K.; Sahayaraj, A.; et al. Curcumin- and natural extract-loaded nanofibres for potential treatment of lung and breast cancer: In vitroefficacy evaluation. J. Biomater. Sci. Polym. Ed. 2014, 25, 985–998. [Google Scholar] [CrossRef]
- Sharifisamani, E.; Mousazadegan, F.; Bagherzadeh, R.; Latifi, M. PEG-PLA-PCL based electrospun yarns with curcumin control release property as suture. Polym. Eng. Sci. 2020, 60, 1520–1529. [Google Scholar] [CrossRef]
- Viju, S.; Thilagavathi, G. Fabrication and characterization of silk braided sutures. Fibers Polym. 2012, 13, 782–789. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kwon, T.-Y.; Kim, K.-H.; Kwon, S.-T.; Cho, D.-H.; Jang, S.H.; Son, J.S.; Lee, K.-B. Anti-inflammatory drug releasing absorbable surgical sutures using poly(lactic-co-glycolic acid) particle carriers. Polym. Bull. 2014, 71, 1933–1946. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Pun, E.Y.B.; Lin, H. Nano-spider-web-like electrospun fibers of europium complexes doped polyvinylpyrrolidone for medical suture. Opt. Mater. 2018, 84, 38–45. [Google Scholar] [CrossRef]
- Deranlot, J.; Maurel, N.; Diop, A.; Pratlong, N.; Roche, L.; Tiemtore, R.; Nourissat, G. Abrasive properties of braided polyblend sutures in cuff tendon repair: An in vitro biomechanical study exploring regular and tape sutures. Arthrosc. J. Arthrosc. Relat. Surg. 2014, 30, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Sheng, D.; Jiang, L.; Shafiq, M.; Khan, A.U.R.; Hashim, R.; Chen, Y.; Li, B.; Xie, X.; Chen, J.; et al. Vascular endothelial growth factor-Capturing aligned electrospun polycaprolactone/gelatin nanofibers promote patellar ligament regeneration. Acta Biomater. 2022, 140, 233–246. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, J.; Li, C.; Han, H.; Lin, J.; Wang, F.; Wang, L. Bamboo-inspired lightweight tape suture with hollow and porous structure for tendon repair. Mater. Des. 2020, 193, 108843. [Google Scholar] [CrossRef]
- Seo, Y.-K.; Kim, J.-H.; Eo, S.-R. Co-effect of silk and amniotic membrane for tendon repair. J. Biomater. Sci. Polym. Ed. 2016, 27, 1232–1247. [Google Scholar] [CrossRef]
- Ye, Y.; Zhou, Y.; Jing, Z.; Xu, Y.; Yin, D. Electrospun heparin-loaded nano-fiber sutures for the amelioration of achilles tendon rupture regeneration: In vivo evaluation. J. Mater. Chem. B 2021, 9, 4154–4168. [Google Scholar] [CrossRef]
- Eke, P.I.; Dye, B.A.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef]
- Meghil, M.M.; Rueggeberg, F.; El-Awady, A.; Miles, B.; Tay, F.; Pashley, D.; Cutler, C.W. Novel coating of surgical suture confers antimicrobial activity against porphyromonas gingivalis and enterococcus faecalis. J. Periodontol. 2015, 86, 788–794. [Google Scholar] [CrossRef]
- Kashiwabuchi, F.; Parikh, K.S.; Omiadze, R.; Zhang, S.; Luo, L.; Patel, H.V.; Xu, Q.; Ensign, L.M.; Mao, H.-Q.; Hanes, J.; et al. Development of absorbable, antibiotic-eluting sutures for ophthalmic surgery. Transl. Vis. Sci. Technol. 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.S.; Omiadze, R.; Josyula, A.; Shi, R.; Anders, N.M.; He, P.; Yazdi, Y.; McDonnell, P.J.; Ensign, L.M.; Hanes, J. Ultra-thin, high strength, antibiotic-eluting sutures for prevention of ophthalmic infection. Bioeng. Transl. Med. 2021, 6, e10204. [Google Scholar] [CrossRef] [PubMed]


| Specification | Nonabsorbable Surgical Sutures/mm | Absorbable Surgical Sutures/mm | ||||||
|---|---|---|---|---|---|---|---|---|
| Class I | Class II—Single Strand | Class II—Many Strands | ||||||
| Width of Sutures Diameter Range | Diameter Difference of Adjacent Coarse Gauge Sutures | Width of Sutures Diameter Range | Diameter Difference of Adjacent Coarse Gauge Sutures | Width of Sutures Diameter Range | Diameter Difference of Adjacent Coarse Gauge Sutures | Width of Sutures Diameter Range | Diameter Difference of Adjacent Coarse Gauge Sutures | |
| 12-0 | 0.008 | 0.009 | —— | —— | 0.008 | 0.009 | —— | —— |
| 11-0 | 0.009 | 0.010 | —— | —— | 0.009 | 0.010 | —— | —— |
| 10-0 | 0.009 | 0.010 | —— | —— | 0.009 | 0.010 | —— | —— |
| 9-0 | 0.009 | 0.010 | 0.009 | 0.010 | 0.009 | 0.010 | —— | —— |
| 8-0 | 0.009 | 0.010 | 0.019 | 0.020 | 0.009 | 0.010 | —— | —— |
| 7-0 | 0.019 | 0.020 | 0.029 | 0.030 | 0.019 | 0.010 | 0.044 | 0.045 |
| 6-0 | 0.029 | 0.030 | 0.049 | 0.050 | 0.029 | 0.030 | 0.054 | 0.055 |
| 5-0 | 0.049 | 0.050 | 0.049 | 0.050 | 0.049 | 0.050 | 0.049 | 0.050 |
| 4-0 | 0.049 | 0.050 | 0.049 | 0.050 | 0.049 | 0.050 | 0.049 | 0.050 |
| 4-0/T | —— | —— | 0.049 | 0.050 | —— | —— | —— | —— |
| 3-0 | 0.049 | 0.050 | 0.049 | 0.050 | 0.049 | 0.050 | 0.089 | 0.090 |
| 2-0/T | 0.049 | 0.050 | —— | —— | 0.049 | 0.050 | —— | —— |
| 2-0 | 0.049 | 0.050 | 0.079 | 0.080 | 0.049 | 0.050 | 0.059 | 0.060 |
| 0 | 0.049 | 0.050 | 0.069 | 0.070 | 0.049 | 0.050 | 0.099 | 0.010 |
| 1 | 0.099 | 0.100 | 0.099 | 0.100 | 0.099 | 0.100 | 0.070 | 0.071 |
| 2 | 0.099 | 0.100 | 0.099 | 0.100 | 0.099 | 0.100 | 0.039 | —— |
| 3 | 0.099 | 0.100 | 0.099 | 0.100 | 0.099 | 0.100 | —— | —— |
| 4 | 0.099 | 0.100 | —— | —— | ||||
| 5 | 0.099 | 0.100 | —— | —— | 0.099 | —— | —— | —— |
| 6 | 0.099 | 0.100 | —— | —— | —— | —— | —— | —— |
| 7 | 0.099 | 0.100 | —— | —— | —— | —— | —— | —— |
| 8 | 0.099 | 0.100 | —— | —— | —— | —— | —— | —— |
| 9 | 0.099 | 0.100 | —— | —— | —— | —— | —— | —— |
| 10 | 0.099 | —— | —— | —— | —— | —— | —— | —— |
| Bioactive Substances | Polymers | Characteristics | Preparation Method | Ref. |
|---|---|---|---|---|
| Silver nanoparticles | PGA-PLGA | Significant antibacterial effect, biocompatibility and degradability | Blend electrospinning | [139] |
| Triclosan | Polylactose 910 | Effective antibacterial avoidance of wound infection | Coating | [141] |
| NO | Acrylonitrile-co-1-vinylimidazole (AN/VIM). | Maintain good mechanical properties, antibacterial and promote healing | Melt spinning | [142] |
| GO | PVA | Good antibacterial properties, low cytotoxicity | Blend electrospinning | [143] |
| Growth factor (VEGF/bFGF/TGF-β) | RSF/BAMG, PCL/collagen, PLGA | Promote cell adhesion and value-add, promote the regeneration of new blood vessels | Coaxial electrospinning | [144,145,146] |
| Curcumin | PEG, PLA and PCL | Good chemical stability, low toxicity, antibacterial and healing | Blend electrospinning | [151] |
| heparin | PLGA, PEO and PgP | Reduces platelet adhesion, anti-thrombosis | Blend electrospinning | [148] |
| Aceclofenac/insulin | PLLA/PLGA | Promote epidermal hyperplasia, cell adhesion migration | Blend electrospinning | [96] |
| Chitosan/tetracycline hydrochloride | Silk | Antibacterial, bleeding | Blend electrospinning | [152] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Liu, Y.; Zhou, W.; Yu, D. Electrospun Medical Sutures for Wound Healing: A Review. Polymers 2022, 14, 1637. https://doi.org/10.3390/polym14091637
Xu L, Liu Y, Zhou W, Yu D. Electrospun Medical Sutures for Wound Healing: A Review. Polymers. 2022; 14(9):1637. https://doi.org/10.3390/polym14091637
Chicago/Turabian StyleXu, Lin, Yanan Liu, Wenhui Zhou, and Dengguang Yu. 2022. "Electrospun Medical Sutures for Wound Healing: A Review" Polymers 14, no. 9: 1637. https://doi.org/10.3390/polym14091637
APA StyleXu, L., Liu, Y., Zhou, W., & Yu, D. (2022). Electrospun Medical Sutures for Wound Healing: A Review. Polymers, 14(9), 1637. https://doi.org/10.3390/polym14091637