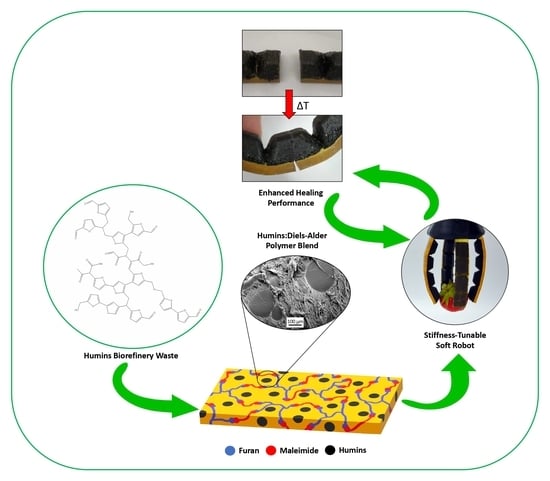
Humins Blending in Thermoreversible Diels–Alder Networks for Stiffness Tuning and Enhanced Healing Performance for Soft Robotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of BMI689-F400 and Humins-DA
2.3. Characterization and Self-Healing Efficiency Determination
2.3.1. ATR-FTIR Spectroscopy
2.3.2. Thermal Analysis
2.3.3. Scanning Electron Microscopy
2.3.4. Rheological and Mechanical Analysis
2.4. Humins-DA Reprocessing via Extrusion
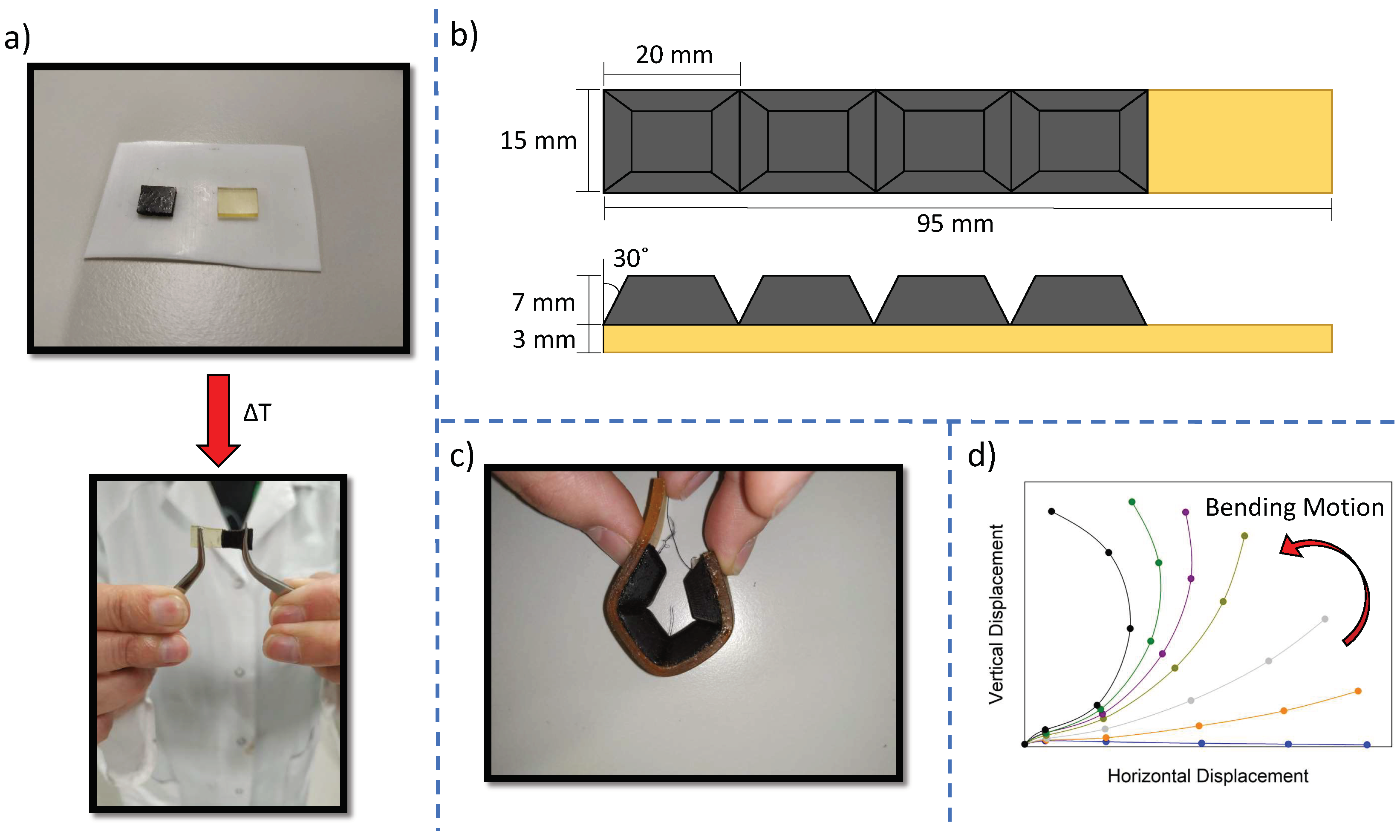
2.5. Characterization of the Soft Robotic Actuator
3. Results and Discussion
3.1. Reactants Employed and Thermal Properties of the Synthesized Materials
3.2. Rheological Behaviour during the Gel Transition
3.3. Mechanical Properties and Self-Healing Efficiency
3.4. Stiffening Effect upon Heating and Healing Mechanism Elucidation
3.5. Humins-DA Reprocessability
3.6. Soft Robotic Gripper as a Multi-Material Demonstrator
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filiciotto, L.; Rothenberg, G. Biodegradable Plastics: Standards, Policies, and Impacts. Chemsuschem 2021, 14, 56–72. [Google Scholar] [CrossRef]
- de Jong, E.; Dam, M.; Sipos, L.; Gruter, G.; Smith, P.; Gross, R. Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters. Biobased Monomers Polym. Mater. 2012, 1105, 1–13. [Google Scholar]
- Nolasco, M.; Araujo, C.; Thiyagarajan, S.; Rudic, S.; Vaz, P.; Silvestre, A.; Ribeiro-Claro, P.; Sousa, A. Asymmetric Monomer, Amorphous Polymer? Structure-Property Relationships in 2,4-FDCA and 2,4-PEF. Macromolecules 2020, 53, 1380–1387. [Google Scholar] [CrossRef]
- Pfab, E.; Filiciotto, L.; Luque, R. The Dark Side of Biomass Valorization: A Laboratory Experiment To Understand Humin Formation, Catalysis, and Green Chemistry. J. Chem. Educ. 2019, 96, 3030–3037. [Google Scholar] [CrossRef]
- van Zandvoort, I.; Wang, Y.; Rasrendra, C.; van Eck, E.; Bruijnincx, P.; Heeres, H.; Weckhuysen, B. Formation, Molecular Structure, and Morphology of Humins in Biomass Conversion: Influence of Feedstock and Processing Conditions. Chemsuschem 2013, 6, 1745–1758. [Google Scholar] [CrossRef]
- Filiciotto, L.; Balu, A.; Romero, A.; Angelici, C.; van der Waal, J.; Luque, R. Reconstruction of humins formation mechanism from decomposition products: A GC-MS study based on catalytic continuous flow depolymerizations. Mol. Catal. 2019, 479, 5–16. [Google Scholar] [CrossRef]
- Dee, S.; Bell, A. A Study of the Acid-Catalyzed Hydrolysis of Cellulose Dissolved in Ionic Liquids and the Factors Influencing the Dehydration of Glucose and the Formation of Humins. Chemsuschem 2011, 4, 1166–1173. [Google Scholar] [CrossRef]
- Hoang, T.; van Eck, E.; Bula, W.; Gardeniers, J.; Lefferts, L.; Seshan, K. Humin based by-products from biomass processing as a potential carbonaceous source for synthesis gas production. Green Chem. 2015, 17, 959–972. [Google Scholar] [CrossRef]
- Hoang, T.; Lefferts, L.; Seshan, K. Valorization of Humin-Based Byproducts from Biomass Processing-A Route to Sustainable Hydrogen. Chemsuschem 2013, 6, 1651–1658. [Google Scholar] [CrossRef]
- Filiciotto, L.; de Miguel, G.; Balu, A.; Romero, A.; van der Waal, J.; Luque, R. Towards the photophysical studies of humin by-products. Chem. Commun. 2017, 53, 7015–7017. [Google Scholar] [CrossRef] [Green Version]
- Mija, A.; van der Waal, J.; Pin, J.; Guigo, N.; de Jong, E. Humins as promising material for producing sustainable carbohydrate-derived building materials. Constr. Build. Mater. 2017, 139, 594–601. [Google Scholar] [CrossRef]
- Pin, J.; Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N.; van der Waal, J.; de Jong, E. Valorization of Biorefinery Side-Stream Products: Combination of Humins with Polyfurfuryl Alcohol for Composite Elaboration. ACS Sustain. Chem. Eng. 2014, 2, 2182–2190. [Google Scholar] [CrossRef]
- Cantarutti, C.; Dinu, R.; Mija, A. Biorefinery Byproducts and Epoxy Biorenewable Monomers: A Structural Elucidation of Humins and Triglycidyl Ether of Phloroglucinol Cross-Linking. Biomacromolecules 2020, 21, 517–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinu, R.; Mija, A. Cross-linked polyfuran networks with elastomeric behaviour based on humins biorefinery by-products. Green Chem. 2019, 21, 6277–6289. [Google Scholar] [CrossRef] [Green Version]
- Tosi, P.; van Klink, G.; Celzard, A.; Fierro, V.; Vincent, L.; de Jong, E.; Mija, A. Auto-Crosslinked Rigid Foams Derived from Biorefinery Byproducts. Chemsuschem 2018, 11, 2797–2809. [Google Scholar] [CrossRef]
- Tosi, P.; van Klink, G.; Hurel, C.; Lomenech, C.; Celzard, A.; Fierro, V.; Delgado-Sanchez, C.; Mija, A. Investigating the properties of humins foams, the porous carbonaceous materials derived from biorefinery by-products. Appl. Mater. Today 2020, 20, 100622. [Google Scholar] [CrossRef]
- Sangregorio, A.; Guigo, N.; van der Waal, J.; Sbirrazzuoli, N. All ′green′ composites comprising flax fibres and humins′ resins. Compos. Sci. Technol. 2019, 171, 70–77. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- White, S.; Sottos, N.; Geubelle, P.; Moore, J.; Kessler, M.; Sriram, S.; Brown, E.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]
- Toohey, K.; Sottos, N.; Lewis, J.; Moore, J.; White, S. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581–585. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Verdejo, R.; Lopez-Manchado, M.; Santana, M. Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: A review. Mater. Horiz. 2020, 7, 2882–2902. [Google Scholar] [CrossRef]
- Diaz, M.; Van Assche, G.; Maurer, F.; Van Mele, B. Thermophysical characterization of a reversible dynamic polymer network based on kinetics and equilibrium of an amorphous furan-maleimide Diels-Alder cycloaddition. Polymer 2017, 120, 176–188. [Google Scholar] [CrossRef]
- Ehrhardt, D.; Van Durme, K.; Jansen, J.; Van Mele, B.; Van den Brande, N. Self-healing UV-curable polymer network with reversible Diels-Alder bonds for applications in ambient conditions. Polymer 2020, 203, 122762. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Z.; Su, X.; Cao, J.; Chen, M.; Liu, R. Stiff UV-Curable self-healing coating based on double reversible networks containing diels-alder cross-linking and hydrogen bonds. Prog. Org. Coat. 2020, 146, 105699. [Google Scholar] [CrossRef]
- Willocq, B.; Khelifa, F.; Odent, J.; Lemaur, V.; Yang, Y.; Leclere, P.; Cornil, J.; Dubois, P.; Urban, M.; Raquez, J. Mechanistic Insights on Spontaneous Moisture-Driven Healing of Urea-Based Polyurethanes. ACS Appl. Mater. Interfaces 2019, 11, 46176–46182. [Google Scholar] [CrossRef]
- Ni, X.; Gao, Y.; Zhang, X.; Lei, Y.; Sun, G.; You, B. An eco-friendly smart self-healing coating with NIR and pH dual-responsive superhydrophobic properties based on biomimetic stimuli-responsive mesoporous polydopamine microspheres. Chem. Eng. J. 2021, 406, 126725. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Dong, Y.; Li, J. Flexible Self-Repairing Materials for Wearable Sensing Applications: Elastomers and Hydrogels. Macromol. Rapid Commun. 2020, 41, 2000444. [Google Scholar] [CrossRef]
- Roels, E.; Terryn, S.; Brancart, J.; Verhelle, R.; Van Assche, G.; Vanderborght, B. Additive Manufacturing for Self-Healing Soft Robots. Soft Robot. 2020, 7, 711–723. [Google Scholar] [CrossRef]
- Terryn, S.; Brancart, J.; Lefeber, D.; Van Assche, G.; Vanderborght, B. Self-healing soft pneumatic robots. Sci. Robot. 2017, 2, eaan4268. [Google Scholar] [CrossRef]
- Terryn, S.; Roels, E.; Brancart, J.; Van Assche, G.; Vanderborght, B. Self-Healing and High Interfacial Strength in Multi-Material Soft Pneumatic Robots via Reversible Diels-Alder Bonds. Actuators 2020, 9, 34. [Google Scholar] [CrossRef]
- Hartmann, F.; Baumgartner, M.; Kaltenbrunner, M. Becoming Sustainable, The New Frontier in Soft Robotics. Adv. Mater. 2021, 33, 2004413. [Google Scholar] [CrossRef] [PubMed]
- Terryn, S.; Langenbach, J.; Roels, E.; Brancart, J.; Bakkali-Hassani, C.; Poutrel, Q.; Georgopoulou, A.; Thuruthel, T.; Safaei, A.; Ferrentino, P.; et al. A review on self-healing polymers for soft robotics. Mater. Today 2021, 47, 187–205. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, J.; Gan, L.; Long, M. Research progress in bio-based self-healing materials. Eur. Polym. J. 2020, 129, 109651. [Google Scholar] [CrossRef]
- Park, K.; Shin, C.; Song, Y.; Lee, H.; Kim, Y. Recyclable and Mendable Cellulose-Reinforced Composites Crosslinked with Diels-Alder Adducts. Polymers 2019, 11, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Kang, M.; Li, K.; Yao, F.; Oderinde, O.; Fu, G.; Xu, L. Polysaccharide-templated preparation of mechanically-tough, conductive and self-healing hydrogels. Chem. Eng. J. 2018, 334, 2222–2230. [Google Scholar] [CrossRef]
- Ghosh, B.; Chellappan, K.; Urban, M. Self-healing inside a scratch of oxetane-substituted chitosan-polyurethane (OXE-CHI-PUR) networks. J. Mater. Chem. 2011, 21, 14473–14486. [Google Scholar] [CrossRef]
- Buono, P.; Duval, A.; Averous, L.; Habibi, Y. Thermally healable and remendable lignin-based materials through Diels—Alder click polymerization. Polymer 2017, 133, 78–88. [Google Scholar] [CrossRef]
- Duval, A.; Lange, H.; Lawoko, M.; Crestini, C. Reversible crosslinking of lignin via the furan-maleimide Diels-Alder reaction. Green Chem. 2015, 17, 4991–5000. [Google Scholar] [CrossRef]
- Thys, M.; Brancart, J.; Van Assche, G.; Vendamme, R.; Van den Brande, N. Reversible Lignin-Containing Networks Using Diels-Alder Chemistry. Macromolecules 2021, 54, 9750–9760. [Google Scholar] [CrossRef]
- Mangialetto, J.; Cuvellier, A.; Verhelle, R.; Brancart, J.; Rahier, H.; Van Assche, G.; Van den Brande, N.; Van Mele, B. Diffusion- and Mobility-Controlled Self-Healing Polymer Networks with Dynamic Covalent Bonding. Macromolecules 2019, 52, 8440–8452. [Google Scholar] [CrossRef]
- Brancart, J.; Verhelle, R.; Mangialetto, J.; Van Assche, G. Coupling the Microscopic Healing Behaviour of Coatings to the Thermoreversible Diels-Alder Network Formation. Coatings 2019, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Scodeller, I.; Mansouri, S.; Morvan, D.; Muller, E.; Vigier, K.; Wischert, R.; Jerome, F. Synthesis of Renewable meta-Xylylenediamine from Biomass-Derived Furfural. Angew. Chem.-Int. Ed. 2018, 57, 10510–10514. [Google Scholar] [CrossRef] [PubMed]
- Terryn, S.; Brancart, J.; Roels, E.; Van Assche, G.; Vanderborght, B. Room Temperature Self-Healing in Soft Pneumatic Robotics Autonomous Self-Healing in a Diels-Alder Polymer Network. IEEE Robot. Autom. Mag. 2020, 27, 44–55. [Google Scholar] [CrossRef]
- Roels, E.; Terryn, S.; Iida, F.; Bosman, A.; Norvez, S.; Clemens, F.; Van Assche, G.; Vanderborght, B.; Brancart, J. Processing of Self-Healing Polymers for Soft Robotics. Adv. Mater. 2022, 34, 2104798. [Google Scholar] [CrossRef]
- Winter, H. Can the gel point of a cross-linking polymer be detected by the G′-G′′ crossover. Polym. Eng. Sci. 1987, 27, 1698–1702. [Google Scholar] [CrossRef]
- Sangregorio, A.; Guigo, N.; de Jong, E.; Sbirrazzuoli, N. Kinetics and Chemorheological Analysis of Cross-Linking Reactions in Humins. Polymers 2019, 11, 1804. [Google Scholar] [CrossRef] [Green Version]
- Sangregorio, A.; Guigo, N.; van der Waal, J.; Sbirrazzuoli, N. Humins from Biorefineries as Thermoreactive Macromolecular Systems. Chemsuschem 2018, 11, 4246–4255. [Google Scholar] [CrossRef]
- Montane, X.; Dinu, R.; Mija, A. Synthesis of Resins Using Epoxies and Humins as Building Blocks: A Mechanistic Study Based on In-Situ FT-IR and NMR Spectroscopies. Molecules 2019, 24, 4110. [Google Scholar] [CrossRef] [Green Version]
- Tsilomelekis, G.; Orella, M.; Lin, Z.; Cheng, Z.; Zheng, W.; Nikolakis, V.; Vlachos, D. Molecular structure, morphology and growth mechanisms and rates of 5-hydroxymethyl furfural (HMF) derived humins. Green Chem. 2016, 18, 1983–1993. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Goulas, K.; Rodriguez, N.; Saha, B.; Vlachos, D. Growth kinetics of humins studied via X-ray scattering. Green Chem. 2020, 22, 2301–2309. [Google Scholar] [CrossRef]
- Cerdan, K.; Van Assche, G.; van Puyvelde, P.; Brancart, J. A novel approach for the closure of large damage in self-healing elastomers using magnetic particles. Polymer 2020, 204, 122819. [Google Scholar] [CrossRef]
- Hayes, S.; Jones, F.; Marshiya, K.; Zhang, W. A self-healing thermosetting composite material. Compos. Part A-Appl. Sci. Manuf. 2007, 38, 1116–1120. [Google Scholar] [CrossRef]
- Rodriguez, E.; Luo, X.; Mather, P. Linear/Network Poly (epsilon-caprolactone) Blends Exhibiting Shape Memory Assisted Self-Healing (SMASH). ACS Appl. Mater. Interfaces 2011, 3, 152–161. [Google Scholar] [CrossRef]
- Alabiso, W.; Hron, T.; Reisinger, D.; Bautista-Anguis, D.; Schlogl, S. Shape memory-assisted self-healing of dynamic thiol-acrylate networks. Polym. Chem. 2021, 12, 5704–5714. [Google Scholar] [CrossRef]
- Chen, T.; Fang, L.; Li, X.; Gao, D.; Lu, C.; Xu, Z. Self-healing polymer coatings of polyurea-urethane/epoxy blends with reversible and dynamic bonds. Prog. Org. Coat. 2020, 147, 105876. [Google Scholar] [CrossRef]
- Barros, L.; Canevarolo, S.; Klein, D.; Maia, J. On-line ATR-MIR for real-time quantification of chemistry kinetics along the barrel in extrusion-based processes. Polym. Test. 2021, 103, 107350. [Google Scholar] [CrossRef]
- Roels, E.; Terryn, S.; Brancart, J.; Van Assche, G.; Vanderborght, B. A Multi-Material Self-Healing Soft Gripper. In Proceedings of the 2019 2nd IEEE International Conference on Soft Robotics (Robosoft 2019), Seoul, Korea, 14–18 April 2019; pp. 316–321. [Google Scholar]
- Zhang, Y.; Yin, X.; Zheng, M.; Moorlag, C.; Yang, J.; Wang, Z. 3D printing of thermoreversible polyurethanes with targeted shape memory and precise in situ self-healing properties. J. Mater. Chem. 2019, 7, 6972–6984. [Google Scholar] [CrossRef]
- Zheng, M.; Guo, Q.; Yin, X.; Getangama, N.; de Bruyn, J.; Xiao, J.; Bai, Y.; Liu, M.; Yang, J. Direct ink writing of recyclable and in situ repairable photothermal polyurethane for sustainable 3D printing development. J. Mater. Chem. 2021, 9, 6981–6992. [Google Scholar] [CrossRef]
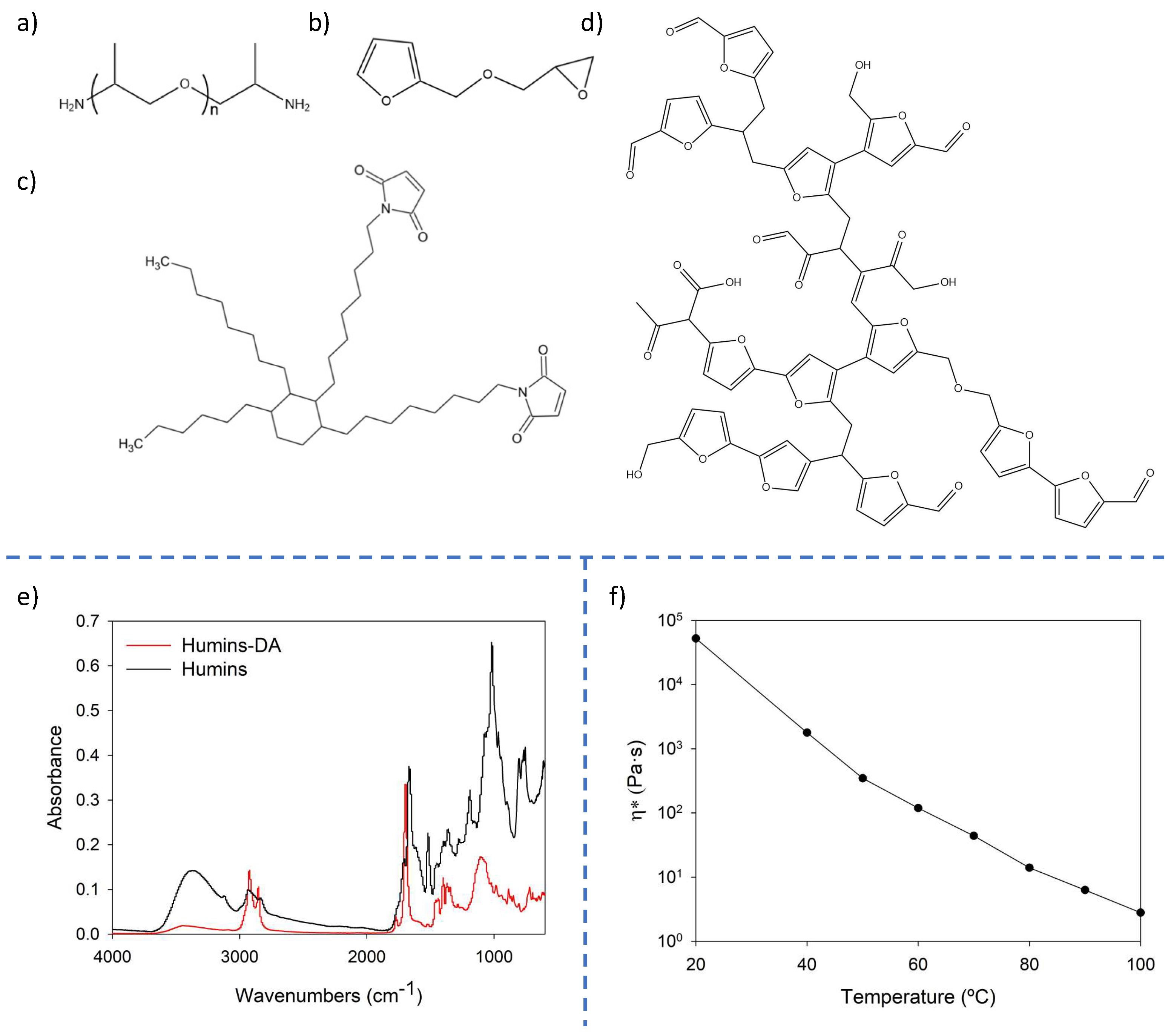
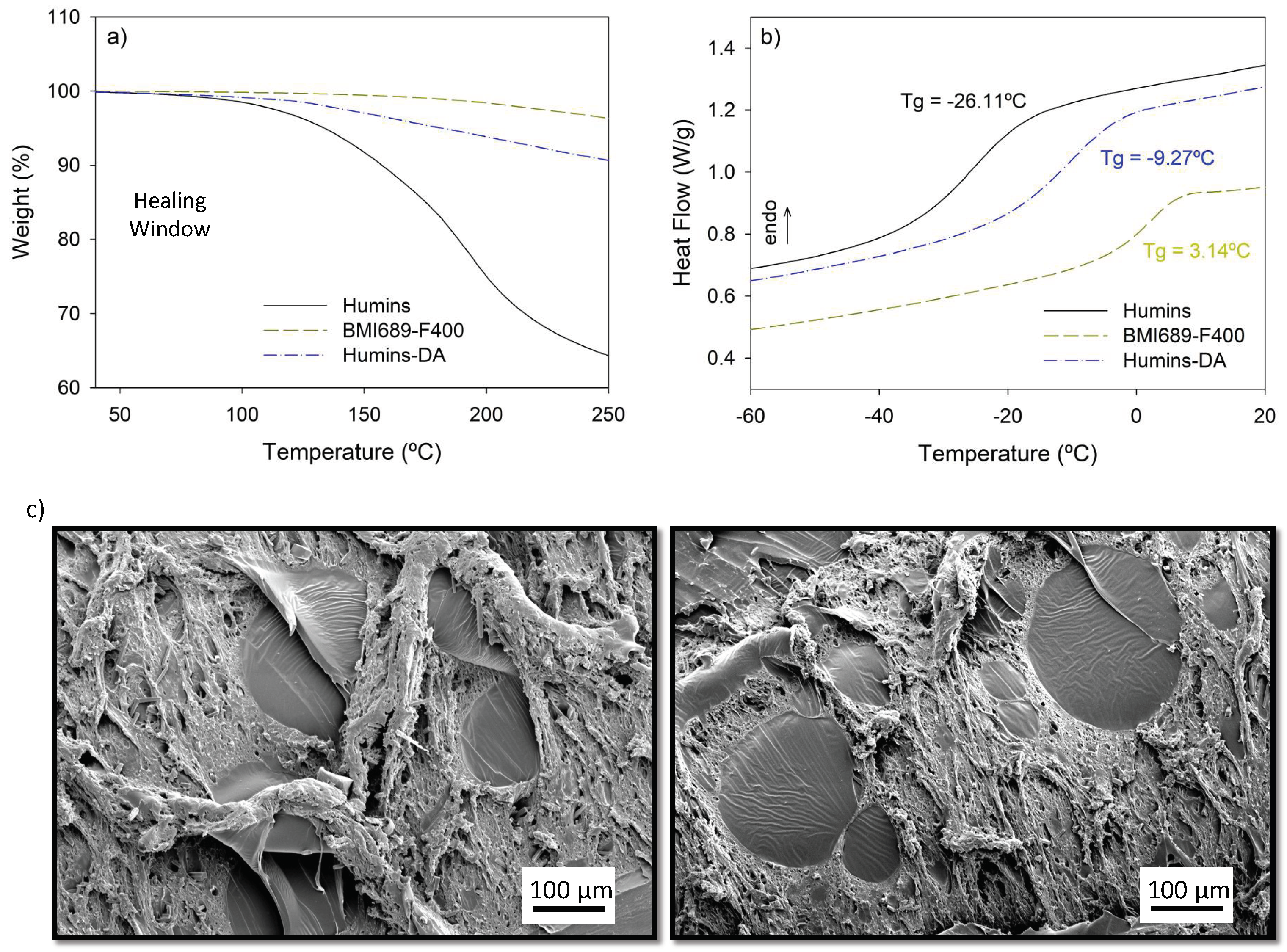




| Wavenumber (cm−1) | Peak Assignments |
|---|---|
| 3370 | O–H stretching |
| 3127 | (C=C)–H stretching |
| 2926, 2829 | C–H (sp3) stretching |
| 1701 | C=O stretching |
| 1671 | C=O stretching (Furan) |
| 1608, 1515 | C=C stretching |
| 1393, 1359 | O–H bending |
| 1281, 1183, 1076, 1017 | C–O stretching C–O stretching (furan) |
| 895 | C=C bending |
| 812–749 | C–H bending out of plane |
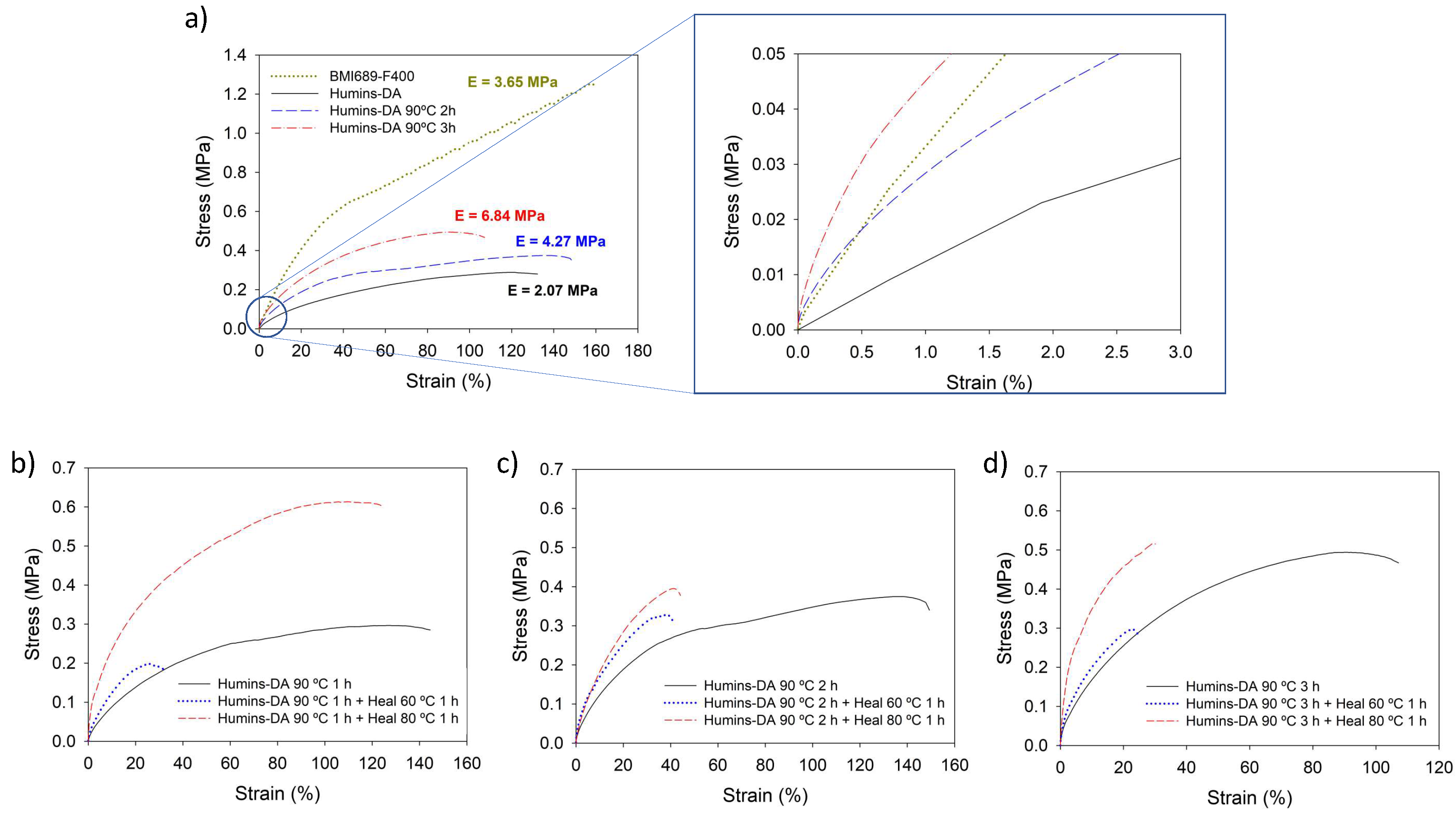
| Sample | σ (MPa) | ησ (%) | ε (%) | ηε (%) | E (MPa) | ηE (%) | |
|---|---|---|---|---|---|---|---|
| BMI689-F400 | Initial | 1.86 ± 0.14 | 0 | 141 ± 22 | 0 | 3.65 ± 0.20 | 0 |
| Healed 1 h 60 °C | Not Healed | Not Healed | Not Healed | ||||
| Humins-DA | Initial | 0.30 ± 0.11 | 82 | 134 ± 36 | 78 | 2.07 ± 0.40 | 177 * |
| Healed 1 h 60 °C | 0.24 ± 0.04 | 104 ± 17 | 3.67 ± 0.73 | ||||
| Sample | σ (MPa) | ησ (%) | ε (%) | ηε (%) | E (MPa) | ηE (%) | |
|---|---|---|---|---|---|---|---|
| Humins-DA 90 °C 1 h | Initial | 0.29 ± 0.03 | - | 110 ± 20 | - | 2.47 ± 0.21 | - |
| Healed 1 h 60 °C | 0.21 ± 0.09 | 71 | 34 ± 15 | 31 | 4.27 ± 0.38 | 172 * | |
| Healed 1 h 80 °C | 0.54 ± 0.17 | 187 * | 93 ± 24 | 84 | 9.06 ± 1.23 | 366 * | |
| Humins-DA 90 °C 2 h | Initial | 0.37 ± 0.01 | - | 119 ± 26 | - | 4.27 ± 0.62 | - |
| Healed 1 h 60 °C | 0.29 ± 0.06 | 77 | 35 ± 8 | 30 | 5.80 ± 0.45 | 136 * | |
| Healed 1 h 80 °C | 0.33 ± 0.10 | 89 | 36 ± 9 | 30 | 4.60 ± 0.20 | 108 * | |
| Humins-DA 90 °C 3 h | Initial | 0.61 ± 0.08 | - | 98 ± 11 | - | 6.84 ± 0.93 | - |
| Healed 1 h 60 °C | 0.25 ± 0.06 | 40 | 19 ± 5 | 19 | 6.49 ± 1.18 | 95 | |
| Healed 1 h 80 °C | 0.43 ± 0.10 | 70 | 31 ± 13 | 32 | 8.48 ± 3.19 | 124 * | |
| Sample | σ (MPa) | ησ (%) | ε (%) | ηε (%) | E (MPa) | ηE (%) | |
|---|---|---|---|---|---|---|---|
| Extruded Humins-DA | Initial | 0.77 ± 0.07 | - | 111 ± 18 | - | 3.40 ± 0.18 | - |
| Healed 1 h 60 °C | 0.36 ± 0.09 | 47 | 27 ± 4 | 24 | 5.00 ± 0.04 | 147 * | |
| Healed 1 h 80 °C | 0.68 ± 0.17 | 88 | 63 ± 22 | 56 | 5.33 ± 1.05 | 157 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerdan, K.; Brancart, J.; Roels, E.; Vanderborght, B.; Van Puyvelde, P. Humins Blending in Thermoreversible Diels–Alder Networks for Stiffness Tuning and Enhanced Healing Performance for Soft Robotics. Polymers 2022, 14, 1657. https://doi.org/10.3390/polym14091657
Cerdan K, Brancart J, Roels E, Vanderborght B, Van Puyvelde P. Humins Blending in Thermoreversible Diels–Alder Networks for Stiffness Tuning and Enhanced Healing Performance for Soft Robotics. Polymers. 2022; 14(9):1657. https://doi.org/10.3390/polym14091657
Chicago/Turabian StyleCerdan, Kenneth, Joost Brancart, Ellen Roels, Bram Vanderborght, and Peter Van Puyvelde. 2022. "Humins Blending in Thermoreversible Diels–Alder Networks for Stiffness Tuning and Enhanced Healing Performance for Soft Robotics" Polymers 14, no. 9: 1657. https://doi.org/10.3390/polym14091657