Preparation and Characterizations of PSS/PDADMAC Polyelectrolyte Complex Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PEC Hydrogel
2.3. Characterization Techniques
3. Results and Discussion
3.1. Mechanism of PSS/PDADMAC PEC Hydrogel Formation
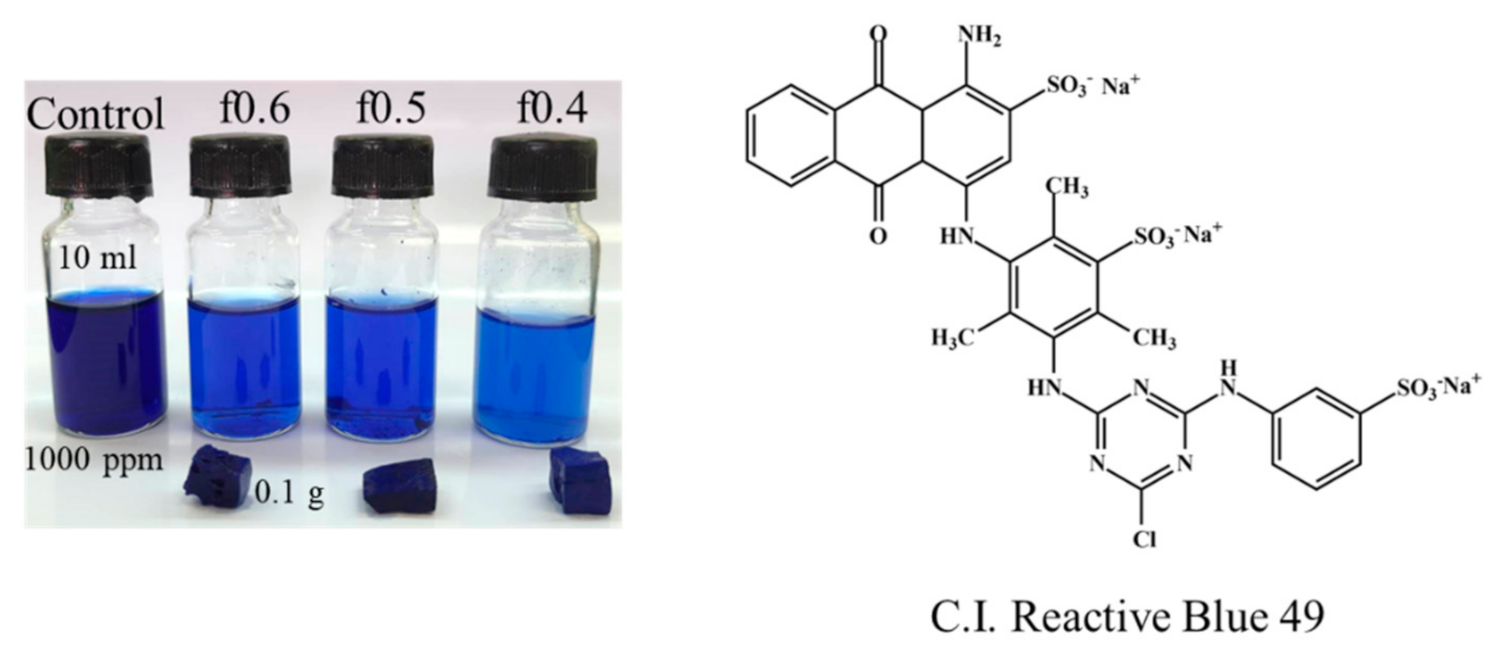
3.2. Physical Appearance of PSS/PDADMAC PEC Hydrogels
3.3. Scanning Electron Microscopy
3.4. Surface Area Determination by BET Analysis
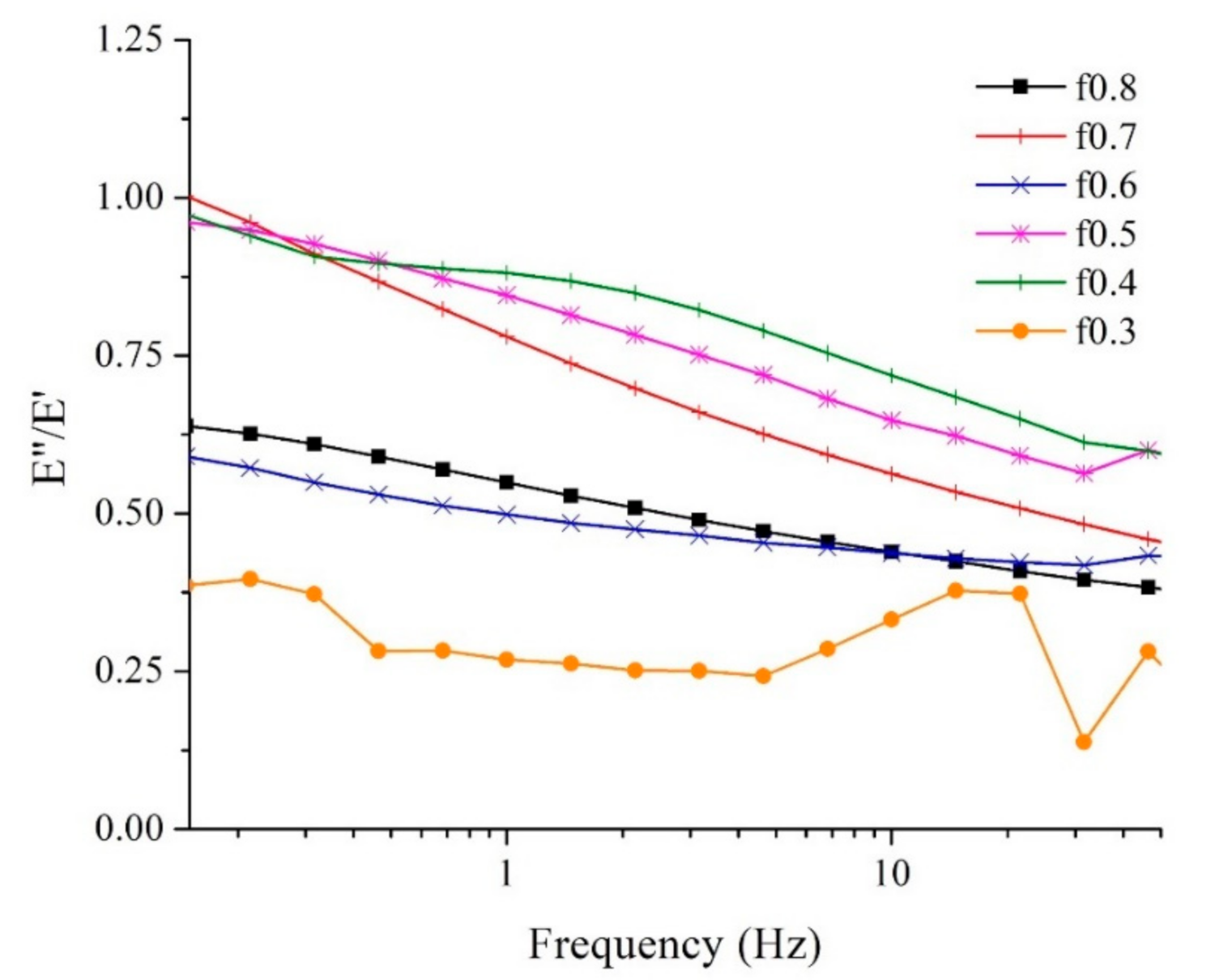
3.5. Compressive Strength by Dynamic Mechanical Analysis
3.6. Water Absorbency
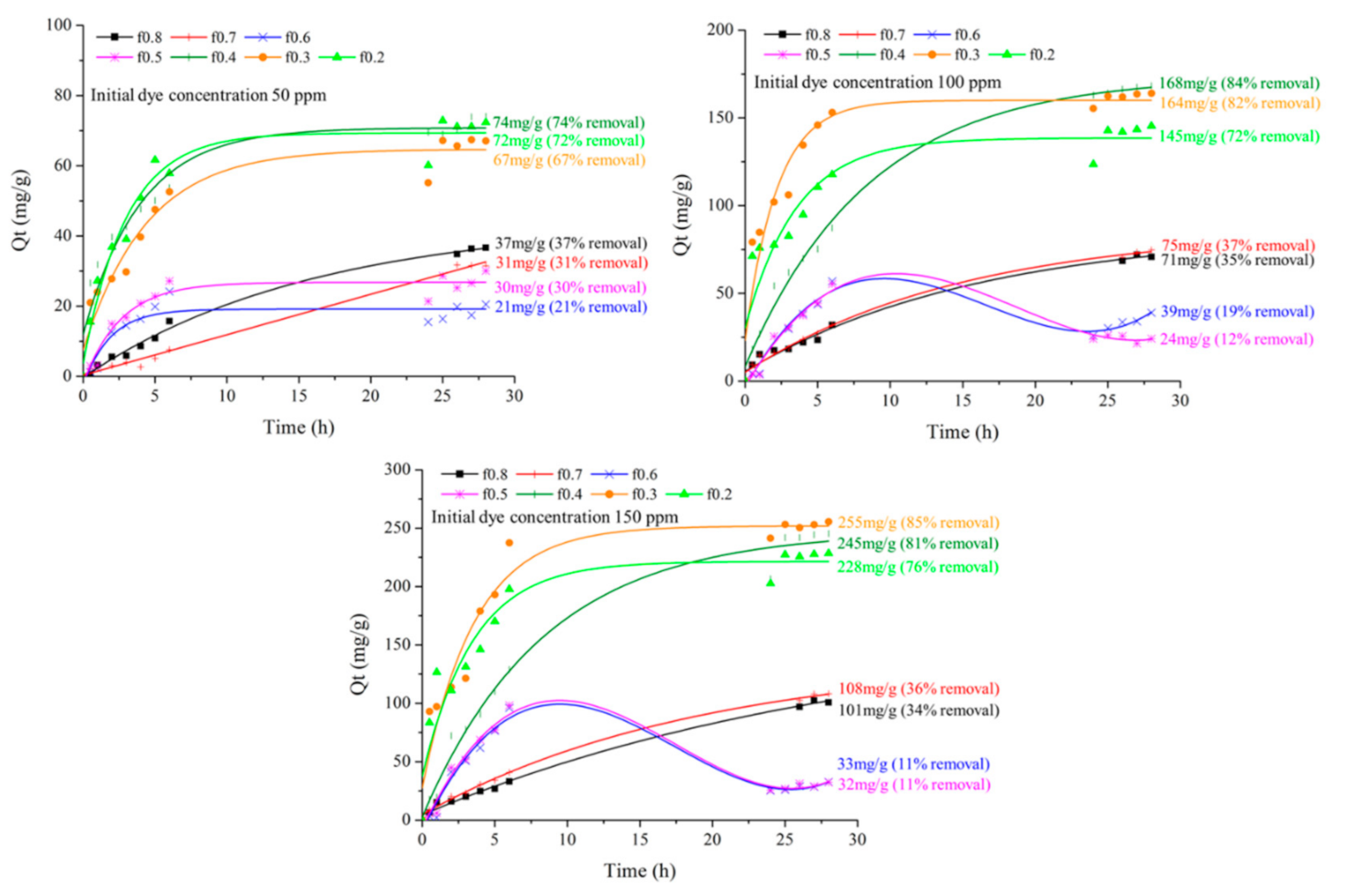
3.7. Textile Dye Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Thakur, S.; Thakur, V.K.; Arotiba, O.A. History, classification, properties and application of hydrogels: An overview. In Hydrogels: Recent Advances; Thakur, V.K., Thakur, M.K., Eds.; Springer: Singapore, 2018; pp. 29–50. [Google Scholar]
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Self-assembly of binary oppositely charged polysaccharides into polyelectrolyte complex hydrogel film for facile and efficient Pb2+ removal. Chem. Eng. J. 2020, 388, 124189. [Google Scholar] [CrossRef]
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Smart and functional polyelectrolyte complex hydrogel composed of salecan and chitosan lactate as superadsorbent for decontamination of nickel ions. Int. J. Biol. Macromol. 2020, 165, 1852–1861. [Google Scholar] [CrossRef]
- Chan, K.; Morikawa, K.; Shibata, N.; Zinchenko, A. Adsorptive removal of heavy metal ions, organic dyes, and pharmaceuticals by dna–chitosan hydrogels. Gels 2021, 7, 112. [Google Scholar] [CrossRef]
- Li, P.; Wang, T.; He, J.; Jiang, J.; Lei, F. Synthesis, characterization, and selective dye adsorption by pH- and ion-sensitive polyelectrolyte galactomannan-based hydrogels. Carbohydr. Polym. 2021, 264, 118009. [Google Scholar] [CrossRef]
- Zhao, J.; Xing, T.; Li, Q.; Chen, Y.; Yao, W.; Jin, S.; Chen, S. Preparation of chitosan and carboxymethylcellulose-based polyelectrolyte complex hydrogel via SD-A-SGT method and its adsorption of anionic and cationic dye. J. Appl. Polym. Sci. 2020, 137, 48980. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Hussain, Z.; Zhang, H.; Wang, H.; Chang, N.; Li, F. Modification and characterization of hydrogel beads and its used as environmentally friendly adsorbent for the removal of reactive dyes. J. Clean. Prod. 2022, 342, 130789. [Google Scholar] [CrossRef]
- Durmaz, E.N.; Baig, M.I.; Willott, J.D.; de Vos, W.M. Polyelectrolyte complex membranes via salinity change induced aqueous phase separation. ACS Appl. Polym. Mater. 2020, 2, 2612–2621. [Google Scholar] [CrossRef]
- Emonds, S.; Kamp, J.; Borowec, J.; Roth, H.; Wessling, M. Polyelectrolyte complex tubular membranes via a salt dilution induced phase inversion process. Adv. Eng. Mater. 2021, 23, 2001401. [Google Scholar] [CrossRef]
- Baig, M.I.; Willott, J.D.; de Vos, W.M. Tuning the structure and performance of polyelectrolyte complexation based aqueous phase separation membranes. J. Membr. Sci. 2020, 615, 118502. [Google Scholar] [CrossRef]
- Davachi, S.M.; Haramshahi, S.M.A.; Akhavirad, S.A.; Bahrami, N.; Hassanzadeh, S.; Ezzatpour, S.; Hassanzadeh, N.; Malekzadeh Kebria, M.; Khanmohammadi, M.; Bagher, Z. Development of Chitosan/Hyaluronic acid hydrogel scaffolds via enzymatic reaction for cartilage tissue engineering. Mater. Today Commun. 2022, 30, 103230. [Google Scholar] [CrossRef]
- Rajaram, A.; Schreyer, D.J.; Chen, D.X.B. Use of the polycation polyethyleneimine to improve the physical properties of Alginate–Hyaluronic acid hydrogel during fabrication of tissue repair scaffolds. J. Biomater. Sci. Polym. Ed. 2015, 26, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, X.; Zhao, J.; Feng, H.; Li, P.; Tong, Z.; Yang, Z.; Li, S.; Yang, J.; Jin, S. Preparation of the Chitosan/Poly(glutamic acid)/Alginate polyelectrolyte complexing hydrogel and study on its drug releasing property. Carbohydr. Polym. 2018, 191, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Umaredkar, A.A.; Dangre, P.V.; Mahapatra, D.K.; Dhabarde, D.M. Fabrication of Chitosan-Alginate polyelectrolyte complexed hydrogel for controlled release of Cilnidipine: A statistical design approach. Mater. Technol. 2020, 35, 697–707. [Google Scholar] [CrossRef]
- Sonawane, R.O.; Patil, S.D. Gelatin–κ-carrageenan polyelectrolyte complex hydrogel compositions for the design and development of extended-release pellets. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 812–823. [Google Scholar] [CrossRef]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Formation of self-assembled polyelectrolyte complex hydrogel derived from Salecan and Chitosan for sustained release of vitamin C. Carbohydr. Polym. 2020, 234, 115920. [Google Scholar] [CrossRef]
- Shu, M.; Long, S.; Huang, Y.; Li, D.; Li, H.; Li, X. High strength and antibacterial polyelectrolyte complex CS/HS hydrogel films for wound healing. Soft Matter 2019, 15, 7686–7694. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Delv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Xue, W.; Liu, B.; Zhang, H.; Ryu, S.; Kuss, M.; Shukla, D.; Hu, G.; Shi, W.; Jiang, X.; Lei, Y.; et al. Controllable fabrication of Alginate/Poly-L-ornithine polyelectrolyte complex hydrogel networks as therapeutic drug and cell carriers. Acta Biomater. 2022, 138, 182–192. [Google Scholar] [CrossRef]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Gull, N.; Haider, B.; Khan, R.U.; Riaz, T. Development and evaluation of pH-sensitive biodegradable ternary blended hydrogel films (Chitosan/Guar gum/PVP) for drug delivery application. Sci. Rep. 2021, 11, 21255. [Google Scholar] [CrossRef]
- Dreiss, C.A. Hydrogel design strategies for drug delivery. Curr. Opin. Colloid Interface Sci. 2020, 48, 1–17. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Liu, M. Conductive hydrogels as smart materials for flexible electronic devices. Chem. Eur. J. 2018, 24, 16930–16943. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, P.; Shojaei, A. A review on the features, performance and potential applications of hydrogel-based wearable strain/pressure sensors. Adv. Colloid Interface Sci. 2021, 298, 102553. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Dong, Z.; Ding, Z.; Hu, Z.; Yu, D.; Hu, Y.; Abidi, N.; Li, W. Electroresponsive homogeneous polyelectrolyte complex hydrogels from naturally derived polysaccharides. ACS Sustain. Chem. Eng. 2018, 6, 7052–7063. [Google Scholar] [CrossRef]
- Shang, J.; Shao, Z.; Chen, X. Electrical behavior of a natural polyelectrolyte hydrogel: Chitosan/Carboxymethylcellulose hydrogel. Biomacromolecules 2008, 9, 1208–1213. [Google Scholar] [CrossRef]
- Li, G.; Zhang, G.; Sun, R.; Wong, C.-P. Dually pH-responsive polyelectrolyte complex hydrogel composed of Polyacrylic acid and Poly (2-(dimthylamino) ethyl methacrylate). Polymer 2016, 107, 332–340. [Google Scholar] [CrossRef]
- Dhanjai; Sinha, A.; Kalambate, P.K.; Mugo, S.M.; Kamau, P.; Chen, J.; Jain, R. Polymer hydrogel interfaces in electrochemical sensing strategies: A review. TrAC-Trends Anal. Chem. 2019, 118, 488–501. [Google Scholar] [CrossRef]
- Song, H.; Sun, Y.; Zhu, J.; Xu, J.; Zhang, C.; Liu, T. Hydrogen-bonded network enables polyelectrolyte complex hydrogels with high stretchability, excellent fatigue resistance and self-healability for human motion detection. Compos. Part B Eng. 2021, 217, 108901. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, H.; Zhang, K.; Shao, J.; Qiu, J.; Wu, J.; Wu, Y.; Yan, L. A high-voltage quasi-solid-state flexible supercapacitor with a wide operational temperature range based on a low-cost “water-in-salt” hydrogel electrolyte. Nanoscale 2021, 13, 3010–3018. [Google Scholar] [CrossRef]
- Peng, K.; Wang, W.; Zhang, J.; Ma, Y.; Lin, L.; Gan, Q.; Chen, Y.; Feng, C. Preparation of Chitosan/Sodium alginate conductive hydrogels with high salt contents and their application in flexible supercapacitors. Carbohydr. Polym. 2022, 278, 118927. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Zhao, F.; Yu, G. Functional hydrogels for next-generation batteries and supercapacitors. Trends Chem. 2019, 1, 335–348. [Google Scholar] [CrossRef]
- Feng, E.; Gao, W.; Yan, Z.; Li, J.; Li, Z.; Ma, X.; Ma, L.; Yang, Z. A multifunctional hydrogel polyelectrolyte based flexible and wearable supercapacitor. J. Power Sources 2020, 479, 229100. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, J.; Su, S.; Jiang, J.; Feng, J.; Wang, Q. Water-deactivated polyelectrolyte hydrogel electrolytes for flexible high-voltage supercapacitors. Chem. Sus. Chem. 2018, 11, 3410–3415. [Google Scholar] [CrossRef] [PubMed]
- Mejía, E.H.; Contreras, H.; Delgado, E.; Quintana, G. Effect of experimental parameters on the formation of hydrogels by polyelectrolyte complexation of Carboxymethylcellulose, Carboxymethyl starch, and Alginic acid with Chitosan. Int. J. Chem. Eng. 2019, 2019, 3085691. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Shen, M.; Wu, T.; Luo, Y.; Li, M.; Wen, H.; Xie, J. Role of salt ions and molecular weights on the formation of Mesona chinensis polysaccharide-Chitosan polyelectrolyte complex hydrogel. Food Chem. 2020, 333, 127493. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Patel, H.M.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Polyelectrolyte complexes: Mechanisms, critical experimental aspects, and applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakawa, K.; King, D.R.; Sun, T.; Guo, H.; Kurokawa, T.; Gong, J.P. Polyelectrolyte complexation via viscoelastic phase separation results in tough and self-recovering porous hydrogels. J. Mater. Chem. B 2019, 7, 5296–5305. [Google Scholar] [CrossRef]
- Starchenko, V.; Müller, M.; Lebovka, N. Sizing of PDADMAC/PSS complex aggregates by polyelectrolyte and salt concentration and PSS molecular weight. J. Phys. Chem. B 2012, 116, 14961–14967. [Google Scholar] [CrossRef]
- Santifuengkul, P.; Srikulkit, K. Salt-free dyeing of cotton cellulose with a model cationic reactive dye. J. Soc. Dye Colour. 2000, 116, 398–402. [Google Scholar]

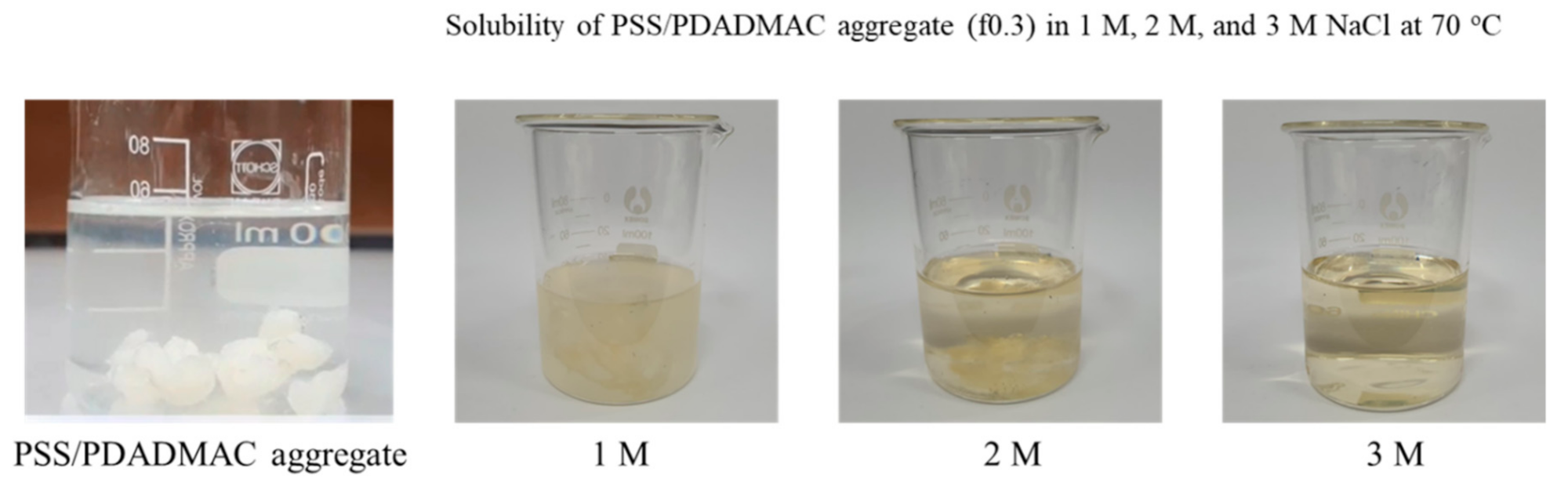

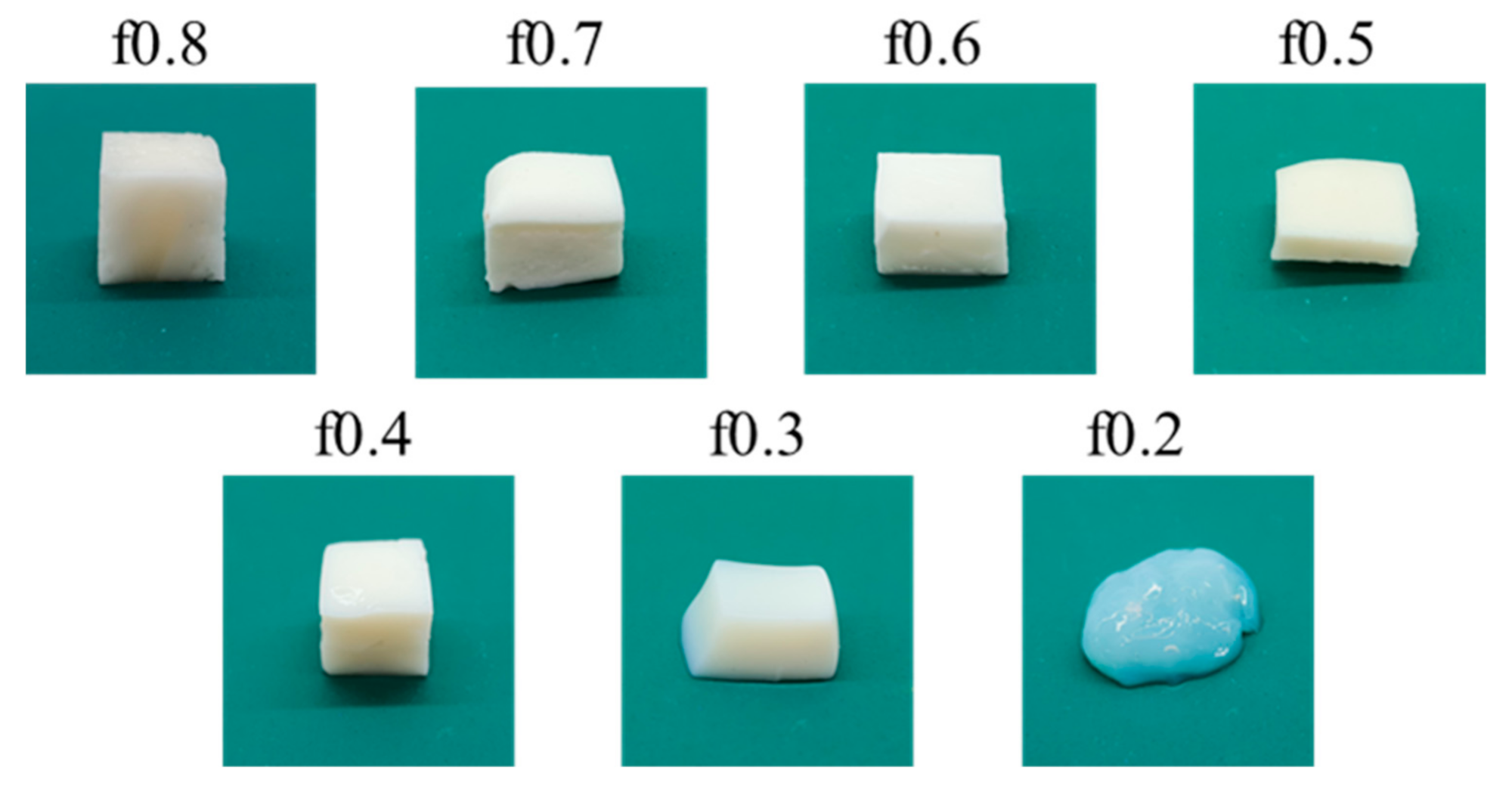

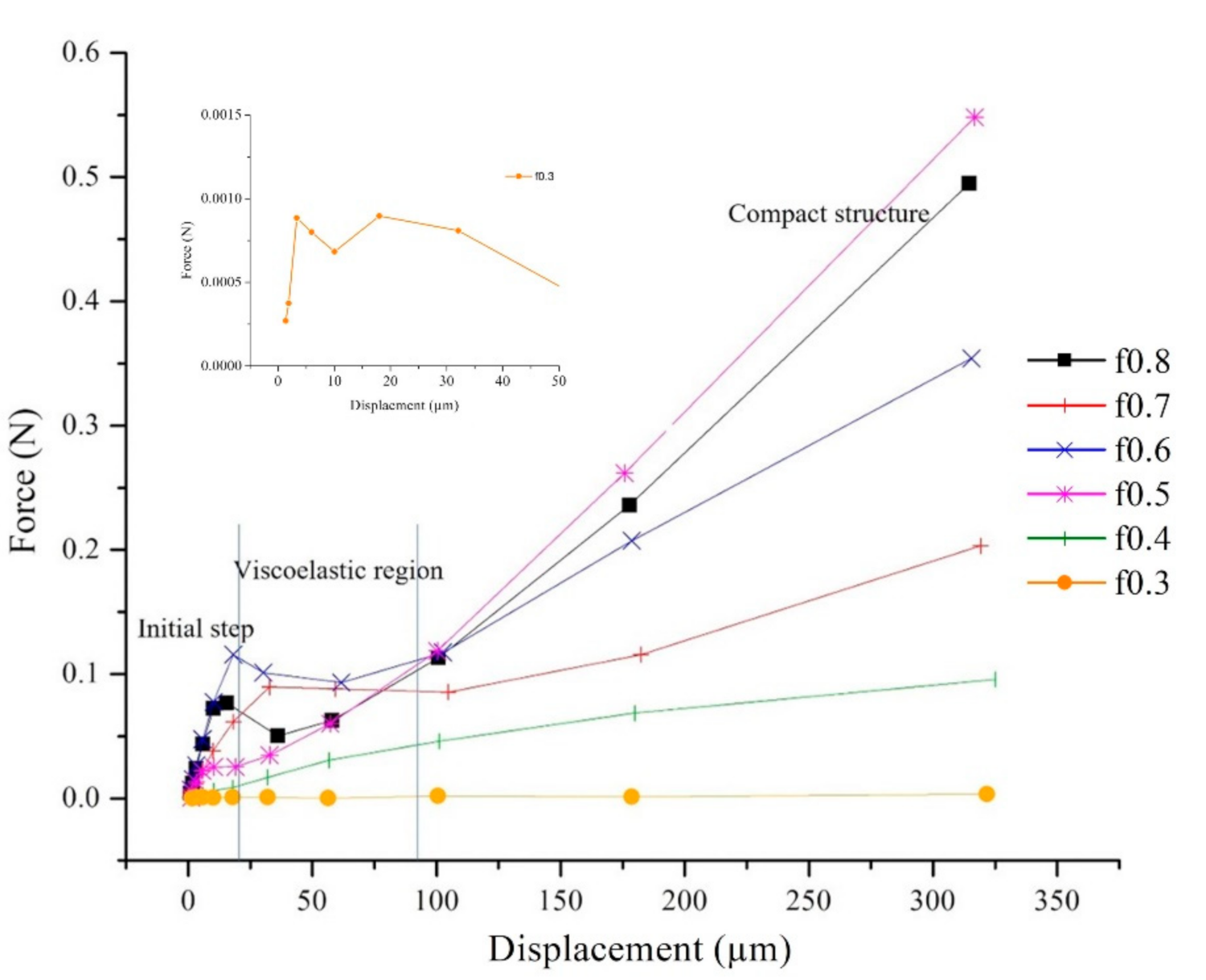

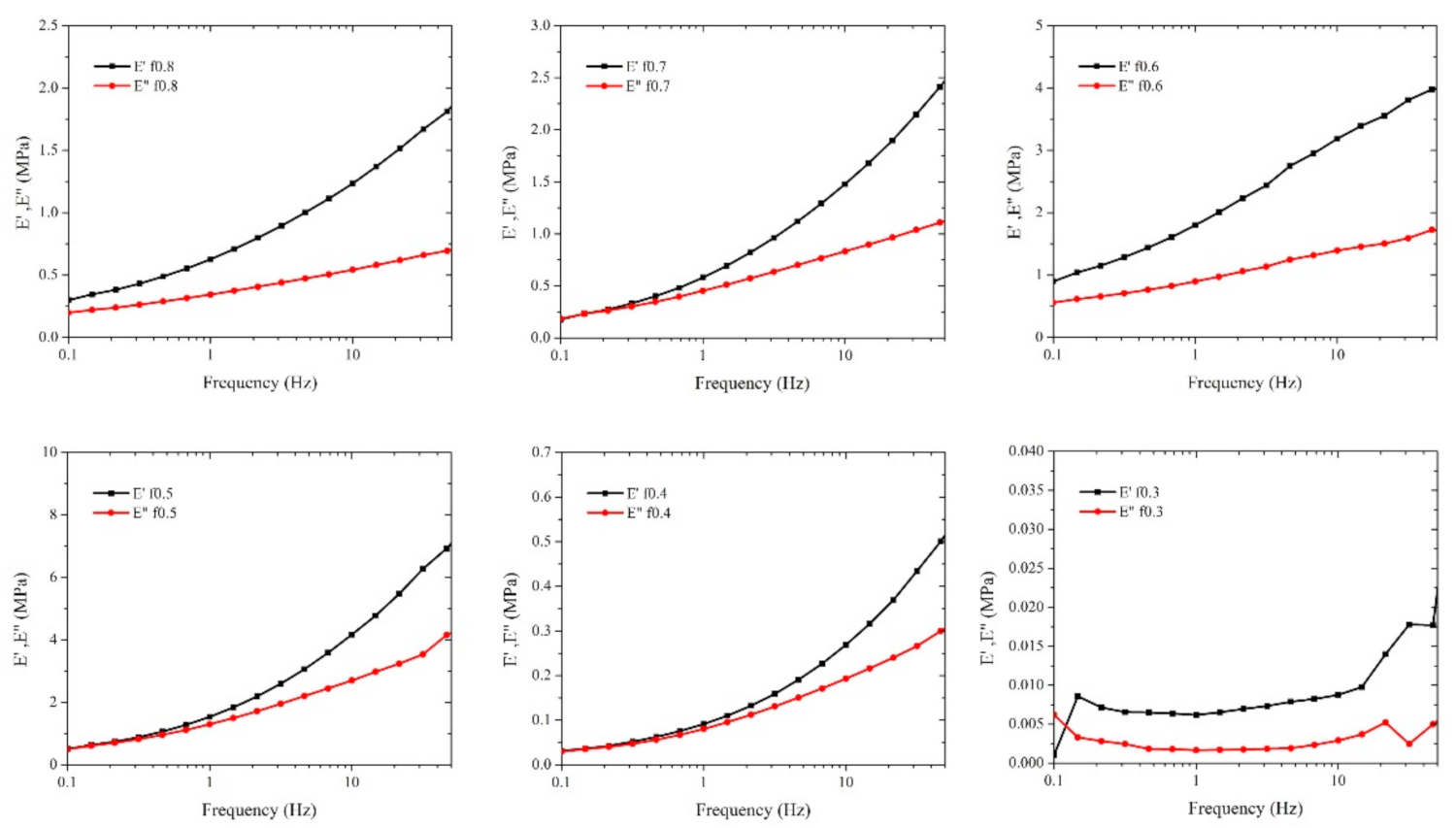

| Sample | Mole Fraction * PSS:PDADMAC | NaCl (M) | PSS (g) | 20% (w/w) PDADMAC (Cal. on Solid Content, g) |
|---|---|---|---|---|
| f0.8 | 0.8:0.2 | 4 | 8.24 | 1.62 |
| f0.7 | 0.7:0.3 | 4 | 7.21 | 2.43 |
| f0.6 | 0.6:0.4 | 4 | 6.18 | 3.23 |
| f0.5 | 0.5:0.5 | 4 | 5.15 | 4.04 |
| f0.4 | 0.4:0.6 | 3 | 4.12 | 4.85 |
| f0.3 | 0.3:0.7 | 3 | 3.09 | 5.66 |
| f0.2 | 0.2:0.8 | 3 | 2.06 | 6.47 |
| Sample | Macropore Size (µm) | SD |
|---|---|---|
| f0.8 | 28.871 | 12.040 |
| f0.7 | 22.573 | 7.666 |
| f0.6 | 5.869 | 2.711 |
| f0.5 | 11.627 | 7.285 |
| f0.4 | 68.768 | 22.203 |
| f0.3 | 32.496 | 17.264 |
| f0.2 | 42.045 | 15.805 |
| Sample | BET Surface Area (m2/g) | Pore Volume | Pore Size | ||
|---|---|---|---|---|---|
| Single Point Adsorption Total Pore Volume (cm3/g) | Single Point Desorption Total Pore Volume (cm3/g) | Adsorption Average Pore Diameter (4V/A by BET) (Å) | Desorption Average Pore Diameter (4V/A by BET) (Å) | ||
| f0.8 | 3.5402 | 0.003848 | 0.004257 | 43.477 | 48.103 |
| f0.7 | 2.1645 | 0.002135 | 0.002555 | 39.450 | 47.217 |
| f0.6 | 1.7297 | 0.002654 | 0.002992 | 61.383 | 69.194 |
| f0.5 | 0.9876 | 0.001358 | 0.007038 | 54.997 | 285.050 |
| f0.4 | 1.1959 | 0.001179 | 0.001671 | 39.424 | 55.885 |
| f0.3 | 0.4858 | 0.000428 | 0.000716 | 35.218 | 58.968 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sungoradee, T.; Srikulkit, K. Preparation and Characterizations of PSS/PDADMAC Polyelectrolyte Complex Hydrogel. Polymers 2022, 14, 1699. https://doi.org/10.3390/polym14091699
Sungoradee T, Srikulkit K. Preparation and Characterizations of PSS/PDADMAC Polyelectrolyte Complex Hydrogel. Polymers. 2022; 14(9):1699. https://doi.org/10.3390/polym14091699
Chicago/Turabian StyleSungoradee, Thichakorn, and Kawee Srikulkit. 2022. "Preparation and Characterizations of PSS/PDADMAC Polyelectrolyte Complex Hydrogel" Polymers 14, no. 9: 1699. https://doi.org/10.3390/polym14091699
APA StyleSungoradee, T., & Srikulkit, K. (2022). Preparation and Characterizations of PSS/PDADMAC Polyelectrolyte Complex Hydrogel. Polymers, 14(9), 1699. https://doi.org/10.3390/polym14091699






