Axial Crystal Growth Evolution and Crystallization Characteristics of Bi-Continuous Polyamide 66 Membranes Prepared via the Cold Non-Solvent-Induced Phase Separation Technique
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of PA66 Flat Sheet Membranes
2.3. Membrane Characterizations
2.3.1. Basic Physicochemical Characterizations
2.3.2. Crystalline Forms
2.3.3. Membrane Morphology and Microstructure
2.3.4. The Hydrophilicity of Membrane
2.3.5. The Permeability, Anti-Fouling Performance of the Membrane
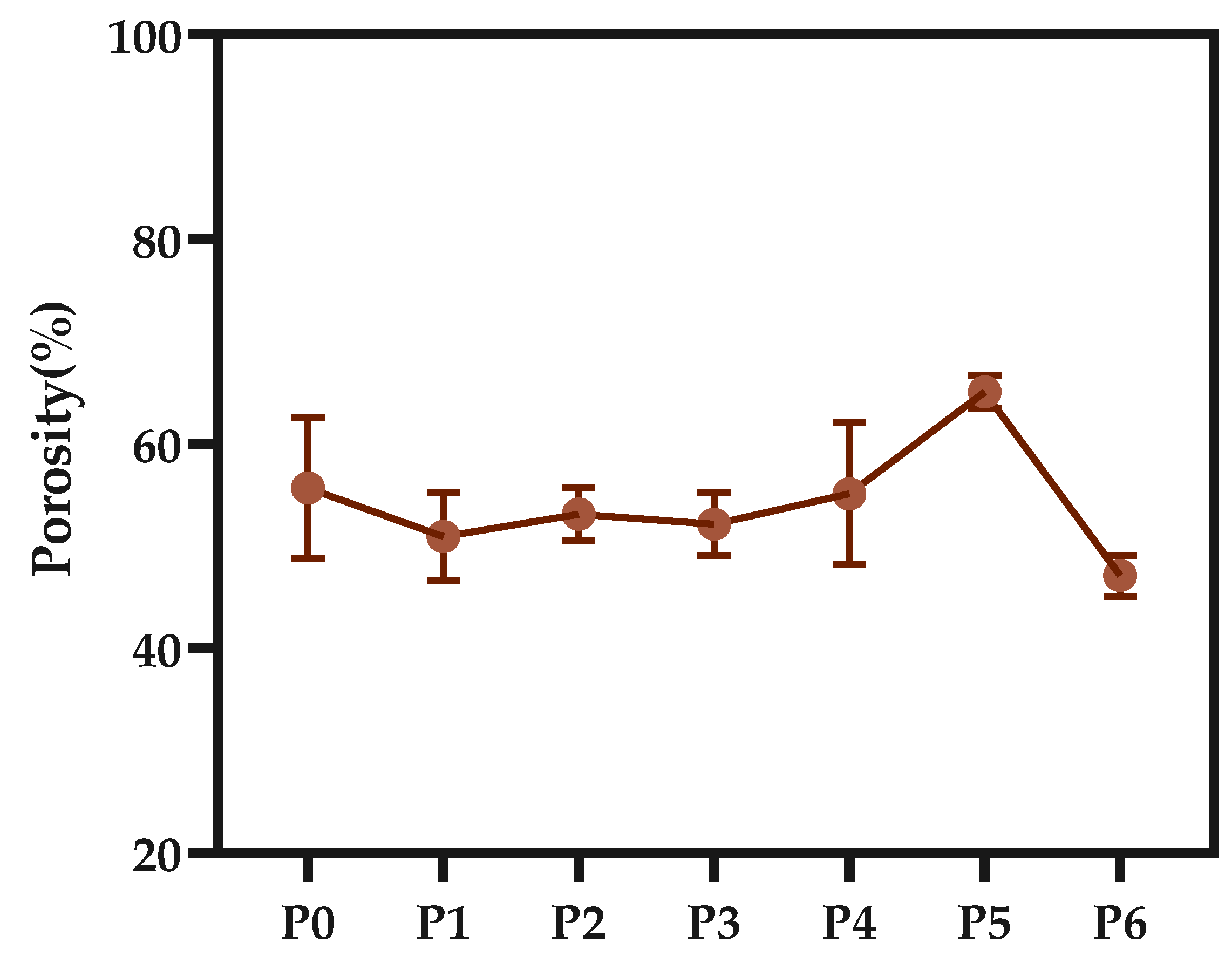
2.3.6. The Porosity of the Membrane
2.3.7. Mechanical Property of Membrane
3. Results and Discussion
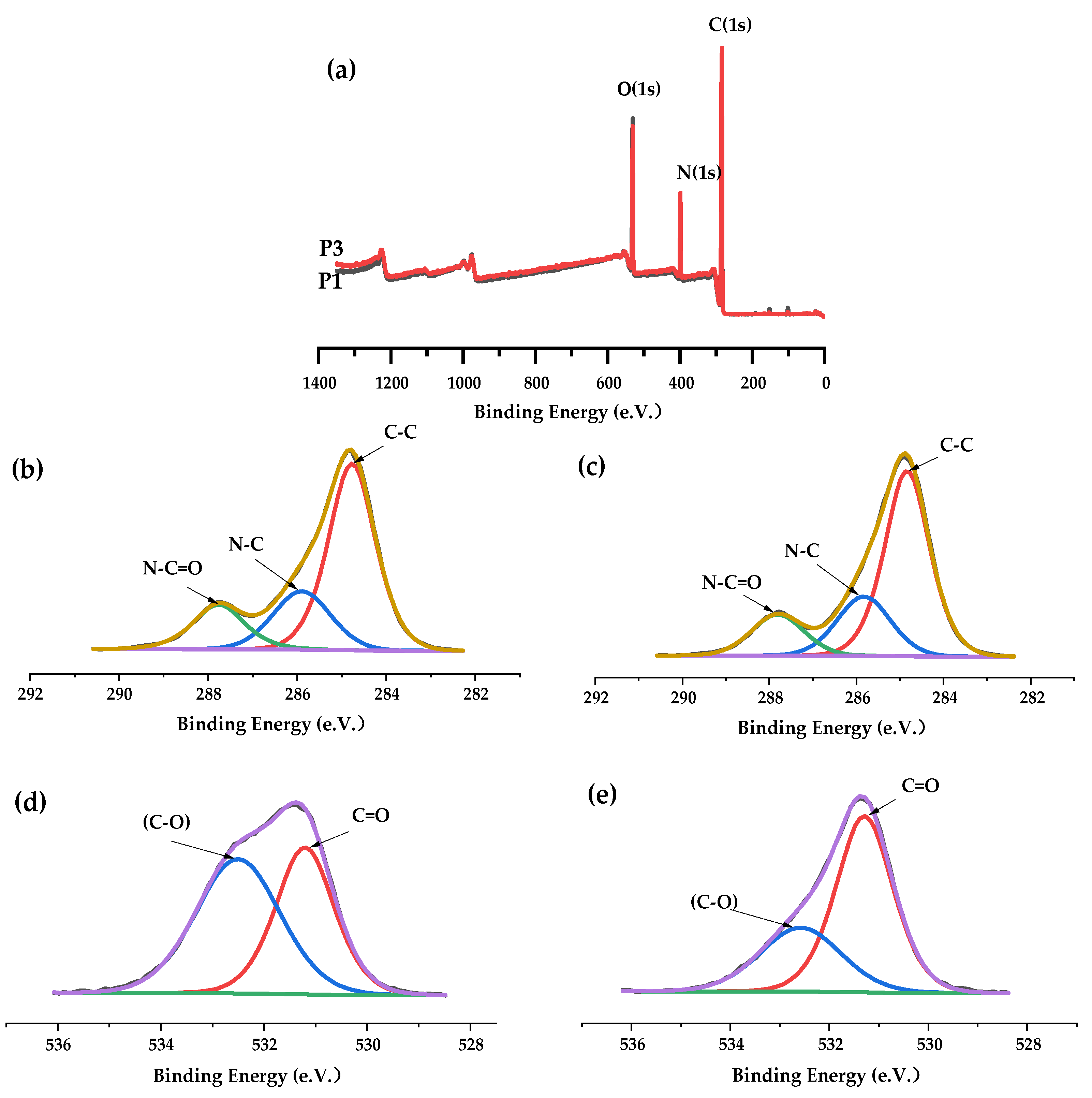
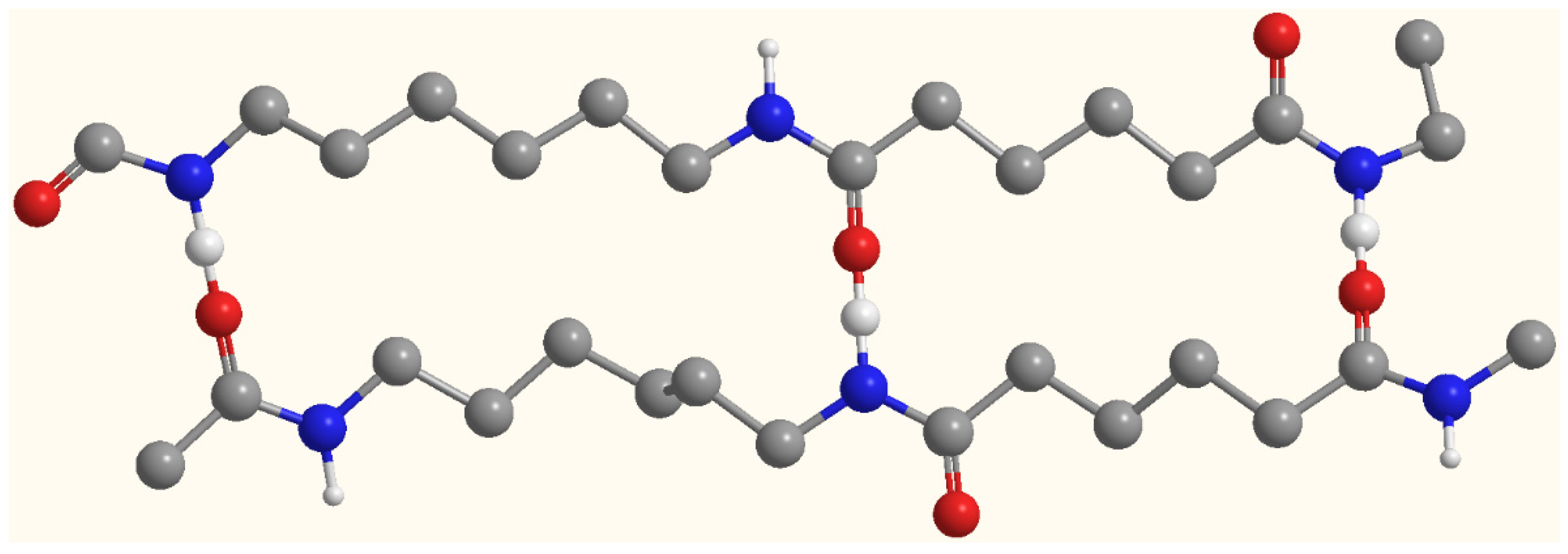
3.1. Chemical Characterizations
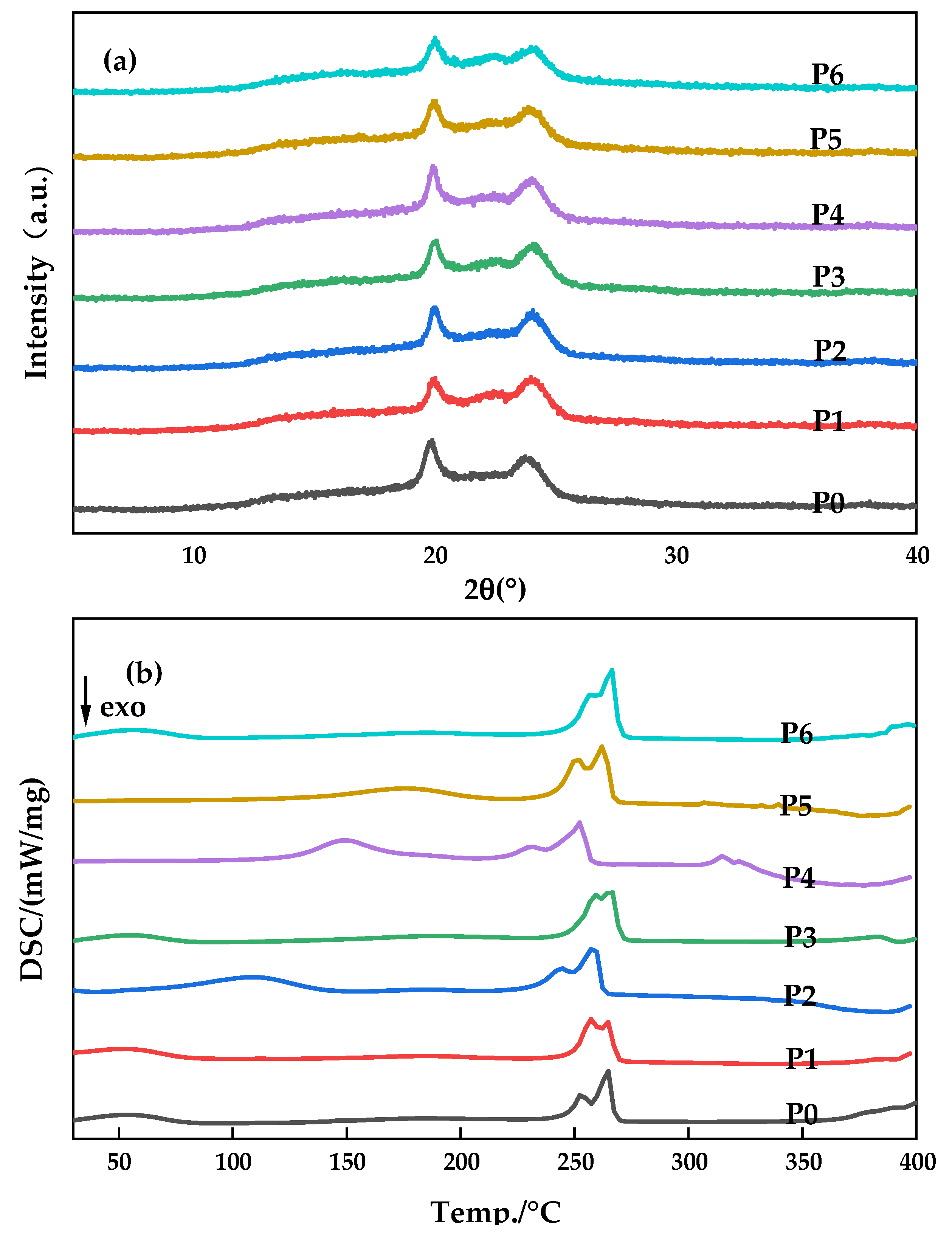
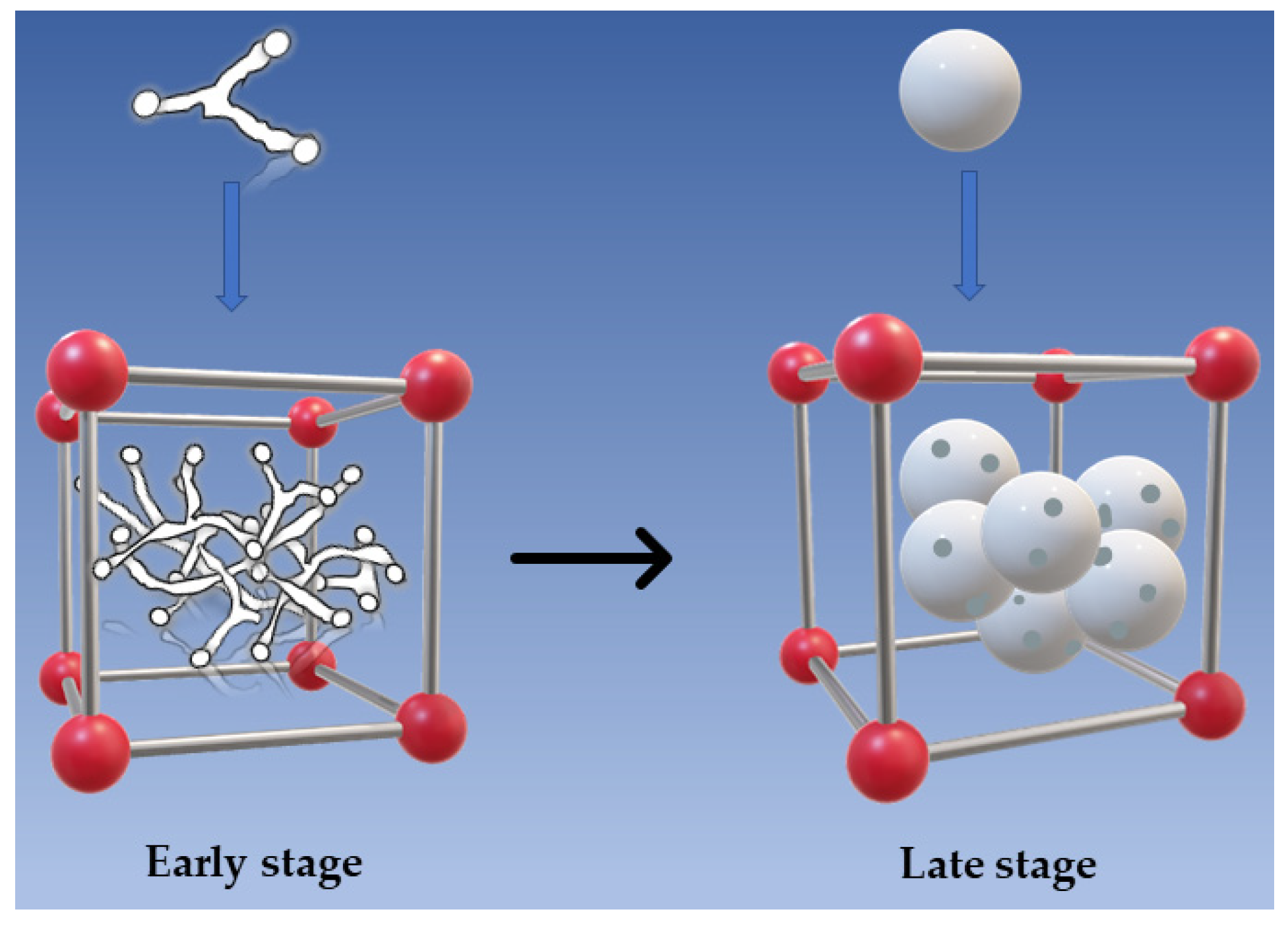
3.2. Crystals of the Membranes
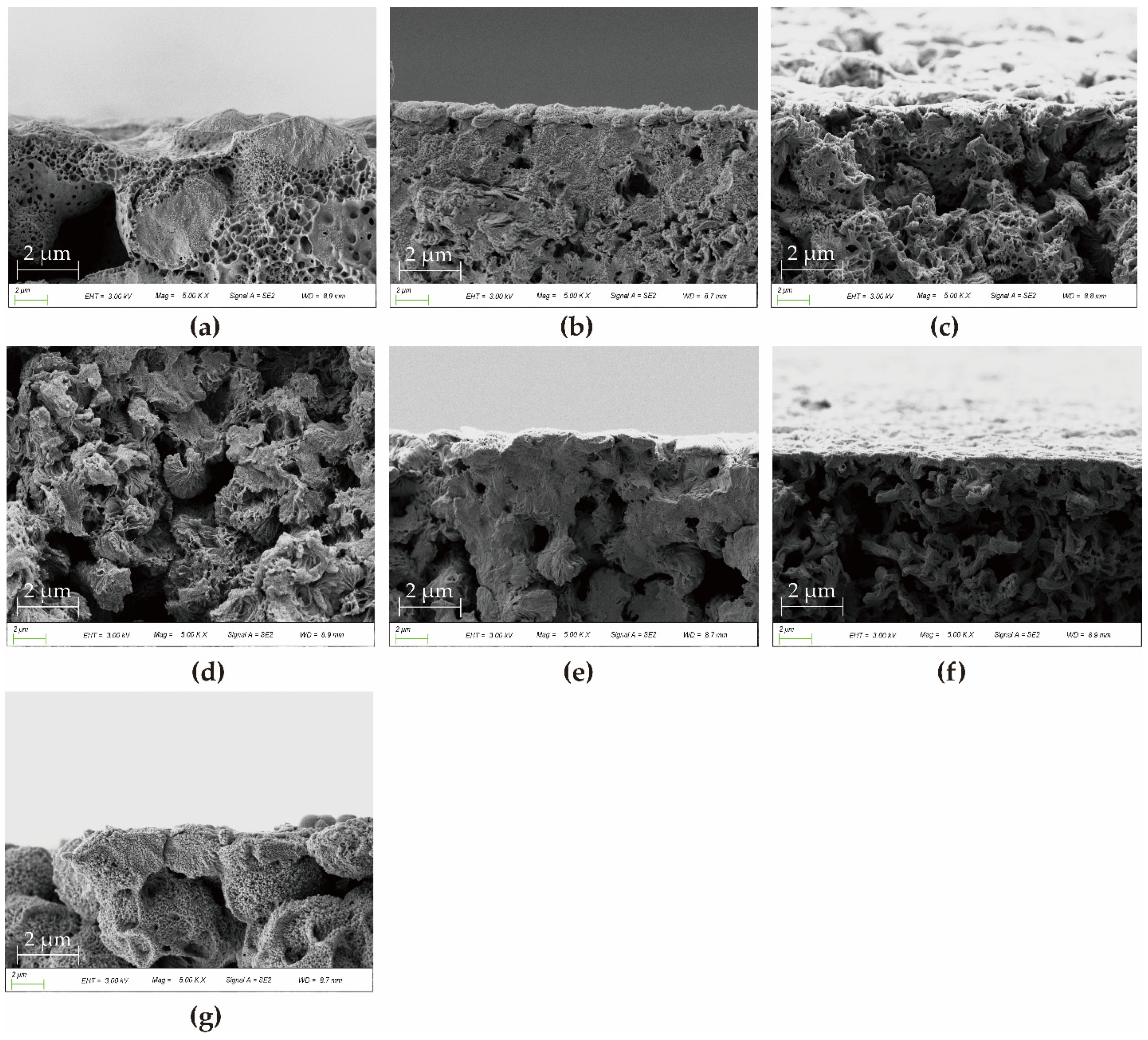
3.3. Morphological Characterizations
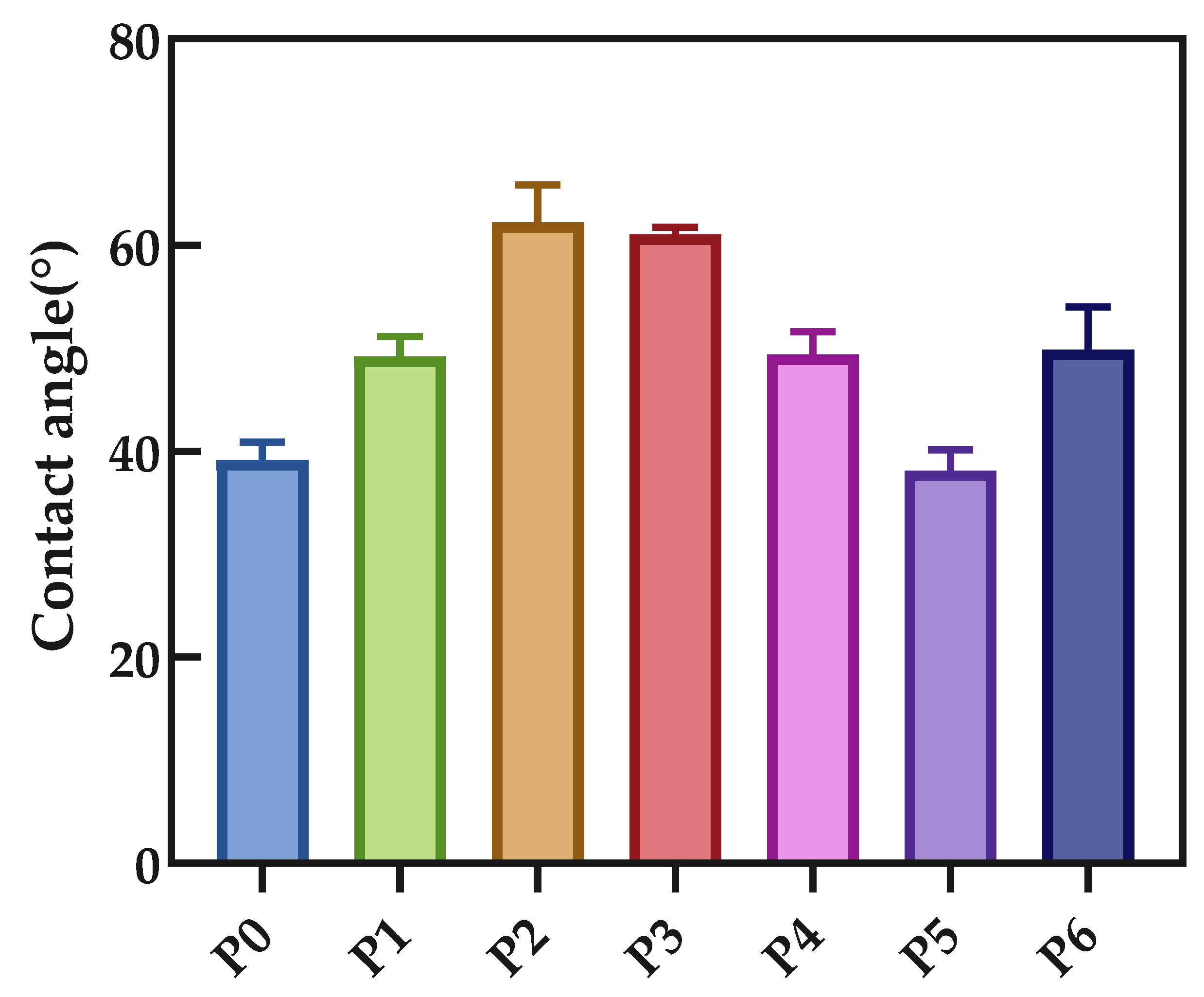
3.4. Contact Angle Characterization
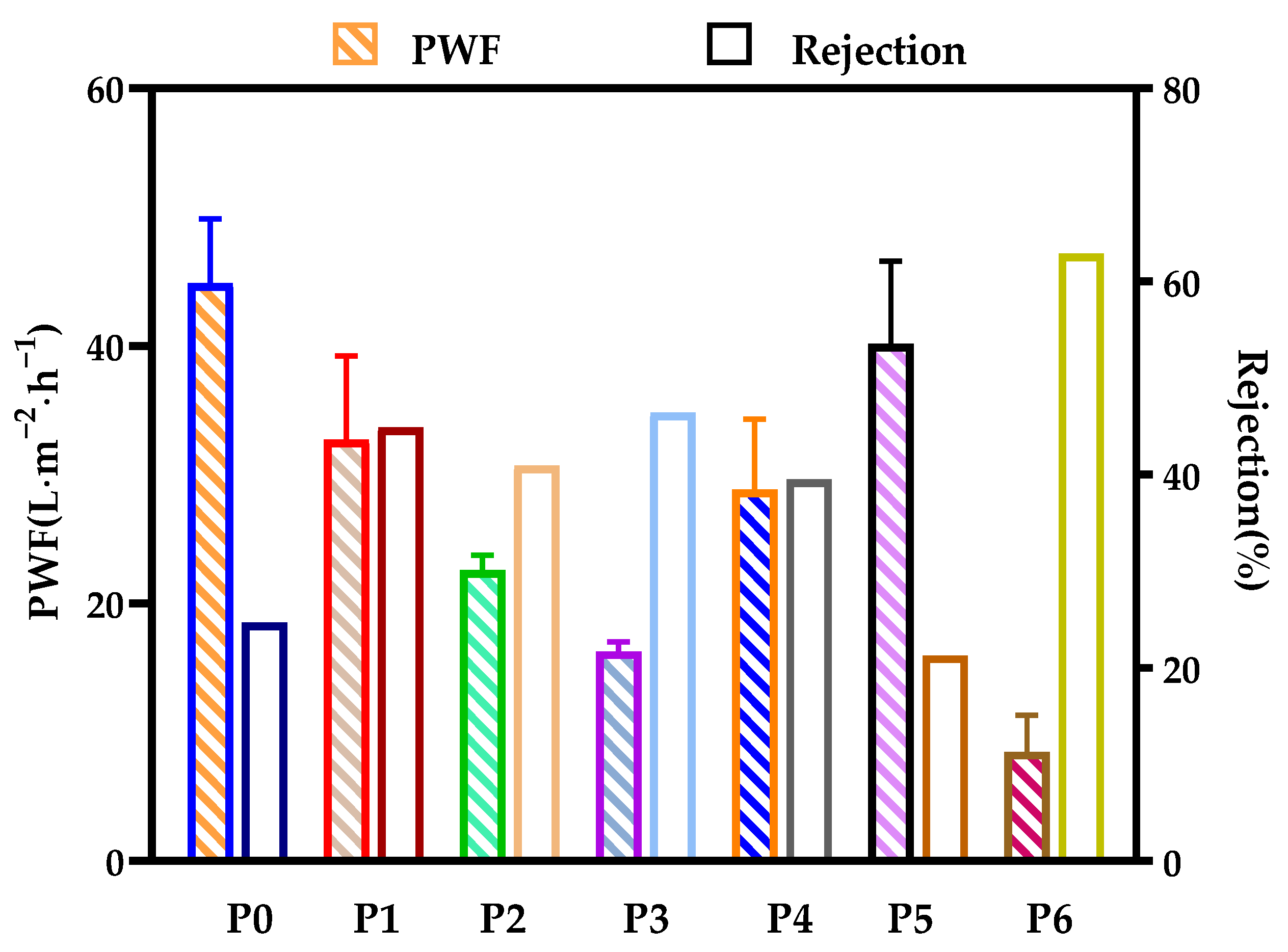
3.5. Membrane Filtration Performance
3.6. Anti-Fouling Performance of Developed Membranes
3.7. Mechanical Performance of the Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, H.-H.; Beltsios, K.; Lin, D.-J.; Cheng, L.-P. Formation of polyamide 12 membranes via thermal-nonsolvent induced phase separation. J. Appl. Polym. Sci. 2013, 130, 14–24. [Google Scholar] [CrossRef]
- Shih, C.-H.; Gryte, C.C.; Cheng, L.-P. Morphology of membranes formed by the isothermal precipitation of polyamide solutions from water/formic acid systems. J. Appl. Polym. Sci. 2005, 96, 944–960. [Google Scholar] [CrossRef]
- Zeni, M.; Riveros, R.; de Souza, J.F.; Mello, K.; Meireles, C.; Filho, G.R. Morphologic analysis of porous polyamide 6,6 membranes prepared by phase inversion. Desalination 2008, 221, 294–297. [Google Scholar] [CrossRef]
- Linggawati, A.; Mohammad, A.W.; Ghazali, Z. Effect of electron beam irradiation on morphology and sieving characteristics of nylon-66 membranes. Eur. Polym. J. 2009, 45, 2797–2804. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Beltsios, K.; Cheng, L.-P. Formation of bicontinuous, hydrophobic nylon 12 membranes via cold-solvent-induced phase separation for membrane distillation application. J. Appl. Polym. Sci. 2019, 136, 47036. [Google Scholar] [CrossRef]
- Lin, D.-J.; Chang, C.-L.; Lee, C.-K.; Cheng, L.-P. Fine structure and crystallinity of porous Nylon 66 membranes prepared by phase inversion in the water/formic acid/Nylon 66 system. Eur. Polym. J. 2006, 42, 356–367. [Google Scholar] [CrossRef]
- Strathmann, H.; Giorno, L.; Drioli, E. Introduction to Membrane Science and Technology; Wiley-VCH: Weinheim, Germany, 2011; Volume 544. [Google Scholar]
- Schüth, F.; Sing, K.S.W.; Weitkamp, J. Handbook of Porous Solids; Wiley-VCH: Hoboken, NJ, USA, 2002. [Google Scholar]
- Wu, Y.-H.; Beltsios, K.G.; Lin, J.-D.; Huang, P.-Y.; Lin, D.-J.; Cheng, L.-P. Effects of bath temperature on the morphology and performance of EVOH membranes prepared by the cold-solvent induced phase separation (CIPS) method. J. Appl. Polym. Sci. 2017, 134, 44553. [Google Scholar] [CrossRef]
- Chang, J.; Niu, H.; He, M.; Sun, M.; Wu, D. Structure-property relationship of polyimide fibers containing ether groups. J. Appl. Polym. Sci. 2015, 132, 42474. [Google Scholar] [CrossRef]
- Jiang, Z.-h.; Xiao, C.-f.; Wang, X.; Hu, X.-y. Structure and performance of polyamide-6 membranes prepared by thermally induced phase separation. Chinese J. Polym. Sci. 2010, 28, 721–729. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Z.; Quan, S.; Xu, Y.; Jiang, Z.; Shao, L. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly(vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl. Surf. Sci. 2014, 316, 537–548. [Google Scholar] [CrossRef]
- Shao, F.; Dong, L.; Dong, H.; Zhang, Q.; Zhao, M.; Yu, L.; Pang, B.; Chen, Y. Graphene oxide modified polyamide reverse osmosis membranes with enhanced chlorine resistance. J. Membr. Sci. 2017, 525, 9–17. [Google Scholar] [CrossRef]
- Xiang, S.; Guo, Z.; Wang, Y.; Liu, H.; Zhang, J.; Cui, Z.; Wang, H.; Li, J. The microstructure regulation, strengthening, toughening and hydrophilicity of polyamide6 in fabricating poly (vinylidene fluoride)-based flat membrane via the thermally induced phase separation technique. Eur. Polym. J. 2020, 126, 109568. [Google Scholar] [CrossRef]
- Wu, Z.; Cui, Z.; Li, T.; Qin, S.; He, B.; Han, N.; Li, J. Fabrication of PVDF-based blend membrane with a thin hydrophilic deposition layer and a network structure supporting layer via the thermally induced phase separation followed by non-solvent induced phase separation process. Appl. Surf. Sci. 2017, 419, 429–438. [Google Scholar] [CrossRef]
- Xu, P.; Hong, J.; Qian, X.; Xu, Z.; Xia, H.; Ni, Q.-Q. “Bridge” graphene oxide modified positive charged nanofiltration thin membrane with high efficiency for Mg2+/Li+ separation. Desalination 2020, 488, 114522. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.T.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Žagar, E.; Češarek, U.; Drinčić, A.; Sitar, S.; Shlyapnikov, I.M.; Pahovnik, D. Quantitative Determination of PA6 and/or PA66 Content in Polyamide-Containing Wastes. ACS Sustain. Chem. Eng. 2020, 8, 11818–11826. [Google Scholar] [CrossRef]
- Cui, Z.; Hassankiadeh, N.T.; Lee, S.Y.; Lee, J.M.; Woo, K.T.; Sanguineti, A.; Arcella, V.; Lee, Y.M.; Drioli, E. Poly(vinylidene fluoride) membrane preparation with an environmental diluent via thermally induced phase separation. J. Membr. Sci. 2013, 444, 223–236. [Google Scholar] [CrossRef]
- Zhao, J.; Chong, J.Y.; Shi, L.; Wang, R. Explorations of combined nonsolvent and thermally induced phase separation (N-TIPS) method for fabricating novel PVDF hollow fiber membranes using mixed diluents. J. Membr. Sci. 2019, 572, 210–222. [Google Scholar] [CrossRef]
- Chang, H.-H.; Chen, S.-C.; Lin, D.-J.; Cheng, L.-P. Preparation of bi-continuous Nylon-66 porous membranes by coagulation of incipient dopes in soft non-solvent baths. Desalination 2013, 313, 77–86. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Zhang, L.; Han, N.; Tan, L.; Qian, Y.; Cui, Z.; Cai, J. Preparation of hydrolysis of poly(acrylonitrile-co-methyl acrylate) membranes via thermally induced phase separation: Effects of hydrolysis conditions and additives. J. Appl. Polym. Sci. 2018, 135, 46380. [Google Scholar] [CrossRef]
- Zhao, W.; Su, Y.; Li, C.; Shi, Q.; Ning, X.; Jiang, Z. Fabrication of antifouling polyethersulfone ultrafiltration membranes using Pluronic F127 as both surface modifier and pore-forming agent. J. Membr. Sci. 2008, 318, 405–412. [Google Scholar] [CrossRef]





| Code | Content (wt.%) | Aging | Coagulation Bath | ||
|---|---|---|---|---|---|
| PA66 | FA | PC | Time (h) | Temperature (°C) | |
| P0 | 20 | 80 | 0 | 2 | 15 |
| P1 | 22 | 78 | 0 | 2 | 15 |
| P2 | 22 | 73 | 5 | 2 | 15 |
| P3 | 22 | 68 | 10 | 2 | 15 |
| P4 | 22 | 63 | 15 | 2 | 15 |
| P5 | 22 | 58 | 20 | 2 | 15 |
| P6 | 24 | 76 | 0 | 2 | 15 |
| Membrane | C (%) | N (%) | O (%) |
|---|---|---|---|
| P1 | 70.52 | 10.69 | 18.79 |
| P3 | 73.75 | 11.60 | 14.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Nie, L.; Li, G.; Zhu, Y.; Gao, M.; Wu, R.; Wang, B. Axial Crystal Growth Evolution and Crystallization Characteristics of Bi-Continuous Polyamide 66 Membranes Prepared via the Cold Non-Solvent-Induced Phase Separation Technique. Polymers 2022, 14, 1706. https://doi.org/10.3390/polym14091706
Yan J, Nie L, Li G, Zhu Y, Gao M, Wu R, Wang B. Axial Crystal Growth Evolution and Crystallization Characteristics of Bi-Continuous Polyamide 66 Membranes Prepared via the Cold Non-Solvent-Induced Phase Separation Technique. Polymers. 2022; 14(9):1706. https://doi.org/10.3390/polym14091706
Chicago/Turabian StyleYan, Jiangyi, Lihong Nie, Guiliang Li, Yuanlu Zhu, Ming Gao, Ruili Wu, and Beifu Wang. 2022. "Axial Crystal Growth Evolution and Crystallization Characteristics of Bi-Continuous Polyamide 66 Membranes Prepared via the Cold Non-Solvent-Induced Phase Separation Technique" Polymers 14, no. 9: 1706. https://doi.org/10.3390/polym14091706
APA StyleYan, J., Nie, L., Li, G., Zhu, Y., Gao, M., Wu, R., & Wang, B. (2022). Axial Crystal Growth Evolution and Crystallization Characteristics of Bi-Continuous Polyamide 66 Membranes Prepared via the Cold Non-Solvent-Induced Phase Separation Technique. Polymers, 14(9), 1706. https://doi.org/10.3390/polym14091706






