Polymer Coating Integrity, Thrombogenicity and Computational Fluid Dynamics Analysis of Provisional Stenting Technique in the Left Main Bifurcation Setting: Insights from an In-Vitro Model
Abstract
:1. Introduction
2. Materials and Methods
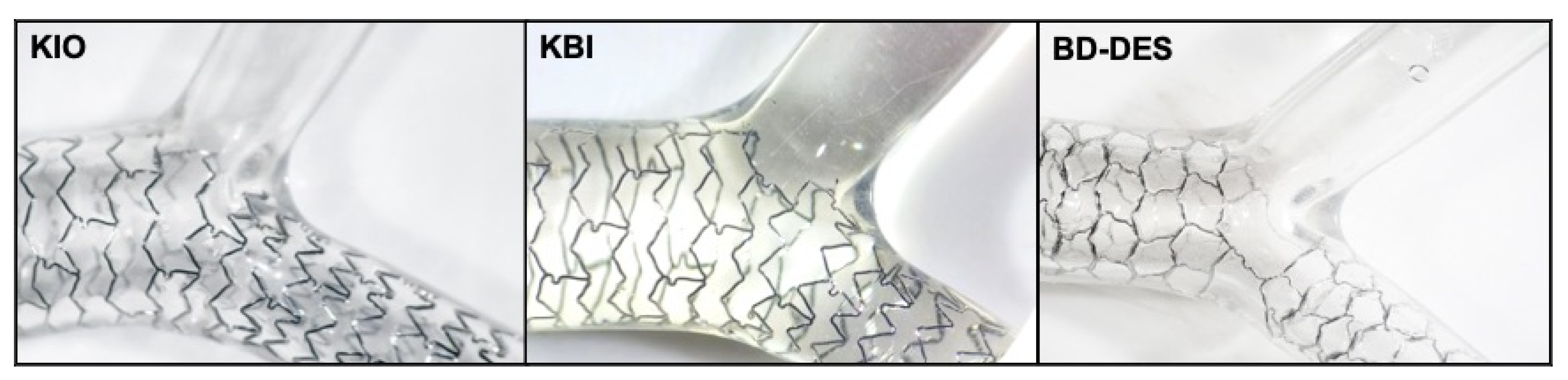
2.1. Device Description
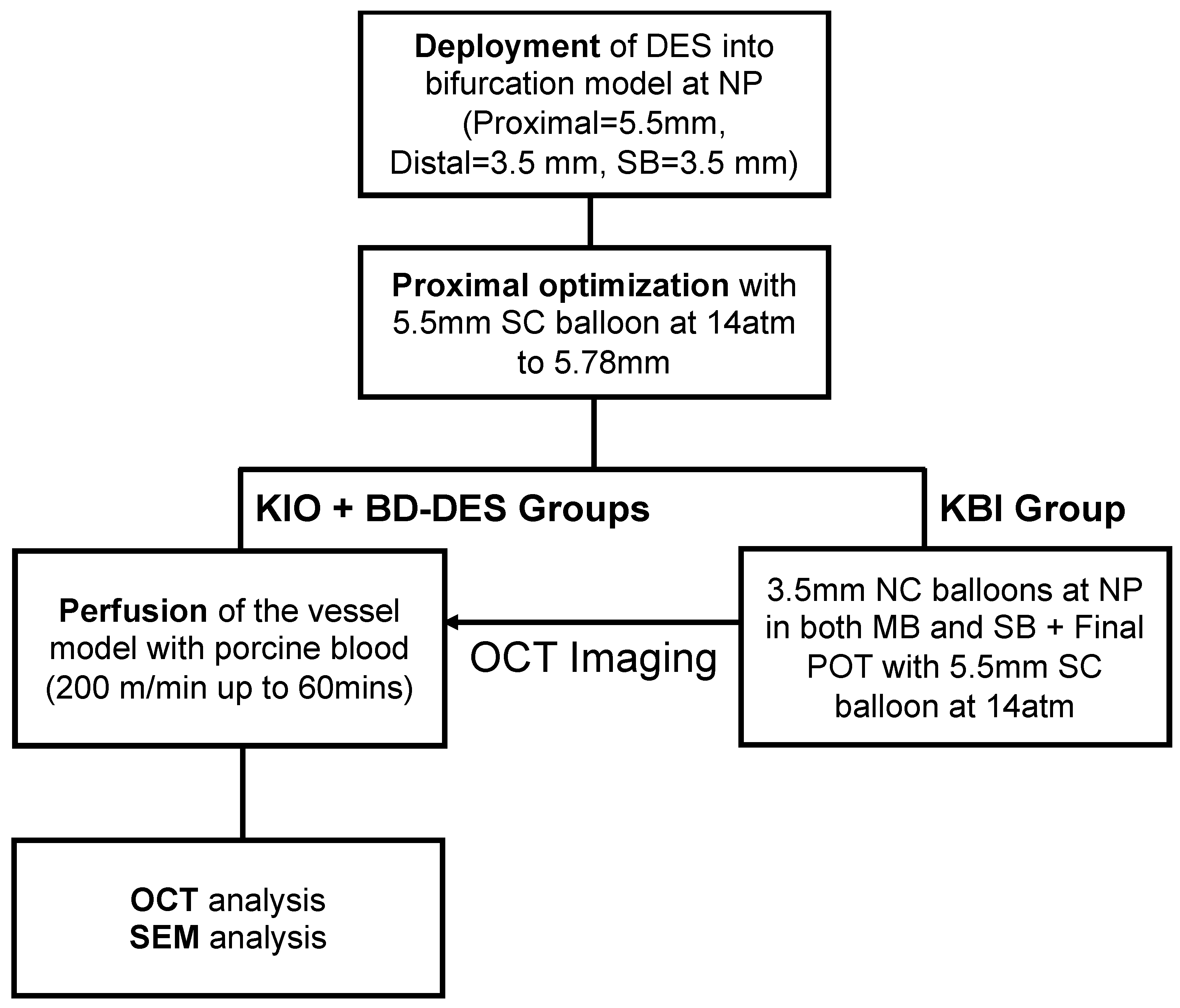
2.2. Deployment of the Stents
2.3. Flow Perfusion
2.4. OCT Analysis
2.5. Computational Fluid Dynamics
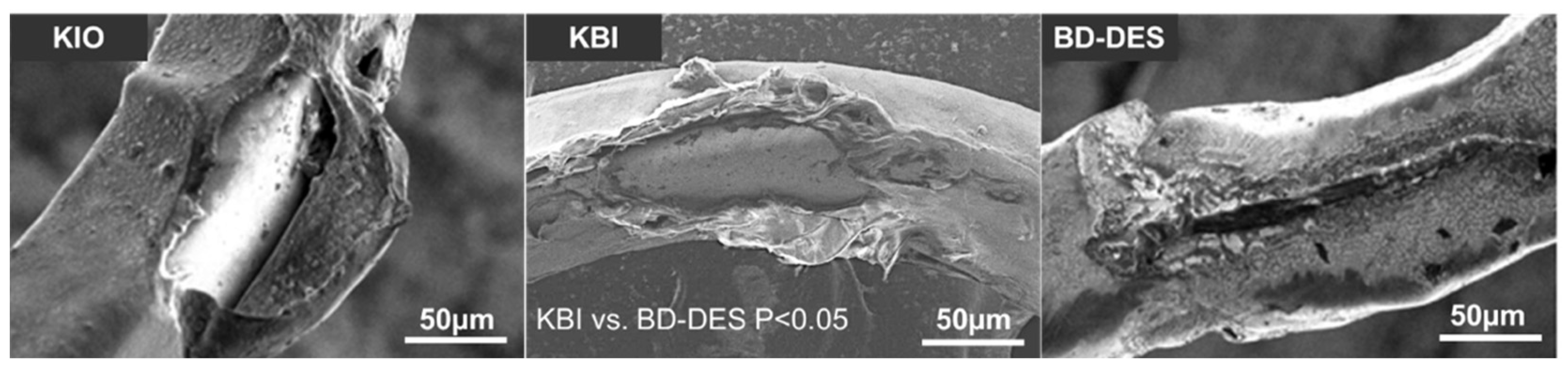
2.6. Drug Coating Integrity
2.7. Statistical Analysis
3. Results
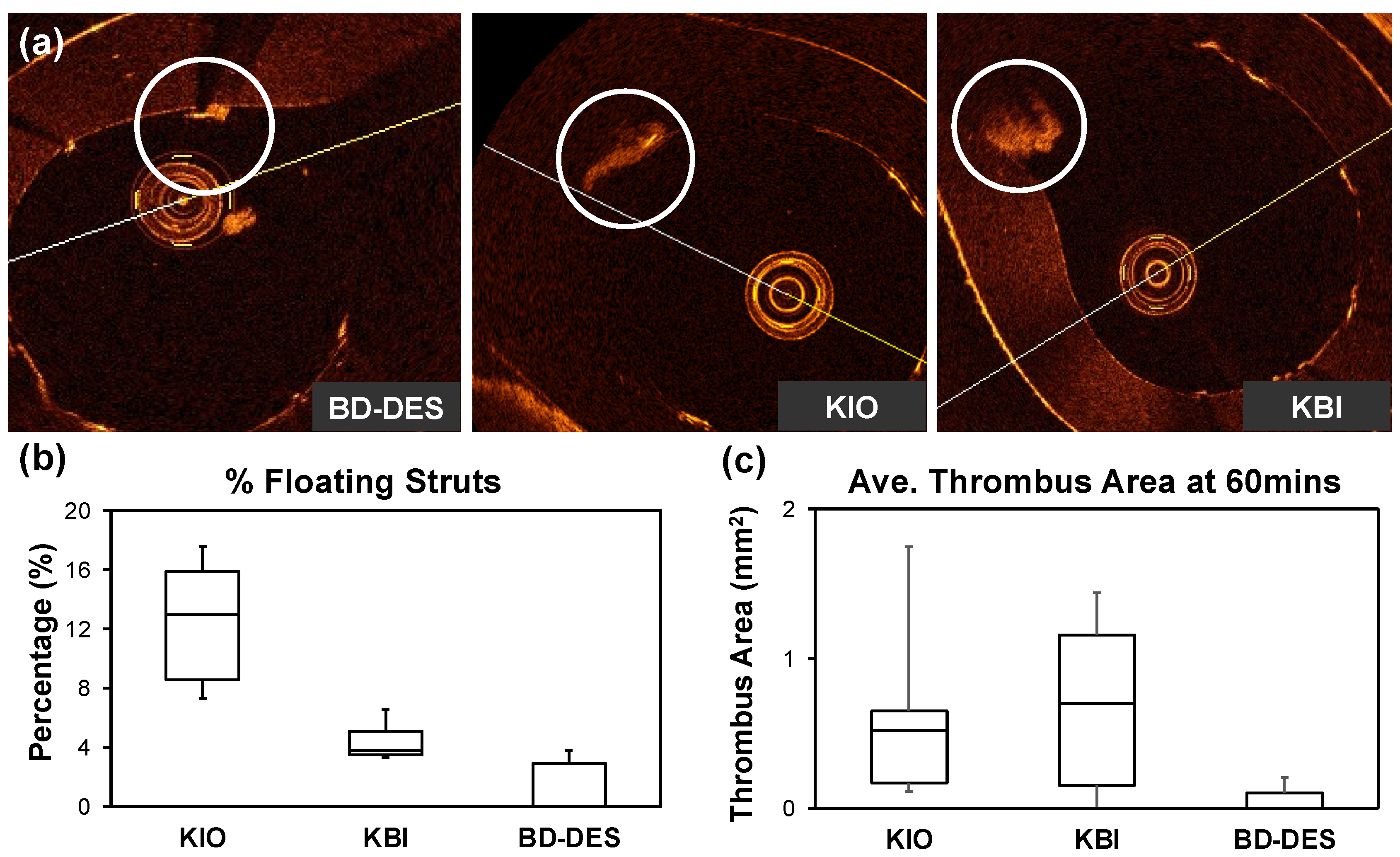
3.1. Optical Coherence Tomography
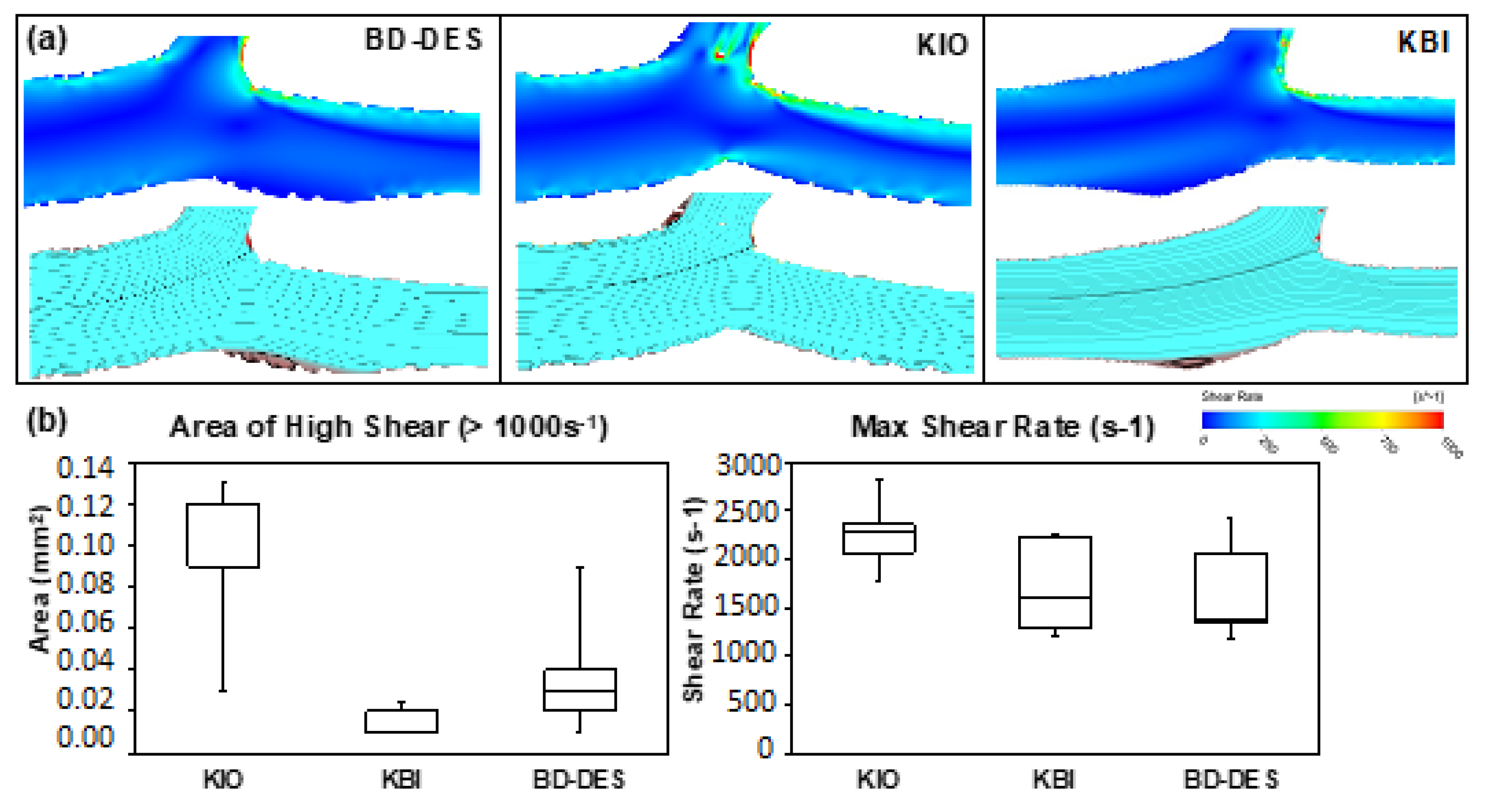
3.2. Computational Fluid Dynamics
3.3. Drug Coating Integrity
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lassen, J.; Burzotta, F.; Banning, A.; Lefevre, T.; Darremont, O.; Hildick-Smith, D.; Chieffo, A.; Pan, M.; Holm, N.; Louvard, Y.; et al. Percutaneous coronary intervention for the left main stem and other bifurcation lesions: 12th consensus document from the European Bifurcation Club. EuroIntervention 2018, 13, 1540–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iakovou, I.; Schmidt, T.; Bonizzoni, E.; Ge, L.; Sangiorgi, G.; Stankovic, G.; Airoldi, F.; Chieffo, A.; Montorfano, M.; Carlino, M.; et al. Incidence, Predictors, and Outcome of Thrombosis After Successful Implantation of Drug-Eluting Stents. JAMA 2005, 293, 2126–2130. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.; Stone, G.W.; Ormiston, J.; Kastrati, A. Coronary balloon angioplasty, stents, and scaffolds. Lancet 2017, 390, 781–792. [Google Scholar] [CrossRef]
- Stefanini, G.G.; Holmes, D.R., Jr. Drug-Eluting Coronary-Artery Stents. N. Engl. J. Med. 2013, 368, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Baber, U.; Mehran, R.; Sharma, S.K.; Brar, S.; Yu, J.; Suh, J.-W.; Kim, H.-S.; Park, S.-J.; Kastrati, A.; de Waha, A.; et al. Impact of the Everolimus-Eluting Stent on Stent Thrombosis: A Meta-Analysis of 13 Randomized Trials. J. Am. Coll. Cardiol. 2011, 58, 1569–1577. [Google Scholar] [CrossRef]
- Nebeker, J.R.; Virmani, R.; Bennett, C.L.; Hoffman, J.M.; Samore, M.H.; Alvarez, J.; Davidson, C.J.; McKoy, J.M.; Raisch, D.W.; Whisenant, B.K.; et al. Hypersensitivity Cases Associated With Drug-Eluting Coronary Stents: A Review of Available Cases From the Research on Adverse Drug Events and Reports (RADAR) Project. J. Am. Coll. Cardiol. 2006, 47, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Commandeur, S.; Van Beusekom, H.M.; Van Der Giessen, W.J. Polymers, Drug Release, and Drug-Eluting Stents. J. Interv. Cardiol. 2006, 19, 500–506. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kang, J.; Hwang, D.; Han, J.-K.; Yang, H.-M.; Kang, H.-J.; Koo, B.-K.; Kim, S.Y.; Park, K.-H.; Rha, S.-W.; et al. Durable Polymer Versus Biodegradable Polymer Drug-Eluting Stents After Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome: The HOST-REDUCE-POLYTECH-ACS Trial. Circulation 2021, 143, 1081–1091. [Google Scholar] [CrossRef]
- El-Hayek, G.; Bangalore, S.; Dominguez, A.C.; Devireddy, C.; Jaber, W.; Kumar, G.; Mavromatis, K.; Tamis-Holland, J.; Samady, H. Meta-Analysis of Randomized Clinical Trials Comparing Biodegradable Polymer Drug-Eluting Stent to Second-Generation Durable Polymer Drug-Eluting Stents. JACC Cardiovasc. Interv. 2017, 10, 462–473. [Google Scholar] [CrossRef]
- Mohr, F.W.; Morice, M.-C.; Kappetein, A.P.; Feldman, E.T.; Ståhle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R.; Morel, M.-A.; Van Dyck, N.; et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013, 381, 629–638. [Google Scholar] [CrossRef]
- Stone, G.W.; Sabik, J.F.; Serruys, P.W.; Simonton, C.A.; Généreux, P.; Puskas, J.; Kandzari, D.E.; Morice, M.-C.; Lembo, N.; Brown, W.M.; et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. New Engl. J. Med. 2016, 375, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-M.; Roh, J.-H.; Kim, Y.-H.; Park, D.-W.; Yun, S.-C.; Lee, P.H.; Chang, M.; Park, H.W.; Lee, S.-W.; Lee, C.W.; et al. Randomized Trial of Stents Versus Bypass Surgery for Left Main Coronary Artery Disease. J. Am. Coll. Cardiol. 2015, 65, 2198–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, A.; Moses, J.W.; Morice, M.C.; Ludwig, J.; Holmes, D.R.; Spanos, V.; Louvard, Y.; Desmedt, B.; Di Mario, C.; Leon, M.B. Randomized Study to Evaluate Sirolimus-Eluting Stents Implanted at Coronary Bifurcation Lesions. Circulation 2004, 109, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Hildick-Smith, D.; Egred, M.; Banning, A.; Brunel, P.; Ferenc, M.; Hovasse, T.; Wlodarczak, A.; Pan, M.; Schmitz, T.; Silvestri, M.; et al. The European bifurcation club Left Main Coronary Stent study: A randomized comparison of stepwise provisional vs. systematic dual stenting strategies (EBC MAIN). Eur. Heart J. 2021, 42, 3829–3839. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Yang, J.H.; Bin Song, Y.; Hahn, J.-Y.; Choi, S.-H.; Choi, J.-H.; Lee, H.J.; Oh, J.H.; Koo, B.-K.; Rha, S.W.; et al. Long-Term Clinical Outcomes of Final Kissing Ballooning in Coronary Bifurcation Lesions Treated With the 1-Stent Technique: Results From the COBIS II Registry (Korean Coronary Bifurcation Stenting Registry). JACC Cardiovasc. Interv. 2015, 8, 1297–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biondi-Zoccai, G.; Sheiban, I.; De Servi, S.; Tamburino, C.; Sangiorgi, G.; Romagnoli, E. To kiss or not to kiss? Impact of final kissing-balloon inflation on early and long-term results of percutaneous coronary intervention for bifurcation lesions. Heart Vessel. 2014, 29, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.W.; Nuschele, S.; Wixforth, A.; Gorzelanny, C.; Alexander-Katz, A.; Netz, R.R.; Schneider, M.F. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc. Natl. Acad. Sci. USA 2007, 104, 7899–7903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bark, D.L.; Para, A.N.; Ku, D.N. Correlation of thrombosis growth rate to pathological wall shear rate during platelet accumulation. Biotechnol. Bioeng. 2012, 109, 2642–2650. [Google Scholar] [CrossRef]
- Jackson, S.P.; Nesbitt, W.; Westein, E. Dynamics of platelet thrombus formation. J. Thromb. Haemost. 2009, 7 (Suppl. S1), 17–20. [Google Scholar] [CrossRef]
- Paradies, V.; Lu, S.; Ng, J.; Ang, H.Y.; Joner, M.; Foin, N. Thrombogenicity at the jailed side branch ostia in the provisional stenting technique: Insights from an in vitro model. EuroIntervention 2018, 14, 826–827. [Google Scholar] [CrossRef]
- Kolandaivelu, K.; Swaminathan, R.; Gibson, W.J.; Kolachalama, V.; Nguyen-Ehrenreich, K.-L.; Giddings, V.L.; Coleman, L.; Wong, G.K.; Edelman, E. Stent Thrombogenicity Early in High-Risk Interventional Settings Is Driven by Stent Design and Deployment and Protected by Polymer-Drug Coatings. Circulation 2011, 123, 1400–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, F.; Cheng, Q.; Yahagi, K.; Acampado, E.; Sheehy, A.; Yazdani, S.K.; Sakakura, K.; Euller, K.; Perkins, L.E.; Kolodgie, F.D.; et al. Acute Thrombogenicity of a Durable Polymer Everolimus-Eluting Stent Relative to Contemporary Drug-Eluting Stents With Biodegradable Polymer Coatings Assessed Ex Vivo in a Swine Shunt Model. JACC Cardiovasc. Interv. 2015, 8, 1248–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.; Ng, J.; Ang, H.Y.; Paradies, V.; Wong, P.E.; Al-Lamee, R.; Al-Lamee, K.; Bullett, N.; Ahmed, N.; Joner, M.; et al. Is There Light at the End of the Thin-Strut Tunnel?: In Vitro Insights on Strut Thickness Impact on Thrombogenicity in Bioresorbable Stents or Scaffolds. JACC Cardiovasc. Interv. 2018, 11, 714–716. [Google Scholar] [CrossRef]
- Foin, N.; Mattesini, A.; Ghione, M.; Dall’Ara, G.; Sen, S.; Nijjer, S.; Petraco, R.; Sgueglia, G.A.; Davies, J.E.; Di Mario, C. Tools & Techniques Clinical: Optimising stenting strategy in bifurcation lesions with insights from in vitro bifurcation models. EuroIntervention 2013, 9, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Ormiston, J.A.; Webster, M.W.; El Jack, S.; Ruygrok, P.N.; Stewart, J.T.; Scott, D.; Currie, E.; Rn, M.J.P.; Shaw, B.; O’Shaughnessy, B. Drug-eluting stents for coronary bifurcations: Bench testing of provisional side-branch strategies. Catheter. Cardiovasc. Interv. 2005, 67, 49–55. [Google Scholar] [CrossRef]
- Piccolo, R.; Franzone, A.; Windecker, S. From bare metal to barely anything: An update on coronary stenting. Heart 2017, 104, 533–540. [Google Scholar] [CrossRef]
- Orlik, B.; Buszman, P.P.; Krauze, A.; Gąsior, P.; Desperak, P.; Pająk, J.; Kasperczyk, J.; Janas, A.; Jelonek, M.; Handzlik-Orlik, G.; et al. A Nuclear Magnetic Resonance Spectroscopy as a Method for Evaluation of In Vivo Poly-l-Lactide Biodegradation Kinetics From Stent-Polymer Matrices: An Experimental Study Utilizing Porcine Model of In-Stent Restenosis. J. Cardiovasc. Pharmacol. Ther. 2015, 21, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Girasis, C.; Farooq, V.; Diletti, R.; Muramatsu, T.; Bourantas, C.V.; Onuma, Y.; Holmes, D.R.; Feldman, T.E.; Morel, M.-A.; van Es, G.-A.; et al. Impact of 3-Dimensional Bifurcation Angle on 5-Year Outcome of Patients After Percutaneous Coronary Intervention for Left Main Coronary Artery Disease: A Substudy of the SYNTAX Trial (Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery). JACC Cardiovasc. Interv. 2013, 6, 1250–1260. [Google Scholar] [CrossRef]
- Juan, Y.-H.; Tsay, P.-K.; Shen, W.-C.; Yeh, C.-S.; Wen, M.-S.; Wan, Y.-L. Comparison of the Left Main Coronary Bifurcating Angle among Patients with Normal, Non-significantly and Significantly Stenosed Left Coronary Arteries. Sci. Rep. 2017, 7, 1515. [Google Scholar] [CrossRef] [Green Version]
- Kern, M.J.; Dupouy, P.; Drury, J.H.; Aguirre, F.V.; Aptecar, E.; Bach, R.G.; Caracciolo, A.E.; Donohue, T.J.; Rande, J.-L.D.; Geschwind, H.J.; et al. Role of Coronary Artery Lumen Enlargement in Improving Coronary Blood Flow After Balloon Angioplasty and Stenting: A Combined Intravascular Ultrasound Doppler Flow and Imaging Study. J. Am. Coll. Cardiol. 1997, 29, 1520–1527. [Google Scholar] [CrossRef]
- Karalis, I.; Ahmed, T.A.H.N.; Jukema, J.W. Late acquired stent malapposition: Why, when and how to handle? Heart 2012, 98, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Boutsianis, E.; Dave, H.; Frauenfelder, T.; Poulikakos, D.; Wildermuth, S.; Turina, M.; Ventikos, Y.; Zund, G. Computational simulation of intracoronary flow based on real coronary geometry. Eur. J. Cardio-Thoracic Surg. 2004, 26, 248–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellnhofer, E.; Osman, J.; Kertzscher, U.; Affeld, K.; Fleck, E.; Goubergrits, L. Flow simulation studies in coronary arteries—Impact of side-branches. Atherosclerosis 2010, 213, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Foin, N.; Gutiérrez-Chico, J.L.; Nakatani, S.; Torii, R.; Bourantas, C.V.; Sen, S.; Nijjer, S.; Petraco, R.; Kousera, C.; Ghione, M.; et al. Incomplete Stent Apposition Causes High Shear Flow Disturbances and Delay in Neointimal Coverage as a Function of Strut to Wall Detachment Distance: Implications for the Management of Incomplete Stent Apposition. Circ. Cardiovasc. Interv. 2014, 7, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Yazdani, S.K.; Sheehy, A.; Pacetti, S.; Rittlemeyer, B.; Kolodgie, F.D.; Virmani, R. Stent Coating Integrity of Durable and Biodegradable Coated Drug Eluting Stents. J. Interv. Cardiol. 2016, 29, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Joner, M.; Nakazawa, G.; Finn, A.V.; Quee, S.C.; Coleman, L.; Acampado, E.; Wilson, P.S.; Skorija, K.; Cheng, Q.; Xu, X.; et al. Endothelial Cell Recovery Between Comparator Polymer-Based Drug-Eluting Stents. J. Am. Coll. Cardiol. 2008, 52, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, G.; Yazdani, S.K.; Finn, A.V.; Vorpahl, M.; Kolodgie, F.D.; Virmani, R. Pathological Findings at Bifurcation Lesions: The Impact of Flow Distribution on Atherosclerosis and Arterial Healing After Stent Implantation. J. Am. Coll. Cardiol. 2010, 55, 1679–1687. [Google Scholar] [CrossRef] [Green Version]
- Souteyrand, G.; Amabile, N.; Mangin, L.; Chabin, X.; Meneveau, N.; Cayla, G.; Vanzetto, G.; Barnay, P.; Trouillet, C.; Rioufol, G.; et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: Insights from the national PESTO French registry. Eur. Heart J. 2016, 37, 1208–1216. [Google Scholar] [CrossRef] [Green Version]
- Prati, F.; Kodama, T.; Romagnoli, E.; Gatto, L.; Di Vito, L.; Ramazzotti, V.; Chisari, A.; Marco, V.; Cremonesi, A.; Parodi, G.; et al. Suboptimal stent deployment is associated with subacute stent thrombosis: Optical coherence tomography insights from a multicenter matched study. From the CLI Foundation investigators: The CLI-THRO study. Am. Heart J. 2015, 169, 249–256. [Google Scholar] [CrossRef]
- Stone, P.H.; Coskun, A.U.; Kinlay, S.; Clark, M.E.; Sonka, M.; Wahle, A.; Ilegbusi, O.J.; Yeghiazarians, Y.; Popma, J.J.; Orav, J.; et al. Effect of Endothelial Shear Stress on the Progression of Coronary Artery Disease, Vascular Remodeling, and In-Stent Restenosis in Humans: In Vivo 6-Month Follow-up Study. Circulation 2003, 108, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Edelman, E.R.; Feldman, C.L.; Stone, P.H. Role of Endothelial Shear Stress in the Natural History of Coronary Atherosclerosis and Vascular Remodeling: Molecular, Cellular, and Vascular Behavior. J. Am. Coll. Cardiol. 2007, 49, 2379–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bluestein, D.; Gutierrez, C.; Londono, M.; Schoephoerster, R.T. Vortex shedding in steady flow through a model of an arterial stenosis and its relevance to mural platelet deposition. Ann. Biomed. Eng. 1999, 27, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Guérin, P.; Pilet, P.; Finet, G.; Gouëffic, Y.; N’Guyen, J.M.; Crochet, D.; Tijou, I.; Pacaud, P.; Loirand, G. Drug-Eluting Stents in Bifurcations: Bench study of strut deformation and coating lesions. Circ. Cardiovasc. Interv. 2010, 3, 120–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, R.J.; Pawłowski, T.; Legutko, J.; Lesiak, M.; Witkowski, A.; Gąsior, M.; Kern, A.; Bil, J. Rationale and design of the randomized, multicenter, open-label, controlled POLBOS 3 trial aimed to compare regular drug-eluting stents versus the dedicated coronary bifurcation sirolimus-eluting BiOSS LIM C stent. Medicine 2019, 98, e15106. [Google Scholar] [CrossRef]
- Iannaccone, M.; D’Ascenzo, F.; Gatti, P.; Cerrato, E.; Nuñez-Gil, I.; Wojakowski, W.; Capodanno, D.; Figini, F.; Wańha, W.; Chieffo, A.; et al. Impact of the metal-to-artery ratio on clinical outcomes in left main and nonleft main bifurcation: Insights the RAIN-CARDIOGROUP VII study (veRy thin stents for patients with left mAIn or bifurcatioN in real life). J. Cardiovasc. Med. 2020, 21, 669–674. [Google Scholar] [CrossRef]
- Jackson, S.P. The growing complexity of platelet aggregation. Blood 2007, 109, 5087–5095. [Google Scholar] [CrossRef] [Green Version]
- Corban, M.T.; Eshtehardi, P.; Suo, J.; McDaniel, M.C.; Timmins, L.H.; Rassoul-Arzrumly, E.; Maynard, C.; Mekonnen, G.; King, S.; Quyyumi, A.A.; et al. Combination of plaque burden, wall shear stress, and plaque phenotype has incremental value for prediction of coronary atherosclerotic plaque progression and vulnerability. Atherosclerosis 2014, 232, 271–276. [Google Scholar] [CrossRef]
- Samady, H.; Eshtehardi, P.; McDaniel, M.C.; Suo, J.; Dhawan, S.S.; Maynard, C.; Timmins, L.H.; Quyyumi, A.A.; Giddens, D.P. Coronary Artery Wall Shear Stress Is Associated With Progression and Transformation of Atherosclerotic Plaque and Arterial Remodeling in Patients With Coronary Artery Disease. Circulation 2011, 124, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Gijsen, F.J.; Mastik, F.; Schaar, J.A.; Schuurbiers, J.C.; Van Der Giessen, W.J.; De Feyter, P.J.; Serruys, P.W.; Van Der Steen, A.F.; Wentzel, J.J. High shear stress induces a strain increase in human coronary plaques over a 6-month period. EuroIntervention 2011, 7, 121–127. [Google Scholar] [CrossRef]
- Costopoulos, C.; Huang, Y.; Brown, A.J.; Calvert, P.A.; Hoole, S.P.; West, N.E.; Gillard, J.H.; Teng, Z.; Bennett, M.R. Plaque Rupture in Coronary Atherosclerosis Is Associated With Increased Plaque Structural Stress. JACC Cardiovasc. Imaging 2017, 10, 1472–1483. [Google Scholar] [CrossRef]
- Kumar, A.; Thompson, E.W.; Lefieux, A.; Molony, D.S.; Davis, E.L.; Chand, N.; Fournier, S.; Lee, H.S.; Suh, J.; Sato, K.; et al. High Coronary Shear Stress in Patients With Coronary Artery Disease Predicts Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 1926–1935. [Google Scholar] [CrossRef] [PubMed]
- Burzotta, F.; Lassen, J.F.; Lefèvre, T.; Banning, A.P.; Chatzizisis, Y.S.; Johnson, T.W.; Ferenc, M.; Rathore, S.; Albiero, R.; Pan, M.; et al. Percutaneous coronary intervention for bifurcation coronary lesions: The 15th consensus document from the European Bifurcation Club. EuroIntervention 2021, 16, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Chronos, N.A.F.; Apkarian, R.P.; Robinson, A.K. Scanning electron microscopic analysis of defects in polymer coatings of three commercially available stents: Comparison of BiodivYsio, Taxus and Cypher stents. J. Invasive Cardiol. 2007, 19, 71–76. [Google Scholar] [PubMed]
- Gasior, P.; Lu, S.; Ng, C.K.J.; Toong, W.Y.D.; Wong, E.H.P.; Foin, N.; Kedhi, E.; Wojakowski, W.; Ang, H.Y. Comparison of overexpansion capabilities and thrombogenicity at the side branch ostia after implantation of four different drug eluting stents. Sci. Rep. 2020, 10, 20791. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.D.R.; Mintz, G.S.; Carlier, S.G.; Costa, R.A.; Fujii, K.; Sano, K.; Kimura, M.; Lui, J.; Weisz, G.; Moussa, I.; et al. Intravascular Ultrasonic Assessment of Stent Diameters Derived from Manufacturer’s Compliance Charts. Am. J. Cardiol. 2005, 96, 74–78. [Google Scholar] [CrossRef]
- Casa, L.D.C.; Ku, D.N. High Shear Thrombus Formation under Pulsatile and Steady Flow. Cardiovasc. Eng. Technol. 2014, 5, 154–163. [Google Scholar] [CrossRef]
- Chesnutt, J.K.W.; Han, H.-C. Computational simulation of platelet interactions in the initiation of stent thrombosis due to stent malapposition. Phys. Biol. 2016, 13, 016001. [Google Scholar] [CrossRef] [Green Version]
- Migliori, S.; Chiastra, C.; Bologna, M.; Montin, E.; Dubini, G.; Aurigemma, C.; Fedele, R.; Burzotta, F.; Mainardi, L.; Migliavacca, F. A framework for computational fluid dynamic analyses of patient-specific stented coronary arteries from optical coherence tomography images. Med Eng. Phys. 2017, 47, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Morris, P.D.; Iqbal, J.; Chiastra, C.; Wu, W.; Migliavacca, F.; Gunn, J.P. Simultaneous kissing stents to treat unprotected left main stem coronary artery bifurcation disease; stent expansion, vessel injury, hemodynamics, tissue healing, restenosis, and repeat revascularization. Catheter. Cardiovasc. Interv. 2018, 92, E381–E392. [Google Scholar] [CrossRef] [Green Version]
| KIO | KBI | BD-DES | p Value | |
|---|---|---|---|---|
| OCT Proximal Diameter (mm) | ||||
| Min | 5.40 (5.23–5.58) | 5.36 (5.12–5.52) | 5.33 (5.23–5.37) | >0.05 |
| Mean | 5.55 (5.42–5.69) | 5.52 (5.30–5.66) | 5.50 (5.40–5.53) | >0.05 |
| Max | 5.75 (5.60–5.79) | 5.65 (5.41–5.75) | 5.59 (5.55–5.64) | >0.05 |
| OCT Proximal Lumen Area (mm2) | ||||
| Area | 24.22 (23.09–25.4) | 23.93 (22.06–25.16) | 23.81 (22.92–24.00) | >0.05 |
| OCT Proximal Eccentricity Index | ||||
| EI | 1.06 (1.05–1.06) | 1.05 (1.04–1.06) | 1.05 (1.04–1.05) | >0.05 |
| OCT Strut Analysis | ||||
| WA (%) | 78.6 (76.1–85.3) | 93.3 (88.1–93.4) | 94.3 (88.5–98.8) | >0.05 |
| Floating (%) | 15.9 (13.0–17.7) | 3.8 (3.5–5.1) | 0.0 (0.0–2.7) | >0.05 |
| MA (%) | 4.9 (4.7–5.3) | 3.3 (1.6–6.0) | 2.7 (1.2–2.9) | >0.05 |
| SEM Coating Analysis | ||||
|---|---|---|---|---|
| KIO | KBI | BD-DES | p Value | |
| Category 1 | 19 (11–21) | 17 (15–27) | 7 (6–14) | >0.05 |
| Category 2 | 7 (7–11) | 21 (18–21) | 1 (0–3) | <0.05 b |
| Category 3 | 4 (3–12) | 11 (7–12) | 0 (0–1) | <0.05 a,b |
| Category 4 | 5 (3–15) | 20 (16–21) | 0 (0–0) | <0.05 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milewski, M.; Ng, C.K.J.; Gąsior, P.; Lian, S.S.; Qian, S.X.; Lu, S.; Foin, N.; Kedhi, E.; Wojakowski, W.; Ang, H.Y. Polymer Coating Integrity, Thrombogenicity and Computational Fluid Dynamics Analysis of Provisional Stenting Technique in the Left Main Bifurcation Setting: Insights from an In-Vitro Model. Polymers 2022, 14, 1715. https://doi.org/10.3390/polym14091715
Milewski M, Ng CKJ, Gąsior P, Lian SS, Qian SX, Lu S, Foin N, Kedhi E, Wojakowski W, Ang HY. Polymer Coating Integrity, Thrombogenicity and Computational Fluid Dynamics Analysis of Provisional Stenting Technique in the Left Main Bifurcation Setting: Insights from an In-Vitro Model. Polymers. 2022; 14(9):1715. https://doi.org/10.3390/polym14091715
Chicago/Turabian StyleMilewski, Marek, Chen Koon Jaryl Ng, Pawel Gąsior, Shaoliang Shawn Lian, Su Xiao Qian, Shengjie Lu, Nicolas Foin, Elvin Kedhi, Wojciech Wojakowski, and Hui Ying Ang. 2022. "Polymer Coating Integrity, Thrombogenicity and Computational Fluid Dynamics Analysis of Provisional Stenting Technique in the Left Main Bifurcation Setting: Insights from an In-Vitro Model" Polymers 14, no. 9: 1715. https://doi.org/10.3390/polym14091715






