Cryogenic Coaxial Printing for 3D Shell/Core Tissue Engineering Scaffold with Polymeric Shell and Drug-Loaded Core
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
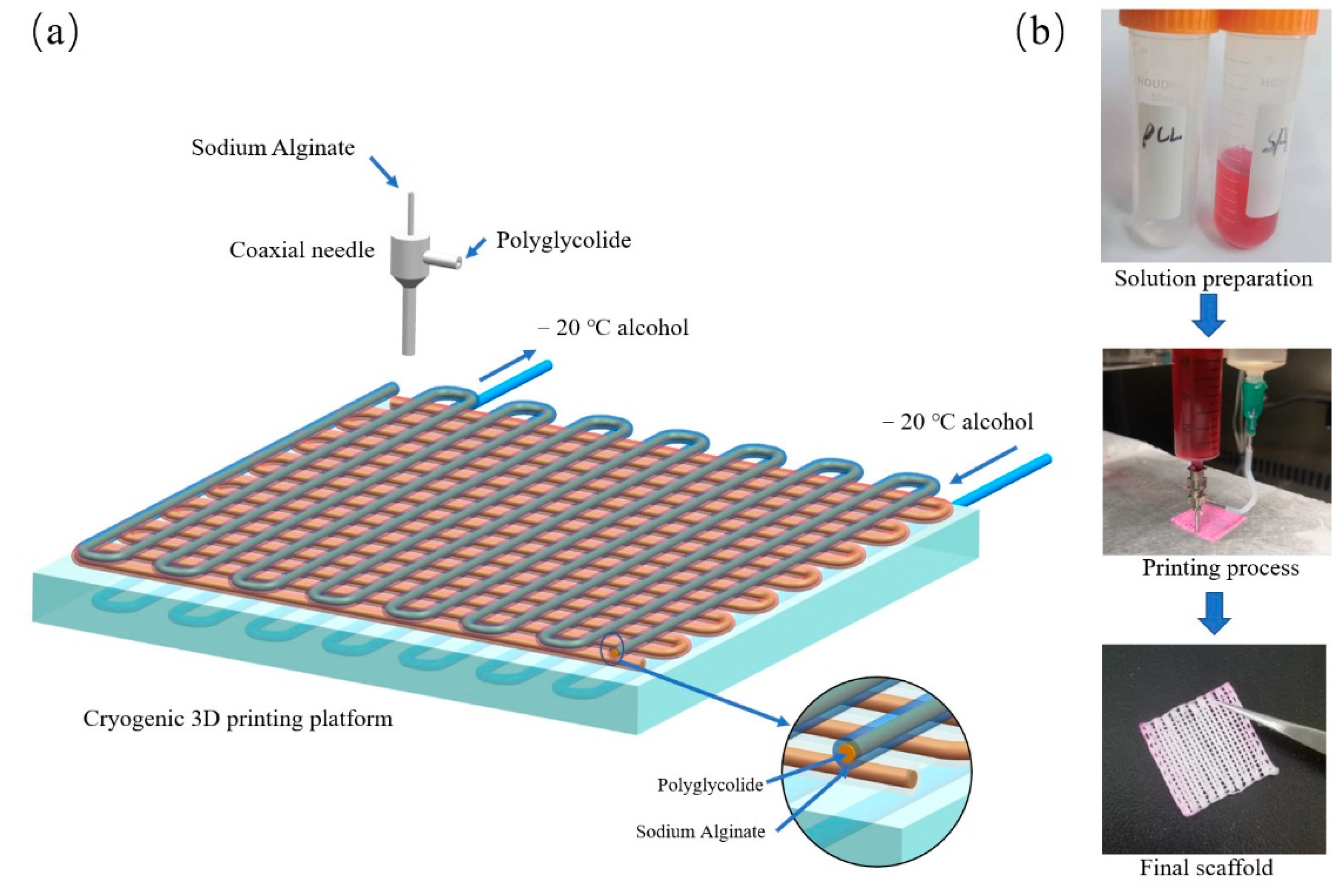
2.2. Cryogenic Coaxial Printing
2.3. Morphology Characterization
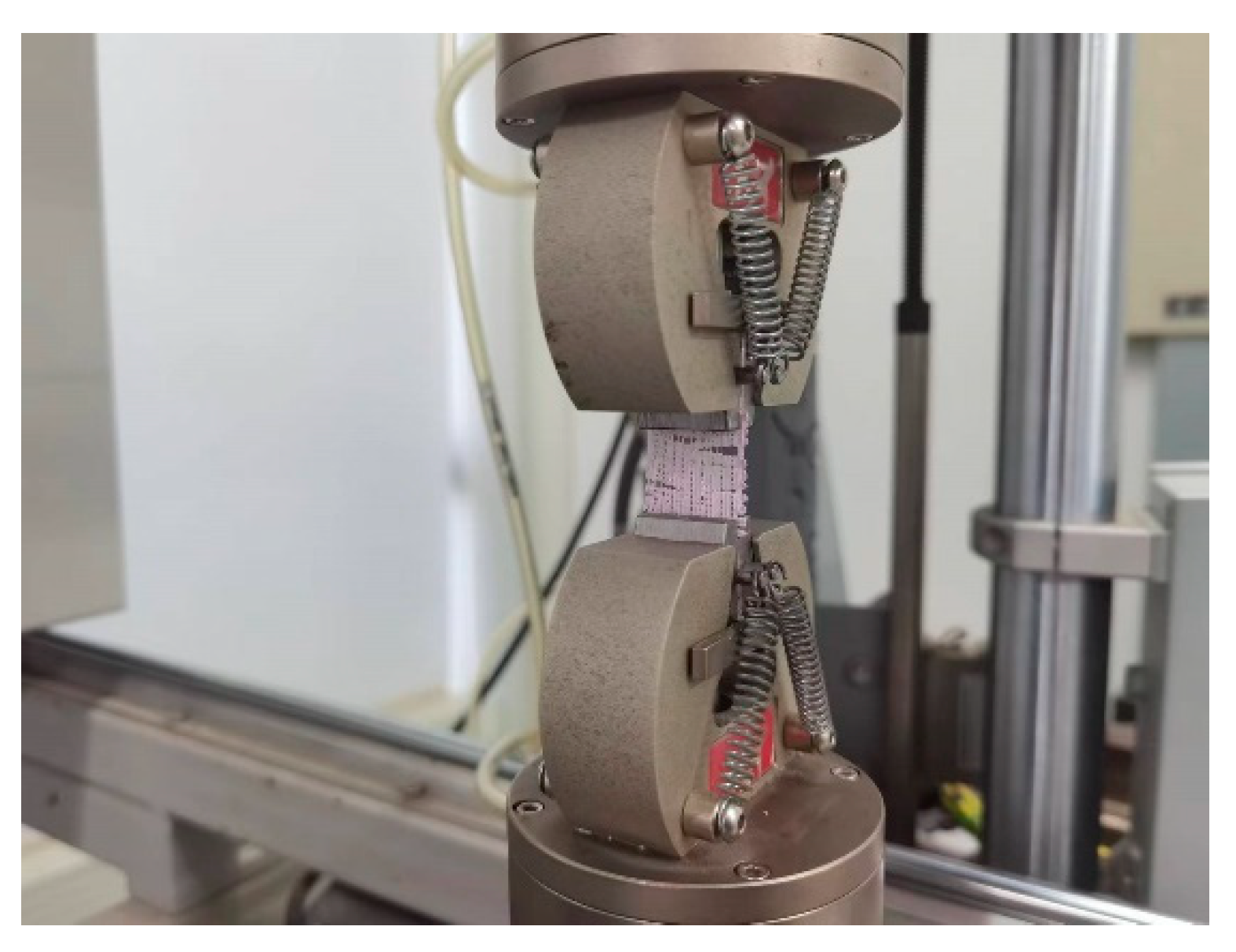
2.4. Mechanical Properties
2.5. Accelerated Acid Degradation
2.6. In Vitro Release of Levofloxacin from Cryogenic Coaxial Scaffolds
2.7. Statistical Analysis
3. Results and Discussion
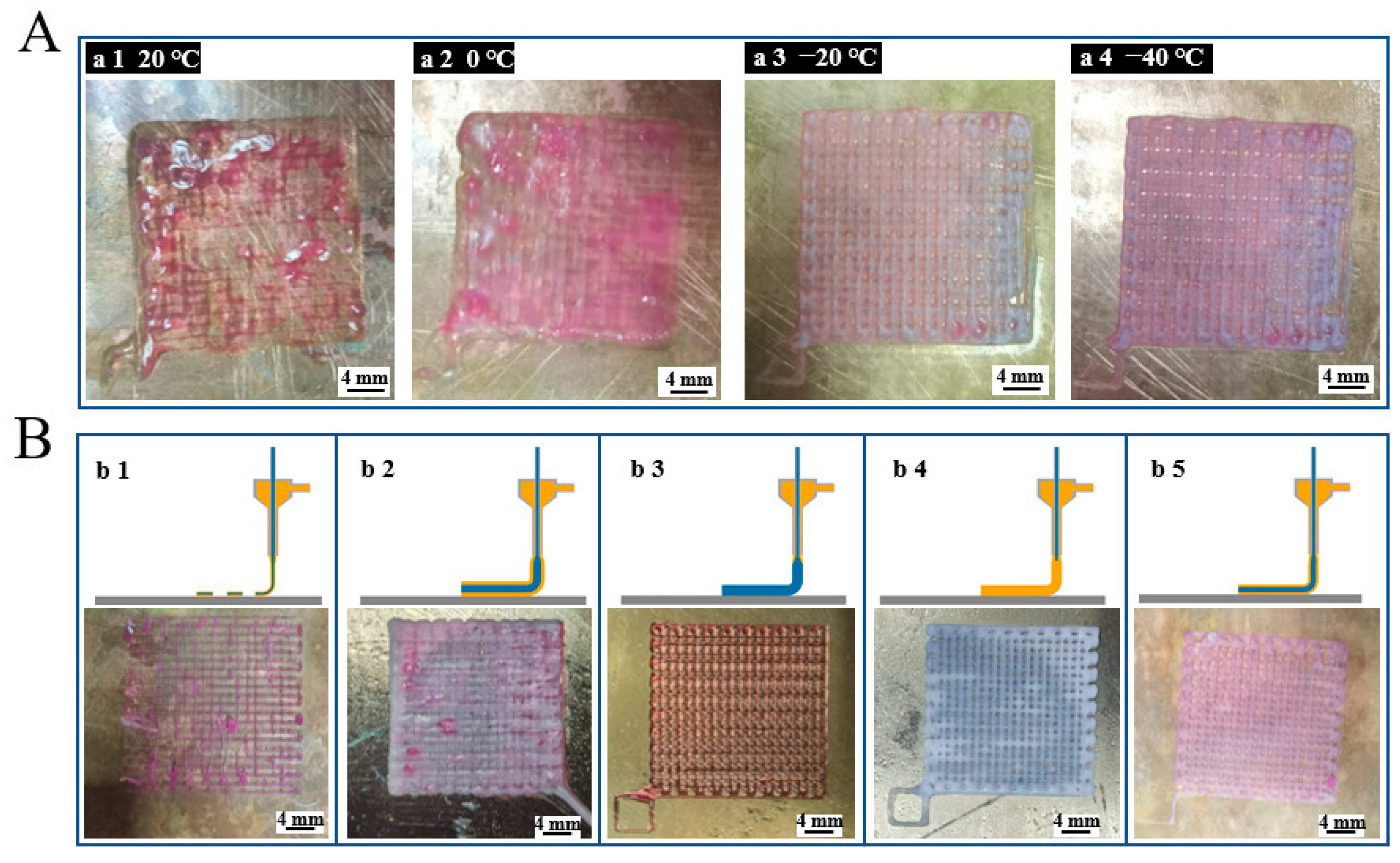
3.1. Optimization of 3D Printing Process
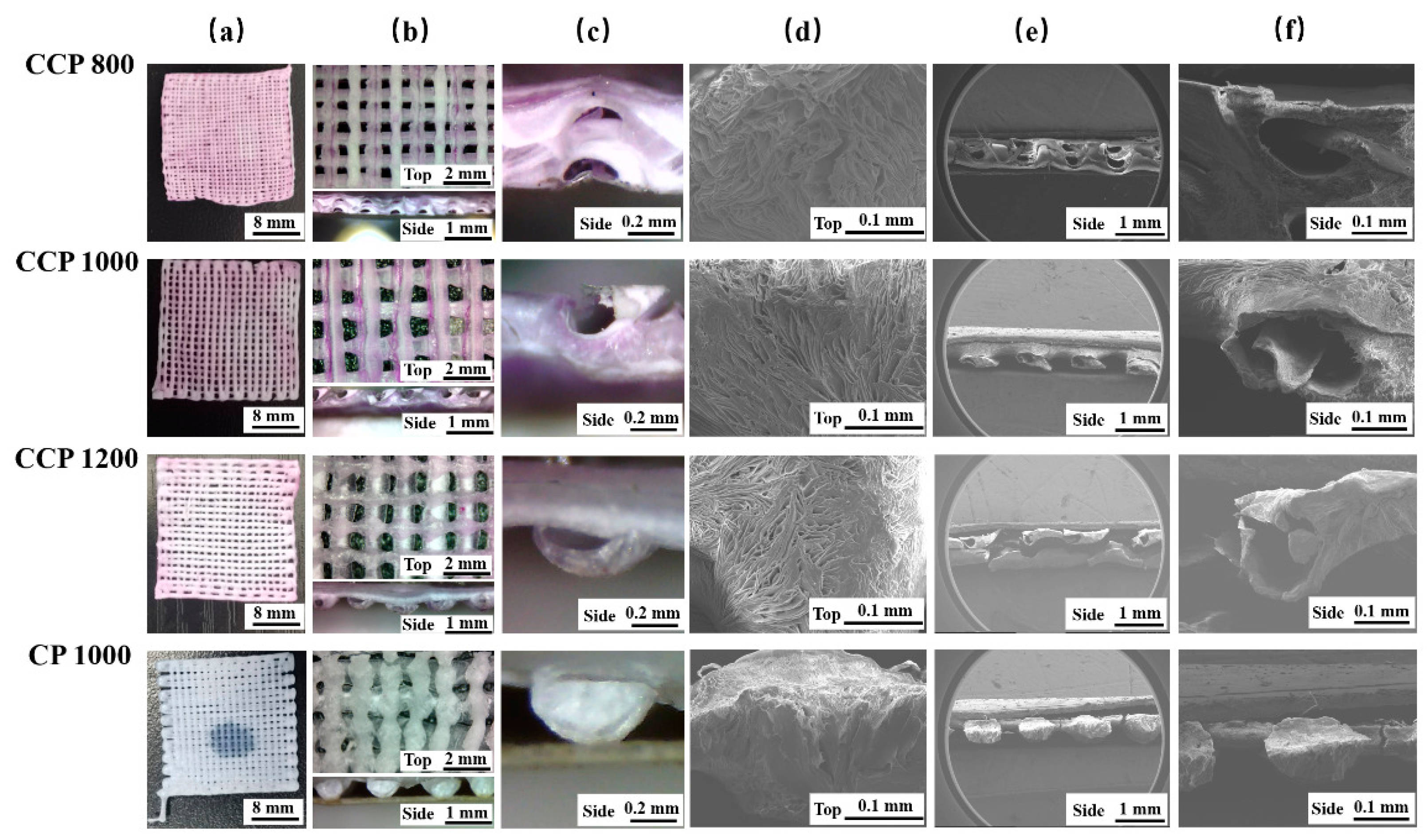
3.2. Morphology of Scaffolds
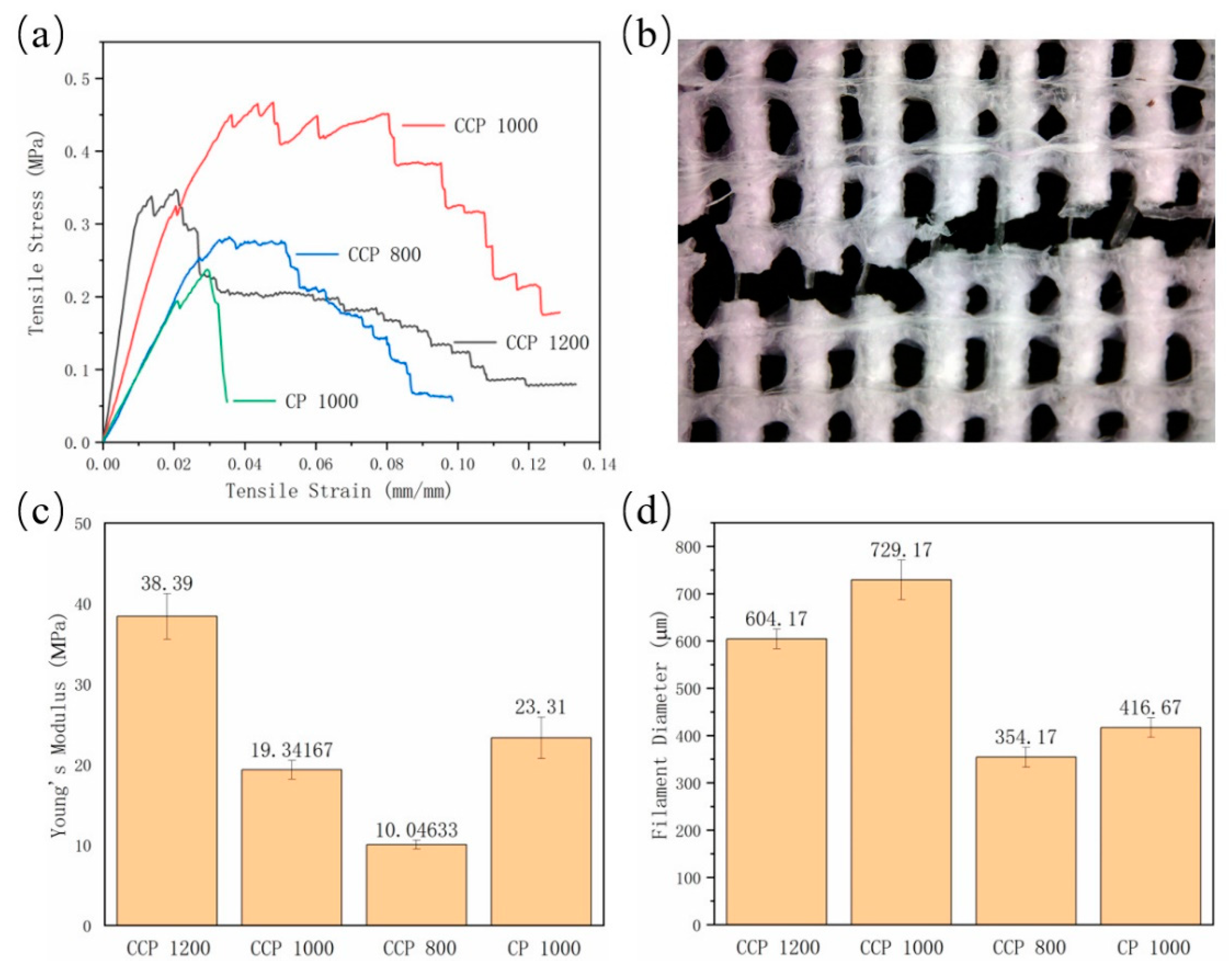
3.3. Tensile Properties of 3D-Printed Coaxial Scaffolds
3.4. Accelerated Acidic Degradation
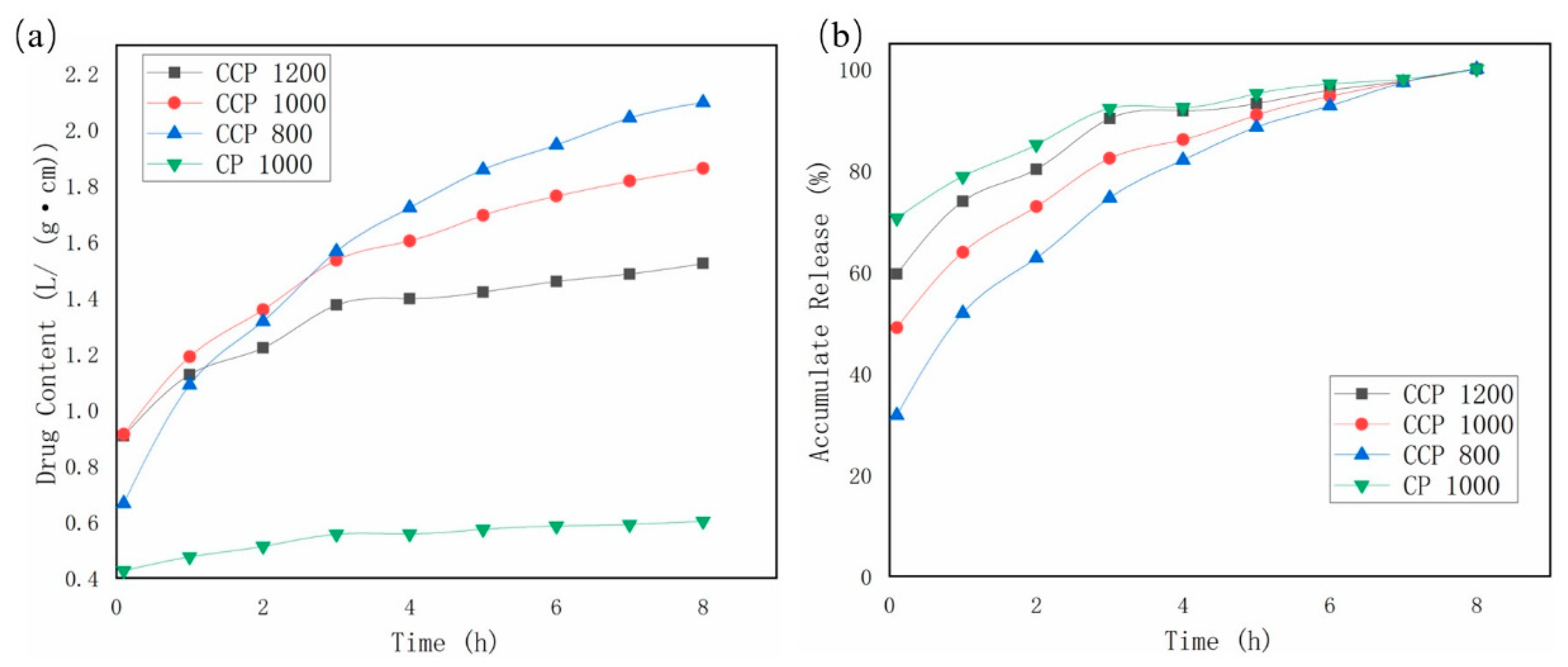
3.5. Drug-Sustained Release Properties of Coaxial Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanaei, H.R.; Khelladi, S.; Deligant, M.; Shirinbayan, M.; Tcharkhtchi, A. Numerical Prediction for Temperature Profile of Parts Manufactured using Fused Filament Fabrication. J. Manuf. Process. 2022, 76, 1526–6125. [Google Scholar] [CrossRef]
- Yang, B.; Liu, T.; Gao, G.; Zhang, X.; Wu, B. Fabrication of 3D GelMA Scaffolds Using Agarose Microgel Embedded Printing. Micromachines 2022, 13, 469. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, K.; Jiang, X.; Hu, Q.; Zhang, C.; Wang, B. Rapid Fabrication of Ready-to-Use Gelatin Scaffolds with Prevascular Networks Using Alginate Hollow Fibers as Sacrificial Templates. ACS Biomater. Sci. Eng. 2020, 6, 2297–2311. [Google Scholar] [CrossRef] [PubMed]
- Paredes, C.; Martínez-Vázquez, F.J.; Pajares, A.; Miranda, P. Development by robocasting and mechanical characterization of hybrid HA/PCL coaxial scaffolds for biomedical applications. J. Eur. Ceram. Soc. 2019, 39, 4375–4383. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, C.; Yang, G.; Li, G.; Ding, X.; Wang, S.; Dou, Y.; Zhang, Z.; Chang, J.; Wu, C.; et al. 3D-printed scaffolds with synergistic effect of hollow-pipe structure and bioactive ions for vascularized bone regeneration. Biomaterials 2017, 135, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Han, W.; Nam, S.A.; Kim, J.W.; Kim, J.Y.; Kim, Y.K.; Cho, D.W. Three-dimensional cell-printing of advanced renal tubular tissue analogue. Biomaterials 2020, 232, 119734. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Nowicki, M.; Sun, H.; Hann, S.Y.; Cui, H.; Esworthy, T.; Lee, J.D.; Plesniak, M.; Zhang, L.G. 3D Bioprinting-Tunable Small-Diameter Blood Vessels with Biomimetic Biphasic Cell Layers. ACS Appl. Mater. Interfaces 2020, 12, 45904–45915. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Pengsuk, C.; Lirdprapamongkol, K.; Techasakul, S.; Svasti, J.; Nooeaid, P. Enhanced Structural Stability and Controlled Drug Release of Hydrophilic Antibiotic-Loaded Alginate/Soy Protein Isolate Core-Sheath Fibers for Tissue Engineering Applications. Fibers Polym. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Yuan, H.; Li, X.; Lee, M.S.; Zhang, Z.; Li, B.; Xuan, H.; Li, W.J.; Zhang, Y. Collagen and chondroitin sulfate functionalized bioinspired fibers for tendon tissue engineering application. Int. J. Biol. Macromol. 2021, 170, 248–260. [Google Scholar] [CrossRef]
- Sang, Q.; Li, H.; Williams, G.; Wu, H.; Zhu, L.M. Core-shell poly(lactide-co-ε-caprolactone)-gelatin fiber scaffolds as pH-sensitive drug delivery systems. J. Biomater. Appl. 2018, 32, 1105–1118. [Google Scholar] [CrossRef]
- Yi, B.; Shen, Y.; Tang, H.; Wang, X.; Li, B.; Zhang, Y. Stiffness of Aligned Fibers Regulates the Phenotypic Expression of Vascular Smooth Muscle Cells. ACS Appl. Mater. Interfaces 2019, 11, 6867–6880. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Nejad, Z.; Zamanian, A.; Saeidifar, M.; Vanaei, H.R.; Amoli, M.S. 3D Bioprinting of Polycaprolactone-Based Scaffolds for Pulp-Dentin Regeneration: Investigation of Physicochemical and Biological Behavior. Polymers 2021, 13, 4442. [Google Scholar] [CrossRef] [PubMed]
- Pi, Q.; Maharjan, S.; Yan, X.; Liu, X.; Singh, B.; van Genderen, A.M.; Robledo-Padilla, F.; Parra-Saldivar, R.; Hu, N.; Jia, W.; et al. Digitally Tunable Microfluidic Bioprinting of Multilayered Cannular Tissues. Adv. Mater. 2018, 30, 1706913. [Google Scholar] [CrossRef] [PubMed]
- Bagnol, R.; Sprecher, C.; Peroglio, M.; Chevalier, J.; Mahou, R.; Büchler, P.; Richards, G.; Eglin, D. Coaxial micro-extrusion of a calcium phosphate ink with aqueous solvents improves printing stability, structure fidelity and mechanical properties. Acta Biomater. 2021, 125, 322–332. [Google Scholar] [CrossRef]
- Cornock, R.; Beirne, S.; Thompson, B.; Wallace, G.G. Coaxial additive manufacture of biomaterial composite scaffolds for tissue engineering. Biofabrication 2014, 6, 025002. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, B.; Xiong, Y.; Tao, R.; Panayi, A.C.; Chen, L.; Tian, W.; Xue, H.; Shi, L.; Zhang, X.; et al. Cryogenic 3D printed hydrogel scaffolds loading exosomes accelerate diabetic wound healing. Chem. Eng. J. 2021, 426, 130634. [Google Scholar] [CrossRef]
- Sadowska, J.M.; Genoud, K.J.; Kelly, D.J.; O'Brien, F.J. Bone biomaterials for overcoming antimicrobial resistance: Advances in non-antibiotic antimicrobial approaches for regeneration of infected osseous tissue. Mater. Today 2021, 46, 136–154. [Google Scholar] [CrossRef]
- Wright, J.A.; Nair, S.P. Interaction of staphylococci with bone. Int. J. Med. Microbiol. 2010, 300, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.; Lelkes, P.I. On the road to smart biomaterials for bone research: Definitions, concepts, advances, and outlook. Bone Res. 2021, 9, 12. [Google Scholar] [CrossRef]
- Xiong, M.H.; Bao, Y.; Yang, X.Z.; Wang, Y.C.; Sun, B.; Wang, J. Lipase-sensitive polymeric triple-layered nanogel for “on-demand” drug delivery. J. Am. Chem. Soc. 2012, 134, 4355–4362. [Google Scholar] [CrossRef]
- Li, L.L.; Xu, J.H.; Qi, G.B.; Zhao, X.; Yu, F.; Wang, H. Core-shell supramolecular gelatin nanoparticles for adaptive and “on-demand” antibiotic delivery. ACS Nano 2014, 8, 4975–4983. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Gao, H.; Cheng, T.; Zhang, Y.; Liu, J.; Huang, F.; Yang, C.; Shi, L.; Liu, J. A charge-adaptive nanosystem for prolonged and enhanced: In vivo antibiotic delivery. Chem. Commun. 2016, 52, 6265–6268. [Google Scholar] [CrossRef] [PubMed]
- Won, J.Y.; Kim, J.; Gao, G.; Kim, J.; Jang, J.; Park, Y.H.; Cho, D.W. 3D printing of drug-loaded multi-shell rods for local delivery of bevacizumab and dexamethasone: A synergetic therapy for retinal vascular diseases. Acta Biomater. 2020, 116, 174–185. [Google Scholar] [CrossRef] [PubMed]
- van de Witte, P.; Dijkstra, P.J.; van den Berg, J.W.A.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Meng, C.; Ding, Q.; Yu, K.; Zhang, X.; Zhang, W.; Tian, W.; Zhang, Q.; Guo, X.; Wu, B.; et al. In situ bone regeneration with sequential delivery of aptamer and BMP2 from an ECM-based scaffold fabricated by cryogenic free-form extrusion. Bioact. Mater. 2021, 6, 4163–4175. [Google Scholar] [CrossRef]
- Kim, G.; Ahn, S.; Kim, Y.; Cho, Y.; Chun, W. Coaxial structured collagen-alginate scaffolds: Fabrication, physical properties, and biomedical application for skin tissue regeneration. J. Mater. Chem. 2011, 21, 6165–6172. [Google Scholar] [CrossRef]
- Zhang, W.; Ullah, I.; Shi, L.; Zhang, Y.; Ou, H.; Zhou, J.; Ullah, M.W.; Zhang, X.; Li, W. Fabrication and characterization of porous polycaprolactone scaffold via extrusion-based cryogenic 3D printing for tissue engineering. Mater. Des. 2019, 180, 107946. [Google Scholar] [CrossRef]
- Pitaluga, L.H.; Souza, M.T.; Zanotto, E.D.; Romero, M.E.S.; Hatton, P.V. Electrospun F18 Bioactive Glass/PCL-Poly (epsilon-caprolactone)-Membrane for Guided Tissue Regeneration. Materials 2018, 11, 400. [Google Scholar] [CrossRef] [Green Version]

| Samples | CCP 1200 | CCP 1000 | CCP 800 | CP 1000 | |
|---|---|---|---|---|---|
| Time (h) | |||||
| 2 | 34.05% ± 8.64% | 31.55% ± 2.14% | 26.51% ± 0.08% | 18.60% ± 5.48% | |
| 4 | 42.49% ± 3.00% | 41.54% ± 11.47% | 35.38% ± 9.73% | 21.58% ± 6.27% | |
| 6 | 49.93% ± 7.70% | 43.88% ± 9.52% | 41.39% ± 2.33% | 31.26% ± 4.71% | |
| 8 | 64.43% ± 6.92% | 100% | 100% | 40.02% ± 1.11% | |
| 10 | 100% | 100% | 100% | 51.43% ± 1.27% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Yang, B.; Tian, W.; Zhang, X.; Wu, B. Cryogenic Coaxial Printing for 3D Shell/Core Tissue Engineering Scaffold with Polymeric Shell and Drug-Loaded Core. Polymers 2022, 14, 1722. https://doi.org/10.3390/polym14091722
Liu T, Yang B, Tian W, Zhang X, Wu B. Cryogenic Coaxial Printing for 3D Shell/Core Tissue Engineering Scaffold with Polymeric Shell and Drug-Loaded Core. Polymers. 2022; 14(9):1722. https://doi.org/10.3390/polym14091722
Chicago/Turabian StyleLiu, Tianqi, Bo Yang, Wenqing Tian, Xianglin Zhang, and Bin Wu. 2022. "Cryogenic Coaxial Printing for 3D Shell/Core Tissue Engineering Scaffold with Polymeric Shell and Drug-Loaded Core" Polymers 14, no. 9: 1722. https://doi.org/10.3390/polym14091722
APA StyleLiu, T., Yang, B., Tian, W., Zhang, X., & Wu, B. (2022). Cryogenic Coaxial Printing for 3D Shell/Core Tissue Engineering Scaffold with Polymeric Shell and Drug-Loaded Core. Polymers, 14(9), 1722. https://doi.org/10.3390/polym14091722







