The Modification of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Melt Blending
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Mixture Preparation
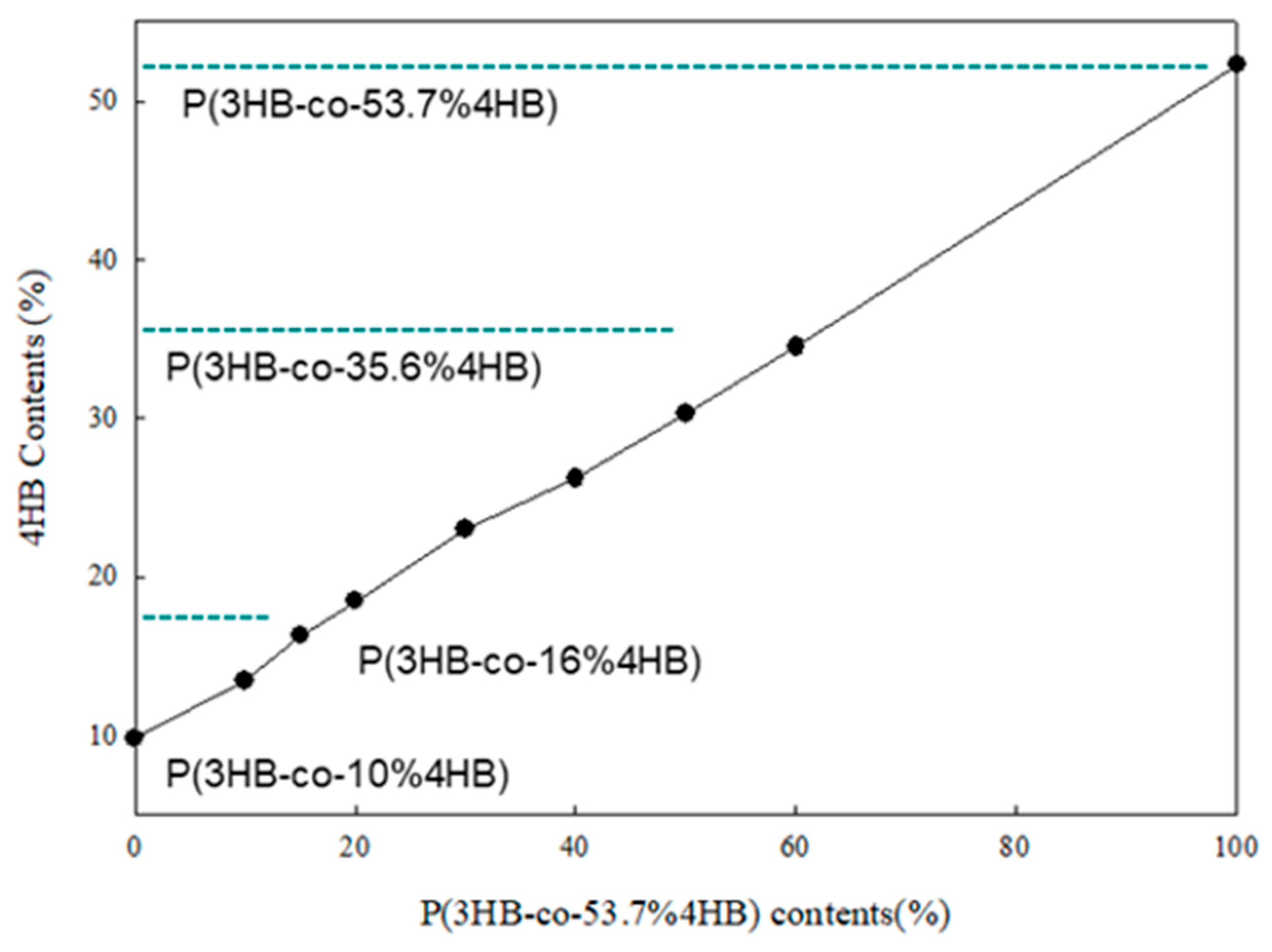
2.2. The 4HB Contents of P(3HB-co-4HB) Mixtures
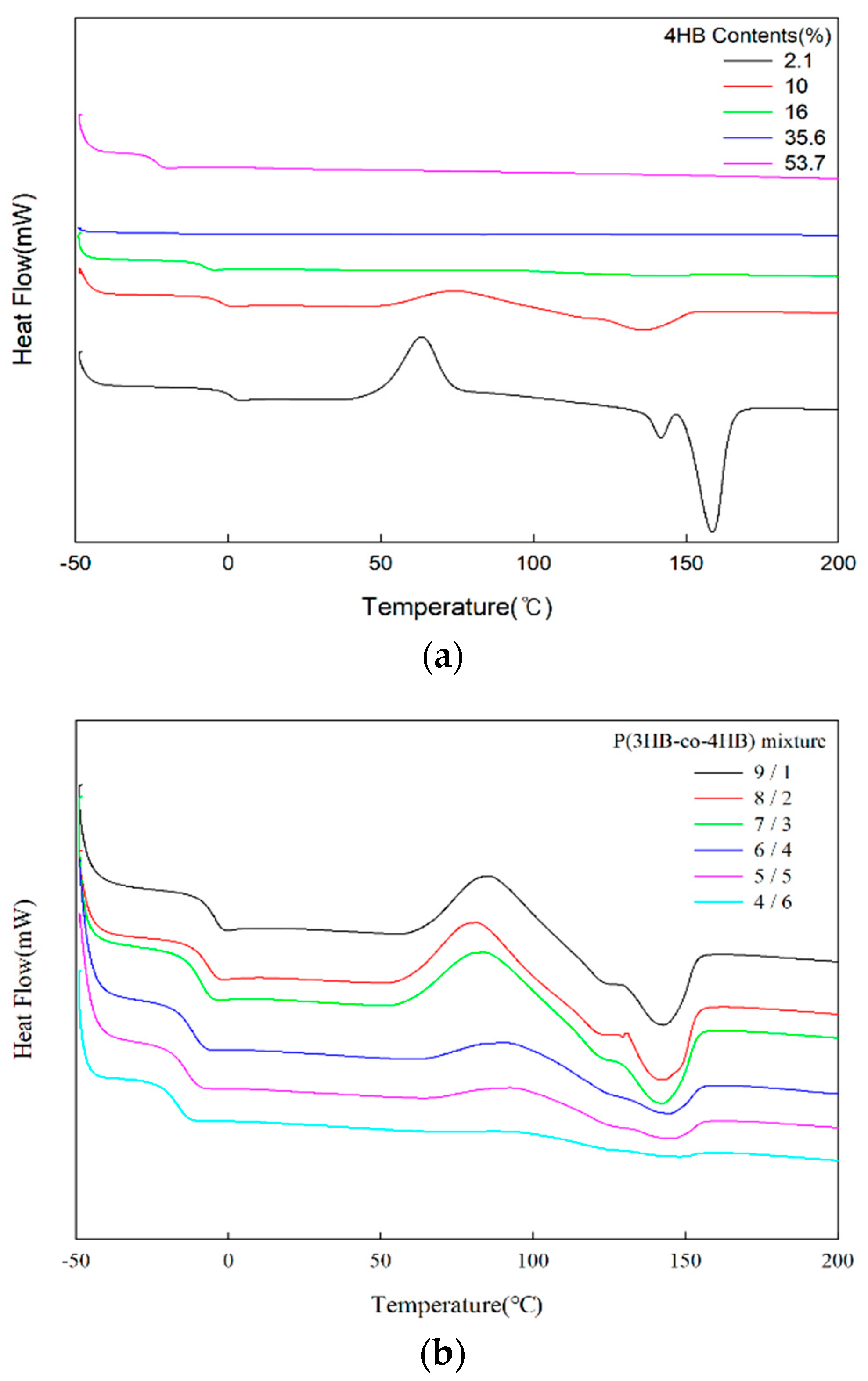
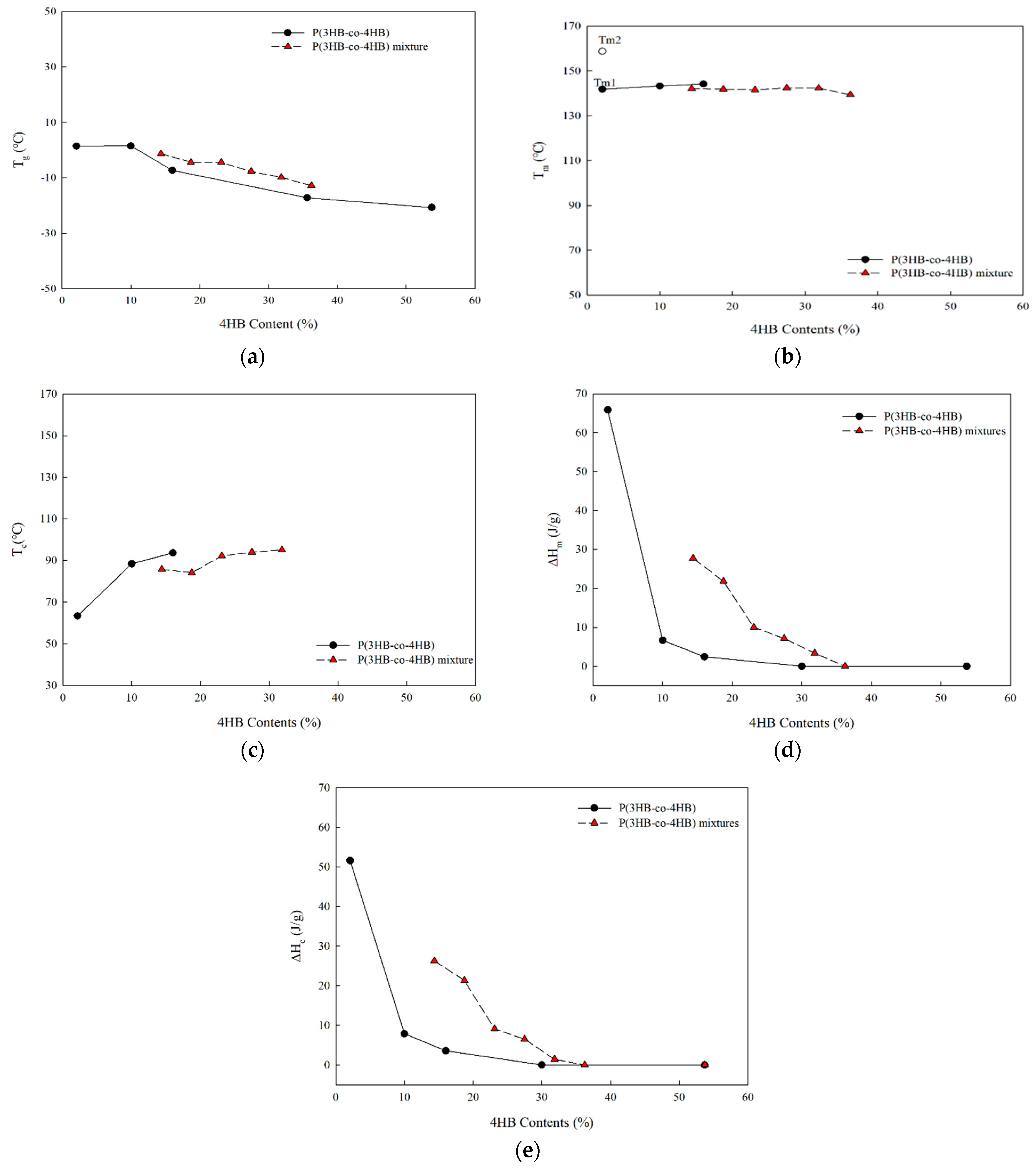
2.3. Thermal Properties of P(3HB-co-4HB) Mixtures
2.4. Rheological Properties of P(3HB-co-4HB) Mixtures
2.5. Mechanical Properties of P(3HB-co-4HB) Mixtures
3. Results and Discussion
3.1. 4HB Contents of P(3HB-co-4HB) Mixtures
3.2. Thermal Properties of P(3HB-co-4HB) Mixtures
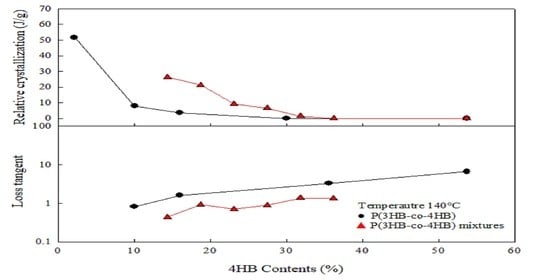
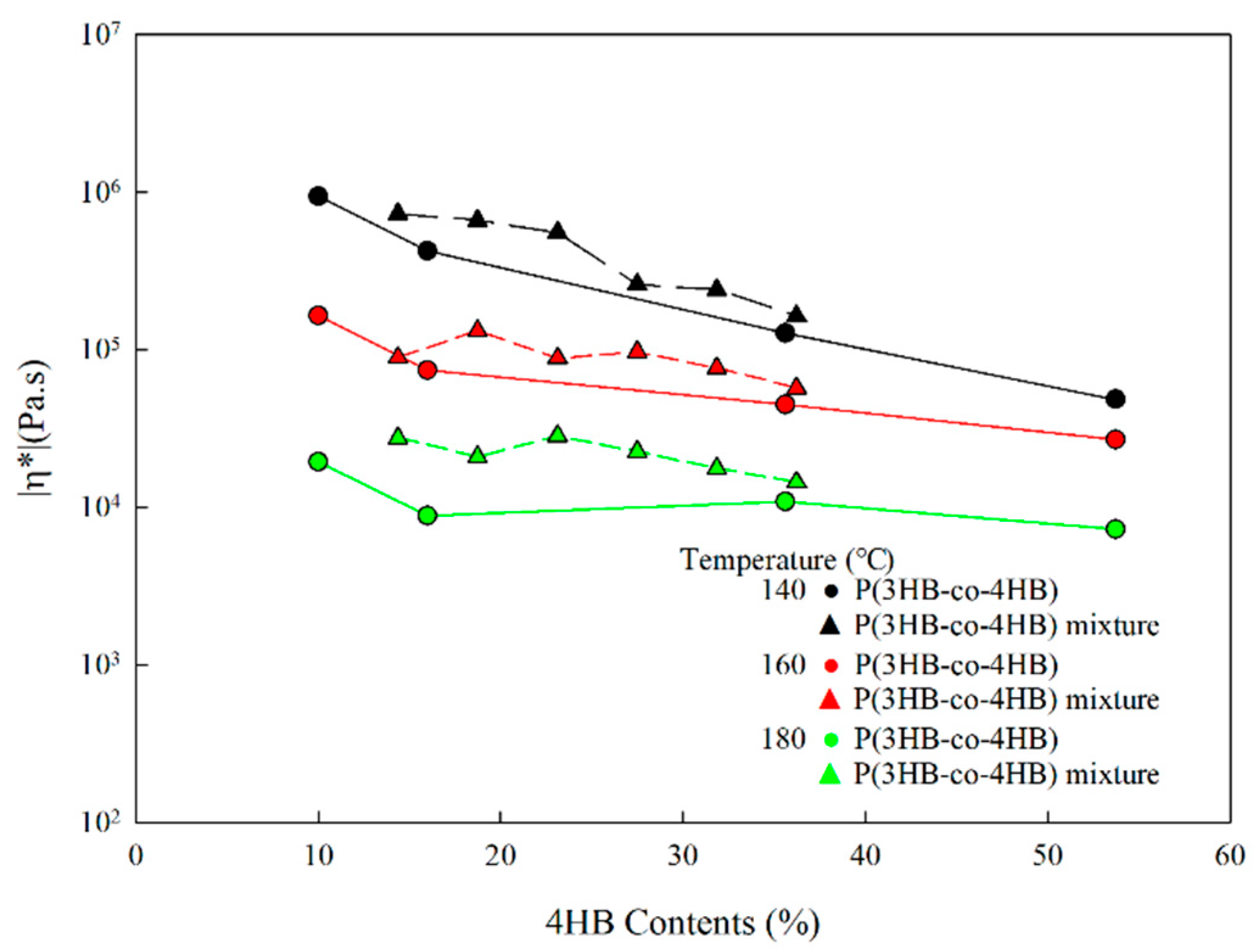
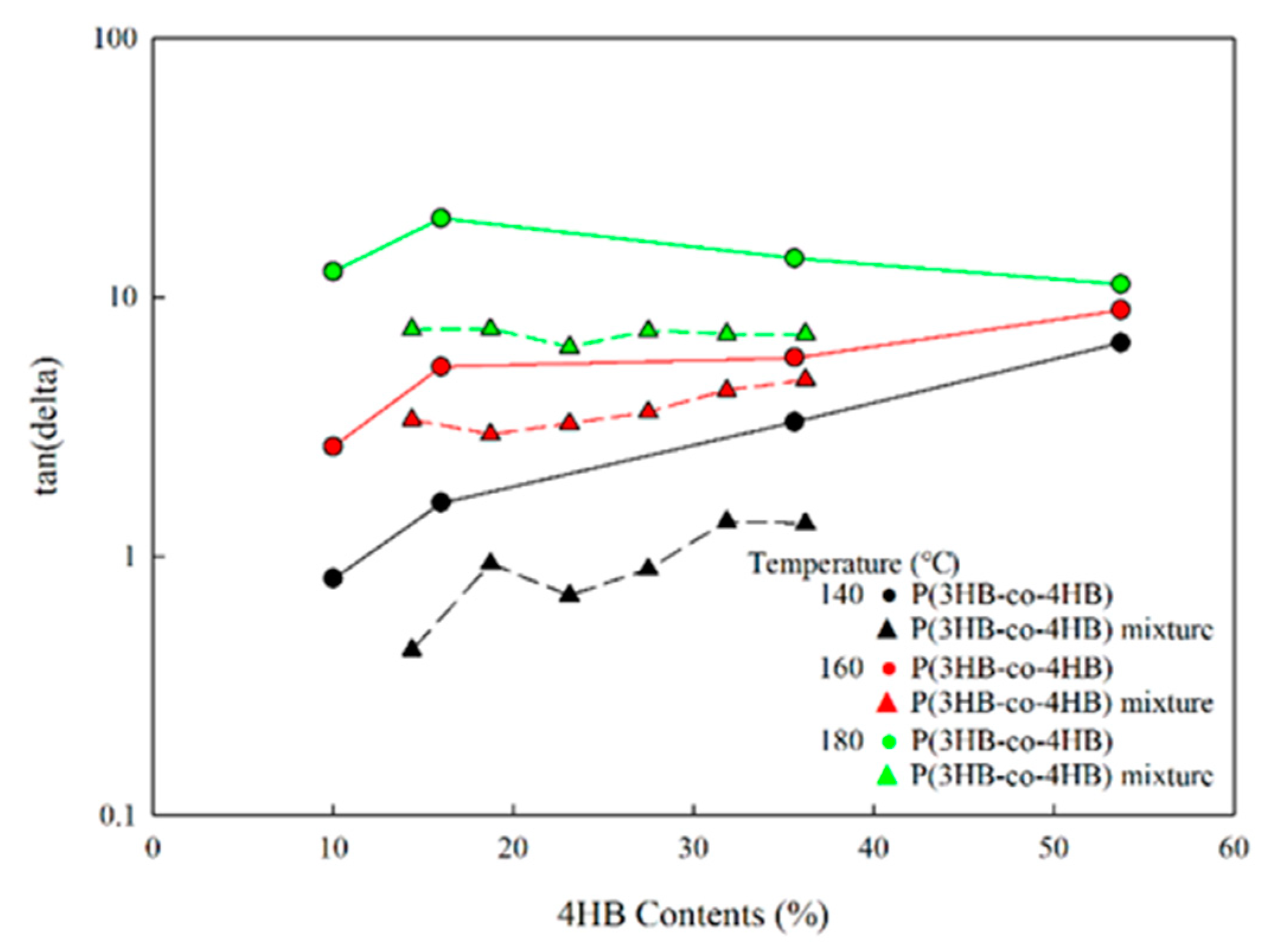
3.3. Rheological Properties of P(3HB-co-4HB) Mixtures
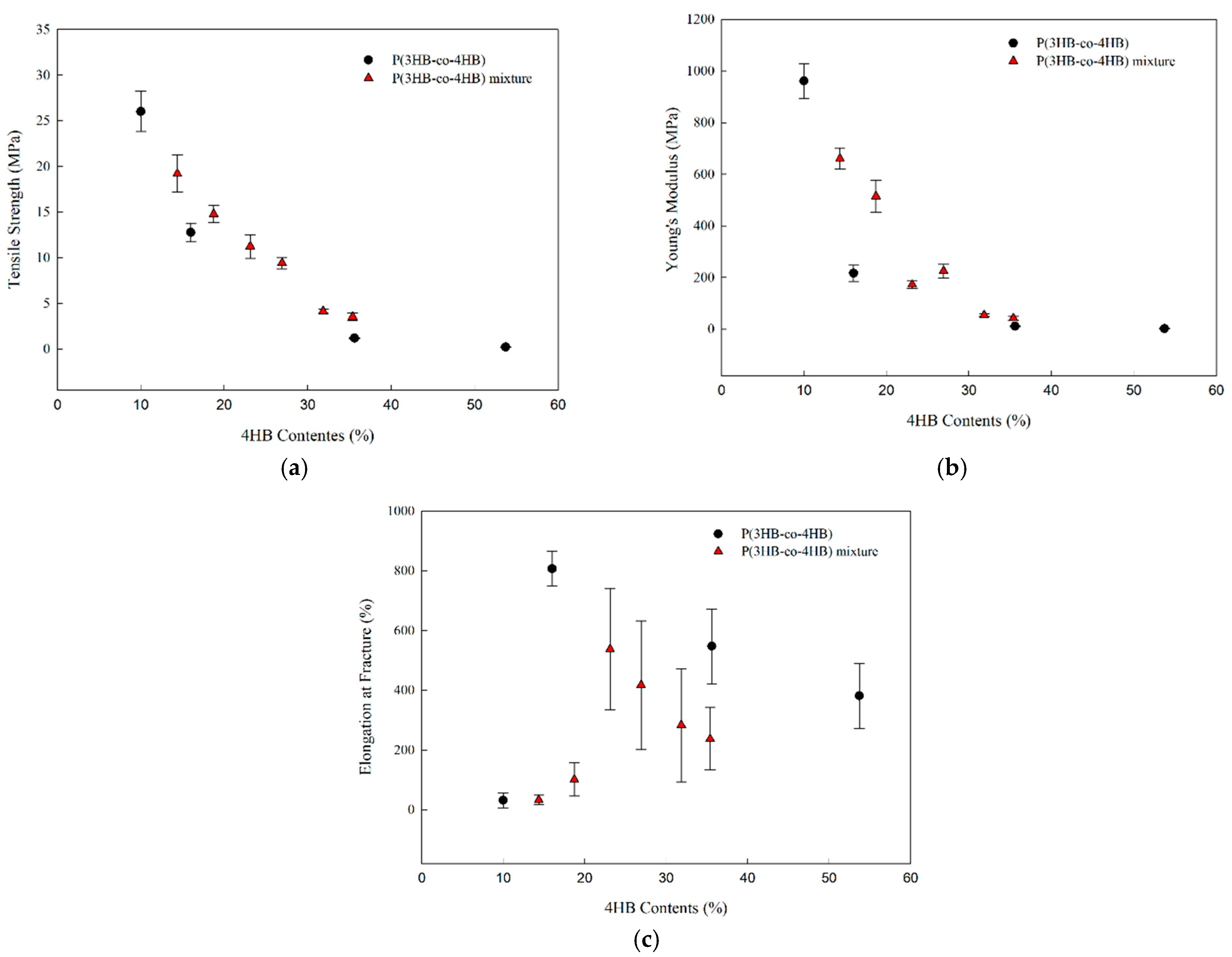
3.4. Mechanical Properties of P(3HB-co-4HB) Mixtures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanesawa, Y.; Tanahashi, N.; Doi, Y. Enzymatic degradation of microbial poly(3-hydroxyalkanoates). Polym. Degrad. Stab. 1994, 45, 179–185. [Google Scholar] [CrossRef]
- Chen, G.-Q. (Ed.) Introduction of bacterial plastics PHA, PLA, PBS, PE, PTT, and PPP. In Plastics from Bacteria: Natural Functions and Applications; Springer: Berlin/Heidelberg, Germany, 2010; Volume 14, pp. 1–16. [Google Scholar]
- Norhafini, H.; Huong, K.H.; Amirul, A.A. High PHA density fed-batch cultivation strategies for 4HB-rich P(3HB-co-4HB) copolymer production by transformant Cupriavidus malaysiensis USMAA1020. Int. J. Biol. Macromol. 2019, 125, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Chanprateep, S.; Buasri, K.; Muangwong, A.P. Utiswannakul, Biosynthesis and biocompatibility of biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Polym. Degrad. Stab. 2010, 95, 2003–2012. [Google Scholar] [CrossRef]
- Chen, G.Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.Y.; Chee, J.Y.; Sudesh, K. Degradation of Polyhydroxyalkanoate (PHA): A Review. J. Sib. Fed. Univ. Biol. 2017, 10, 211–225. [Google Scholar] [CrossRef]
- Raquez, J.M.; Habili, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Liu, L.F.; Yu, J.Y.; Cheng, L.D.; Yang, X.J. Biodegradability of poly(butylene succinate) (PBS) composite reinforced with jute fibre. Polym. Degrad. Stab. 2009, 94, 90–94. [Google Scholar] [CrossRef]
- Xu, D.J.; Guo, B.H. Poly(butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- El-Hadi, A.; Schnabel, R.; Straube, E.; Müller, G.; Henning, S. Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly(3-hydroxyalkanoate) PHAs and their blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Erich, M.; Hannes, G.; Maximilian, L. PHB-bio based and biodegradable replacement for PP: A Review. Nov. Tech. Nutr. Food Sci. 2018, 2, 206–209. [Google Scholar]
- Savenkova, L.; Gercberga, Z.; Nikolaeva, V.; Dzene, A.; Bibers, I.; Kalnin, M. Mechanical properties and biodegradation characteristics of PHB-based films. Process. Biochem. 2000, 35, 573–579. [Google Scholar] [CrossRef]
- Patricia, P.S.O.; Pereira, F.V.; Santos, M.C.; Souza, P.P.; Roa, J.P.B.; Orefice, R.L. Increasing the elongation at break of polyhydroxybutyrate biopolymer: Effect of cellulose nanowhiskers on mechanical and thermal properties. J. Appl. Polym. Sci. 2013, 127, 3613–3621. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Popa, M.S.; Raditoiu, V.; Frone, A.N.; Sacarescu, L.; Gabor, A.R.; Teodorescu, M. Effect of calcium stearate as a lubricant and catalyst on the thermal degradation of poly (3-hydroxybutyrate). Int. J. Biol. Macromol. 2021, 190, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Pachekoski, W.M.; Dalmolin, C.; Agnelli, J.A.M. The Influence of the Industrial Processing on the Degradation of Poly(hidroxybutyrate)—PHB. Macromol. Res. 2013, 16, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Koyama, N.; Doi, Y. Morphology and biodegradability of a binary blend of poly ((R)-3-hydroxybutyric acid) and poly ((R, S)-lactic acid). Can. J. Microbiol. 1995, 41, 316–322. [Google Scholar] [CrossRef]
- Wang, Y.X.; Huang, G.C.; Luo, L.Q. Thermal Properties of Poly(Lactic Acid) Modified Poly(3-Hydroxybutyrate-co-4-Hydroxbutyrate). Mater. Sci. Forum 2016, 852, 677–685. [Google Scholar]
- Kennouche, S.; Moigne, N.L.; Kaci, M.; Quantin, J.C.; Caro-Bretelle, A.S.; Delaite, C.; Lopez-Cuesta, J.M. Morphological characterization and thermal properties of compatibilized poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/poly(butylene succinate) (PBS)/halloysite ternary nanocomposites. Eur. Polym. J. 2016, 75, 142–162. [Google Scholar] [CrossRef]
- Bian, Y.; Han, L.; Han, C.; Lin, H.; Zhang, H.; Bian, J.; Dong, L. Intriguing crystallization behavior and rheological properties of radical-based crosslinked biodegradable poly (3-hydroxybutyrate-co-4-hydroxybutyrate). CrystEngComm 2014, 16, 2702–2714. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.Q.; Xu, J.; Wei, D.S.; Zhu, H.W.; Li, D. Thermal property, morphology, mechanical and rheological properties of a modified bio-polymers prepared by blending poly (3-hydrobutyrate-co-4-hydrobutyrate) with chain extenders. Open J. Adv. Mater. Res. 2011, 152, 924–930. [Google Scholar] [CrossRef]
- Xiang, H.; Chen, Z.; Zheng, N.; Zhang, X.; Zhu, L.; Zhou, Z.; Zhu, M. Melt-spun microbial poly (3-hydroxybutyrate-co-3-hydroxyvalerate) fibers with enhanced toughness: Synergistic effect of heterogeneous nucleation, long-chain branching and drawing process. Int. J. Biol. Macromol. 2019, 122, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.; Chen, C.; Chen, S.; Peng, S.; Zhuang, Y.; An, Y.; Dong, L. Crosslinking of poly [(3-hydroxybutyrate)-co-(3-hydroxyvalerate)] using dicumyl peroxide as initiator. Polym. Int. 2004, 53, 937–943. [Google Scholar] [CrossRef]
- Rupp, B.; Ebner, C.; Rossegger, E.; Slugovc, C.; Stelzer, F.; Wiesbrock, F. UV-induced crosslinking of the biopolyester poly (3-hydroxybutyrate)-co-(3-hydroxyvalerate). Curr. Green Chem. 2010, 12, 1796–1802. [Google Scholar] [CrossRef]
- Bhubalan, K.; Lee, W.H.; Loo, C.Y.; Yamamoto, T.; Tsuge, T.; Doi, Y.; Sudesh, K. Controlled biosynthesis and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) from mixtures of palm kernel oil and 3HV-precursors. Polym. Degrad. Stab. 2008, 93, 17–23. [Google Scholar] [CrossRef]
- Loo, C.Y.; Sudesh, K. Biosynthesis and native granule characteristics of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) in Delftia acidovorans. Int. J. Biol. Macromol. 2007, 40, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, G.; Zhou, X.R.; Chen, G.Q. Biosynthesis of poly (3-hydroxydecanoate) and 3-hydroxydodecanoate dominating polyhydroxyalkanoates by β-oxidation pathway inhibited Pseudomonas putida. Metab. Eng. 2011, 13, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, B.H.; Kim, B.S. Production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) by Ralstonia eutropha. Biochem. Eng. J. 2005, 23, 169–174. [Google Scholar] [CrossRef]
- Saito, Y.; Nakamura, S.; Hiramitsu, M.; Doi, Y. Microbial synthesis and properties of poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Polym. Int. 1996, 39, 169–174. [Google Scholar] [CrossRef]
- Valentin, H.E.; Dennis, D. Production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) in recombinant Escherichia coli grown on glucose. J. Biotechnol. 1997, 58, 33–38. [Google Scholar] [CrossRef]
- Al-Kaddo, K.B.; Mohamad, F.; Murugan, P.; Tan, J.S.; Sudesh, K.; Samian, M.R. Production of P(3HB-co-4HB) copolymer with high 4HB molar fraction by Burkholderia contaminans Kad1 PHA synthase. Biochem. Eng. J. 2020, 153, 107394–107400. [Google Scholar] [CrossRef]
- Yun, O.Y.; Min, X.; Li, Y. Properties analysis of biodegradable material P(3HB-co-4HB). Open J. Adv. Mater. Res. 2011, 380, 168–172. [Google Scholar]
- Wen, X.; Lu, X.; Peng, Q.; Zhu, F.; Zheng, N. Crystallization behaviors and morphology of biodegradablepoly(3-hydroxybutyrate-co-4-hydroxybutyrate). J. Therm. Anal. Calorim. 2012, 109, 959–966. [Google Scholar] [CrossRef]
- Omura, T.; Gato, T.; Maehara, A.; Kimura, S.; Abe, H.; Iwata, T. Thermal degradation behavior of poly [(R)-3-hydroxybutyrate-co-4-hydroxybutyrate]. Polym. Degrad. Stab. 2021, 183, 109460. [Google Scholar] [CrossRef]
- Doi, Y.; Segawa, A.; Kunioka, M. Biosynthesis and characterization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in Alcaligenes eutrophus. Int. J. Biol. Macromol. 1990, 12, 106–111. [Google Scholar] [CrossRef]
- Yang, S.Z.; Madbouly, S.A.; Schrader, J.A.; Grewell, D.; Kessler, M.R.; Graves, W.R. Processing and characterization of bio-based poly (hydroxyalkanoate)/poly(amide) blends: Improved flexibility and impact resistance of PHA-based plastics. J. Appl. Polym. Sci. 2015, 132, 42209–42218. [Google Scholar] [CrossRef] [Green Version]
- Harrison, G.M.; Melik, D.H. Application of degradation kinetics to the rheology of poly(hydroxyalkanoates). J. Appl. Polym. Sci. 2006, 102, 1794–1802. [Google Scholar] [CrossRef]





| Name | 4HB (%) | MW (k) |
|---|---|---|
| P(3HB-co-2.1% 4HB) | 2.1 | 200 |
| P(3HB-co-10% 4HB) | 10 | 600 |
| P(3HB-co-16% 4HB) | 16 | 1000 |
| P(3HB-co-35.6% 4HB) | 35.6 | 580 |
| P(3HB-co-53.7% 4HB) | 53.7 | 695 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, M.; Jang, Y.; Lee, E.; Shin, S.; Kang, H.-J. The Modification of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Melt Blending. Polymers 2022, 14, 1725. https://doi.org/10.3390/polym14091725
Jo M, Jang Y, Lee E, Shin S, Kang H-J. The Modification of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Melt Blending. Polymers. 2022; 14(9):1725. https://doi.org/10.3390/polym14091725
Chicago/Turabian StyleJo, Minki, Yunjae Jang, Eunhye Lee, Sooan Shin, and Ho-Jong Kang. 2022. "The Modification of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Melt Blending" Polymers 14, no. 9: 1725. https://doi.org/10.3390/polym14091725
APA StyleJo, M., Jang, Y., Lee, E., Shin, S., & Kang, H.-J. (2022). The Modification of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Melt Blending. Polymers, 14(9), 1725. https://doi.org/10.3390/polym14091725