Adsorption Mechanism of Chloropropanol by Crystalline Nanocellulose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Configuration
2.3. QCM Detection
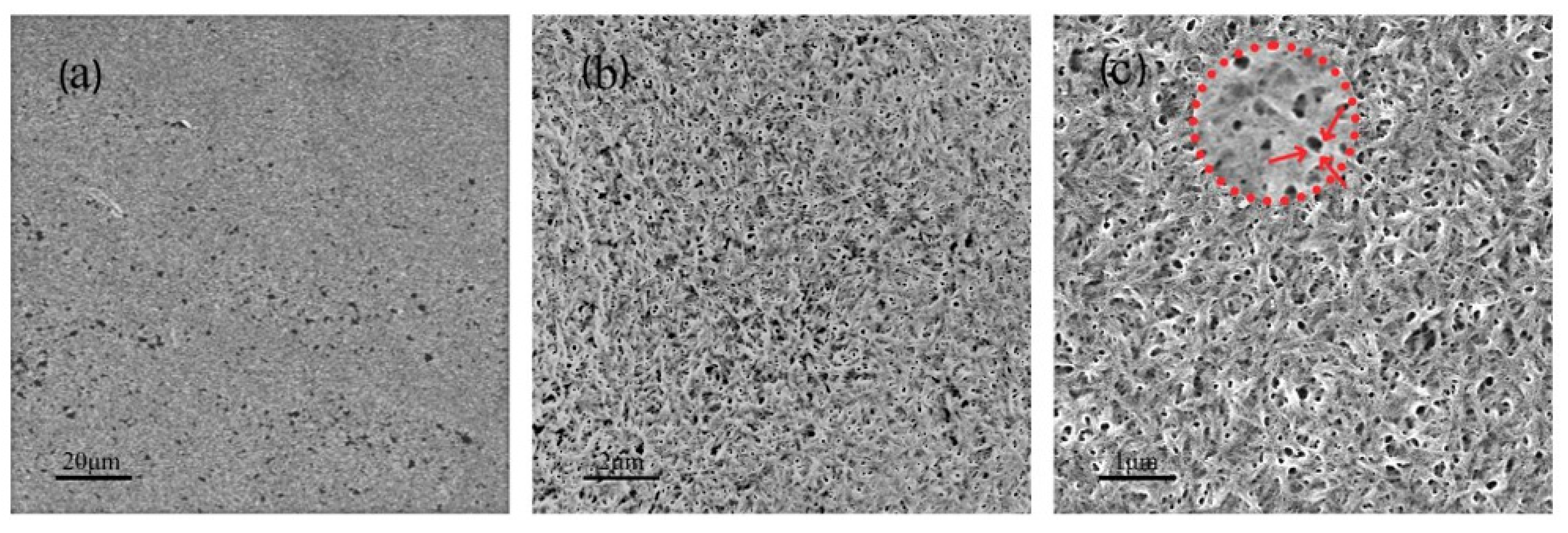
2.4. SEM Characterization
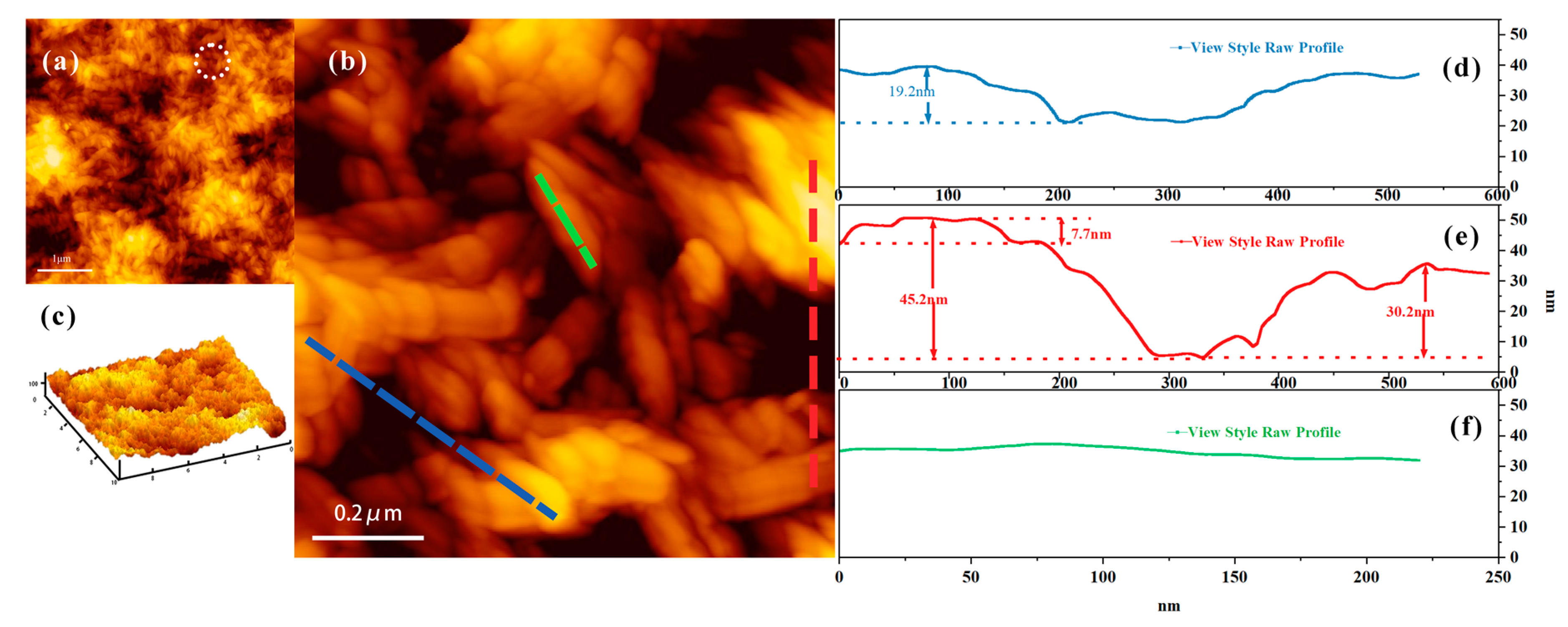
2.5. AFM Characterization
2.6. Zeta Potential Measurement
3. Results and Discussion
3.1. Surface Analysis before and after CNC Adsorption
3.2. Effect of Chloropropanol Concentration on the Adsorption Capacity of CNC
3.3. Effect of CNC Dosage pH on the Adsorption Capacity
3.4. Effect of pH on the Adsorption Capacity
3.5. Adsorption Kinetics
3.6. Saturated Adsorption Capacity of Chloropropanol by CNC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Taherimehr, M.; YousefniaPasha, H.; Tabatabaeekoloor, R.; Pesaranhajiabbas, E. Trends and challenges of biopolymer-based nanocomposites in food packaging. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5321–5344. [Google Scholar] [CrossRef] [PubMed]
- Humbert, S.; Rossi, V.; Margni, M.; Jolliet, O.; Loerincik, Y. Life cycle assessment of two baby food packaging alternatives: Glass jars vs. plastic pots. Int. J. Life Cycle Assess. 2009, 14, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wu, Y.; Han, Y.; Qin, C.; Nie, S.; Liu, S.; Wang, S.; Yao, S. Effect of hydrothermal pretreatment on the demineralization and thermal degradation behavior of eucalyptus. Bioresour. Technol. 2020, 307, 123246. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic Bionanocomposites: A Review of Preparation, Properties and Applications. Polymers 2010, 2, 728. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.J.; Haugaard, V.; Festersen, R.; Bertelsen, G. Production and applications of biobased packaging materials for the food industry. Food Addit. Contam. 2002, 19, 172–177. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food packaging-Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Yao, S.; Nie, S.; Zhu, H.; Wang, S.; Song, X.; Qin, C. Extraction of hemicellulose by hot water to reduce adsorbable organic halogen formation in chlorine dioxide bleaching of bagasse pulp. Ind. Crops Prod. 2017, 96, 178–185. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Y.; Cao, L.; Zhu, J.; Deng, B.; Hou, Y.; Liang, C.; Huang, C.; Qin, C.; Yao, S. High efficiency and clean separation of eucalyptus components by glycolic acid pretreatment. Bioresour. Technol. 2021, 341, 125757. [Google Scholar] [CrossRef] [PubMed]
- Schilter, B.; Scholz, G.; Seefelder, W. Fatty acid esters of chloropropanols and related compounds in food: Toxicological aspects. Eur. J. Lipid Sci. Technol. 2011, 113, 309–313. [Google Scholar] [CrossRef]
- Mezouari, S.; Liu, W.Y.; Pace, G.; Hartman, T.G. Development and validation of an improved method for the determination of chloropropanols in paperboard food packaging by GC-MS. Food Addit. Contam. Part. A-Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 768–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, G.V.; Hartman, T.G. Migration studies of 3-chloro-1,2-propanediol (3-MCPD) in polyethylene extrusion-coated paperboard food packaging. Food Addit. Contam. Part. A-Chem. Anal. Control. Expo. Risk Assess. 2010, 27, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Korte, R.; Schulz, S.; Brauer, B. Chloropropanols (3-MCPD, 1,3-DCP) from food contact materials: GC-MS method improvement, market survey and investigations on the effect of hot water extraction. Food Addit. Contam. Part. A-Chem. Anal. Control. Expo. Risk Assess. 2021, 38, 904–913. [Google Scholar] [CrossRef]
- Koljonen, K.; Mustranta, A.; Stenius, P. Surface characterisation of mechanical pulps by polyelectrolyte adsorption. Nord. Pulp Pap. Res. J. 2004, 19, 495–505. [Google Scholar] [CrossRef]
- Avitsland, G.A.; Wagberg, L. Flow resistance of wet and dry sheets used for preparation of liquid packaging board. Nord. Pulp Pap. Res. J. 2005, 20, 345–353. [Google Scholar] [CrossRef]
- Dong, C.; Wang, B.; Meng, Y.; Pang, Z. Preparation, structural changes and adsorption performance of heavy metal ions on sulfonated cellulose with varying degrees of substitution. Holzforschung 2019, 73, 501–507. [Google Scholar] [CrossRef]
- De Nino, A.; Tallarida, M.A.; Algieri, V.; Olivito, F.; Costanzo, P.; De Filpo, G.; Maiuolo, L. Sulfonated Cellulose-Based Magnetic Composite as Useful Media for Water Remediation from Amine Pollutants. Appl. Sci.-Basel 2020, 10, 8155. [Google Scholar] [CrossRef]
- Tang, F.; Yu, H.; Abdalkarim, S.Y.H.; Sun, J.; Fan, X.; Li, Y.; Zhou, Y.; Tam, K.C. Green acid-free hydrolysis of wasted pomelo peel to produce carboxylated cellulose nanofibers with super absorption/flocculation ability for environmental remediation materials. Chem. Eng. J. 2020, 395, 125070. [Google Scholar] [CrossRef]
- Ahola, S.; Osterberg, M.; Laine, J. Cellulose nanofibrils-adsorption with poly(amideamine) epichlorohydrin studied by QCM-D and application as a paper strength additive. Cellulose 2008, 15, 303–314. [Google Scholar] [CrossRef]
- Marx, K.A. Quartz crystal microbalance: A useful tool for studying thin polymer films and complex biomolecular systems at the solution-surface interface. Biomacromolecules 2003, 4, 1099–1120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhu, Y.; Wang, X.; Wang, P.; Tian, J.; Zhu, W.; Song, J.; Xiao, H. Revealing Adsorption Behaviors of Amphoteric Polyacrylamide on Cellulose Fibers and Impact on Dry Strength of Fiber Networks. Polymers 2019, 11, 1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.; Zhang, Y.; Wang, P.; Wu, S.; Jin, Y.; Song, J. Comparison of the interactions between fungal cellulases from different origins and cellulose nanocrystal substrates with different polymorphs. Cellulose 2018, 25, 1185–1195. [Google Scholar] [CrossRef]
- Larsson, C.; Rodahl, M.; Hook, F. Characterization of DNA immobilization and subsequent hybridization on a 2D arrangement of streptavidin on a biotin-modified lipid bilayer supported on SiO2. Anal. Chem. 2003, 75, 5080–5087. [Google Scholar] [CrossRef]
- Julien, F.; Baudu, M.; Mazet, M. Relationship between chemical and physical surface properties of activated carbon. Water Res. 1998, 32, 3414–3424. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Bai, L.; Tian, W.; Zhang, L. Research on the Porous Structures and Properties of Composite Membranes of Polysulfone and Nanocrystalline Cellulose. In Proceedings of the 7th International Forum on Advanced Material Science and Technology, Dalian, China, 26–28 June 2011; pp. 391–394. [Google Scholar]
- Soni, B.; Hassan, E.B.; Mahmoud, B. Chemical isolation and characterization of different cellulose nanofibers from cotton stalks. Carbohydr. Polym. 2015, 134, 581–589. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, H.; Pang, Z.; Liu, Y.; Zhang, F. Sulfonated modification of cotton linter and its application as adsorbent for high-efficiency removal of lead(II) in effluent. Bioresour. Technol. 2013, 146, 512–518. [Google Scholar] [CrossRef]
- Barnea, O.; Gillon, G. Cavernosometry: A theoretical analysis. Int. J. Impot. Res. 2004, 16, 154–159. [Google Scholar] [CrossRef] [Green Version]





| Carboxylated CNC | Sulfonated CNC | ||
|---|---|---|---|
| pH | Chloropropanol ng/CNC ng | pH | Chloropropanol ng/CNC ng |
| 5.2 | 7 × 10−2 | 5.7 | 8.0 × 10−2 |
| 7.3 | 3 × 10−1 | 7.3 | 4.0 × 10−1 |
| 9.0 | 4 × 10−1 | 8.3 | 3.6 × 10−1 |
| 10.3 | 2 × 10−1 | 9.3 | 1.4 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Gong, Z.; Chen, C.; Liang, C.; Huang, L.; Huang, M.; Qin, C.; Wang, S. Adsorption Mechanism of Chloropropanol by Crystalline Nanocellulose. Polymers 2022, 14, 1746. https://doi.org/10.3390/polym14091746
Zhao J, Gong Z, Chen C, Liang C, Huang L, Huang M, Qin C, Wang S. Adsorption Mechanism of Chloropropanol by Crystalline Nanocellulose. Polymers. 2022; 14(9):1746. https://doi.org/10.3390/polym14091746
Chicago/Turabian StyleZhao, Jinwei, Zhiqiang Gong, Can Chen, Chen Liang, Lin Huang, Meijiao Huang, Chengrong Qin, and Shuangfei Wang. 2022. "Adsorption Mechanism of Chloropropanol by Crystalline Nanocellulose" Polymers 14, no. 9: 1746. https://doi.org/10.3390/polym14091746







