Improvement of Oil and Water Barrier Properties of Food Packaging Paper by Coating with Microcrystalline Wax Emulsion
Abstract
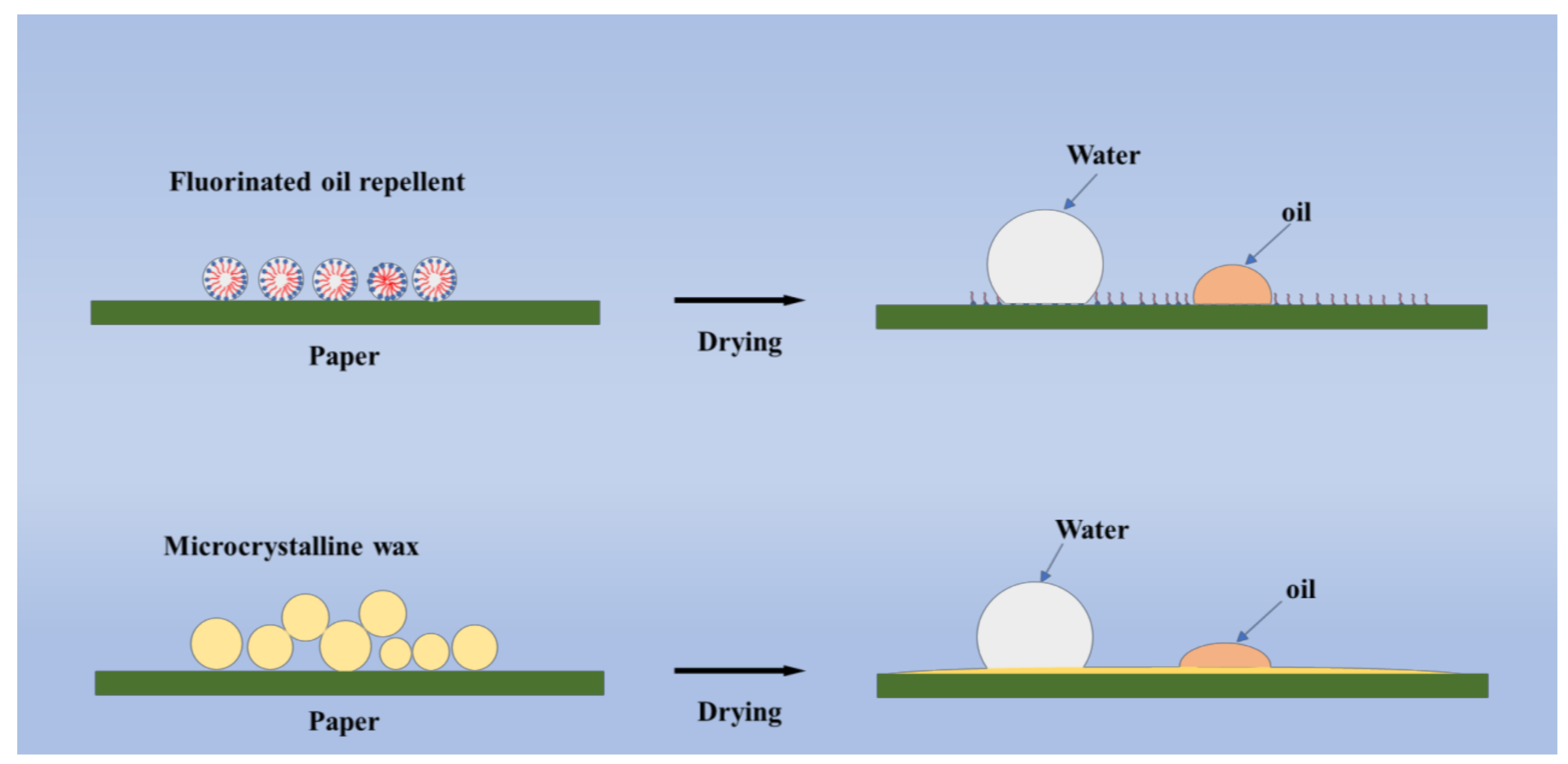
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Preparation of Microcrystalline Wax Emulsion
2.2.2. Original Paper Pretreatment
2.2.3. Microcrystalline Wax Emulsion Surface Coating
2.2.4. Emulsion Property Testing
2.2.5. Oil and Water Resistance Testing of Paper
2.2.6. Scanning Electron Microscopy (SEM)
2.2.7. Water Vapor Permeability (WVP)
2.2.8. The Overall Migration of Paper
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Factors Influencing the Preparation of Microcrystalline Wax Emulsions
- (a)
- Emulsifier ratio
- (b)
- Emulsifier dosage
3.2. Microcrystalline Wax Coating
3.3. Paper Pretreatment
3.4. Surface Morphology of the Paper
3.5. Water Vapor Permeability (WVP) of the Paper
3.6. The Overall Migration of Paper
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Boban, M.; Snyder, S.A.; Kobaku, S.P.R.; Kwon, G.; Mehta, G.; Tuteja, A. Paper-Based Surfaces with Extreme Wettabilities for Novel, Open-Channel Microfluidic Devices. Adv. Funct. Mater. 2016, 26, 6121–6131. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Tuteja, A. The design and applications of superomniphobic surfaces. NPG Asia Mater. 2014, 6, e109. [Google Scholar] [CrossRef] [Green Version]
- Trombetta, T.; Iengo, P.; Turri, S. Fluorinated segmented polyurethane anionomers for water-oil repellent surface treatments of cellulosic substrates. J. Appl. Polym. Sci. 2005, 98, 1364–1372. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, G.; Wang, Q.; Zhang, Q.; Zhan, X.; Chen, F. Novel Fluorinated Polymers Containing Short Perfluorobutyl Side Chains and Their Super Wetting Performance on Diverse Substrates. ACS Appl. Mater. Interfaces 2016, 8, 10513–10523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, J.; Luo, Z. Toward efficient water/oil separation material: Effect of copolymer composition on pH-responsive wettability and separation performance. AIChE J. 2016, 62, 1758–1771. [Google Scholar] [CrossRef]
- Nair, A.; Kansal, D.; Khan, A.; Rabnawaz, M. Oil- and water-resistant paper substrate using blends of chitosan-graft-polydimethylsiloxane and poly(vinyl alcohol). J. Appl. Polym. Sci. 2021, 138, 50494. [Google Scholar] [CrossRef]
- Dassuncao, C.; Hu, X.C.; Nielsen, F.; Weihe, P.; Grandjean, P.; Sunderland, E.M. Shifting Global Exposures to Poly- and Perfluoroalkyl Substances (PFASs) Evident in Longitudinal Birth Cohorts from a Seafood-Consuming Population. Environ. Sci. Technol. 2018, 52, 3738–3747. [Google Scholar] [CrossRef]
- Begley, T.H.; Hsu, W.; Noonan, G.; Diachenko, G. Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Addit. Contam. Part A 2008, 25, 384–390. [Google Scholar] [CrossRef]
- Gong, X.; Yang, C.; Hong, Y.; Chung, A.C.; Cai, Z. PFOA and PFOS promote diabetic renal injury in vitro by impairing the metabolisms of amino acids and purines. Sci. Total Environ. 2019, 676, 72–86. [Google Scholar] [CrossRef]
- Guillaume, C.; Pinte, J.; Gontard, N.; Gastaldi, E. Wheat gluten-coated papers for bio-based food packaging: Structure, surface and transfer properties. Food Res. Int. 2010, 43, 1395–1401. [Google Scholar] [CrossRef]
- Khwaldia, K.; Arab-Tehrany, E.; Desobry, S. Biopolymer Coatings on Paper Packaging Materials. Compr. Rev. Food Sci. Food Saf. 2010, 9, 82–91. [Google Scholar] [CrossRef]
- Nechita, P.; Roman, M. Review on Polysaccharides Used in Coatings for Food Packaging Papers. Coatings 2020, 10, 566. [Google Scholar] [CrossRef]
- Wang, F.-J.; Wang, L.-Q.; Zhang, X.-C.; Ma, S.-F.; Zhao, Z.-C. Enhancement of oil resistance of cellulose packaging paper for food application by coating with materials derived from natural polymers. J. Food Eng. 2022, 111039. [Google Scholar] [CrossRef]
- Xie, J.; Xu, J.; Cheng, Z.; Chen, J.; Zhang, Z.; Chen, T.; Yang, R.; Sheng, J. Facile synthesis of fluorine-free cellulosic paper with excellent oil and grease resistance. Cellulose 2020, 27, 7009–7022. [Google Scholar] [CrossRef]
- De Freitas, C.A.S.; de Sousa, P.H.M.; Soares, D.J.; da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. Carnauba wax uses in food—A review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Negrete, D.; Mahn, A.; Sepúlveda, F. Design and evaluation of a heat exchanger that uses paraffin wax and recycled materials as solar energy accumulator. Energy Convers. Manag. 2014, 88, 391–398. [Google Scholar] [CrossRef]
- Saji, V.S. Wax-based artificial superhydrophobic surfaces and coatings. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125132. [Google Scholar] [CrossRef]
- Han, J.; Salmieri, S.; Le Tien, C.; Lacroix, M. Improvement of Water Barrier Property of Paperboard by Coating Application with Biodegradable Polymers. J. Agric. Food Chem. 2010, 58, 3125–3131. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, G.; Long, Z.; Xiao, H.; Qian, L. Bio-Wax Latex-Modified Paper as Antimicrobial and Water-Vapor-Resistant Packaging Material. J. Wood Chem. Technol. 2015, 36, 182–191. [Google Scholar] [CrossRef]
- Khwaldia, K. Water vapor barrier and mechanical properties of paper-sodium caseinate and paper-sodium caseinate-paraffin wax films. J. Food Biochem. 2010, 34, 998–1013. [Google Scholar] [CrossRef]
- Naderizadeh, S.; Heredia-Guerrero, J.A.; Caputo, G.; Grasselli, S.; Malchiodi, A.; Athanassiou, A.; Bayer, I.S. Superhydrophobic Coatings from Beeswax-in-Water Emulsions with Latent Heat Storage Capability. Adv. Mater. Interfaces 2019, 6, 1801782. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, P.; Qian, L.; Xiao, H. Fabrication of superhydrophobic paper surface via wax mixture coating. Chem. Eng. J. 2014, 250, 431–436. [Google Scholar] [CrossRef]
- Kurniawan, M.; Subramanian, S.; Norrman, J.; Paso, K.G. Influence of Microcrystalline Wax on the Properties of Model Wax-Oil Gels. Energy Fuels 2018, 32, 5857–5867. [Google Scholar] [CrossRef]
- Gaur, G.; Sharma, Y. Preparation and Properties of Wax Emulsions, Wax Dispersion and Their Applications in Coatings. Int. Res. J. Eng. IT Sci. Res. 2018, 6, 118–124. [Google Scholar]
- Zhao, X.; Hu, T.; Zhang, J. Superhydrophobic coatings with high repellency to daily consumed liquid foods based on food grade waxes. J. Colloid Interface Sci. 2018, 515, 255–263. [Google Scholar] [CrossRef]
- EPoFAaNSatF (ANS). Scientific Opinion on the re-evaluation of microcrystalline wax (E 905) as a food additive. EFSA J. 2013, 11, 3146. [Google Scholar]
- Hasan, I.; Wang, J.; Tajvidi, M. Tuning physical, mechanical and barrier properties of cellulose nanofibril films through film drying techniques coupled with thermal compression. Cellulose 2021, 28, 11345–11366. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, H.; Xu, G.; Tan, Y.; Zhang, J.; Lv, X. Influence of CTAB and SDS on the properties of oil-in-water nano-emulsion with paraffin and span 20/Tween 20. Colloids Surf. A Physicochem. Eng. Asp. 2013, 418, 60–67. [Google Scholar] [CrossRef]
- Li, C.; Mei, Z.; Liu, Q.; Wang, J.; Xu, J.; Sun, D. Formation and properties of paraffin wax submicron emulsions prepared by the emulsion inversion point method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 356, 71–77. [Google Scholar] [CrossRef]
- Liu, W.; Sun, D.; Li, C.; Liu, Q.; Xu, J. Formation and stability of paraffin oil-in-water nano-emulsions prepared by the emulsion inversion point method. J. Colloid Interface Sci. 2006, 303, 557–563. [Google Scholar] [CrossRef]
- Vilasau, J.; Solans, C.; Gómez, M.; Dabrio, J.; Mújika-Garai, R.; Esquena, J. Influence of a mixed ionic/nonionic surfactant system and the emulsification process on the properties of paraffin emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 38–44. [Google Scholar] [CrossRef]
- Vilasau, J.; Solans, C.; Gómez, M.; Dabrio, J.; Mújika-Garai, R.; Esquena, J. Phase behaviour of a mixed ionic/nonionic surfactant system used to prepare stable oil-in-water paraffin emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 473–481. [Google Scholar] [CrossRef]
- Kansal, D.; Hamdani, S.S.; Ping, R.; Sirinakbumrung, N.; Rabnawaz, M. Food-Safe Chitosan–Zein Dual-Layer Coating for Water- and Oil-Repellent Paper Substrates. ACS Sustain. Chem. Eng. 2020, 8, 6887–6897. [Google Scholar] [CrossRef]
- Li, Z.; Rabnawaz, M.; Khan, B. Response Surface Methodology Design for Biobased and Sustainable Coatings for Water- and Oil-Resistant Paper. ACS Appl. Polym. Mater. 2020, 2, 1378–1387. [Google Scholar] [CrossRef]
- Kjellgren, H.; Engström, G. Influence of base paper on the barrier properties of chitosan-coated papers. Nord. Pulp Pap. Res. J. 2006, 21, 685–689. [Google Scholar] [CrossRef]
- Reis, A.B.; Yoshida, C.M.; Reis, A.P.C.; Franco, T.T. Application of chitosan emulsion as a coating on Kraft paper. Polym. Int. 2011, 60, 963–969. [Google Scholar] [CrossRef]
- Despond, S.; Espuche, E.; Cartier, N.; Domard, A. Barrier properties of paper–chitosan and paper–chitosan–carnauba wax films. J. Appl. Polym. Sci. 2005, 98, 704–710. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, H.; Qian, L. Enhanced water vapour barrier and grease resistance of paper bilayer-coated with chitosan and beeswax. Carbohydr. Polym. 2014, 101, 401–406. [Google Scholar] [CrossRef]





| w(Span-80):w(Tween-80) | Average Particle Size/nm | Stability | HLB Value |
|---|---|---|---|
| 3:7 | 401.9 ± 8.7 a | Stratified | 11.79 |
| 4:6 | 321.0 ± 6.5 b | Unstratified | 10.72 |
| 5:5 | 304.8 ± 5.4 bc | Unstratified | 9.65 |
| 6:4 | 316.4 ± 3.9 bcd | Unstratified | 8.58 |
| 7:3 | 369.6 ± 2.5 ae | Stratified | 7.51 |
| Emulsifier Dosage (Compared to Microcrystalline Wax)/wt% | Average Particle Size/nm | Stability | Viscosity /mPa·s |
|---|---|---|---|
| 10 | 402.9 ± 7.9 a | Stratified | 35.1 ± 0.7 a |
| 15 | 316.3 ± 2.1 b | Stratified | 29.7 ± 0.7 b |
| 20 | 304.8 ± 4.5 bc | Unstratified | 30.2 ± 0.4 bc |
| 25 | 297.2 ± 9.3 bcd | Unstratified | 33.5 ± 0.8 ad |
| 30 | 320.3 ± 1.6 bce | Unstratified | 48.0 ± 0.3 e |
| 35 | 336.1 ± 6.9 df | Unstratified | 57.5 ± 1.9 f |
| Microcrystalline Wax Coating Load (g/m2) | The Overall Migration of Unpretreated Paper (mg/dm2) | The Overall Migration of Pretreated Paper (mg/dm2) |
|---|---|---|
| 2 | 5 ± 0.8 a | 5.2 ± 0.4 a |
| 4 | 7.1 ± 1.1 ab | 7.5 ± 0.8 ab |
| 6 | 9.8 ± 0.5 bc | 9.4 ± 1.2 abc |
| 8 | 15.8 ± 2.7 d | 16.7 ± 1.8 d |
| 10 | 35.1 ± 5.4 e | 33.9 ± 4.7 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Duan, Y.; Wang, S.; Gong, M.; Dai, H. Improvement of Oil and Water Barrier Properties of Food Packaging Paper by Coating with Microcrystalline Wax Emulsion. Polymers 2022, 14, 1786. https://doi.org/10.3390/polym14091786
Liu D, Duan Y, Wang S, Gong M, Dai H. Improvement of Oil and Water Barrier Properties of Food Packaging Paper by Coating with Microcrystalline Wax Emulsion. Polymers. 2022; 14(9):1786. https://doi.org/10.3390/polym14091786
Chicago/Turabian StyleLiu, Dongyang, Yuqing Duan, Shumei Wang, Murong Gong, and Hongqi Dai. 2022. "Improvement of Oil and Water Barrier Properties of Food Packaging Paper by Coating with Microcrystalline Wax Emulsion" Polymers 14, no. 9: 1786. https://doi.org/10.3390/polym14091786
APA StyleLiu, D., Duan, Y., Wang, S., Gong, M., & Dai, H. (2022). Improvement of Oil and Water Barrier Properties of Food Packaging Paper by Coating with Microcrystalline Wax Emulsion. Polymers, 14(9), 1786. https://doi.org/10.3390/polym14091786






