Carbohydrate-Binding Modules of Potential Resources: Occurrence in Nature, Function, and Application in Fiber Recognition and Treatment
Abstract
:1. Introduction
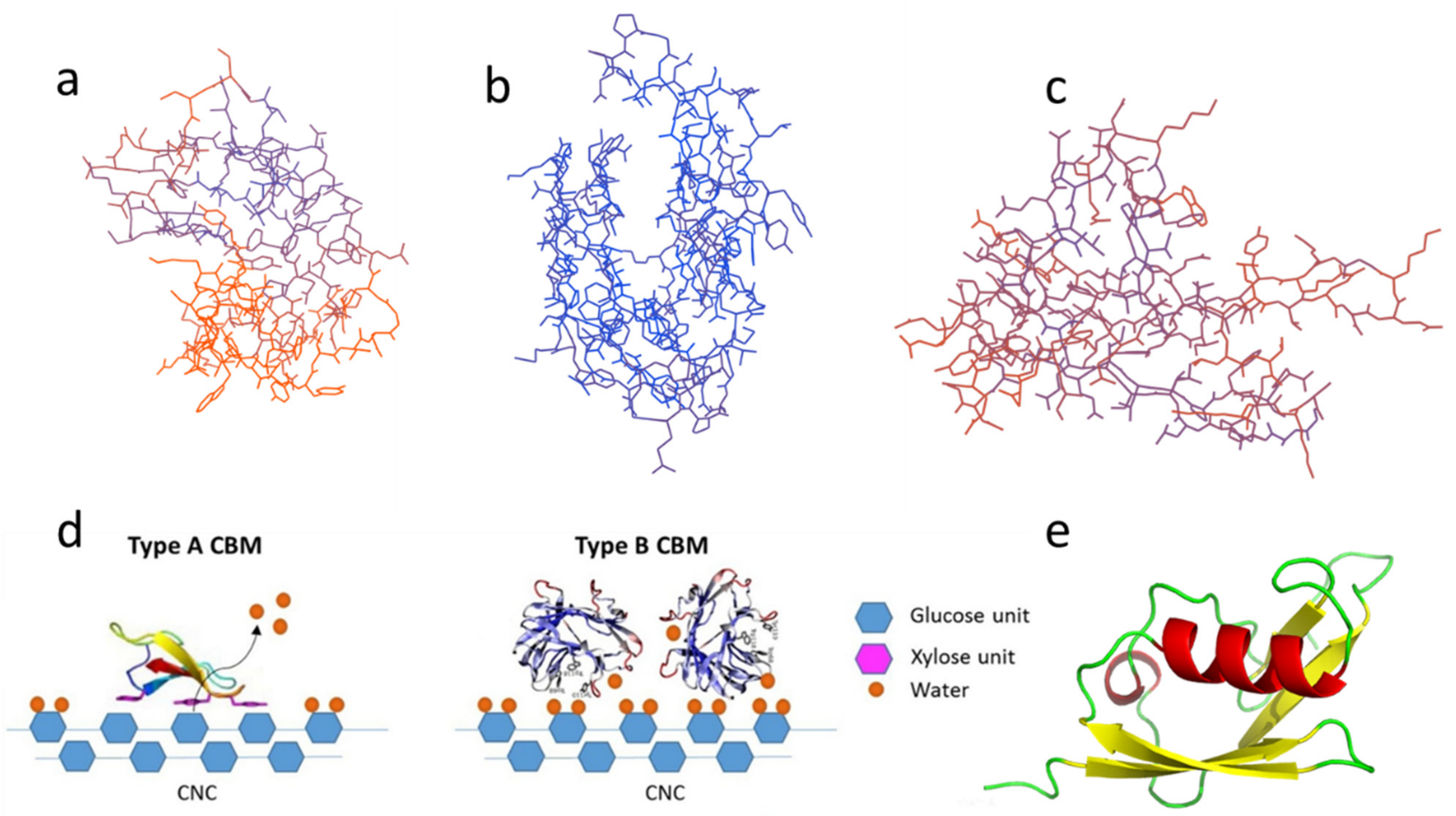
2. CBMs: Classification, Sources, Structures, and Functions
2.1. Type-A CBMs
2.2. Type-B CBMs
2.3. Type-C CBMs
2.4. Other Classification Methods
| CBM | Organism (Representative Example) | Common Ligands | Sequence | Gene Bank | Ref. | |
|---|---|---|---|---|---|---|
| A | 1 | Cel6A, Cel7A | Cellulose, hemicellulose, chitin | Cel6A: ACSSVWGQCGGQNWSGPTCCASGSTCVYSNDYYSQCL Cel7A: TQSHYGQCGGIGYSGPTVCASGTTCQVLNPYYSQCL | AAA34212.1 CAM98445.1 | [66] |
| 3 | A. thermocellum | Cellulose, chitin | TPTKGATPTNTATPTKSATATPTRPSVPTNTPTNTPANTP VSGNLKVEFYNSNPSDTTNSINPQFKVTNTGSSAIDLSKLTLRYYYTVDGQKDQTFWCDHAAIDLSKLTLRYYYTVDGQKDQTFW QFVEWDQVTAYLNGVLVWGKEHHHHHH | CAP78917.1 | [67] | |
| 10 | T. reesei. 7B | NM | MCNWYGSLTPLCVTTTSGWGYENGKSCV…CNWYGTLYPLCVTTQSGWGWWENSQSCIS | NM | [68] | |
| 20 | β-amylase, B. cereus | Starch, cyclodextrins | TPVMQTIVVKNVPTTIGDTVYITGNRAELGSWDTKQYPIQLYYDSHSNDWRGNVVLPAERNIEFKAFIKSKDGTVKSWQTIQQSWNPVPLKTTSHTSSW | BAA34650.1 | [62] | |
| B | 4 | Cellulase K Clostridium thermocellum | Xylan, β-1,3, glucan, β-1,3-1,4-glucan, β-1,6-glucan, amorphous cellulose | NDLLYERTFDEGLCYPWHTCEDSGGKCSFDVVDVPGQPGNKAFAVTVLDKGQNRWSVQMRHRGLTLEQGHTYRVRLKIWADASCKVYIKIGQMGEPYAEYW NNKWSPYTLTAGKVLEIDETFVM | ABN51650.1 | [69] |
| 11 | Endo-β-1,4- glucanase, C.fimi;xylanase, Rhodothermus Marinus; Laminarinase, Thermotoga maritima MSB8 | Xylan, β-1,3-glucan, β-1,3-1,4-glucan, β-1,6-glucan and amorphous cellulose | YGEQLIEDFEGAMQWAAYSGVDATASCKISSGKSNNGLEITYAGSSNGYWGVVDNEHRNQDWEKWQ KISFDIKSSNTNEVRLLIAEQSKIEGEDGEHWTYVIKPSTSWTTIEIPFSSFTKRMDYQPPAQDGSETFD LYKVGSLHFMYSNSNSGTLNIDNIKLIGL | ACL75216.1 | [47,59] | |
| 17 | Endo-β-1,4-glucanase, C. cellulovorans | Amorphous cellulose, oligosaccharides | ATPIVQLLRNKGNENLIIVGNPFWSQRPDLAADNPINDSNTMYSVHFYSGTNPISTVDTNRDNAMSNVRYALNHGAAVFATEWGTSLATGTTGPYLAKADAWLDFLNGNNISWCNFSISNKDEKAAALNSLTSLDPGSDKLWADNELTTSGQYVRARIKGAYYATPVDPVTNQPTAPKDFSSGFWDFNDGTTQGFGVNPDSPITAINVENANNALKISNLNSKGSNDLSEGNFWANVRISADIWGQSINIYGDTKLTMDVIAPTPVNVSIAAIPQSSTHGWGNPTRAIRVWTNNFVAQTDGTYKATLTISTNDSPNFNTIATDAADSVVTNMILFVGSNSDNISLDNIKFTK | AAB40891.1 | [70] | |
| 44 | Endoglucanase J. Clostridium thermocellum | Cellulose, xyloglucan, β-glucan, lichenan | SRWKEVKFEKGAPFSLTPDTEDDYVYMDEFVNYLVNKYGNASTPTGIKGYSIDNEPALWSHTHPRIHPDNVTAKELIEKSVALSKAVKKVDPYAEIFGPALYGFAAYETLQSAPDWGTEGEGYRWFIDYYLDKMKKASDEEGKRLLDVLDVHWYPEA | BAA12070.1 | [45,71] | |
| 9 | Xylanase A, T.maritima MSB8 | Glucose, cellobiose | 56-166: SFEGTTEGVVPFGKDVVLTASQDVAADGEYSLKVENRTSPWDGVEIDLTGKVKSGADYLLSFQVYQSSDAPQLFNVVARTEDEKGERYDVILDKVVVSDHWKEILVPFSPT 205-339: VIYETSFENGVGDWQPRGDVNIEASSEVAHSGKSSLFISNRQKGWQGAQINLKGILKTGKTYAFEAWVYQNSGQDQTIIMTMQRKYSSDASTQYEWIKSATVPSGQWVQLSGTYTIPAGVTVEDLTLYFESQNPT | AAD35155.1 | [3] | |
| C | 13 | Actinohivin, Actinomycete K97; Xylanase 10A, S.lividans | α (1-2)mannobiose/lactose, galactose | ASVTIRNAQTGRLLDSNYNGNVYTLPANGGNYQRWTGPGDGTVRNAQTGRCLDSNYDGAVYTLPCNGGSYQKWLFYSNGYIQNVETGRVLDSNYNGNVYTLPANGGNYQKW | BAA97578.1 | [50] |
| 14 | Chitinase, Aedes aegypti, Homo sapiens | Chitotriose | CTGDGLFPDPDSCKKYYVCSNGHIFEFSCPDGLLFDQQNQICNWPEMVDC | AAZ39947.1 | [72] | |
3. Substrate Recognition and Binding by CBMs
3.1. Substrate Recognition and Binding by CBMs as ‘Probes’

3.2. Use AFM to Explore the CBM-Substrate Interactions
3.3. Other Methods to Study CBM-Substrate Interactions
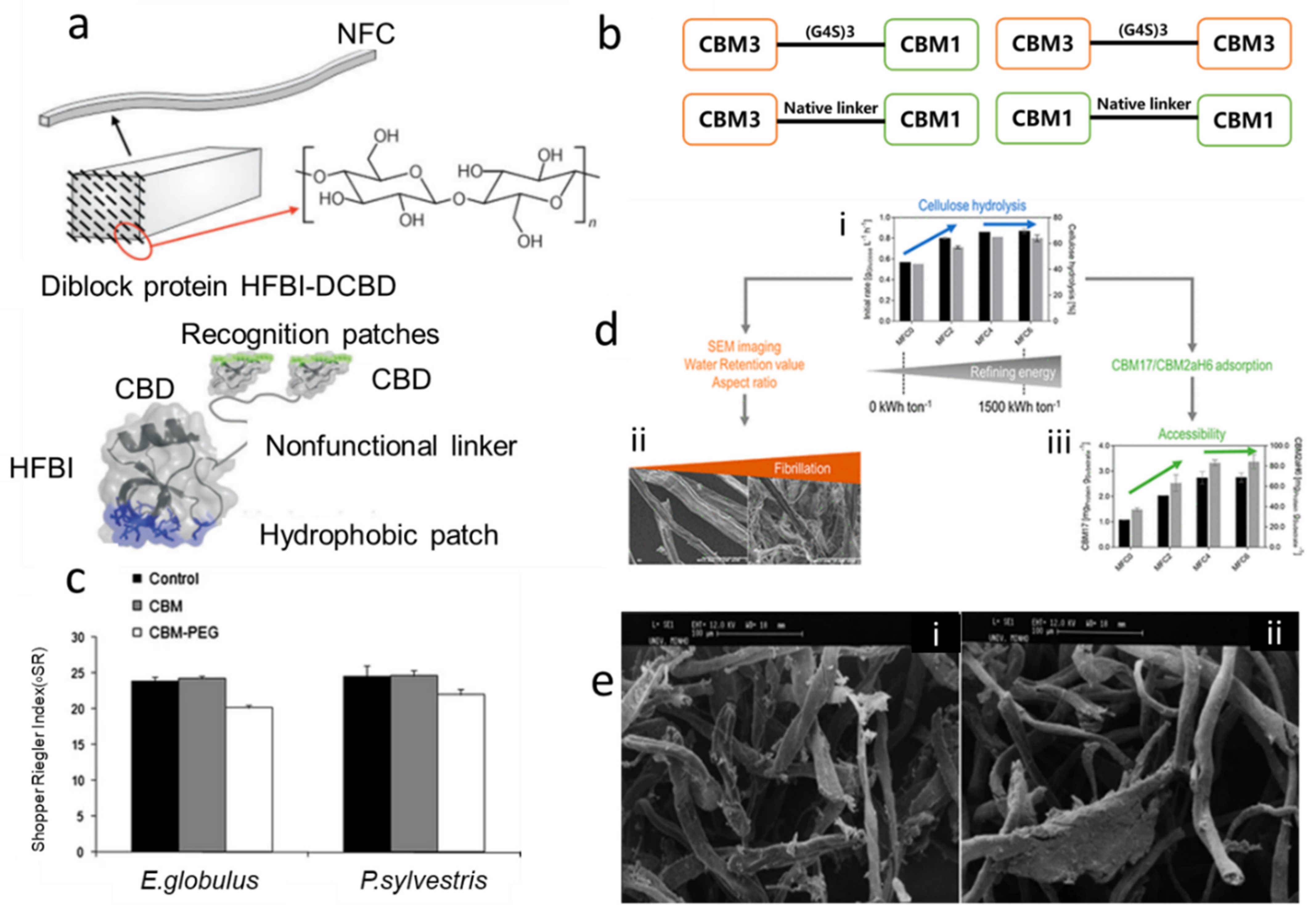
4. Fiber Treatment Using CBMs
4.1. Use CBMs Alone in Fiber Treatment
4.2. CBMs Conjugated with Other Polymers for Fiber Treatment
4.3. Other Functions
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Sanoja, R.; Oviedo, N.; Escalante, L.; Ruiz, B.; Sanchez, S. A single residue mutation abolishes attachment of the CBM26 starch-binding domain from Lactobacillus amylovorus alpha-amylase. J. Ind. Microbio. Biotechnol. 2009, 36, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Aissa, K.; Novy, V.; Nielsen, F.; Saddler, J. Use of carbohydrate binding modules to elucidate the relationship between fibrillation, hydrolyzability, and accessibility of cellulosic substrates. ACS Sustain. Chem. Eng. 2019, 7, 1113–1119. [Google Scholar] [CrossRef]
- Notenboom, V.; Boraston, A.B.; Kilburn, D.G.; Rose, D.R. Crystal structures of the family 9 carbohydrate-binding module from Thermotoga maritima Xylanase 10A in native and ligand-bound forms. Biochemistry 2001, 40, 6248–6256. [Google Scholar] [CrossRef]
- Jagadeeswaran, G.; Gainey, L.; Mort, A.J. An AA9-LPMO containing a CBM1 domain in Aspergillus nidulans is active on cellulose and cleaves cello-oligosaccharides. AMB Expr. 2018, 8, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazy Database. Available online: http://www.cazy.org (accessed on 24 March 2022).
- Tomme, P.; Warren, R.A.J.; Miller, R.C.; Kilburn, D.G.; Gilkes, N.R. Cellulose-Binding Domains: Classification and Properties; ACS Symposium Series No. 618; ACS Publications: Washington, DC, USA, 1996. [Google Scholar]
- Zerillo, M.M.; Adhikari, B.N.; Hamilton, J.P.; Buell, C.R.; Levesque, C.A.; Tisserat, N. Carbohydrate-active enzymes in pythium and their role in plant cell wall and storage polysaccharide degradation. PLoS ONE 2013, 8, e72572. [Google Scholar] [CrossRef] [PubMed]
- Zhan, N.; Wang, Z.; Xie, Y.; Shang, X.; Liu, G.; Wu, Z. Expression Patterns and Regulation of Non-Coding RNAs during Synthesis of Cellulose in Eucalyptus grandis Hill. Forests 2021, 12, 1565. [Google Scholar] [CrossRef]
- Jin, E.; Guo, J.; Yang, F.; Zhu, Y.; Song, J.; Jin, Y.; Rojas, O.J. On the polymorphic and morphological changes of cellulose nanocrystals (CNC-I) upon mercerization and conversion to CNC-II. Carbohydr. Polym. 2016, 143, 327–335. [Google Scholar] [CrossRef]
- Jin, E.; Zhang, Y.; Hu, F.; Yang, F.; Wu, S.; Jin, Y.; Song, J. To understand the superior hydrolytic activity after polymorphic conversion from cellulose I to II from the adsorption behaviors of enzymes. Cellulose 2017, 24, 1371–1381. [Google Scholar] [CrossRef]
- Liang, M.C.; Fu, C.G.; Xiao, B.Q.; Luo, L.; Wang, Z.K. A fractal study for the effective electrolyte diffusion through charged porous media. Int. J. Heat Mass Transfer 2019, 137, 365–371. [Google Scholar] [CrossRef]
- Lu, Z.H.; Zhang, B.W.; Gong, H.; Li, J.S. Fabrication of hierarchical porous poly (L-lactide) (PLLA) fibrous membrane by electrospinning. Polymer 2021, 226, 123797. [Google Scholar] [CrossRef]
- Shou, D.; Ye, L.; Fan, J. Longitudinal permeability determination of dual-scale fibrous materials. Compos. Part A 2015, 68, 42–46. [Google Scholar] [CrossRef]
- Xiao, B.Q.; Wang, W.; Fan, J.T.; Chen, H.X.; Hu, X.L.; Zhao, D.S.; Zhang, X.; Ren, W. Optimization of the fractal-like architecture of porous fibrous materials related to premeability, diffusivity and thermal conductivity. Fractals 2017, 25, 1750030. [Google Scholar] [CrossRef]
- Xiao, B.Q.; Wang, W.; Zhang, X.; Long, G.B.; Fan, J.T.; Chen, H.X.; Deng, L. A novel fractal solution for permeability and Kozeny-Carman constant of fibrous porous media made up of solid particles and porous fibers. Powder Technol. 2019, 349, 92–98. [Google Scholar] [CrossRef]
- Iwamoto, S.; Nakagaito, A.N.; Yano, H.; Nogi, M. Optically transparent composites reinforced with plant fiber-based nanofibers. Appl. Phys. A 2005, 81, 1109–1112. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl. Phys. A 2004, 78, 547–552. [Google Scholar] [CrossRef]
- Suardana, N.P.G.; Piao, Y.; Lim, J.K. Mechanical properties of hemp fibers and hemp/PP composites: Effects of chemical surface treatment. Mater. Phys. Mech. 2011, 11, 1–8. [Google Scholar]
- Shaidurova, G.; Gatina, E.; Shevyakov, Y. Practice, Prospects for the Use of Secondary Carbon Fibers. Bull. Sci. Prac. 2020, 6, 39–43. [Google Scholar] [CrossRef]
- Wang, X.; Hu, F.; Lu, X.; Wang, Q.; Zhang, X.; Tian, J.; Guo, J.; Song, J.; Jin, Y.; Xiao, H. Impact of degree of substitution of cationic xylan on strength of cellulose fiber networks along with medium conductivity. Ind. Crop. Prod. 2021, 159, 113058. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Wang, X.; Wang, P.; Tian, J.; Zhu, W.; Song, J.; Xiao, H. Revealing Adsorption Behaviors of Amphoteric Polyacrylamide on Cellulose Fibers and Impact on Dry Strength of Fiber Networks. Polymers 2019, 11, 1886. [Google Scholar] [CrossRef] [Green Version]
- Sutrisno, S.; Soenoko, R.; Irawan, Y.S.; Widodo, T.D. Effect of coconut fiber treatment with limestone water media on the fiber surface, wettability, and interface shear strength. East. Eur. J. Enterp. Technol. 2021, 1, 48–56. [Google Scholar] [CrossRef]
- Fu, H.; Mo, W.; Shen, X.; Li, B. Impact of centrifugation treatment on enzymatic hydrolysis of cellulose and xylan in poplar fibers with high lignin content. Bioresour. Technol. 2020, 316, 123866. [Google Scholar] [CrossRef]
- Beyene, D.; Chae, M.; Dai, J.; Danumah, C.; Tosto, F.; Demesa, A.G.; Bressler, D.C. Characterization of Cellulase-Treated Fibers and Resulting Cellulose Nanocrystals Generated through Acid Hydrolysis. Materials 2018, 11, 1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garajova, S.; Patel, I.; Lomascolo, A.; Legee, F.; Cezard, L.; Cottyn, B.; Lecourt, M.; Bertrand, E.; Sciara, G.; Tapin-Lingua, S.; et al. Treatment of wood fibres with laccases: Improved hardboard properties through phenolic oligomerization. Eur. J. Wood Wood Prods. 2021, 79, 1369–1382. [Google Scholar] [CrossRef]
- Zhou, H.; St John, F.; Zhu, J.Y. Xylanase pretreatment of wood fibers for producing cellulose nanofibrils: A comparison of different enzyme preparations. Cellulose 2019, 26, 543–555. [Google Scholar] [CrossRef]
- Solala, I.; Volperts, A.; Andersone, A.; Dizhbite, T.; Mironova-Ulmane, N.; Vehniainen, A.; Pere, J.; Vuorinen, T. Mechanoradical formation and its effects on birch kraft pulp during the preparation of nanofibrillated cellulose with Masuko refining. Holzforschung 2012, 66, 477–483. [Google Scholar] [CrossRef]
- Payne, C.M.; Knott, B.C.; Mayes, H.B.; Hansson, H.; Himmel, M.E.; Sandgren, M.; Stahlberg, J.; Beckham, G.T. Fungal Cellulases. Chem. Rev. 2015, 115, 1308–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendland, H. Computational Aspects of Radial Basis Function Approximation. Stud. Comput. Math. 2006, 12, 231–256. [Google Scholar] [CrossRef]
- Nakanishi, A.; Bae, J.; Kuroda, K.; Ueda, M. Construction of a novel selection system for endoglucanases exhibiting carbohydrate-binding modules optimized for biomass using yeast cell-surface engineering. AMB Expr. 2012, 2, 56. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Ortiz, V.; Heins, R.A.; Cheng, G.; Kim, E.Y.; Vernon, B.C.; Elandt, R.B.; Adams, P.D.; Sale, K.L.; Hadi, M.Z.; Simmons, B.A.; et al. Addition of a carbohydrate-binding module enhances cellulase penetration into cellulose substrates. Biotechnol. Biofuels 2013, 6, 93. [Google Scholar] [CrossRef] [Green Version]
- Capri, B. The Turkish Adaptation of the Burnout Measure-Short Version (BMS) and Couple Burnout Measure-Short Version (CBMS) and the Relationship between Career and Couple Burnout Based on Psychoanalytic-Existential Perspective. Kuram Ve Uygulamada Egitim Bilimleri 2013, 13, 1408–1417. [Google Scholar]
- Pires, V.M.R.; Pereira, P.M.M.; Bras, J.L.A.; Correia, M.; Cardoso, V.; Bule, P.; Alves, V.D.; Najmudin, S.; Venditto, I.; Ferreira, L.M.A.; et al. Stability and Ligand Promiscuity of Type A Carbohydrate-binding Modules Are Illustrated by the Structure of Spirochaeta thermophila StCBM64C. J. Biolog. Chem. 2017, 292, 4847–4860. [Google Scholar] [CrossRef] [Green Version]
- Credou, J.; Berthelot, T. Cellulose: From biocompatible to bioactive material. J. Mater. Chem. B 2014, 2, 4767–4788. [Google Scholar] [CrossRef] [Green Version]
- Hua, S.; Wang, A. Synthesis, characterization and swelling behaviors of sodium alginate-g-poly(acrylic acid)/sodium humate superabsorbent. Carbohydr. Polym. 2009, 75, 79–84. [Google Scholar] [CrossRef]
- Guillen, D.; Sanchez, S.; Rodriguez-Sanoja, R. Carbohydrate-binding domains: Multiplicity of biological roles. Appl. Microbiol. Biotechnol. 2010, 85, 1241–1249. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Y.; Hu, Q.; Zhang, Y.; Shao, T.; Fan, B.; Jiang, Y.; Chen, Z.; Zhao, M. Coalbed Methane Production Model Based on Random Forests Optimized by a Genetic Algorithm. ACS Omega 2022, 7, 13083–13094. [Google Scholar] [CrossRef]
- Varghese, B.; McKee, G.; Alexandrov, V. Automating fault tolerance in high-performance computational biological jobs using multi-agent approaches. Comput. Biolog. Medicine 2014, 48, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Minami, A.; Ozaki, T.; Liu, C.; Oikawa, H. Cyclopentane-forming di/sesterterpene synthases: Widely distributed enzymes in bacteria, fungi, and plants. Nat. Prod. Rep. 2018, 35, 1330–1346. [Google Scholar] [CrossRef]
- Stojakowska, A.; Michalska, K.; Malarz, J. Simultaneous quantification of eudesmanolides and thymol derivatives from tissues of Inula helenium and I-royleana by reversed-phase high-performance liquid chromatography. Phytochem. Analys. 2006, 17, 157–161. [Google Scholar] [CrossRef]
- Bae, B.; Ohene-Adjei, S.; Kocherginskaya, S.; Mackie, R.I.; Spies, M.A.; Cann, I.K.O.; Nair, S.K. Molecular basis for the selectivity and specificity of ligand recognition by the family 16 carbohydrate-binding modules from Thermoanaerobacterium polysaccharolyticum ManA. J. Biolog. Chem. 2008, 283, 12415–12425. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-N.; Lai, Y.-T.; Chou, W.-I.; Chang, M.D.-T.; Lyu, P.-C. Solution structure of family 21 carbohydrate-binding module from Rhizopus oryzae glucoamylase. Biochem. J. 2007, 403, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Jamal-Talabani, S.; Boraston, A.B.; Turkenburg, J.P.; Tarbouriech, N.; Ducros, V.M.A.; Davies, G.J. Ab initio structure determination and functional characterization of CBM36: A new family of calcium-dependent carbohydrate binding modules. Structure 2004, 12, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Varnai, A.; Makela, M.R.; Djajadi, D.T.; Rahikainen, J.; Hatakka, A.; Viikari, L. Carbohydrate-binding modules of fungal cellulases: Occurrence in nature, function, and relevance in industrial biomass conversion. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 88, pp. 103–165. [Google Scholar]
- Kljun, A.; Benians, T.A.S.; Goubet, F.; Meulewaeter, F.; Knox, J.P.; Blackburn, R.S. Comparative Analysis of Crystallinity Changes in Cellulose I Polymers Using ATR-FTIR, X-ray Diffraction, and Carbohydrate-Binding Module Probes. Biomacromolecules 2011, 12, 4121–4126. [Google Scholar] [CrossRef] [PubMed]
- Boraston, A.B.; Revett, T.J.; Boraston, C.M.; Nurizzo, D.; Davies, G.J. Structural and thermodynamic dissection of specific mannan recognition by a carbohydrate binding module, TmCBM27. Structure 2003, 11, 665–675. [Google Scholar] [CrossRef]
- Simpson, P.J.; Jamieson, S.J.; Abou-Hachem, M.; Karlsson, E.N.; Gilbert, H.J.; Holst, O.; Williamson, M.P. The solution structure of the CBM4-2 carbohydrate binding module from a thermostable Rhodothermus marinus xylanase. Biochemistry 2002, 41, 5712–5719. [Google Scholar] [CrossRef]
- Boraston, A.B.; Healey, M.; Klassen, J.; Ficko-Blean, E.; van Bueren, A.L.; Law, V. A structural and functional analysis of alpha-glucan recognition by family 25 and 26 carbohydrate-binding modules reveals a conserved mode of starch recognition. J. Biolog. Chem. 2006, 281, 587–598. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, H.; Tamai, Y.; Okazaki, F.; Tamaru, Y.; Shimizu, T.; Araki, T.; Sato, M. The first crystal structure of a family 31 carbohydrate-binding module with affinity to beta-1, 3-xylan. FEBS Lett. 2005, 579, 4324–4328. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Fujimoto, Z.; Kim, Y.-M.; Momma, M.; Kishine, N.; Suzuki, R.; Suzuki, S.; Kitamura, S.; Kobayashi, M.; Kimura, A.; et al. Structural Elucidation of the Cyclization Mechanism of alpha-1,6-Glucan by Bacillus circulans T-3040 Cycloisomaltooligosaccharide Glucanotransferase. J. Biolo. Chem. 2014, 289, 12040–12051. [Google Scholar] [CrossRef] [Green Version]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochemical J. 2004, 382, 769–781. [Google Scholar] [CrossRef]
- Li, Y.; Guan, X.; Chaffey, P.K.; Ruan, Y.; Ma, B.; Shang, S.; Himmel, M.E.; Beckham, G.T.; Long, H.; Tan, Z. Carbohydrate-binding moduleO-mannosylation alters binding selectivity to cellulose and lignin. Chem. Sci. 2020, 11, 9262–9271. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.; Lu, X.; Wang, P.; Zhang, X.; Tian, J.; Wang, Q.; Song, J.; Jin, Y.; Xiao, H. Binding affinity of family 4 carbohydrate binding module on cellulose films of nanocrystals and nanofibrils. Carbohydr. Polym. 2021, 251, 116725. [Google Scholar] [CrossRef]
- Linder, M.; Lindeberg, G.; Reinikainen, T.; Teeri, T.T.; Pettersson, G.R. The difference in affinity between two fungal cellulose-binding domains is dominated by a single amino acid substitution. FEBS Lett. 1995, 372, 96–98. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.C.; Phan, N.N.; Chen, Y.; Reilly, P.J. Carbohydrate-binding module tribes. Biopolymers 2015, 103, 203–214. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wang, P.; Song, J.; Jin, Y.; Rojas, O.J. Interactions between type A carbohydrate binding modules and cellulose studied with a quartz crystal microbalance with dissipation monitoring. Cellulose 2020, 27, 3661–3675. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, F.; Hu, F.; Song, J.; Wu, S.; Jin, Y. Binding preference of family 1 carbohydrate binding module on nanocrystalline cellulose and nanofibrillar cellulose films assessed by quartz crystal microbalance. Cellulose 2018, 25, 3327–3337. [Google Scholar] [CrossRef]
- Kraulis, J.; Clore, G.M.; Nilges, M.; Jones, T.A.; Pettersson, G.; Knowles, J.; Gronenborn, A.M. Determination of the three-dimensional solution structure of the C-terminal domain of cellobiohydrolase I from Trichoderma reesei. A study using nuclear magnetic resonance and hybrid distance geometry-dynamical simulated annealing. Biochemistry 1989, 28, 7241–7257. [Google Scholar] [CrossRef]
- Boraston, A.B.; Nurizzo, D.; Notenboom, V.; Ducros, V.; Rose, D.R.; Kilburn, D.G.; Davies, G.J. Differential Oligosaccharide Recognition by Evolutionarily-related β-1,4 and β-1,3 Glucan-binding Modules. J. Molecul. Biol. 2002, 319, 1143–1156. [Google Scholar] [CrossRef]
- Jamal, S.; Nurizzo, D.; Boraston, A.B.; Davies, G.J. X-ray crystal structure of a non-crystalline cellulose-specific carbohydrate-binding module: CBM28. J. Mol. Biol. 2004, 339, 253–258. [Google Scholar] [CrossRef]
- Alahuhta, M.; Xu, Q.; Bomble, Y.J.; Brunecky, R.; Adney, W.S.; Ding, S.-Y.; Himmel, M.E.; Lunin, V.V. The Unique Binding Mode of Cellulosomal CBM4 from Clostridium thermocellum Cellobiohydrolase A. J. Mol. Biol. 2010, 402, 374–387. [Google Scholar] [CrossRef]
- Alahuhta, M.; Luo, Y.; Ding, S.Y.; Himmel, M.E.; Lunin, V.V. Structure of CBM4 from Clostridium thermocellum cellulase K. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 527–530. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. Lectins as cell recognition molecules. Science 1989, 246, 227–234. [Google Scholar] [CrossRef]
- Freeman, H.; Srinivasan, S.; Das, D.; Stayton, P.S.; Convertine, A.J. Fully synthetic macromolecular prodrug chemotherapeutics with EGFR targeting and controlled camptothecin release kinetics. Polym. Chem. 2018, 9, 5224–5233. [Google Scholar] [CrossRef]
- Thiers, F.A.; Burgess, B.J.; Nadol, J.B., Jr. Reciprocal innervation of outer hair cells in a human infant. J. Assoc. Res. Otolaryngol. 2002, 3, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiiba, H.; Hayashi, S.; Yui, T. Molecular simulation study with complex models of the carbohydrate binding module of Cel6A and the cellulose Iα crystal. Cellulose 2012, 19, 635–645. [Google Scholar] [CrossRef]
- Machado, J.; Araújo, A.; Pinto, R.; Gama, F.M. Studies on the interaction of the carbohydrate binding module 3 from the Clostridium thermocellum CipA scaffolding protein with cellulose and paper fibres. Cellulose 2009, 16, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.; Petrovic, D.; Strodel, B.; Smits, S.H.J.; Kolkenbrock, S.; Leggewie, C.; Jaeger, K.-E. Interaction of carbohydrate-binding modules with poly(ethylene terephthalate). Appl. Microbiol. Biotechnol. 2019, 103, 4801–4812. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Quirk, A.; Lipkowski, J.; Dutcher, J.R.; Hill, C.; Mark, A.; Clarke, A.J. Real-Time Observation of the Swelling and Hydrolysis of a Single Crystalline Cellulose Fiber Catalyzed by Cellulase 7B from Trichoderma reesei. Langmuir 2012, 28, 9664–9672. [Google Scholar] [CrossRef]
- Guo, X.; Fan, Y.; Liu, H.; Hou, Y.; Wang, Y.; Sun, J.; Chen, X.; Liu, Y.; Li, X. Prediction of Cellulose Crystallinity in Liquid Phase Using CBM-GFP Probe. Macromol Res. 2019, 27, 377–385. [Google Scholar] [CrossRef]
- Najmudin, S.; Guerreiro, C.I.; Carvalho, A.L.; Prates, J.A.; Correia, M.A.; Alves, V.D.; Ferreira, L.M.; Romao, M.J.; Gilbert, H.J.; Bolam, D.N.; et al. Xyloglucan is recognized by carbohydrate-binding modules that interact with beta-glucan chains. J. Biol. Chem. 2006, 281, 8815–8828. [Google Scholar] [CrossRef] [Green Version]
- Fadel, F.; Zhao, Y.; Cousido-Siah, A.; Ruiz, F.X.; Mitschler, A.; Podjarny, A. X-ray Crystal Structure of the Full Length Human Chitotriosidase (CHIT1) Reveals Features of Its Chitin Binding Domain. PLoS ONE 2016, 11, e0154190. [Google Scholar] [CrossRef]
- Sidar, A.; Albuquerque, E.D.; Voshol, G.P.; Ram, A.F.J.; Vijgenboom, E.; Punt, P.J. Carbohydrate Binding Modules: Diversity of Domain Architecture in Amylases and Cellulases from Filamentous Microorganisms. Front. Bioeng. Biotechnol. 2020, 8, 871. [Google Scholar] [CrossRef]
- Han, Q.; Gong, W.; Zhang, Z.; Wang, L.; Wang, B.; Cai, L.; Meng, Q.; Li, Y.; Liu, Q.; Yang, Y.; et al. Orientated Immobilization of FAD-Dependent Glucose Dehydrogenase on Electrode by Carbohydrate-Binding Module Fusion for Efficient Glucose Assay. Int. J. Mol. Sci. 2021, 22, 5529. [Google Scholar] [CrossRef]
- Eroglu, E.; Wijihastuti, R.S.; Grenik, E.; Vadiveloo, A.; Moheimani, N.R.; Lou, X. Application of poly(2-hydroxyethyl methacrylate) hydrogel disks for the immobilization of three different microalgal species. J. Chem. Technol. Biotechnol. 2018, 93, 2887–2897. [Google Scholar] [CrossRef]
- Endo, R.; Aoyagi, H. Adsorption preference for divalent metal ions by Lactobacillus casei JCM1134. Appl. Microbiol. Biotechnol. 2018, 102, 6155–6162. [Google Scholar] [CrossRef]
- Turkova, J. Oriented immobilization of biologically active proteins as a tool for revealing protein interactions and function. J. Chromatogr. B 1999, 722, 11–31. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, M.; Kelly, A.; Cheng, A.J.B.R. The carbohydrate-binding domain of overexpressed STBD1 is important for its stability and protein–protein interactions. Biosci. Rep. 2014, 34, 311–320. [Google Scholar] [CrossRef]
- Horn, C.; Schmid, B.G.M.; Pogoda, F.S.; Wimmer, E.A. Fluorescent transformation markers for insect transgenesis. Insect Biochem. Mol. Biol. 2002, 32, 1221–1235. [Google Scholar] [CrossRef]
- Gao, S.; You, C.; Renneckar, S.; Bao, J.; Zhang, Y.-H.P. New insights into enzymatic hydrolysis of heterogeneous cellulose by using carbohydrate-binding module 3 containing GFP and carbohydrate-binding module 17 containing CFP. Biotechnol. Biofuels 2014, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yue, Y.; Zhang, Z.-J.; Wang, Z.-Y.; Tan, T.-W.; Fan, L.-H. Site-Specific and High-Loading Immobilization of Proteins by Using Cohesin-Dockerin and CBM-Cellulose Interactions. Bioconj. Chem. 2016, 27, 1579–1583. [Google Scholar] [CrossRef]
- Bombeck, P.L.; Khatri, V.; Meddeb-Mouelhi, F.; Montplaisir, D.; Richel, A.; Beauregard, M. Predicting the most appropriate wood biomass for selected industrial applications: Comparison of wood, pulping, and enzymatic treatments using fluorescent-tagged carbohydrate-binding modules. Biotechnol. Biofuels 2017, 10, 293. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.M.; Jess, P.; Jambusaria, R.B.; Moo, G.M.; Liphardt, J.; Clark, D.S.; Blanch, H.W. A single-molecule analysis reveals morphological targets for cellulase synergy. Nat. Chem. Biol. 2013, 9, 356–361. [Google Scholar] [CrossRef]
- Badruna, L.; Burlat, V.; Montanier, C.Y. CBMs as Probes to Explore Plant Cell Wall Heterogeneity Using Immunocytochemistry. Methods Mol. Biol. 2017, 1588, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.P.S.; Bhat, A.H.; Yusra, A.F.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Progr. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aissa, K.; Karaaslan, M.A.; Renneckar, S.; Saddler, J.N. Functionalizing Cellulose Nanocrystals with Click Modifiable Carbohydrate-Binding Modules. Biomacromolecules 2019, 20, 3087–3093. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, A.; Artzi, L.; Cazade, P.-A.; Gunnoo, M.; Bayer, E.A.; Thompson, D. On the distinct binding modes of expansin and carbohydrate-binding module proteins on crystalline and nanofibrous cellulose: Implications for cellulose degradation by designer cellulosomes. Phys. Chem. Chem. Phys. 2018, 20, 8278–8293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardes, A. Carbohydrate binding modules enhance cellulose enzymatic hydrolysis by increasing access of cellulases to the substrate. Carbohydr. Polym. 2019, 211, 57–68. [Google Scholar] [CrossRef]
- Jung, H.; Wilson, D.B.; Walker, L.P. Binding and reversibility of thermobifida fusca Cel5A, Cel6B, and Cel48A and their respective catalytic domains to bacterial microcrystalline cellulose. Biotechnol. Bioeng. 2003, 84, 151–159. [Google Scholar] [CrossRef]
- Guilliams, A.; Pattathil, S.; Willies, D.; Richards, M.; Pu, Y.; Kandemkavil, S.; Wiswall, E. Physical and chemical differences between one-stage and two-stage hydrothermal pretreated hardwood substrates for use in cellulosic ethanol production. Biotechnol. Biofuels 2016, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Donohoe, B.S.; Resch, M.G. Mechanisms employed by cellulase systems to gain access through the complex architecture of lignocellulosic substrates. Curr. Opin. Chem. Biol. 2015, 29, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.X.; Kim, J.-T. Application of Kevin-Voigt Model in Quantifying Whey Protein Adsorption on Polyethersulfone Using QCM-D. Jala 2009, 14, 213–220. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, M.; Duan, Y.; Huang, R.; Su, R.; Qi, W.; Thielemans, W.; He, Z. Real-Time Adsorption of Exo- and Endoglucanases on Cellulose: Effect of pH, Temperature, and Inhibitors. Langmuir 2018, 34, 13514–13522. [Google Scholar] [CrossRef]
- Arslan, B.C.M.; Ju, X.; Zhang, X.; Kostyukova, A.; Abu-Lail, N.I. The Effects of Noncellulosic Compounds on the Nanoscale Interaction Forces Measured between Carbohydrate-Binding Module and Lignocellulosic Biomass. Biomacromolecules 2016, 17, 1705–1715. [Google Scholar] [CrossRef]
- Currie, M.A.; Cameron, K.; Dias, F.M.; Spencer, H.L.; Bayer, E.A.; Fontes, C.M.; Smith, S.P.; Jia, Z. Small angle X-ray scattering analysis of Clostridium thermocellum cellulosome N-terminal complexes reveals a highly dynamic structure. J. Biol. Chem. 2013, 288, 7978–7985. [Google Scholar] [CrossRef] [Green Version]
- Griffo, A.; Hahl, H.; Grandthyll, S.; Muller, F.; Paananen, A.; Ilmen, M.; Szilvay, G.R.; Landowski, C.P.; Penttila, M.; Jacobs, K.; et al. Single-Molecule Force Spectroscopy Study on Modular Resilin Fusion Protein. ACS Omega 2017, 2, 6906–6915. [Google Scholar] [CrossRef]
- Sokolov, I.; Dokukin, M.E.; Guz, N.V. Method for quantitative measurements of the elastic modulus of biological cells in AFM indentation experiments. Methods 2013, 60, 202–213. [Google Scholar] [CrossRef]
- Milhiet, P.E.; Gubellini, F.; Berquand, A.; Dosset, P.; Rigaud, J.L.; Le Grimellec, C.; Levy, D. High-resolution AFM of membrane proteins directly incorporated at high density in planar lipid bilayer. Biophys. J. 2006, 91, 3268–3275. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wu, S.-C.; Zhou, W.; Xu, B. Imaging and Measuring Single-Molecule Interaction between a Carbohydrate-Binding Module and Natural Plant Cell Wall Cellulose. J. Phys. Chem. B 2012, 116, 9949–9956. [Google Scholar] [CrossRef]
- Peng, Y.; Dong, Y.-M.; Dong, M.; Wang, Y.-W. A Selective, Sensitive, Colorimetric, and Fluorescence Probe for Relay Recognition of Fluoride and Cu(II) Ions with “Off-On-Off” Switching in Ethanol Water Solution. J. Org. Chem. 2012, 77, 9072–9080. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, B.; Xu, B. Mapping single molecular binding kinetics of carbohydrate-binding module with crystalline cellulose by atomic force microscopy recognition imaging. J. Phys. Chem. B 2014, 118, 6714–6720. [Google Scholar] [CrossRef]
- Habibi, Y.; Thibodeaux, C.J. A Hydrogen-Deuterium Exchange Mass Spectrometry (HDX-MS) Platform for Investigating Peptide Biosynthetic Enzymes. Jove-J. Visualized Experiments 2020, 159, e61053. [Google Scholar] [CrossRef]
- Roda, S.; Santiago, G.; Guallar, V. Mapping enzyme-substrate interactions: Its potential to study the mechanism of enzymes. In Advances in Protein Chemistry and Structural Biology; Karabencheva Christova, T., Christov, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 122, pp. 1–31. [Google Scholar]
- Wang, Y.; Manu, V.S.; Kim, J.; Li, G.; Ahuja, L.G.; Aoto, P.; Taylor, S.S.; Veglia, G. Globally correlated conformational entropy underlies positive and negative cooperativity in a kinase’s enzymatic cycle. Nat. Commun. 2019, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.-L.; Otting, G.; Su, X.-C. Conversion of an amide to a high-energy thioester by Staphylococcus aureus sortase A is powered by variable binding affinity for calcium. Sci. Rep. 2018, 8, 16371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Mauff, F.; Bamford, N.C.; Alnabelseya, N.; Zhang, Y.; Baker, P.; Robinson, H.; Codee, J.D.C.; Howell, P.L.; Sheppard, D.C. Molecular mechanism of Aspergillus fumigatus biofilm disruption by fungal and bacterial glycoside hydrolases. J. Biolog. Chem. 2019, 294, 10760–10772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, B.; Xu, B. Measurements of single molecular affinity interactions between carbohydrate-binding modules and crystalline cellulose fibrils. Phys. Chem. Chem. Phys. 2013, 15, 6508–6515. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Kofoed, C.; Espersen, R.; Hojgaard, C.; Winther, J.R.; Willemoes, M.; Wedin, I.; Nuopponen, M.; Vilske, S.; Aimonen, K.; et al. Visualization of Nanofibrillar Cellulose in Biological Tissues Using a Biotinylated Carbohydrate Binding Module of beta-1,4-Glycanase. Chem. Res. Toxicol. 2015, 28, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Shoseyov, O.; Shani, Z.; Levy, I. Carbohydrate binding modules: Biochemical properties and novel applications. Microbiol. Mol. Biol. Rev. 2006, 70, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Saw, S.K.; Akhtar, K.; Yadav, N.; Singh, A.K. Hybrid Composites Made from Jute/Coir Fibers: Water Absorption, Thickness Swelling, Density, Morphology, and Mechanical Properties. J. Nat. Fibers 2014, 11, 39–53. [Google Scholar] [CrossRef]
- Pala, H. Enzymatic upgrade of old paperboard containers. Enzym. Microb. Technol. 2001, 29, 2274–2279. [Google Scholar] [CrossRef] [Green Version]
- Pinto, R.; Moreira, S.; Mota, M.; Gama, M. Studies on the cellulose-binding domains adsorption to cellulose. Langmuir 2004, 20, 1409–1413. [Google Scholar] [CrossRef] [Green Version]
- Shoseyov, O.; Levy, I.; Shani, Z.; Mansfield, S.D. Modulation of wood fibers and paper by cellulose-binding domains. In Applications of Enzymes to Lignocellulosics; Mansfield, S.D., Saddler, J.N., Eds.; ACS Publications: Washington, DC, USA, 2003; Volume 855, pp. 116–131. [Google Scholar]
- Laaksonen, P.; Walther, A.; Malho, J.-M.; Kainlauri, M.; Ikkala, O.; Linder, M.B. Genetic Engineering of Biomimetic Nanocomposites: Diblock Proteins, Graphene, and Nanofibrillated Cellulose. Angew. Chem. Int. Ed. 2011, 50, 8688–8691. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, F.; Pan, R.; Wang, J.; Ding, S. Engineering and Comparative Characteristics of Double Carbohydrate Binding Modules as a Strength Additive for Papermaking Applications. Bioresources 2014, 9, 3117–3131. [Google Scholar] [CrossRef]
- Clementi, C.; Garcı́a, A.E.; Onuchic, J.N. Interplay Among Tertiary Contacts, Secondary Structure Formation and Side-chain Packing in the Protein Folding Mechanism: All-atom Representation Study of Protein L. J. Mol. Biol. 2003, 326, 933–954. [Google Scholar] [CrossRef]
- Sam, S.; Touahir, L.; Salvador Andresa, J.; Allongue, P.; Chazalviel, J.N.; Gouget-Laemmel, A.C.; Henry de Villeneuve, C.; Moraillon, A.; Ozanam, F.; Gabouze, N.; et al. Semiquantitative study of the EDC/NHS activation of acid terminal groups at modified porous silicon surfaces. Langmuir 2010, 26, 809–814. [Google Scholar] [CrossRef]
- Kitaoka, T.; Tanaka, H. Novel paper strength additive containing cellulose-binding domain of cellulase. J. Wood Sci. 2001, 47, 322–324. [Google Scholar] [CrossRef]
- Vincent, F.; Dal Molin, D.; Weiner, R.M.; Bourne, Y.; Henrissat, B. Structure of a polyisoprenoid binding domain from Saccharophagus degradans implicated in plant cell wall breakdown. FEBS Lett. 2010, 584, 1577–1584. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, M.; Simoes, H.; Pinto, S.N.; Macedo, A.S.; Fonte, P.; Prazeres, D.M.F. Fusions of a carbohydrate binding module with the small cationic hexapeptide RWRWRW confer antimicrobial properties to cellulose-based materials. Acta Biomater. 2022, 143, 216–232. [Google Scholar] [CrossRef]
- Koskela, S.; Wang, S.; Xu, D.; Yang, X.; Li, K.; Berglund, L.A.; McKee, L.S.; Bulone, V.; Zhou, Q. Lytic polysaccharide monooxygenase (LPMO) mediated production of ultra-fine cellulose nanofibres from delignified softwood fibres. Green Chem. 2019, 21, 5924–5933. [Google Scholar] [CrossRef] [Green Version]
- Griffo, A.; Rooijakkers, B.J.M.; Haehl, H.; Jacobs, K.; Linder, M.B.; Laaksonen, P. Binding Forces of Cellulose Binding Modules on Cellulosic Nanomaterials. Biomacromolecules 2019, 20, 769–777. [Google Scholar] [CrossRef]
- Li, Q.; Ma, Z.; Meng, D.; Sui, X.; You, C. Facile biosynthesis of synthetic crystalline cellulose nanoribbon from maltodextrin through a minimized two-enzyme phosphorylase cascade and its application in emulsion. J. Biotechnol. 2021, 332, 54–60. [Google Scholar] [CrossRef]
- Mautner, A.; Bismarck, A. Bacterial nanocellulose papers with high porosity for optimized permeance and rejection of nm-sized pollutants. Carbohydr. Polym. 2021, 251, 117130. [Google Scholar] [CrossRef]
- Mautner, A.; Lee, K.-Y.; Tammelin, T.; Mathew, A.P.; Nedoma, A.J.; Li, K.; Bismarck, A. Cellulose nanopapers as tight aqueous ultra-filtration membranes. React. Funct. Polym. 2015, 86, 209–214. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, F.; Xu, X.; Kuang, Y.; Fu, K.; Hitz, E.; Hu, L. Super-Strong, Super-Stiff Macrofibers with Aligned, Long Bacterial Cellulose Nanofibers. Adv. Mater. 2017, 29, 1702498. [Google Scholar] [CrossRef] [PubMed]
- Pierre, B.; Labonte, J.W.; Xiong, T.; Aoraha, E.; Williams, A.; Shah, V.; Chau, E.; Helal, K.Y.; Gray, J.J.; Kim, J.R. Molecular Determinants for Protein Stabilization by Insertional Fusion to a Thermophilic Host Protein. Chembiochem 2015, 16, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.F.; Furtado, G.P.; Lourenzoni, M.R.; Costa-Filho, A.J.; Santos, C.R.; Peixoto Nogueira, S.C.; Betini, J.A.; Polizeli, M.d.L.T.M.; Murakami, M.T.; Ward, R.J. Engineering Bifunctional Laccase-Xylanase Chimeras for Improved Catalytic Performance. J. Biol. Chem. 2011, 286, 43026–43038. [Google Scholar] [CrossRef] [Green Version]
- Chhabra, S.R.; Kelly, R.M. Biochemical characterization of Thermotoga maritima endoglucanase Ce174 with and without a carbohydrate binding module (CBM). FEBS Lett. 2002, 531, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Kavoosi, M.; Creagh, A.L.; Kilburn, D.G.; Haynes, C.A. Strategy for selecting and characterizing linker peptides for CBM9-tagged fusion proteins expressed in Escherichia coli. Biotechnol. Bioeng. 2007, 98, 599–610. [Google Scholar] [CrossRef]
- Liu, Z.; Bartlow, P.; Dilmore, R.M.; Soong, Y.; Pan, Z.; Koepsel, R.; Ataai, M. Production, Purification, and Characterization of a Fusion Protein of Carbonic Anhydrase from Neisseria gonorrhoeae and Cellulose Binding Domain from Clostridium thermocellum. Biotechnol. Prog. 2009, 25, 68–74. [Google Scholar] [CrossRef]
- Kittur, F.S.; Mangala, S.L.; Rus’d, A.A.; Kitaoka, M.; Tsujibo, H.; Hayashi, K. Fusion of family 2b carbohydrate-binding module increases the catalytic activity of a xylanase fromThermotoga maritimato soluble xylan. FEBS Lett. 2003, 549, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Guo, Y.; Lin, X.; You, M.; Lin, C.; Chen, L.; Chen, J. Fusion of a family 20 carbohydrate-binding module (CBM20) with cyclodextrin glycosyltransferase of Geobacillus sp CHB1 improves catalytic efficiency. J. Basic Microbiol. 2017, 57, 471–480. [Google Scholar] [CrossRef]
- Ara, K.Z.G.; Lundemo, P.; Fridjonsson, O.H.; Hreggvidsson, G.O.; Adlercreutz, P.; Karlsson, E.N. A CGTase with high coupling activity using γ-cyclodextrin isolated from a novel strain clustering under the genus Carboxydocella. Glycobiology 2015, 25, 514–523. [Google Scholar] [CrossRef]
- Pelus, A.; Bordes, G.; Barbe, S.; Bouchiba, Y.; Burnard, C.; Cortes, J.; Enjalbert, B.; Esque, J.; Estana, A.; Faure, R.; et al. A tripartite carbohydrate-binding module to functionalize cellulose nanocrystals. Biomater. Sci. 2021, 9, 7444–7455. [Google Scholar] [CrossRef]
- Hinkley, T.C.; Garing, S.; Singh, S.; Le Ny, A.L.M.; Nichols, K.P.; Peters, J.E.; Talbert, J.N.; Nugen, S.R. Reporter bacteriophage T7(NLC) utilizes a novel NanoLuc::CBM fusion for the ultrasensitive detection of Escherichia coli in water. Analyst 2018, 143, 4074–4082. [Google Scholar] [CrossRef] [Green Version]
- Connaris, H.; Govorkova, E.A.; Ligertwood, Y.; Dutia, B.M.; Yang, L.; Tauber, S.; Taylor, M.A.; Alias, N.; Hagan, R.; Nash, A.A.; et al. Prevention of influenza by targeting host receptors using engineered proteins. Proc. Nat. Acad. Sci. USA 2014, 111, 6401–6406. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xing, X.; Liu, Y.; Li, S.; Li, W. Comprehensive Network Analysis of Different Subtypes of Molecular Disorders in Lung Cancer. Oncologie 2020, 22, 107–116. [Google Scholar] [CrossRef]
- Levy, I.; Paldi, T.; Shoseyov, O. Engineering a bifunctional starch-cellulose cross-bridge protein. Biomaterials 2004, 25, 1841–1849. [Google Scholar] [CrossRef]
- Lombardo, S.; Thielemans, W. Thermodynamics of adsorption on nanocellulose surfaces. Cellulose 2019, 26, 249–279. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, P.; Tian, J.; Seidi, F.; Guo, J.; Zhu, W.; Xiao, H.; Song, J. Carbohydrate-Binding Modules of Potential Resources: Occurrence in Nature, Function, and Application in Fiber Recognition and Treatment. Polymers 2022, 14, 1806. https://doi.org/10.3390/polym14091806
Liu Y, Wang P, Tian J, Seidi F, Guo J, Zhu W, Xiao H, Song J. Carbohydrate-Binding Modules of Potential Resources: Occurrence in Nature, Function, and Application in Fiber Recognition and Treatment. Polymers. 2022; 14(9):1806. https://doi.org/10.3390/polym14091806
Chicago/Turabian StyleLiu, Yena, Peipei Wang, Jing Tian, Farzad Seidi, Jiaqi Guo, Wenyuan Zhu, Huining Xiao, and Junlong Song. 2022. "Carbohydrate-Binding Modules of Potential Resources: Occurrence in Nature, Function, and Application in Fiber Recognition and Treatment" Polymers 14, no. 9: 1806. https://doi.org/10.3390/polym14091806
APA StyleLiu, Y., Wang, P., Tian, J., Seidi, F., Guo, J., Zhu, W., Xiao, H., & Song, J. (2022). Carbohydrate-Binding Modules of Potential Resources: Occurrence in Nature, Function, and Application in Fiber Recognition and Treatment. Polymers, 14(9), 1806. https://doi.org/10.3390/polym14091806







