Scaffolds Loaded with Dialdehyde Chitosan and Collagen—Their Physico-Chemical Properties and Biological Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining the Scaffolds
2.3. Structural Studies—Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (FTIR-ATR)
2.4. Thermal Stability
2.5. Determination of Density, Porosity and Water Content
2.6. Scanning Electron Microscopy Imaging
2.7. Cell Culture and Proliferation Ratio Assessment
2.8. Statistics
3. Results and Discussion
3.1. Structural Studies—FTIR-ATR
3.2. Thermal Stability
3.3. Density, Porosity and Water Content
3.4. Morphological Studies—SEM
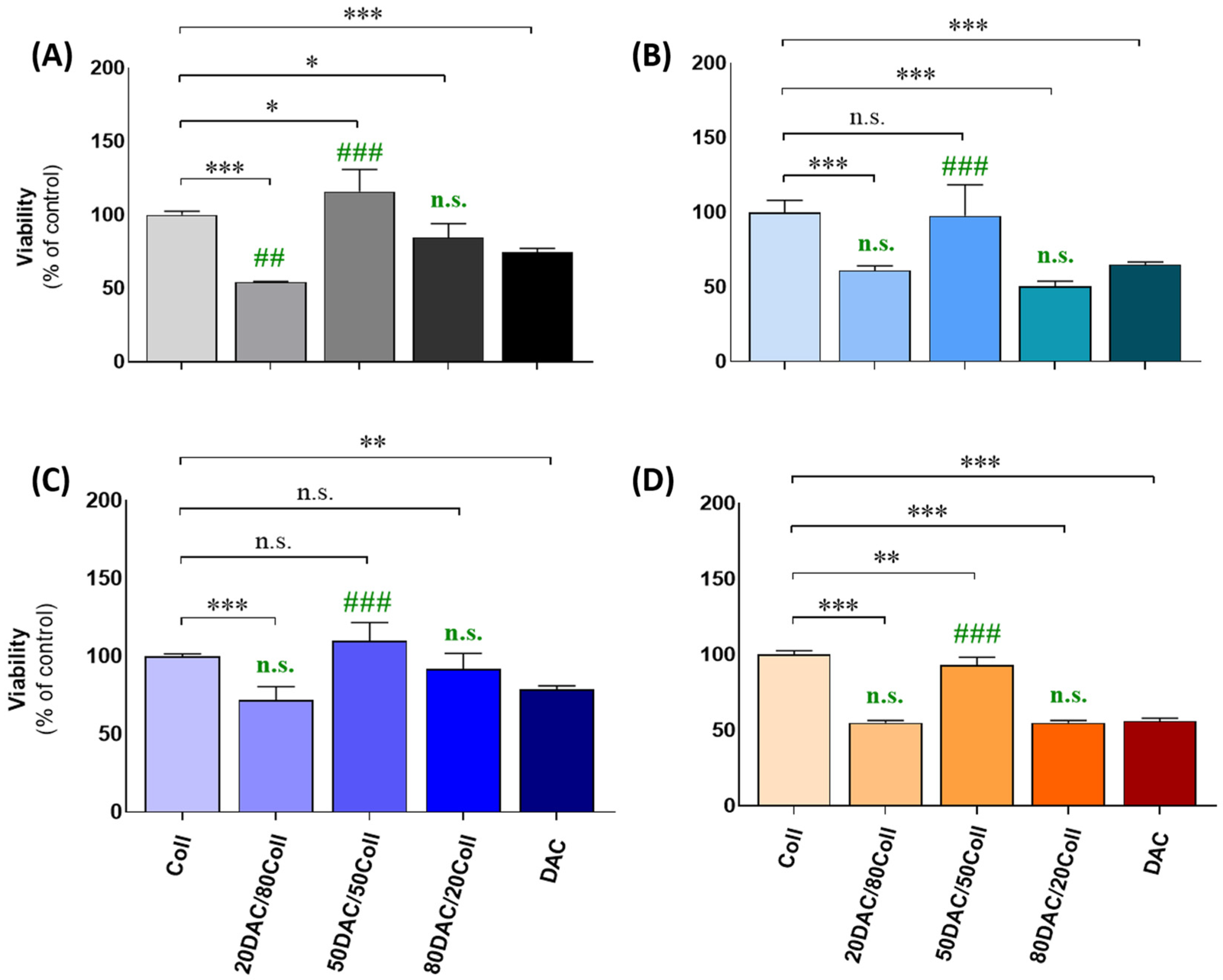
3.5. Cellular Assessments Using Cutaneous Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troy, E.; Tilbury, M.A.; Power, A.M.; Wall, J.G. Nature-based biomaterials and their application in biomedicine. Polymers 2021, 13, 3321. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Adamiak, K.; Musial, K.; Gadomska, M. Collagen based materials in cosmetic applications: A review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Seal, B.L.; Otero, T.C.; Panitch, A. Polymeric biomaterials for tissue and organ regeneration. Mater. Sci. Eng. Rep. 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A. Chitosan/collagen blends with inorganic and organic additive-a review. Adv. Polym. Technol. 2018, 37, 2367–2376. [Google Scholar] [CrossRef]
- Sionkowska, A.; Gadomska, M.; Musiał, K.; Piatek, J. Hyaluronic acid as a component of natural polymer blends for biomedical applications: A review. Molecules 2020, 25, 4035. [Google Scholar] [CrossRef]
- Sionkowska, A.; Grabska, S.; Lewandowska, K.; Andrzejczyk, A. Polymer films based on silk fibroin and collagen-the physico-chemical properties. Mol. Cryst. Liq. Cryst. 2016, 640, 13–20. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Sionkowska, A.; Kaminska, A.; Kaznica, A.; Jachimiak, R.; Drewa, T. Surface characterization of collagen/elastin based biomaterials for tissue regeneration. Appl. Surf. Sci. 2009, 255, 8286–8292. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen blended with natural polymers: Recent advances and trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Sionkowska, A. Interaction of collagen and poly(vinyl pyrrolidone) in blends. Eur. Polym. J. 2003, 39, 2135–2140. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skopińska, J.; Wisniewski, M. Photochemical stability of collagen/poly(vinyl alcohol) blends. Polym. Degrad. Stab. 2004, 83, 117–125. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skopinska-Wisniewska, J.; Wisniewski, M. Collagen–synthetic polymer interactions in solution and in thin films. J. Mol. Liq. 2009, 145, 135–138. [Google Scholar] [CrossRef]
- Yang, C. Enhanced physicochemical properties of collagen by using EDC/NHS-crosslinking. Bull. Mater. Sci. 2012, 35, 913–918. [Google Scholar] [CrossRef]
- Mu, C.; Liu, F.; Cheng, Q.; Li, H.; Wu, B.; Zhang, G.; Lin, W. Collagen cryogel cross-linked by dialdehyde starch. Macromol. Mater. Eng. 2010, 295, 100–107. [Google Scholar] [CrossRef]
- Nimni, M.E.; Cheung, D.; Strates, B.; Kodama, M.; Sheikh, K. Chemically modified collagen: A natural biomaterial for tissue replacement. J. Biomed. Mater. Res. 1987, 21, 741–771. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Monteiro, G.A.; Lapin, N.A.; Chabal, Y.J.; Miksan, J.R.; Shreiber, D.I. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. A 2008, 87, 308–320. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozłowska, J. Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int. J. Biol. Macromol. 2013, 52, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Weadock, K.S.; Miller, E.J.; Bellincampi, L.D.; Zawadsky, J.P.; Dunn, M.G. Physical crosslinking of collagen fibers: Comparison of ultraviolet irradiation and dehydrothermal treatment. J. Biomed. Mater. Res. 1995, 29, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.N.; Ho, H.O.; Sheu, M.T. Characterization of collagen matrices crosslinked using microbial transglutaminase. Biomaterials 2005, 26, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- Bam, P.; Bhatta, A.; Krishnamoorthy, G. Design of biostable scaffold based on collagen crosslinked by dialdehyde chitosan with presence of gallic acid. Int. J. Biol. Macromol. 2019, 130, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Wegrzynowska-Drzymalska, K.; Grebicka, P.; Mlynarczyk, D.T.; Chelminiak-Dudkiewicz, D.; Kaczmarek, H.; Goslinski, T.; Ziegler-Borowska, M. Crosslinking of chitosan with dialdehyde chitosan as a new approach for biomedical applications. Materials 2020, 13, 3413. [Google Scholar] [CrossRef]
- He, X.; Tao, R.; Zhou, T.; Wang, C.; Xie, K. Structure and properties of cotton fabrics treated with functionalized dialdehyde chitosan. Carbohydr. Polym. 2014, 103, 558–565. [Google Scholar] [CrossRef]
- Liu, X.; Dan, N.; Dan, W.; Gong, J. Feasibility study of the natural derived chitosan dialdehyde for chemical modification of collagen. Int. J. Biol. Macromol. 2016, 82, 989–997. [Google Scholar] [CrossRef]
- Langmaier, F.; Mládek, M.; Mokrejš, P. Hydrogels of collagen hydrolysate cross-linked with dialdehyde starch. J. Therm. Anal. Calor. 2009, 98, 807–812. [Google Scholar] [CrossRef]
- Jayakumar, G.; Kanth, S.; Rao, J.R.; Nair, B.U. A molecular level investigation of dialdehyde starch interaction with collagen for eco-friendly stabilization. J. Am. Leath. Chem. Assoc. 2015, 110, 145–151. [Google Scholar]
- Tan, H.; Wu, B.; Li, C.; Mu, C.; Li, H.; Lin, W. Collagen cryogel cross-linked by naturally derived dialdehyde carboxymethyl cellulose. Carbohydr. Polym. 2015, 129, 17–24. [Google Scholar] [CrossRef]
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Comp. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Pietrucha, K.; Safandowska, M. Dialdehyde cellulose-crosslinked collagen and its physicochemical properties. Proc. Biochem. 2015, 50, 2105–2111. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, L.; Gu, Z.; Dan, W.; Dan, N.; Yu, X. Modification of collagen with a natural derived cross-linker, alginate dialdehyde. Carbohydr. Polym. 2014, 102, 324–332. [Google Scholar] [CrossRef]
- Yu, X.; Yuan, Q.; Yang, M.; Liu, R.; Zhu, S.; Li, J.; Zhang, W.; You, J.; Xiong, S.; Hu, Y. Development of biocompatible and antibacterial collagen hydrogels via dialdehyde polysaccharide modification and tetracycline hydrochloride loading. Macromol. Mater. Eng. 2019, 304, 1800755. [Google Scholar] [CrossRef]
- Wanli, H.; Zihan, Y.; Yanan, W.; Bi, S. Preparation of dialdehyde chitosan and its application for crosslinking collagen fiber. Leath. Sci. Eng. 2021, 31, 1–5. [Google Scholar]
- Grabska-Zielińska, S.; Sosik, A.; Małkowska, A.; Olewnik-Kruszkowska, E.; Steinbrink, K.; Kleszczyński, K.; Kaczmarek-Szczepańska, B. The characterization of scaffolds based on dialdehyde chitosan/hyaluronic acid. Materials 2021, 14, 4993. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, K.; Sionkowska, A.; Grabska, S.; Kaczmarek, B.; Michalska, M. The miscibility of collagen/hyaluronic acid/chitosan blends investigated in dilute solutions and solids. J. Mol. Liq. 2016, 220, 726–730. [Google Scholar] [CrossRef]
- Sionkowska, A.; Grabska, S. Preparation and characterization of 3D collagen materials with magnetic properties. Polym. Test. 2017, 62, 382–391. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Lewandowska, K. Modification of collagen and chitosan mixtures by the addition of tannic acid. J. Mol. Liq. 2014, 199, 318–323. [Google Scholar] [CrossRef]
- Sionkowska, A.; Wiśniewski, M.; Skopińska, J.; Mantovani, D. Effects of solar radiation on collagen-based biomaterials. Int. J. Phot. 2006, 2006, 1–6. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozłowska, J.; Skorupska, M.; Michalska, M. Isolation and characterization of collagen from the skin of brama australis. Int. J. Biol. Macromol. 2015, 80, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Keshk, S.M.A.S.; Ramadan, A.M.; Al-Sehemi, A.G.; Irfan, A.; Bondock, S. An unexpected reactivity during periodate oxidation of chitosan and the affinity of its 2,3-di-aldehyde toward sulfa drugs. Carbohydr. Polym. 2017, 175, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Long, Z.; Song, C.; Dai, L.; He, H.; Wang, P. Preparation of dialdehyde chitosan and its application in green synthesis of silver nanoparticles. BioResources 2013, 8, 6161–6172. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Wang, S.; Liu, B.; Yao, S.; Dai, Y.; Zhou, L.; Qin, C.; Fatehi, P. Sustainable chitosan-dialdehyde cellulose nanocrystal film. Materials 2021, 14, 5851. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A. The physical and chemical properties of hydrogels based on natural polymers. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Cambridge, MA, USA, 2020; pp. 151–172. [Google Scholar]
- Carey, S.P.; Kraning-Rush, C.M.; Williams, R.M.; Reinhart-King, C.A. Biophysical control of invasive tumor cell behavior by extracellular matrix microarchitecture. Biomaterials 2012, 33, 4157–4165. [Google Scholar] [CrossRef] [Green Version]
- Grabska-Zielińska, S.; Sionkowska, A.; Reczyńska, K.; Pamuła, E. Physico-chemical characterization and biological tests of collagen/silk fibroin/chitosan scaffolds cross-linked by dialdehyde starch. Polymers 2020, 12, 372. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Zhang, Y.; Yuan, B.; Yang, X.; Shi, R.; Zhang, M. The preparation of nano-SiO2/dialdehyde cellulose hybrid materials as a novel cross-linking agent for collagen solutions. Polymers 2018, 10, 550. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, B.; Miłek, O.; Michalska-Sionkowska, M.; Zasada, L.; Twardowska, M.; Warżyńska, O.; Kleszczyński, K.; Osyczka, A.M. Novel eco-friendly tannic acid-enriched hydrogels-preparation and characterization for biomedical application. Materials 2020, 13, 4572. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Ostrowska, J.; Kozłowska, J.; Szota, Z.; Brożyna, A.A.; Dreier, R.; Reiter, R.J.; Slominski, A.T.; Steinbrink, K.; Kleszczyński, K. Evaluation of polymeric matrix loaded with melatonin for wound dressing. Int. J. Mol. Sci. 2021, 22, 5658. [Google Scholar] [CrossRef]
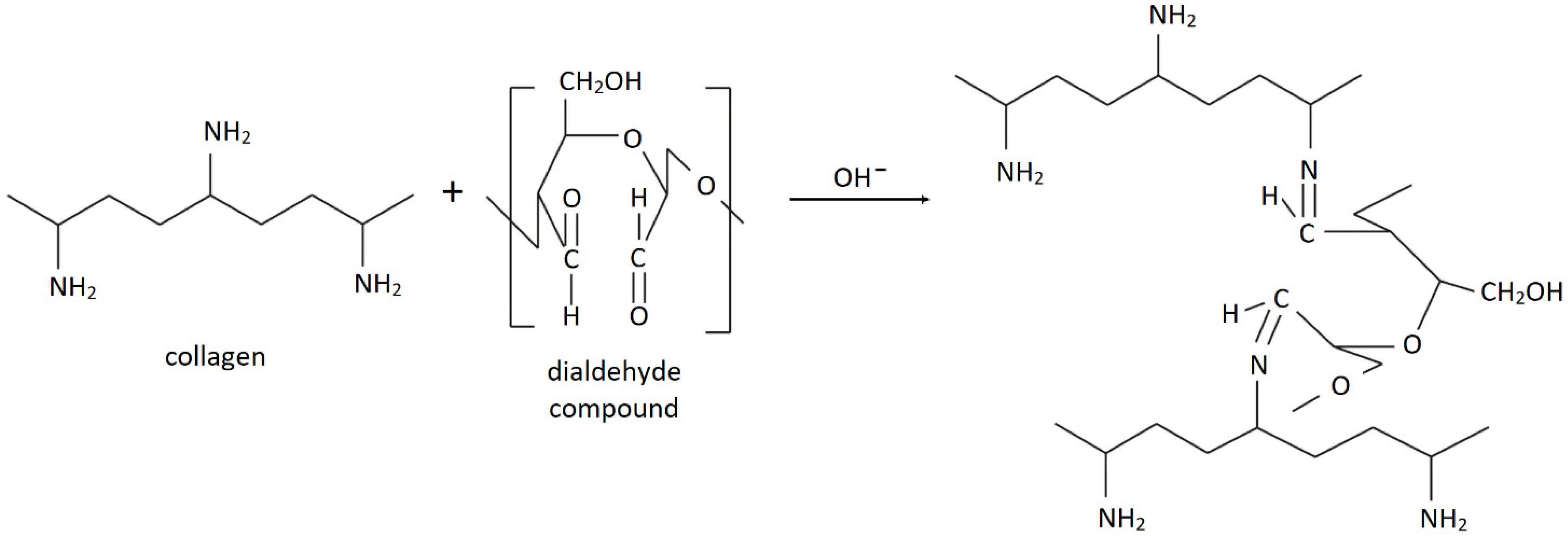



| Specimen | Amide A | Amide B | CH3 | C–OH | Amide I | C=N | Amide II | Amide III |
|---|---|---|---|---|---|---|---|---|
| 20DAC/80Coll | 3313 | 3077 | 2932 | − | 1656 | − | 1556 | 1241 |
| 50DAC/50Coll | 3306 | 3078 | 2934 | 1730 | 1656 | − | 1556 | 1241 |
| 80DAC/20Coll | 3322 | 3086 | 2938 | 1731 | 1658 | 1632 | 1556 | 1240 |
| Specimen | Tmax (1) (°C) | Tmax (2) (°C) |
|---|---|---|
| 100Coll | 47.5 | 324.3 |
| 20DAC/80Coll | 147.0 | 326.7 |
| 50DAC/50Coll | 147.1 | 314.3 |
| 80DAC/20Coll | 147.6 | 307.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabska-Zielińska, S.; Pin, J.M.; Kaczmarek-Szczepańska, B.; Olewnik-Kruszkowska, E.; Sionkowska, A.; Monteiro, F.J.; Steinbrink, K.; Kleszczyński, K. Scaffolds Loaded with Dialdehyde Chitosan and Collagen—Their Physico-Chemical Properties and Biological Assessment. Polymers 2022, 14, 1818. https://doi.org/10.3390/polym14091818
Grabska-Zielińska S, Pin JM, Kaczmarek-Szczepańska B, Olewnik-Kruszkowska E, Sionkowska A, Monteiro FJ, Steinbrink K, Kleszczyński K. Scaffolds Loaded with Dialdehyde Chitosan and Collagen—Their Physico-Chemical Properties and Biological Assessment. Polymers. 2022; 14(9):1818. https://doi.org/10.3390/polym14091818
Chicago/Turabian StyleGrabska-Zielińska, Sylwia, Judith M. Pin, Beata Kaczmarek-Szczepańska, Ewa Olewnik-Kruszkowska, Alina Sionkowska, Fernando J. Monteiro, Kerstin Steinbrink, and Konrad Kleszczyński. 2022. "Scaffolds Loaded with Dialdehyde Chitosan and Collagen—Their Physico-Chemical Properties and Biological Assessment" Polymers 14, no. 9: 1818. https://doi.org/10.3390/polym14091818
APA StyleGrabska-Zielińska, S., Pin, J. M., Kaczmarek-Szczepańska, B., Olewnik-Kruszkowska, E., Sionkowska, A., Monteiro, F. J., Steinbrink, K., & Kleszczyński, K. (2022). Scaffolds Loaded with Dialdehyde Chitosan and Collagen—Their Physico-Chemical Properties and Biological Assessment. Polymers, 14(9), 1818. https://doi.org/10.3390/polym14091818












