Water-Triggered Self-Healing Composite Coating: Fabrication and Anti-Corrosion Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
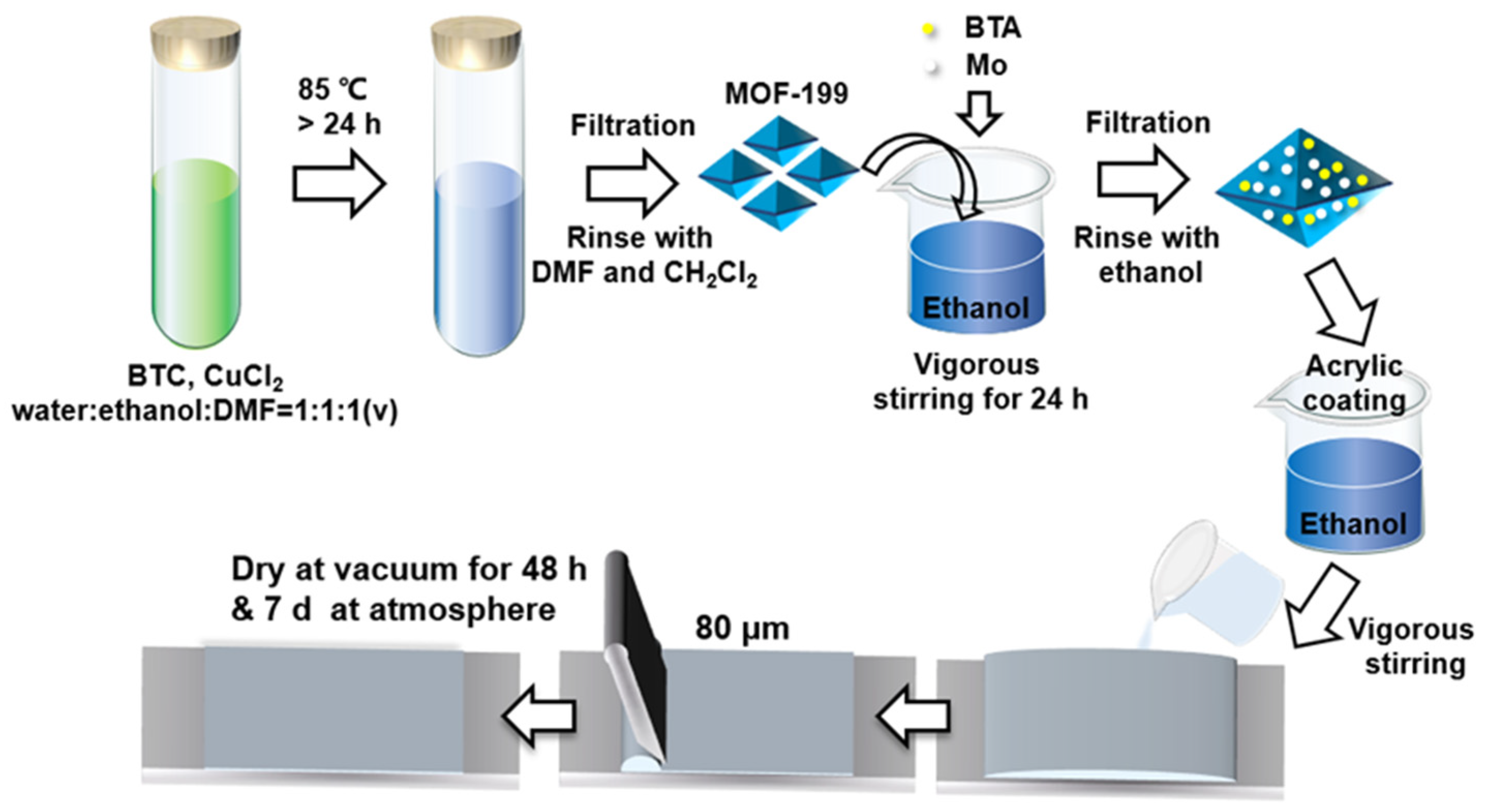
2.2. Fabrication of MOF-199
2.3. Fabrication of BTA-Mo@MOF-199
2.4. Fabrication of Self-Healing Acrylic Coating
2.5. Characterization
3. Results and Discussion
3.1. Characterization of the Synthesized MOF-199
3.2. Characterization of the BTA-Mo@MOF-199
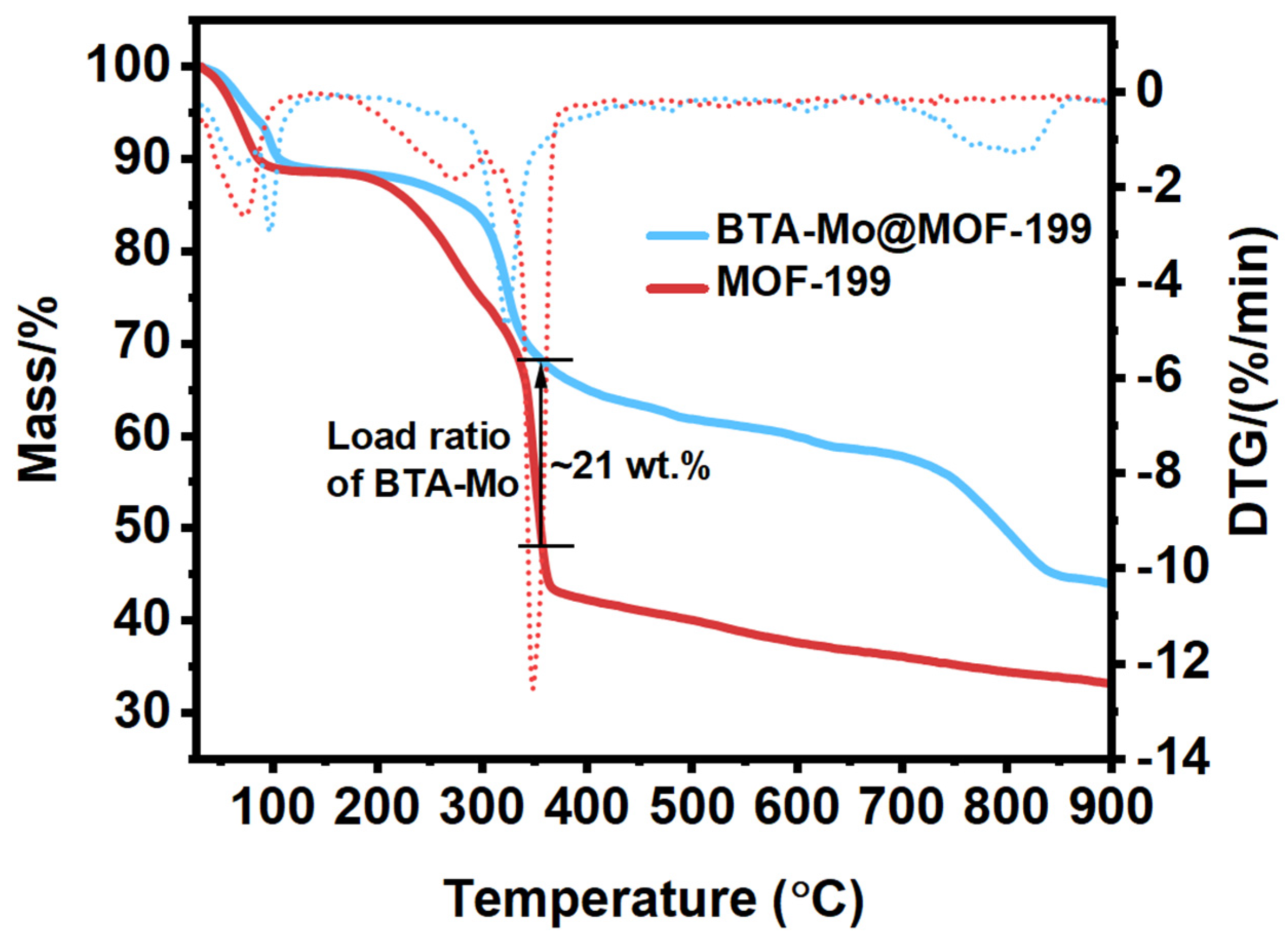
3.3. Thermostability of MOF-199 and BTA-Mo@MOF-199
3.4. Water Sensitivity of MOF-199
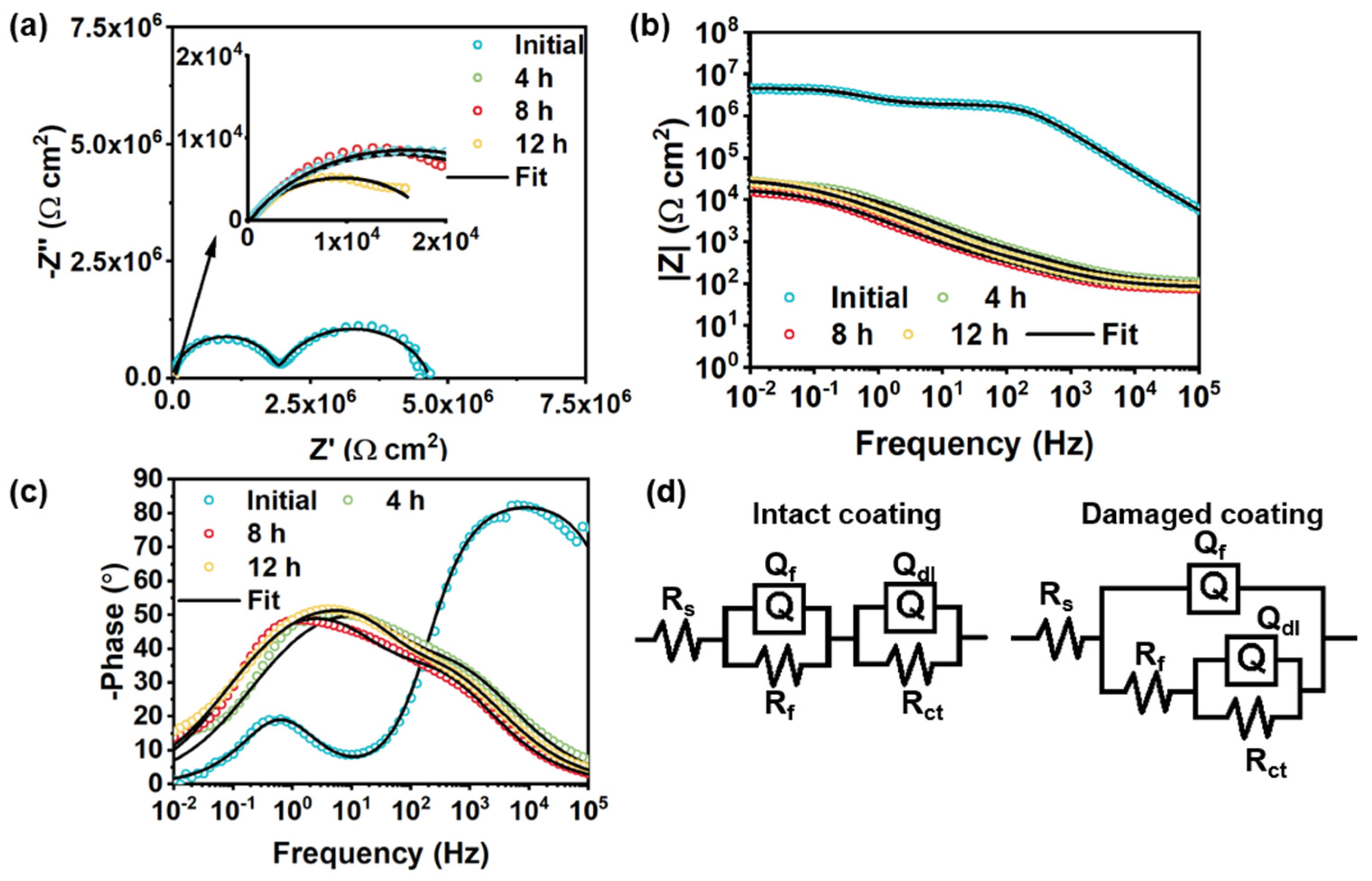
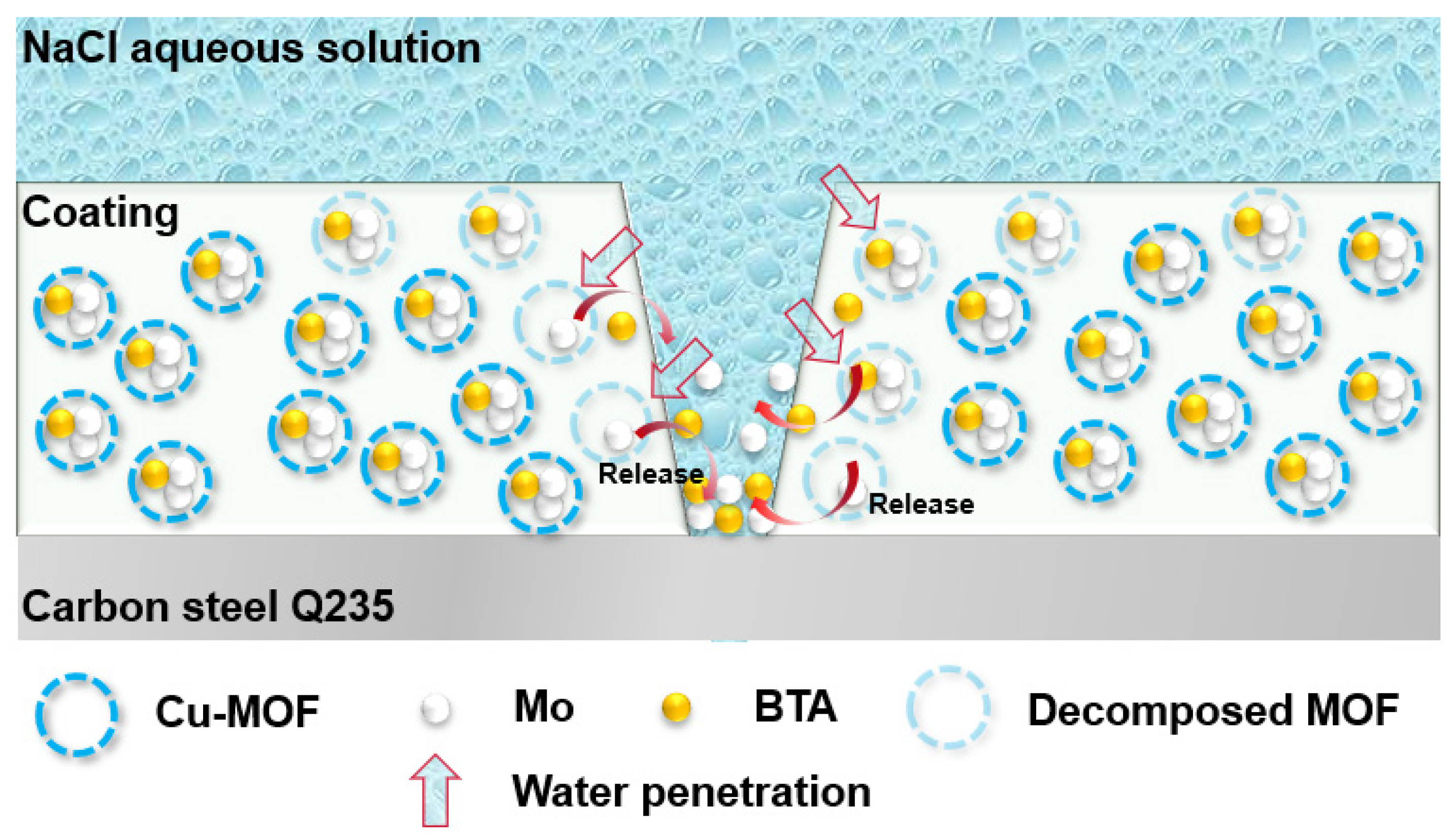
3.5. Self-Healing Performance of Acrylic Coating Doped with BTA-Mo@MOF-199
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.H.; Yang, W.Z.; Chen, Y.; Yin, X.S.; Liu, Y. Smart coatings embedded with polydopamine-decorated layer-by-layer assembled SnO2 nanocontainers for the corrosion protection of 304 stainless steels. J. Colloid Interface Sci. 2020, 579, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Yang, W.Z.; Yin, X.S.; Chen, Y.; Liu, Y.; Xu, B. Corrosion protection of 304 stainless steel from a smart conducting polypyrrole coating doped with pH-sensitive molybdate-loaded TiO2 nanocontainers. Prog. Org. Coat. 2020, 146, 105750. [Google Scholar] [CrossRef]
- Dong, Y.; Geng, C.; Wang, X.; Zhou, Q. Porous polystyrene nanoparticles as nanocontainers of inhibitors for corrosion protection of low-alloy steel. Pigment Resin Technol. 2020, 49, 305–313. [Google Scholar] [CrossRef]
- Dong, Y.H.; Geng, C.D.; Liu, C.M.; Gao, J.; Zhou, Q. Near-infrared light photothermally induced shape memory and self-healing effects of epoxy resin coating with polyaniline nanofibers. Synth. Met. 2020, 266, 116417. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Fugivara, C.S.; Taryba, M.; Montemor, M.F.; Benedetti, A.V. Self-healing ability based on hydrogen bonds in organic coatings for corrosion protection of AA1200. Corros. Sci. 2020, 177, 108984. [Google Scholar] [CrossRef]
- Akhondi, M.; Jamalizadeh, E. Fabrication of beta-cyclodextrin modified halloysite nanocapsules for controlled release of corrosion inhibitors in self-healing epoxy coatings. Prog. Org. Coat. 2020, 145, 105676. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. Construction of a sustainable/controlled-release nano-container of non-toxic corrosion inhibitors for the water-based siliconized film: Estimating the host-guest interactions/desorption of inclusion complexes of cerium acetylacetonate (CeA) with beta-cyclodextrin (beta-CD) via detailed electronic/atomic-scale computer modeling and experimental methods. J. Hazard. Mater. 2020, 399, 123046. [Google Scholar]
- Chen, L.G.; Yu, Z.X.; Yin, D.; Cao, K.Y. Preparation and anticorrosion properties of BTA@HNTs-GO nanocomposite smart coatings. Compos. Interfaces 2021, 28, 1–16. [Google Scholar] [CrossRef]
- Hao, Y.S.; Zhao, Y.F.; Li, B.; Song, L.X.; Guo, Z. Self-healing effect of graphene@PANI loaded with benzotriazole for carbon steel. Corros. Sci. 2020, 163, 108246. [Google Scholar] [CrossRef]
- Javidparvar, A.A.; Naderi, R.; Ramezanzadeh, B. Manipulating graphene oxide nanocontainer with benzimidazole and cerium ions: Application in epoxy-based nanocomposite for active corrosion protection. Corros. Sci. 2020, 165, 108379. [Google Scholar] [CrossRef]
- Liu, C.B.; Jin, Z.Y.; Zhao, H.C.; Wang, L.P. Triple-action smart nanocontainers enhanced protective coatings with superior active and passive properties. Prog. Org. Coat. 2020, 148, 105887. [Google Scholar] [CrossRef]
- Amini, M.; Naderi, R.; Mandavian, M.; Badiei, A. Effect of piperazine functionalization of mesoporous silica type SBA-15 on the loading efficiency of 2-mercaptobenzothiazole corrosion inhibitor. Ind. Eng. Chem. Res. 2020, 59, 3394–3404. [Google Scholar] [CrossRef]
- Atta, A.M.; Ezzat, A.O.; El-Saeed, A.M.; Tawfeek, A.M.; Sabeela, N.I. Self-healing of chemically bonded hybrid silica/epoxy for steel coating. Prog. Org. Coat. 2020, 141, 105549. [Google Scholar] [CrossRef]
- Castro, Y.; Ozmen, E.; Duran, A. Integrated self-healing coating system for outstanding corrosion protection of AA2024. Surf. Coat. Technol. 2020, 387, 125521. [Google Scholar] [CrossRef]
- Khan, A.; Hassanein, A.; Habib, S.; Nawaz, M.; Shakoor, R.A.; Kahraman, R. Hybrid Halloysite Nanotubes a Smart Carriers for Corrosion Protection. ACS Appl. Mater. Interfaces 2020, 12, 37571–37584. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.S.; He, K.H.; Wei, R.Z.; Lv, Y.J.; Liu, Z.; Han, G.C. Synthesis of Mn-MOFs loaded zinc phosphate composite for water-based acrylic coatings with durable anticorrosion performance on mild steel. RSC Adv. 2021, 11, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Yu, Z.; Yin, D. Preparation of Ce-MOF@TEOS to enhance the anti-corrosion properties of epoxy coatings. Prog. Org. Coat. 2019, 135, 613–621. [Google Scholar] [CrossRef]
- Chen, G.M.; Wen, S.F.; Ma, J.C.; Sun, Z.Y.; Lin, C.G.; Yue, Z.F.; Mol, J.M.C.; Liu, M. Optimization of intrinsic self-healing silicone coatings by benzotriazole loaded mesoporous silica. Surf. Coat. Technol. 2021, 421, 127388. [Google Scholar] [CrossRef]
- Anh, H.T.; Vu, N.S.H.; Huyen, L.T.; Tran, N.Q.; Thu, H.T.; Bach, L.X.; Trinh, Q.T.; Prabhakar Vattikuti, S.V.; Nam, N.D. Ficus racemosa leaf extract for inhibiting steel corrosion in a hydrochloric acid medium. Alex. Eng. J. 2020, 59, 4449–4462. [Google Scholar] [CrossRef]
- Van Cong, T.; Hung, N.D.; Tran Thi, N.D.; Van Hoang, N.; Vattikuti, S.V.P.; Dang, N.N. Electrochemical Plasma for Treating 2,4,5-Trichlorophenoxyacetic Acid in a Water Environment Using Iron Electrodes. ACS Omega 2021, 6, 26329–26337. [Google Scholar] [CrossRef]
- Zidov, B.; Lin, Z.F.; Stojanovic, I.; Xu, L.K. Impact of inhibitor loaded mesoporous silica nanoparticles on waterborne coating performance in various corrosive environments. J. Appl. Polym. Sci. 2021, 138, 49614. [Google Scholar] [CrossRef]
- Tang, J.B.; Yao, Y.H.; Guo, M.L.; Jiang, J.B.; Cong, H.S.; Chen, Y.K.; Sun, Y.X.; Kong, Y.; Han, S. Anticorrosion and repair behavior of REC-SiO2 silane film on carbon steel. Surf. Rev. Lett. 2021, 28, 21500980. [Google Scholar] [CrossRef]
- Kirdeikiene, A.; Girciene, O.; Gudaviciute, L.; Jasulaitiene, V.; Selskis, A.; Tutliene, S.; Skruodiene, M.; Pilipavicius, J.; Juodkazyte, J.; Ramanauskas, R. Self-Healing Properties of Cerium-Modified Molybdate Conversion Coating on Steel. Coatings 2021, 11, 194. [Google Scholar] [CrossRef]
- Thi, T.V.N.; Luu, C.L.; Hoang, T.C.; Nguyen, T.; Bui, T.H.; Nguyen, P.H.D.; Thi, T.P.P. Synthesis of MOF-199 and application to CO2 adsorption. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 035016. [Google Scholar]
- Sanaei, M.; Fazaeli, R.; Aliyan, H. Pd/MOF-199: As an efficient heterogeneous catalyst for the Suzukie Miyaura cross-coupling reaction. J. Chin. Chem. Soc. 2019, 66, 1290–1295. [Google Scholar] [CrossRef]
- Nguyen, L.T.L.; Nguyen, T.T.; Nguyen, K.D.; Phan, N.T.S. Metal–organic framework MOF-199 as an efficient heterogeneous catalyst for the aza-Michael reaction. Appl. Catal. A Gen. 2012, 425–426, 44–52. [Google Scholar] [CrossRef]
- Hassan, A.F. Synthesis of carbon nano-onion embedded metal-organic frameworks as an efficient adsorbent for cadmium ions: Kinetic and thermodynamic studies. Environ. Sci. Pollut. Res. Int. 2019, 26, 24099–24111. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Concernng the conversion of the constant phase element parameter Y0 into a capacitance. CORROSION 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Lashgari, S.M.; Yari, H.; Mahdavian, M.; Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Application of nanoporous cobalt-based ZIF-67 metal-organic framework (MOF) for construction of an epoxy-composite coating with superior anti-corrosion properties. Corros. Sci. 2021, 178, 109099. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, J.; Zhang, D.; Qi, T.; Li, G.L. pH-responsive self-healing anticorrosion coatings based on benzotriazole-containing zeolitic imidazole framework. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 1–8. [Google Scholar] [CrossRef]
- Javadian, S.; Ahmadpour, Z.; Yousefi, A. Polypyrrole nanocapsules bearing quaternized alkyl pyridine in a green self-healing coating for corrosion protection of zinc. Prog. Org. Coat. 2020, 147, 105678. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.H.; Hu, W.B. Preparation and corrosion behavior of Cu-8-HQ@HNTs/epoxy coating. Prog. Org. Coat. 2020, 139, 105434. [Google Scholar] [CrossRef]




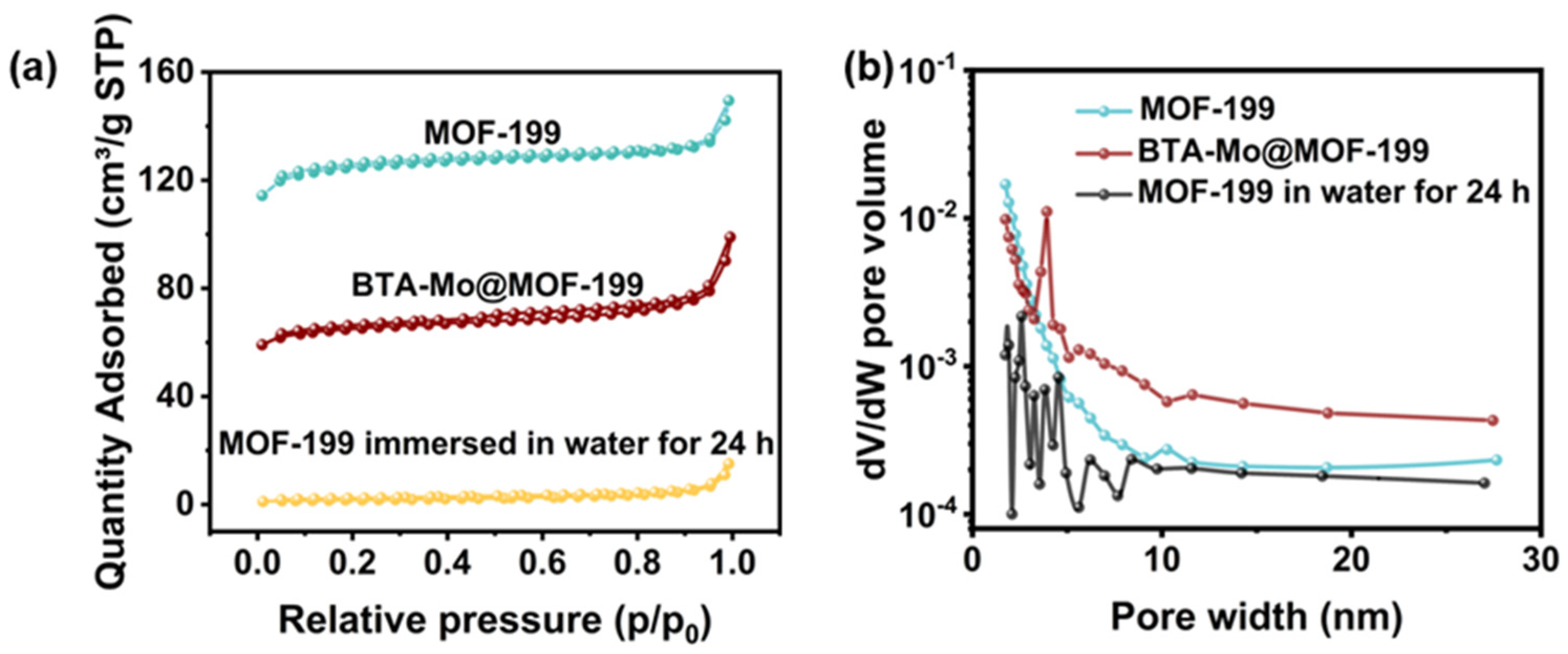


| MOF-199 | BTA-Mo@MOF-199 | MOF-199 in Water | |
|---|---|---|---|
| Langmuir specific area (m2/g) | 598.75 | 341.39 | 31.65 |
| Pore Volume (cm3/g) | 0.21 | 0.12 | 0.01 |
| Average pore size (nm) | 2.20 | 2.05 | 7.38 |
| Bare CS | Initial Intact Coating | Damaged Pure Coating | |||
|---|---|---|---|---|---|
| 4 h | 8 h | 12 h | |||
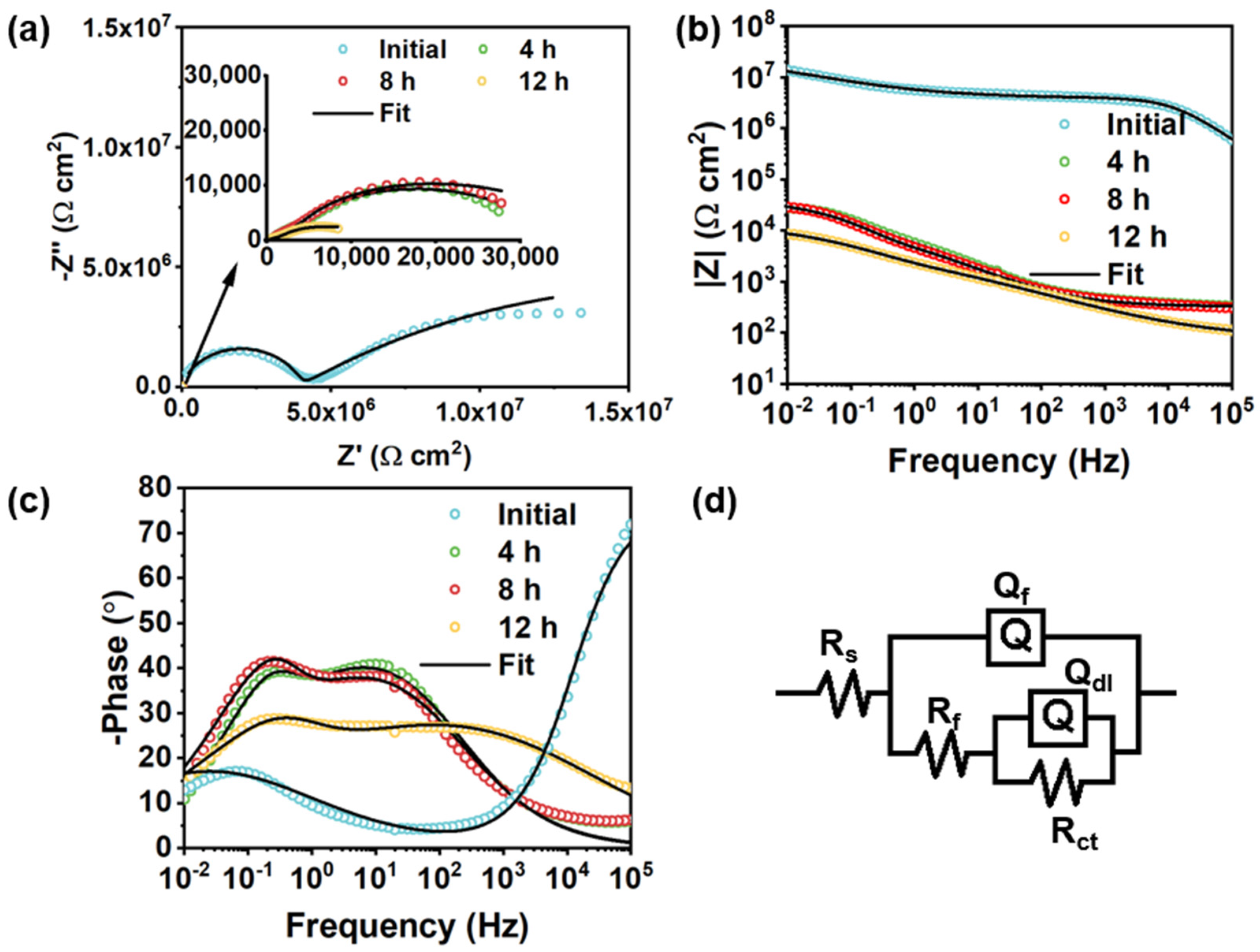
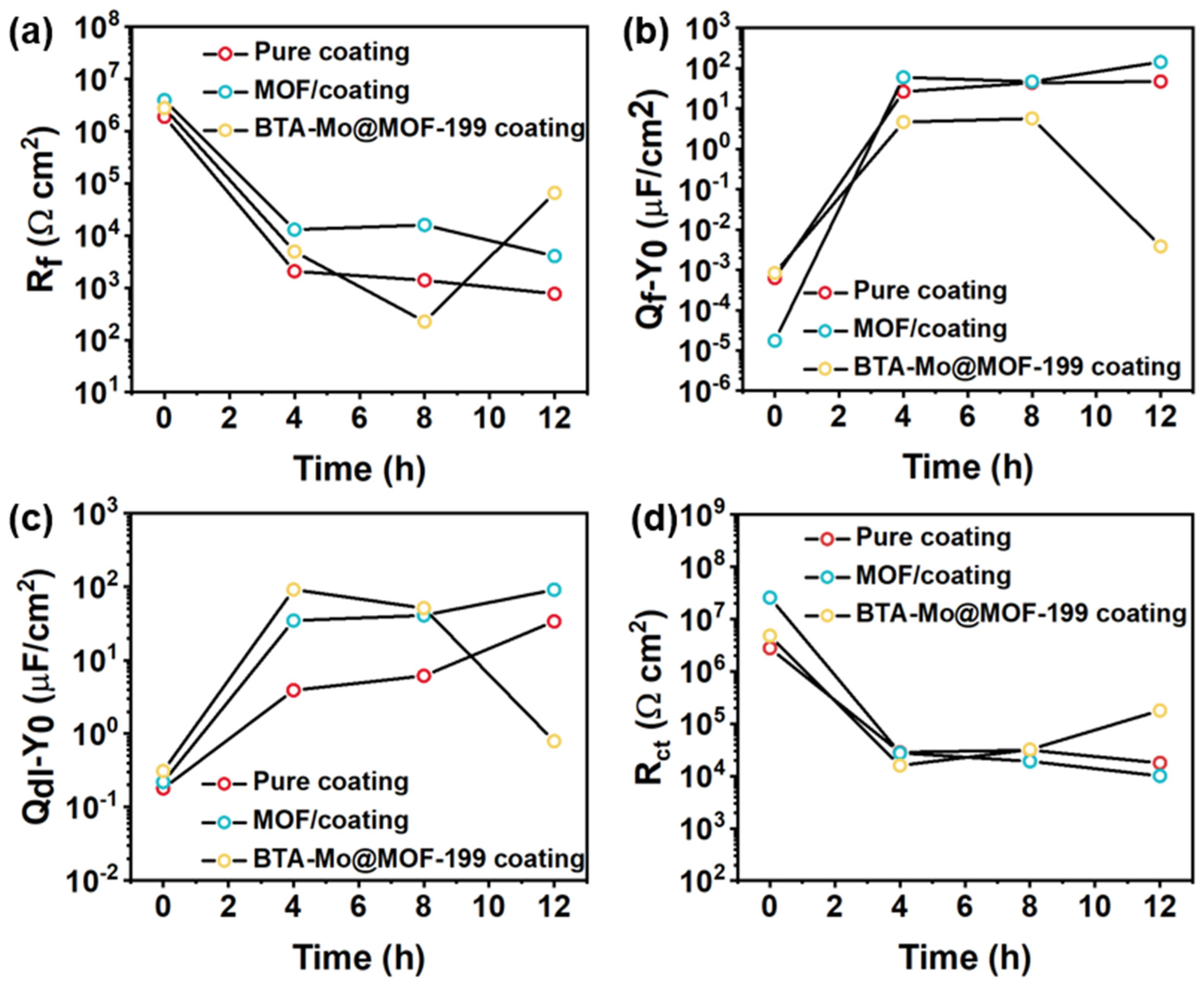
| Rf (Ω cm2) | \ | 1.89 × 106 | 2077 | 1395 | 768.8 |
| Qf−Y0 (μF/cm2) | \ | 6.28 × 10−4 | 26.42 | 43.55 | 47 |
| Qf−n | \ | 0.94 | 0.59 | 0.60 | 0.63 |
| Rct (Ω cm2) | 390.4 | 2.78 × 106 | 2.85 × 104 | 3.13 × 104 | 1.78 × 104 |
| Qdl−Y0 (μF/cm2) | 3.75 × 104 | 0.18 | 3.89 | 6.13 | 33.88 |
| Qdl−n | 0.80 | 0.82 | 0.80 | 0.82 | 0.7 |
| Bare CS | Initial Intact Coating | Damaged MOF-199/Acrylic Coating | |||
|---|---|---|---|---|---|
| 4 h | 8 h | 12 h | |||
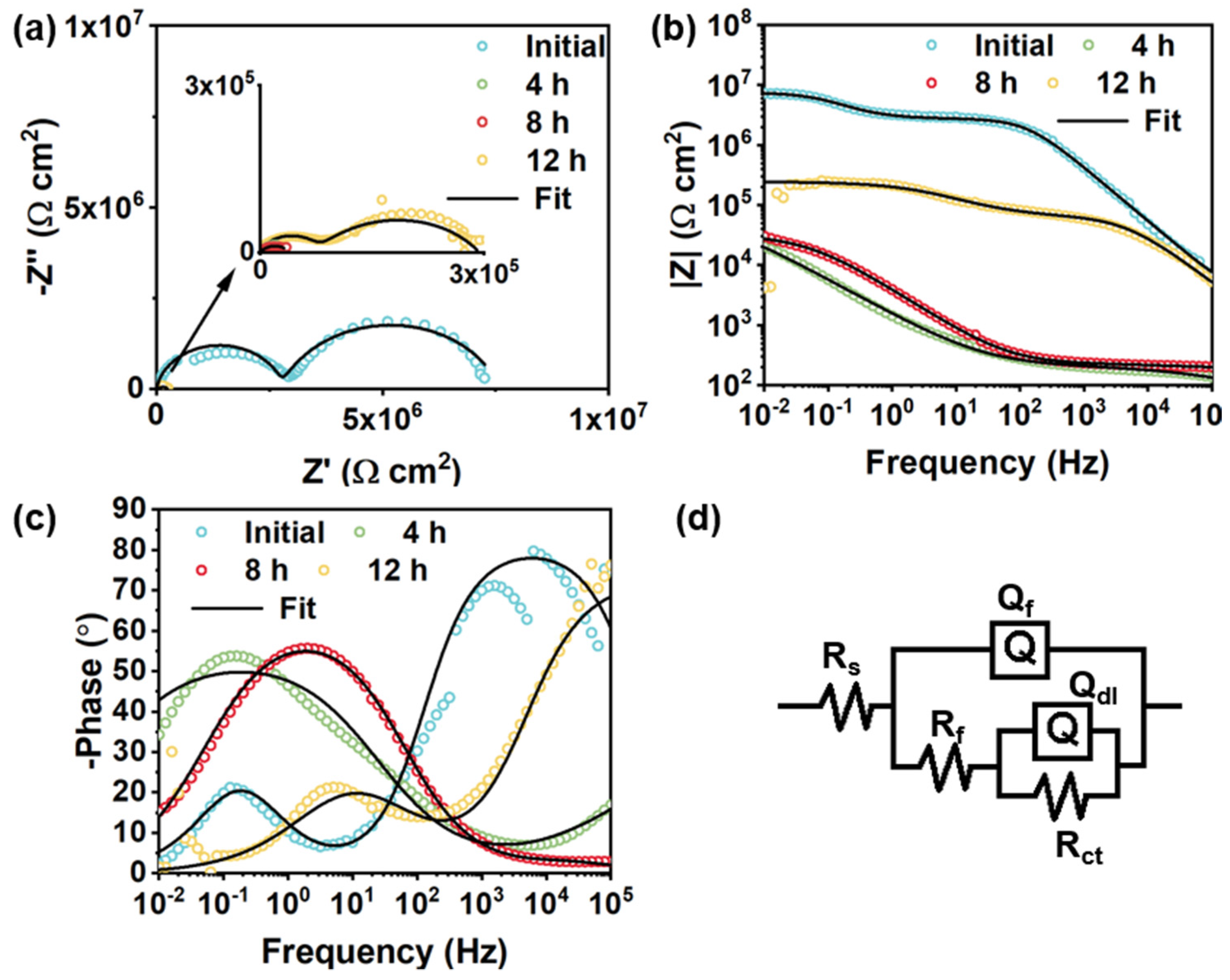
| Rf (Ω cm2) | \ | 3.95 × 106 | 12,980 | 16,060 | 4605 |
| Qf−Y0 (μF/cm2) | \ | 1.75 × 10−5 | 59.7 | 46.93 | 143.5 |
| Qf−n | \ | 0.85 | 0.56 | 0.58 | 0.39 |
| Rct(Ω cm2) | 390.4 | 2.58 × 107 | 27,560 | 19,130 | 10,070 |
| Qdl−Y0 (μF/cm2) | 37,500 | 0.22 | 34.72 | 40.76 | 91.36 |
| Qdl−n | 0.8 | 0.4 | 1 | 1 | 0.74 |
| Bare CS | Initial Intact Coating | Damaged Self-Healing Coating | |||
|---|---|---|---|---|---|
| 4 h | 8 h | 12 h | |||
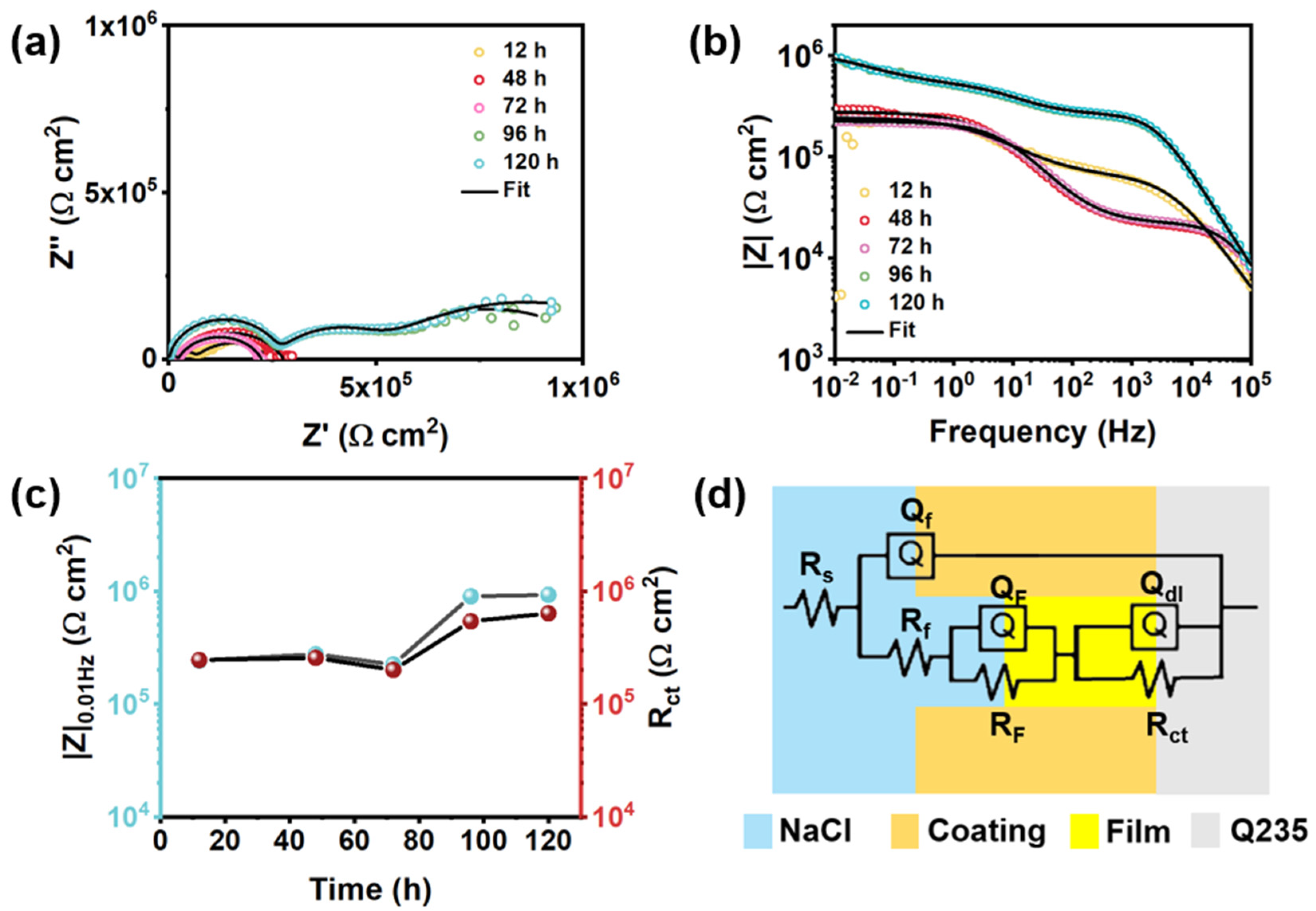
| Rf (Ω cm2) | \ | 2.77 × 106 | 4.97 × 103 | 2.25 × 102 | 2.35 × 103 |
| Qf−Y0 (μF/cm2) | \ | 8.32 × 10−4 | 4.64 | 5.69 | 7.93 |
| Qf−n | \ | 0.91 | 0.46 | 0.35 | 0.44 |
| Rct(Ω cm2) | 390.4 | 4.82 × 106 | 1.59 × 104 | 3.22 × 104 | 2.45 × 105 |
| Qdl−Y0 (μF/cm2) | 3.75 × 104 | 0.31 | 91.85 | 51.24 | 2.17 |
| Qdl−n | 0.8 | 0.8 | 0.44 | 0.72 | 0.7 |
| Stimulus | Container | Response Time | Inhibitor | Ref. |
|---|---|---|---|---|
| pH | TiO2 | 20 d | Mo | [2] |
| pH | Graphene@ polyaniline | 14 d | BTA | [9] |
| pH | Halloysite nanotubes | 3 d | Imidazole | [15] |
| pH | ZIF-67 | 30 d | 3-Aminopropyl Triethoxysilane | [30] |
| pH | Zeolitic imidazole framework | 5 d | Benzotriazole | [31] |
| pH | Polypyrrole | 10 d | Quaternized alkyl Pyridine | [32] |
| pH | Halloysite nanotubes | 2.5 d | Hydroxyquinoline | [33] |
| water | MOF−199 | 12 h | BTA and Mo | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Chen, S.; Chen, Z.; Lin, Z.; Li, W. Water-Triggered Self-Healing Composite Coating: Fabrication and Anti-Corrosion Application. Polymers 2022, 14, 1847. https://doi.org/10.3390/polym14091847
Hao Z, Chen S, Chen Z, Lin Z, Li W. Water-Triggered Self-Healing Composite Coating: Fabrication and Anti-Corrosion Application. Polymers. 2022; 14(9):1847. https://doi.org/10.3390/polym14091847
Chicago/Turabian StyleHao, Zhentao, Si Chen, Zhiwei Chen, Zhifeng Lin, and Weihua Li. 2022. "Water-Triggered Self-Healing Composite Coating: Fabrication and Anti-Corrosion Application" Polymers 14, no. 9: 1847. https://doi.org/10.3390/polym14091847






