Extraction and Characterization of Microcrystalline Cellulose from Lagenaria siceraria Fruit Pedicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Cellulose Fibers from L. siceraria Pedicle
2.2.1. Water Treatment
2.2.2. Alkali Treatment
2.2.3. Bleaching
2.3. Fabrication of MCC
2.4. Characterization
2.4.1. Fourier Transform Infrared Spectroscopy
2.4.2. X-ray Diffraction (XRD) Analysis
2.4.3. Scanning Electron Microscopy and Energy-Dispersion X-ray Analyses
2.4.4. Thermal Analysis
3. Results and Discussion
3.1. Extraction of MCC from Lagenaria siceraria Pedicle
3.2. Characterization
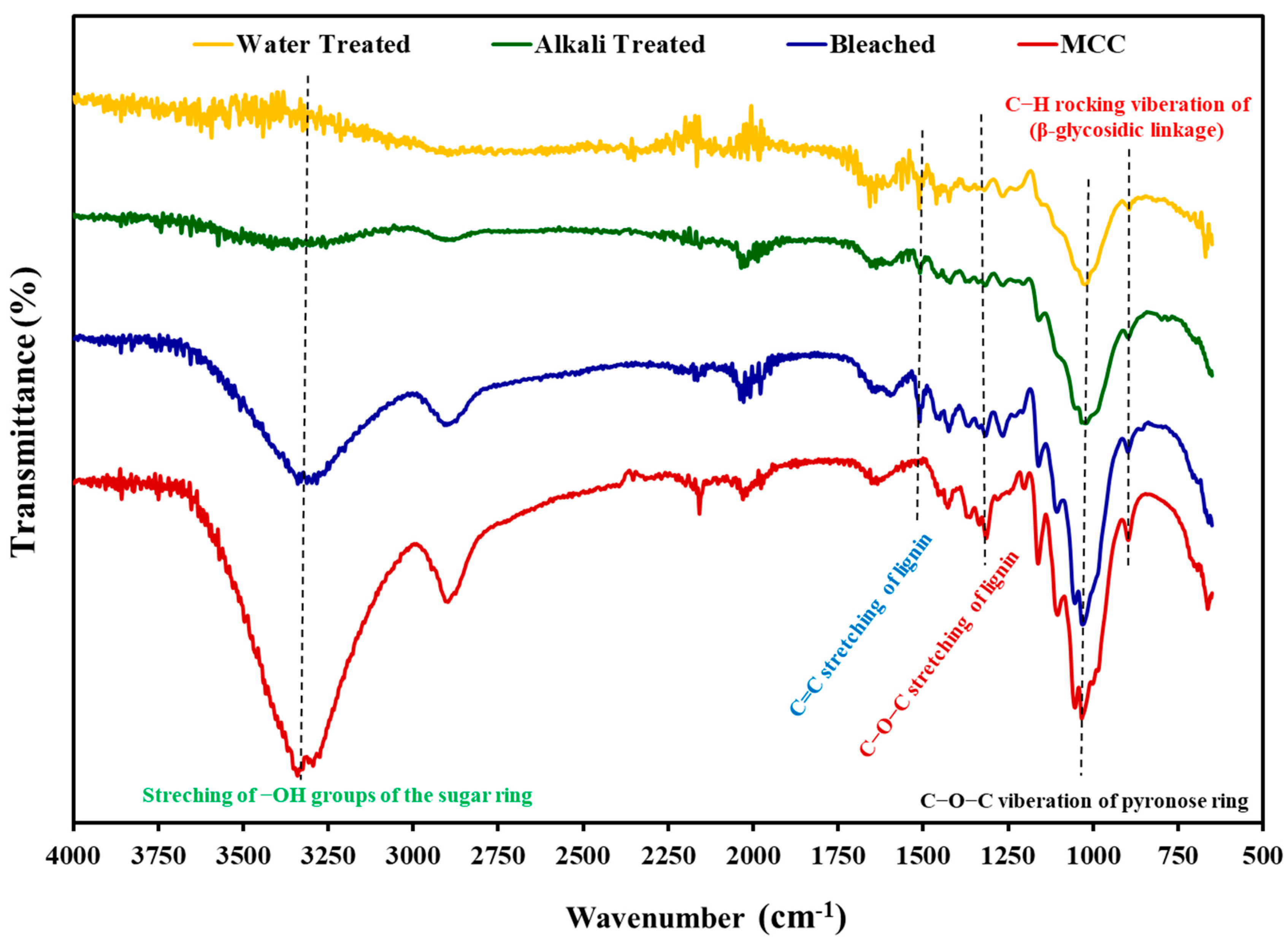
3.2.1. Fourier Transform Infrared Spectroscopy
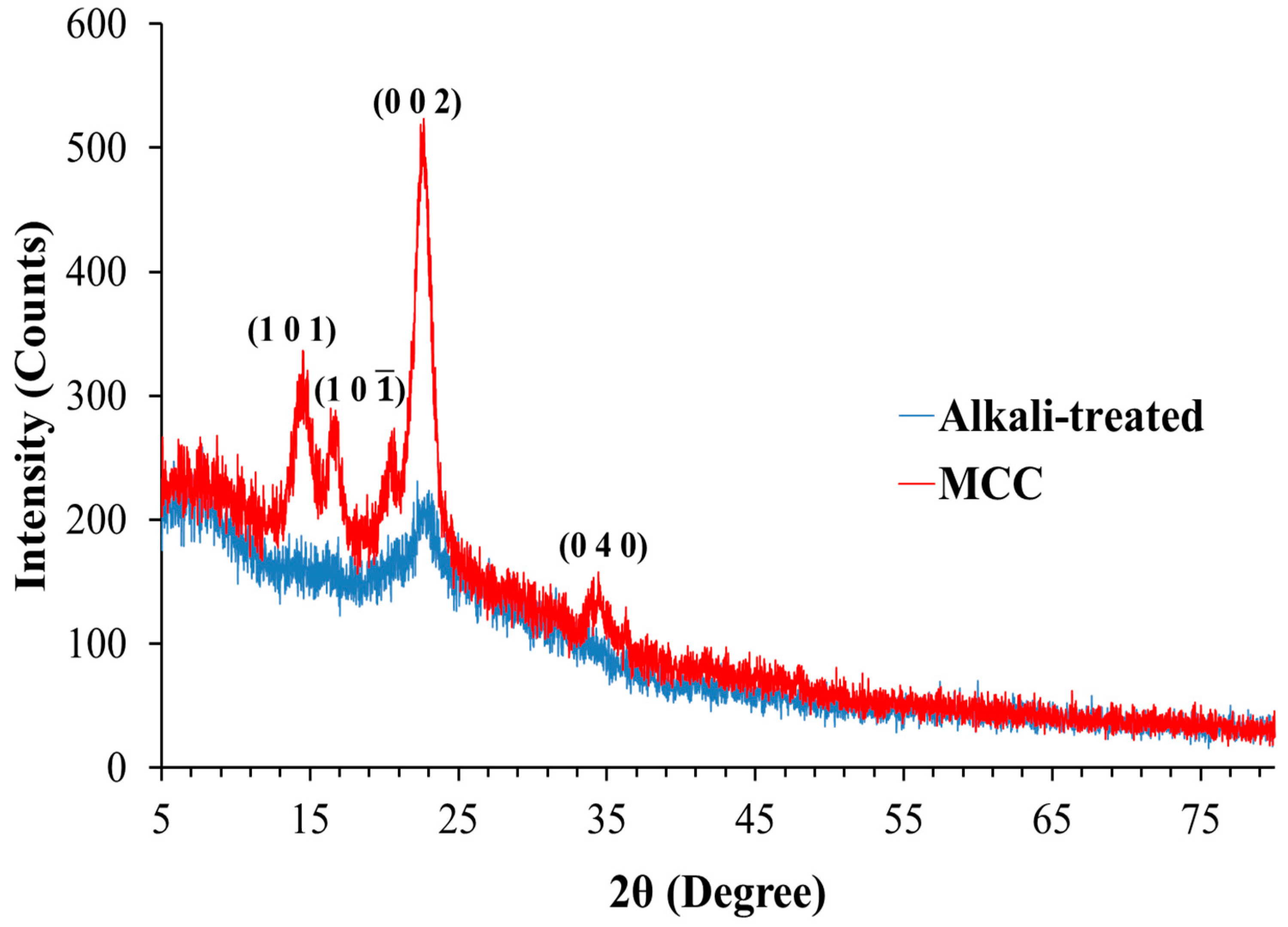
3.2.2. X-ray Diffraction Analysis
3.2.3. Scanning Electron Microscopy and Energy-Dispersion X-ray Analyses
3.2.4. Thermal Analysis (TGA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, W.; Ling, C.; Shi, S.; Yan, Z. Preparation and characterization of microcrystalline cellulose from waste cotton fabrics by using phosphotungstic acid. Int. J. Biol. Macromol. 2019, 123, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Pachuau, L.; Dutta, R.S.; Hauzel, L.; Devi, T.B.; Deka, D. Evaluation of novel microcrystalline cellulose from Ensete glaucum (Roxb.) Cheesman biomass as sustainable drug delivery biomaterial. Carbohydr. Polym. 2019, 206, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Cruz, S.; Tecante, A. Nanocellulose and microcrystalline cellulose from agricultural waste: Review on isolation and application as reinforcement in polymeric matrices. Food Hydrocoll. 2021, 118, 106771. [Google Scholar] [CrossRef]
- FAOSTAT. FAO Statistics “Area Harvested, Production/Crops, Pumpkins, Squash and Gourds, Year 2020”. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 18 April 2022).
- Ahmed, D.; Ashiq, N. In vitro analysis of anti-diabetic and anti-oxidative potential of pedicles of fruit-vegetable bottle gourd. Pak. J. Pharm. Sci. 2018, 31, 2497–2501. [Google Scholar] [PubMed]
- Atique, I.; Ahmed, D.; Maqsood, M.; Malik, W. Solvents for extraction of antidiabetic, iron chelating, and antioxidative properties from bottle gourd fruit. Int. J. Veg. Sci. 2018, 24, 212–226. [Google Scholar] [CrossRef]
- Chaudhery, R.; Ahmed, D.; Liaqat, I.; Dar, P.; Shaban, M. Study of bioactivities of lipid content of fresh Lagenaria siceraria seeds pulp and identification of its chemical constituents. J. Med. Plants Res. 2014, 8, 1014–1020. [Google Scholar]
- Ahmed, D.; Ashiq, N. Lagenaria siceraria Fruit Pedicle Extracts as a Remedy against Microbial Infections. Int. J. Veg. Sci. 2018, 24, 539–549. [Google Scholar] [CrossRef]
- Kale, R.D.; Bansal, P.S.; Gorade, V.G. Extraction of microcrystalline cellulose from cotton sliver and its comparison with commercial microcrystalline cellulose. J. Polym. Environ. 2018, 26, 355–364. [Google Scholar] [CrossRef]
- Haafiz, M.M.; Eichhorn, S.; Hassan, A.; Jawaid, M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr. Polym. 2013, 93, 628–634. [Google Scholar] [CrossRef]
- Thomas, S.; Paul, S.; Pothan, L.; Deepa, B. Natural fibres: Structure, properties and applications. In Cellulose Fibers: Bio-and Nano-Polymer Composites; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–42. [Google Scholar]
- Sen, S.; Martin, J.D.; Argyropoulos, D.S. Review of cellulose non-derivatizing solvent interactions with emphasis on activity in inorganic molten salt hydrates. ACS Sustain. Chem. Eng. 2013, 1, 858–870. [Google Scholar] [CrossRef]
- Wohlert, M.; Benselfelt, T.; Wågberg, L.; Furó, I.; Berglund, L.A.; Wohlert, J. Cellulose and the role of hydrogen bonds: Not in charge of everything. Cellulose 2022, 29, 1–23. [Google Scholar] [CrossRef]
- Owolabi, A.F.; Haafiz, M.K.M.; Hossain, M.S.; Hussin, M.H.; Fazita, M.R.N. Influence of alkaline hydrogen peroxide pre-hydrolysis on the isolation of microcrystalline cellulose from oil palm fronds. Int. J. Biol. Macromol. 2017, 95, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.N.; Thomas, L.H.; Altaner, C.M.; Callow, P.; Forsyth, V.T.; Apperley, D.C.; Kennedy, C.J.; Jarvis, M.C. Nanostructure of cellulose microfibrils in spruce wood. Proc. Natl. Acad. Sci. USA 2011, 108, E1195–E1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Qiang, D.; Zhang, M.; Xiu, H.; Zhang, X. Joint action of ultrasonic and Fe3+ to improve selectivity of acid hydrolysis for microcrystalline cellulose. Carbohydr. Polym. 2015, 129, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Ray, D.; Bandyopadhyay, N.; Sengupta, S. Study of the properties of microcrystalline cellulose particles from different renewable resources by XRD, FTIR, nanoindentation, TGA and SEM. J. Polym. Environ. 2010, 18, 355–363. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Hui Chuin, C.T.; Sabar, S.; Fazita, M.R.N.; Taiwo, O.F.A.; Hassan, T.M.; Haafiz, M.K.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Uesu, N.Y.; Pineda, E.A.G.; Hechenleitner, A.A.W. Microcrystalline cellulose from soybean husk: Effects of solvent treatments on its properties as acetylsalicylic acid carrier. Int. J. Pharm. 2000, 206, 85–96. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, Z.; Lin, X.; Ren, Z.; Li, B.; Zhang, Y. Preparation and characterization of microcrystalline cellulose (MCC) from tea waste. Carbohydr. Polym. 2018, 184, 164–170. [Google Scholar] [CrossRef]
- Chuayjuljit, S.; Su-uthai, S.; Charuchinda, S. Poly (vinyl chloride) film filled with microcrystalline cellulose prepared from cotton fabric waste: Properties and biodegradability study. Waste Manag. Res. 2010, 28, 109–117. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Pujiasih, S.; Kurnia; Masykur, A.; Kusumaningsih, T.; Saputra, O.A. Silylation and characterization of microcrystalline cellulose isolated from indonesian native oil palm empty fruit bunch. Carbohydr. Polym. 2018, 184, 74–81. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.L.; da Silva Barud, H.; de Assunçao, R.M.; da Silva Meireles, C.; Carvalho, G.O.; Rodrigues Filho, G.; Messaddeq, Y.; Ribeiro, S.J.L. Synthesis and characterization of microcrystalline cellulose produced from bacterial cellulose. J. Therm. Anal. Calorim. 2011, 106, 703–709. [Google Scholar] [CrossRef]
- Bae, D.H.; Choi, H.J.; Choi, K.; Nam, J.D.; Islam, M.S.; Kao, N. Fabrication of phosphate microcrystalline rice husk based cellulose particles and their electrorheological response. Carbohydr. Polym. 2017, 165, 247–254. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, A.; Ibrahim, S.A.; Yang, H.; Huang, W. Isolation and characterization of microcrystalline cellulose from pomelo peel. Int. J. Biol. Macromol. 2018, 111, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Abu-Thabit, N.Y.; Judeh, A.A.; Hakeem, A.S.; Ul-Hamid, A.; Umar, Y.; Ahmad, A. Isolation and characterization of microcrystalline cellulose from date seeds (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2020, 155, 730–739. [Google Scholar] [CrossRef]
- Kian, L.K.; Saba, N.; Jawaid, M.; Fouad, H. Characterization of microcrystalline cellulose extracted from olive fiber. Int. J. Biol. Macromol. 2020, 156, 347–353. [Google Scholar] [CrossRef]
- Azum, N.; Jawaid, M.; Kian, L.K.; Khan, A.; Alotaibi, M.M. Extraction of Microcrystalline Cellulose from Washingtonia Fibre and Its Characterization. Polymers 2021, 13, 3030. [Google Scholar] [CrossRef]
- Fouad, H.; Kian, L.K.; Jawaid, M.; Alotaibi, M.D.; Alothman, O.Y.; Hashem, M. Characterization of Microcrystalline Cellulose Isolated from Conocarpus Fiber. Polymers 2020, 12, 2926. [Google Scholar] [CrossRef]
- Adel, A.M.; Abd El-Wahab, Z.H.; Ibrahim, A.A.; Al-Shemy, M.T. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part II: Physicochemical properties. Carbohydr. Polym. 2011, 83, 676–687. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Hassan, M.L. Physical and mechanical properties of microcrystalline cellulose prepared from agricultural residues. Carbohydr. Polym. 2007, 67, 1–10. [Google Scholar] [CrossRef]
- Sheltami, R.M.; Abdullah, I.; Ahmad, I.; Dufresne, A.; Kargarzadeh, H. Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carbohydr. Polym. 2012, 88, 772–779. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Hachaichi, A.; Kouini, B.; Kian, L.K.; Asim, M.; Jawaid, M. Extraction and Characterization of Microcrystalline Cellulose from Date Palm Fibers using Successive Chemical Treatments. J. Polym. Environ. 2021, 29, 1990–1999. [Google Scholar] [CrossRef]
- Rasheed, M.; Jawaid, M.; Karim, Z.; Abdullah, L.C. Morphological, Physiochemical and Thermal Properties of Microcrystalline Cellulose (MCC) Extracted from Bamboo Fiber. Molecules 2020, 25, 2824. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Saeed, A.; He, Z.; Ni, Y. Jute as raw material for the preparation of microcrystalline cellulose. Cellulose 2011, 18, 451–459. [Google Scholar] [CrossRef]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Ouajai, S.; Shanks, R.A. Composition, structure and thermal degradation of hemp cellulose after chemical treatments. Polym. Degrad. Stabil. 2005, 89, 327–335. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M. Microcrystalline cellulose from Posidonia oceanica brown algae: Extraction and characterization. Int. J. Biol. Macromol. 2019, 138, 837–845. [Google Scholar] [CrossRef]
- Chen, H.; Yu, Y.; Zhong, T.; Wu, Y.; Li, Y.; Wu, Z.; Fei, B. Effect of alkali treatment on microstructure and mechanical properties of individual bamboo fibers. Cellulose 2017, 24, 333–347. [Google Scholar] [CrossRef]
- Beroual, M.; Trache, D.; Mehelli, O.; Boumaza, L.; Tarchoun, A.F.; Derradji, M.; Khimeche, K. Effect of the Delignification Process on the Physicochemical Properties and Thermal Stability of Microcrystalline Cellulose Extracted from Date Palm Fronds. Waste Biomass Valorization 2021, 12, 2779–2793. [Google Scholar] [CrossRef]
- Beroual, M.; Boumaza, L.; Mehelli, O.; Trache, D.; Tarchoun, A.F.; Khimeche, K. Physicochemical Properties and Thermal Stability of Microcrystalline Cellulose Isolated from Esparto Grass Using Different Delignification Approaches. J. Polym. Environ. 2021, 29, 130–142. [Google Scholar] [CrossRef]



| Sample | Weight% | Atomic% | ||
|---|---|---|---|---|
| Carbon | Oxygen | Carbon | Oxygen | |
| Alkali-treated | 45.79 | 54.21 | 52.95 | 47.05 |
| Bleached | 46.77 | 53.23 | 53.92 | 46.08 |
| MCC | 50.42 | 49.58 | 57.53 | 42.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asif, M.; Ahmed, D.; Ahmad, N.; Qamar, M.T.; Alruwaili, N.K.; Bukhari, S.N.A. Extraction and Characterization of Microcrystalline Cellulose from Lagenaria siceraria Fruit Pedicles. Polymers 2022, 14, 1867. https://doi.org/10.3390/polym14091867
Asif M, Ahmed D, Ahmad N, Qamar MT, Alruwaili NK, Bukhari SNA. Extraction and Characterization of Microcrystalline Cellulose from Lagenaria siceraria Fruit Pedicles. Polymers. 2022; 14(9):1867. https://doi.org/10.3390/polym14091867
Chicago/Turabian StyleAsif, Muhammad, Dildar Ahmed, Naveed Ahmad, Muhammad Tariq Qamar, Nabil K. Alruwaili, and Syed Nasir Abbas Bukhari. 2022. "Extraction and Characterization of Microcrystalline Cellulose from Lagenaria siceraria Fruit Pedicles" Polymers 14, no. 9: 1867. https://doi.org/10.3390/polym14091867








