Self-Healing Silsesquioxane-Based Materials
Abstract
:1. Introduction
2. POSS-Based Self-Healing Nanocomposites
2.1. POSS Used as Specialty Additives
2.2. Hydrophobic Coatings
2.3. Silsesquioxane Structures Inserted in Main Backbones of Self-Healing Polymers
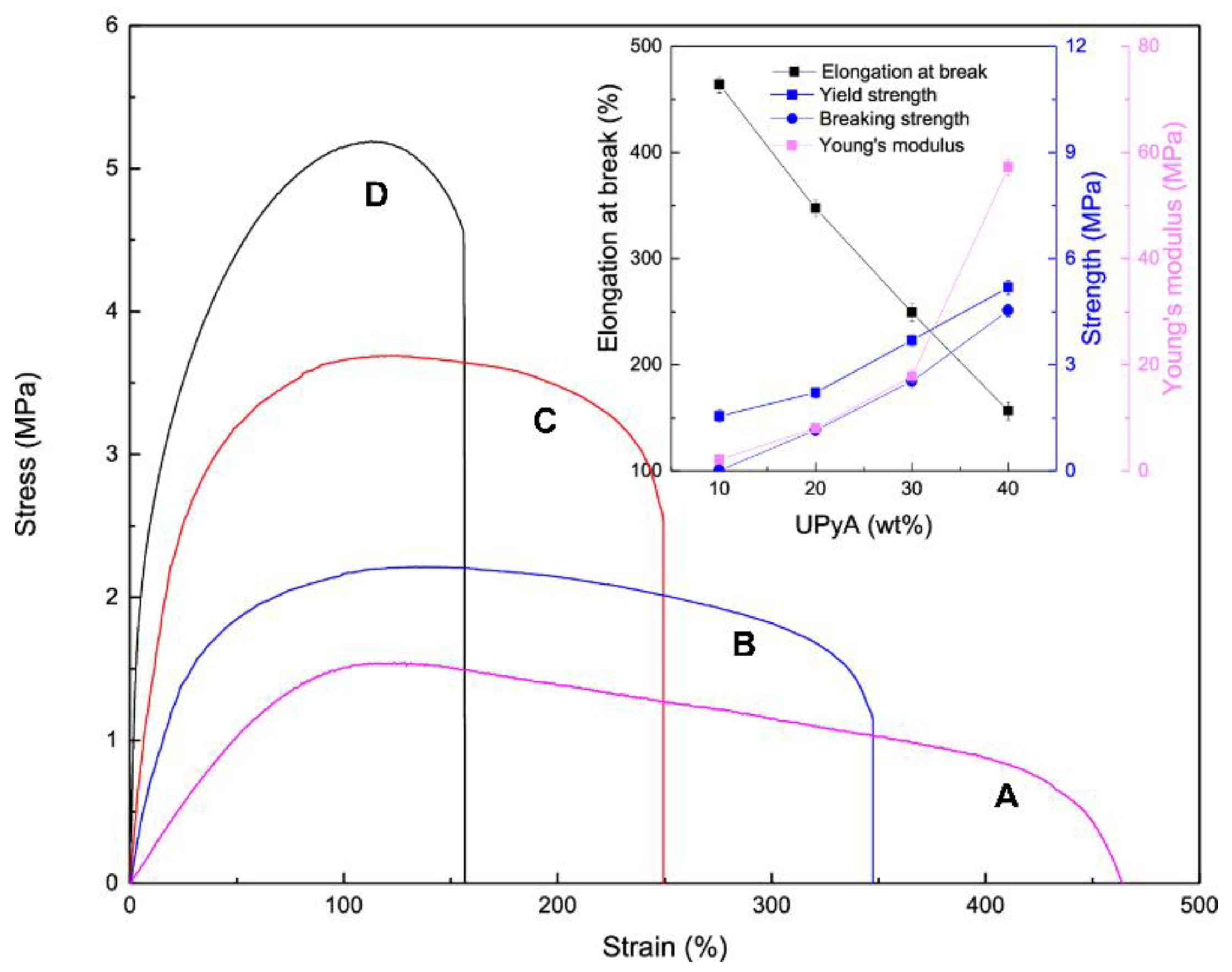
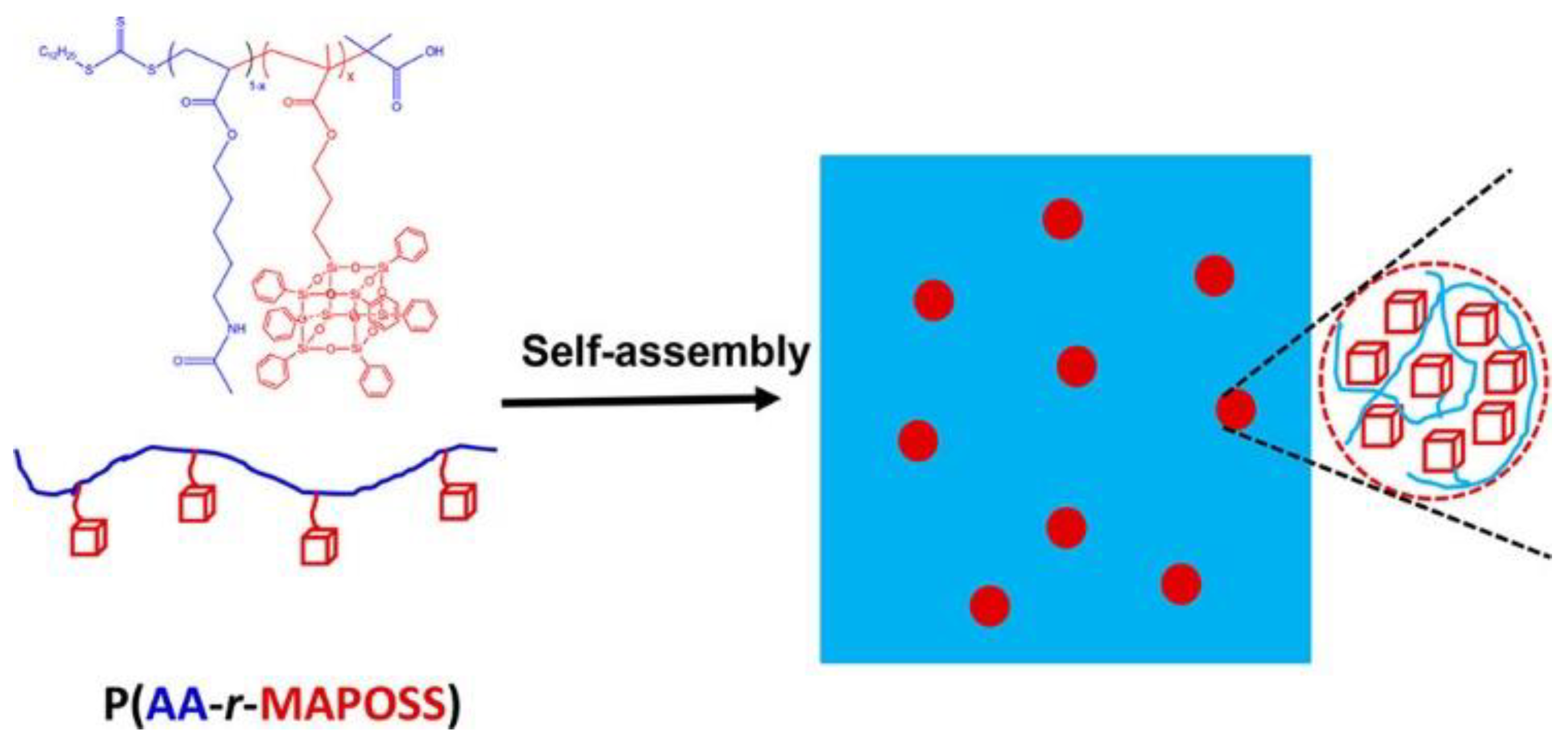
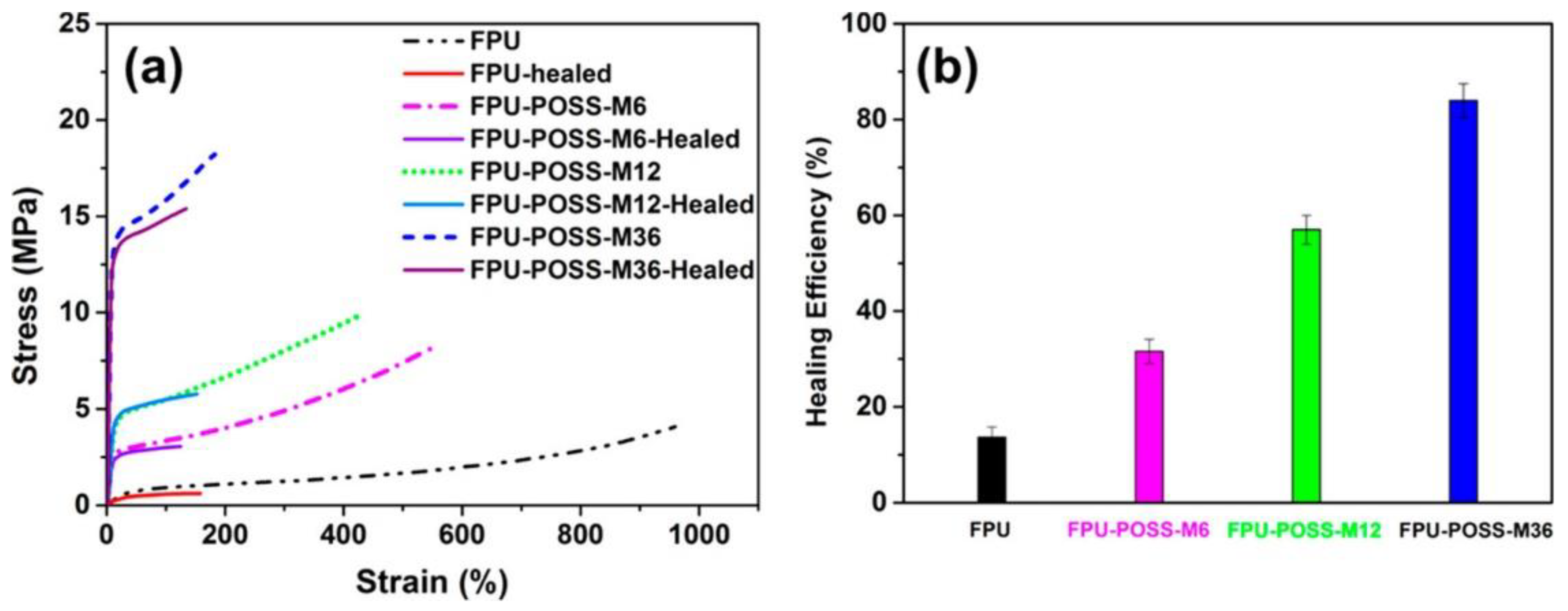
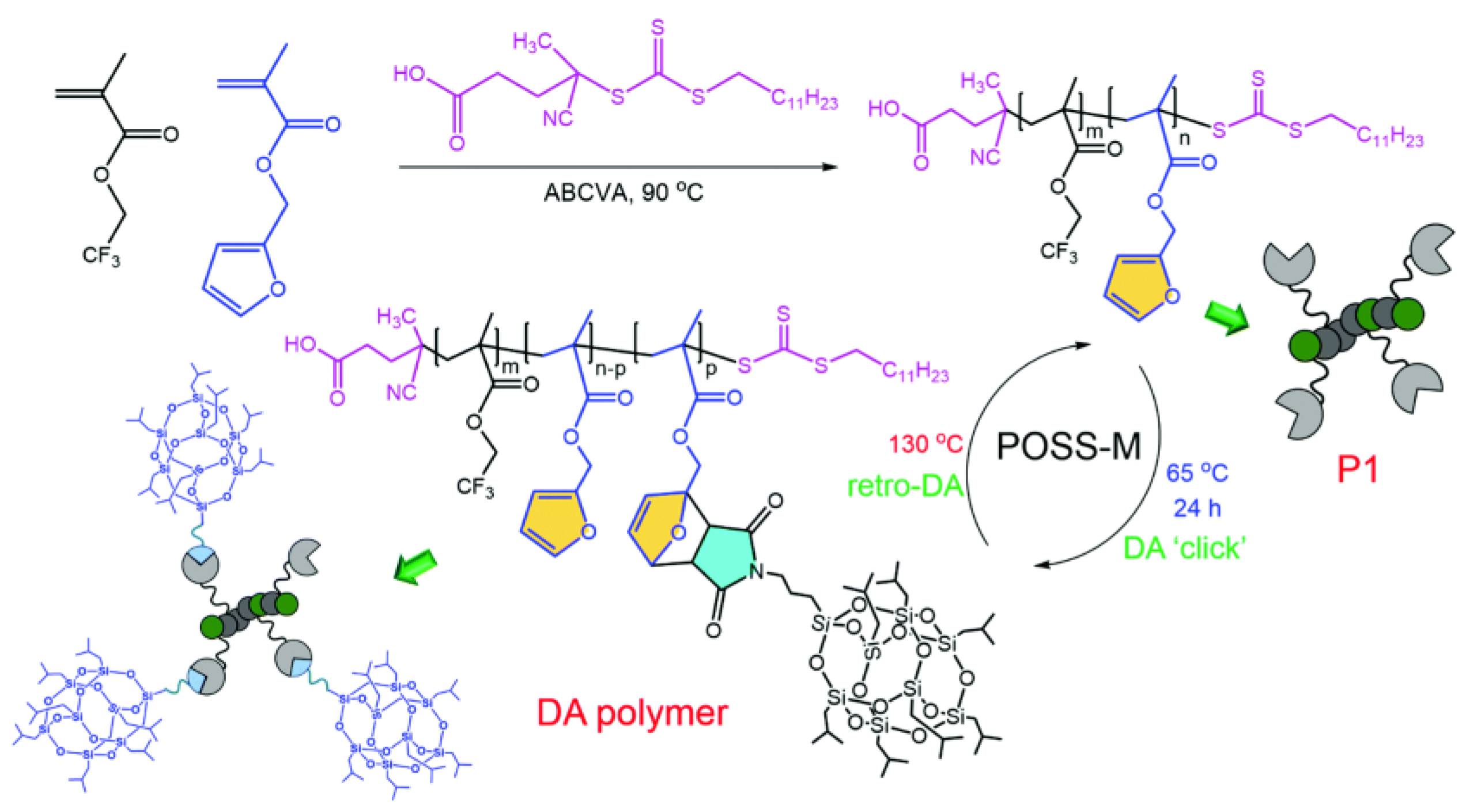
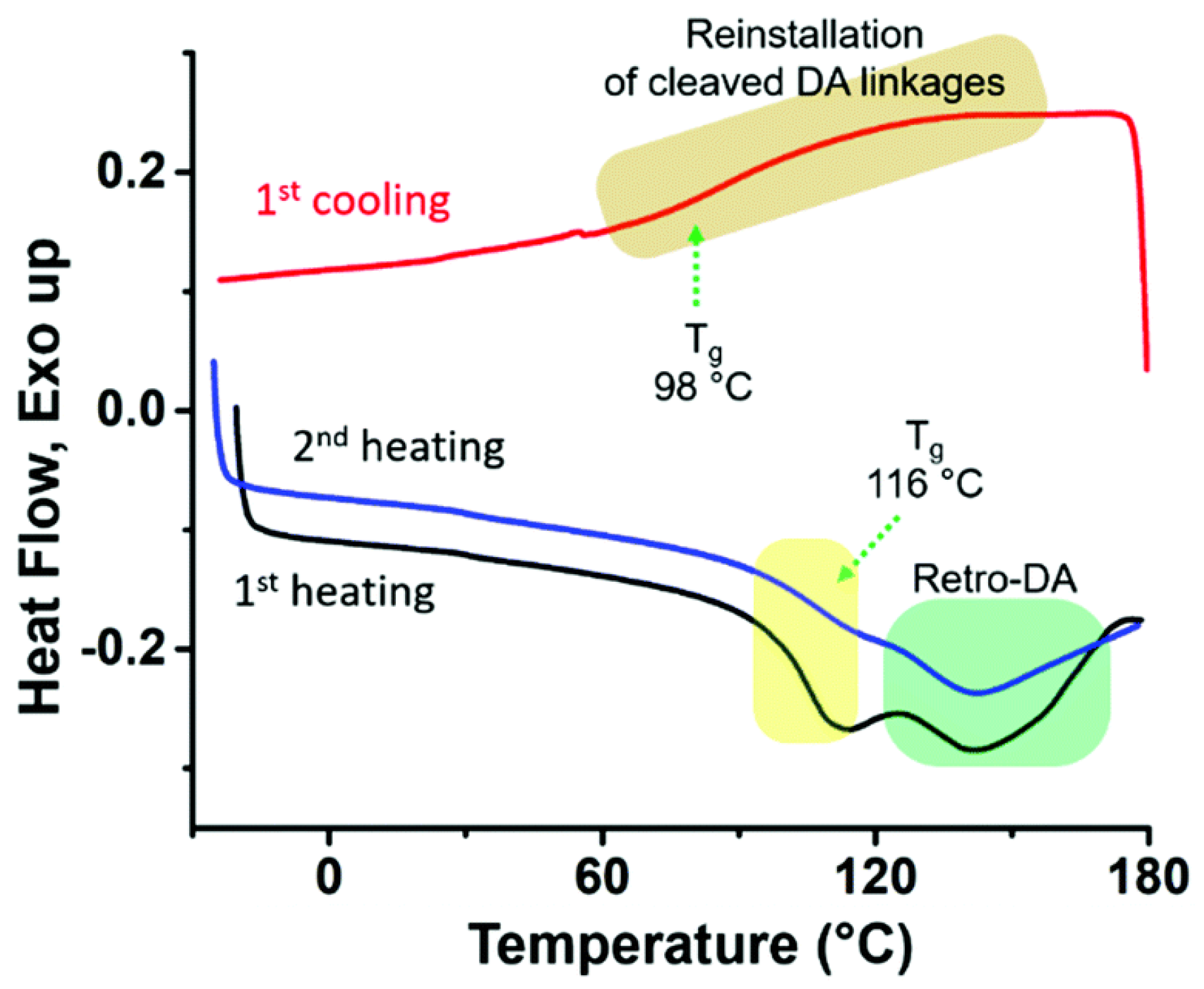
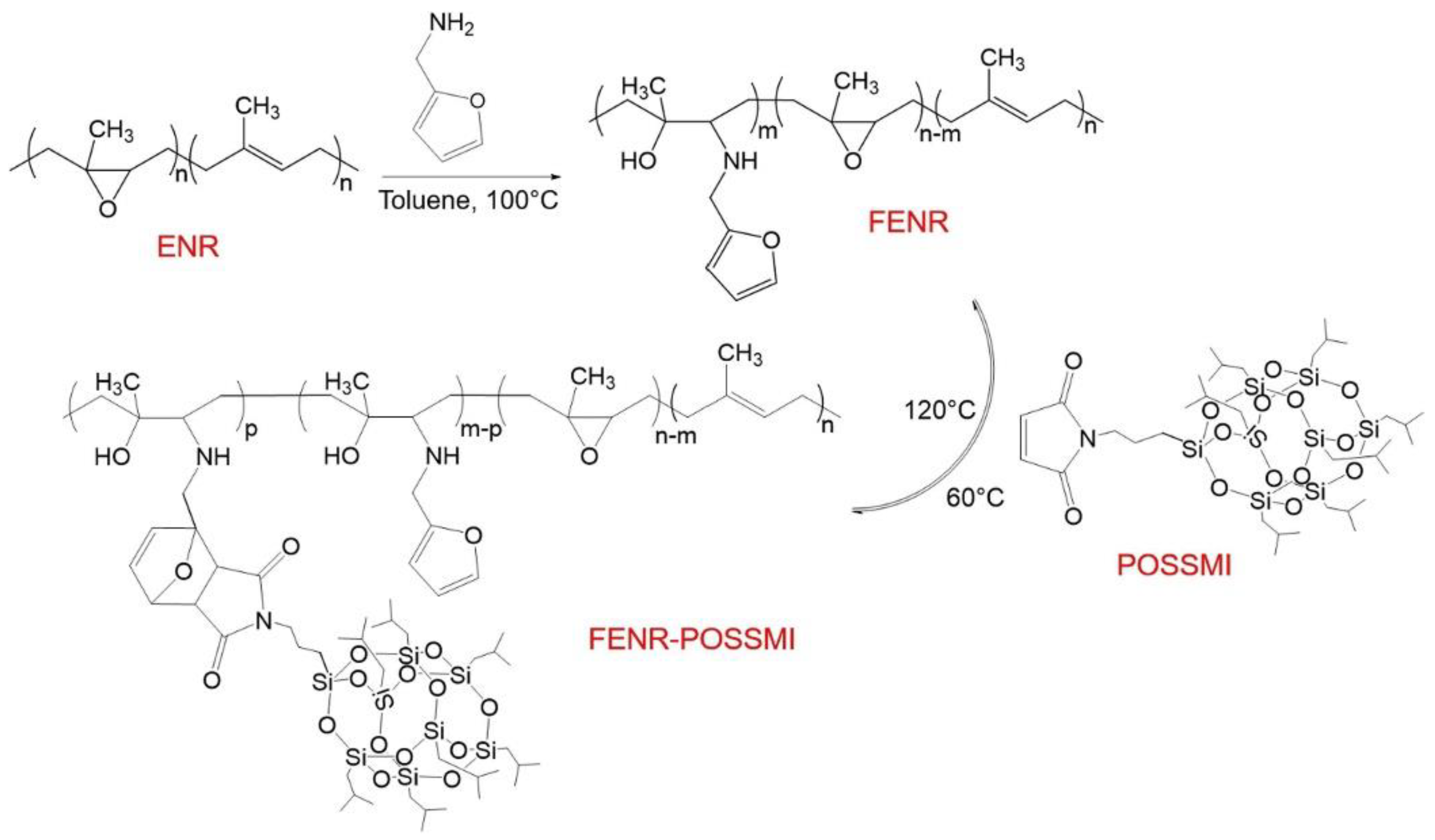
3. POSS Grafted to Polymers through Dynamic Bonds
4. Self-Healing Crosslinked Materials Based on Dynamic Interactions with Octafunctional POSS
5. POSS-Containing Self-Healing Hydrogels
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Zwaag, S. (Ed.) Self Healing Materials: An Alternative Approach to 20 Centuries of Materials Science; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Zhang, W.; Jiang, H.; Chang, Z.; Wu, W.; Wu, G.; Wu, R.; Li, J. Recent achievements in self-healing materials based on ionic liquids: A review. J. Mater. Sci. 2020, 55, 13543–13558. [Google Scholar] [CrossRef]
- Rahman, M.W.; Shefa, N.R. Minireview on Self-Healing Polymers: Versatility, Application, and Prospects. Adv. Polym. Techn. 2021, 2021, 7848088. [Google Scholar] [CrossRef]
- Ekeocha, J.; Ellingford, C.; Pan, M.; Wemyss, A.M.; Bowen, C.; Wan, C. Challenges and Opportunities of Self-Healing Polymers and Devices for Extreme and Hostile Environments. Adv. Mater. 2021, 33, 2008052. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, J.; Song, J.; Gao, C.; Wang, Z.; Song, C.; Wu, Y.; Liu, Y. Self-Healing Ti3C2 MXene/PDMS Supramolecular Elastomers Based on Small Biomolecules Modification for Wearable Sensors. ACS Appl. Mater. Interf. 2020, 12, 45306–45314. [Google Scholar] [CrossRef] [PubMed]
- Tepper, R.; Bode, S.; Geitner, R.; Jäger, M.; Görls, H.; Vitz, J.; Dietzek, B.; Schmitt, M.; Popp, J.; Hager, M.D.; et al. Polymeric Halogen-Bond-Based Donor Systems Showing Self-Healing Behavior in Thin Films. Angew. Chem.-Int. Ed. 2017, 56, 4047–4051. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A Thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, A.A.; Singha, N.K. “Click chemistry” in tailor-made polymethacrylates bearing reactive furfuryl functionality: A new class of self-healing polymeric material. ACS Appl. Mater. Interfaces 2009, 1, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Behera, P.K.; Singha, N.K. A healable thermo-reversible functional polymer prepared via RAFT polymerization and ultrafast ‘click’ chemistry using a triazolinedione derivative. Chem. Commun. 2017, 53, 8715–8718. [Google Scholar] [CrossRef]
- Fawcett, A.S.; Brook, M.A. Thermoplastic Silicone Elastomers through Self-Association of Pendant Coumarin Groups. Macromolecules 2014, 47, 1656–1663. [Google Scholar] [CrossRef]
- Jin, J.; Cai, L.; Jia, Y.-G.; Liu, S.; Chen, Y.; Ren, L. Progress in self-healing hydrogels assembled by host–guest interactions: Preparation and biomedical applications. J. Mater. Chem. B 2019, 7, 1637–1651. [Google Scholar] [CrossRef]
- Rambarran, T.; Bertrand, A.; Gonzaga, F.; Boisson, F.; Bernard, J.; Fleury, E.; Ganachaud, F.; Brook, M.A. Sweet supramolecular elastomers from α,ω-(β-cyclodextrin terminated) PDMS. Chem. Commun. 2016, 52, 6681–6684. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Du, J.; Zhang, C.; Zhou, M.; Cheng, G.; Zhu, H.; Zhang, Q.; Zhu, S. All-Solid-State Self-Healing Ionic Conductors Enabled by Ion–Dipole Interactions within Fluorinated Poly(Ionic Liquid) Copolymers. ACS Appl. Mater. Interfaces 2021, 13, 41140–41148. [Google Scholar] [CrossRef] [PubMed]
- Voorhaar, L.; Diaz, M.M.; Leroux, F.; Rogers, S.; Abakumov, A.M.; Van Tendeloo, G.; Van Assche, G.; Van Mele, B.; Hoogenboom, R. Supramolecular thermoplastics and thermoplastic elastomer materials with self-healing ability based on oligomeric charged triblock copolymers. NPG Asia Mater. 2017, 9, e385. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-H.; Zuo, J.-L. Self-Healing Polymers Based on Coordination Bonds. Adv. Mater. 2020, 32, 1903762. [Google Scholar] [CrossRef]
- Mozhdehi, D.; Ayala, S.; Cromwell, O.R.; Guan, Z. Self-Healing Multiphase Polymers via Dynamic Metal−Ligand Interactions. J. Am. Chem. Soc. 2014, 136, 16128–16131. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Sari, M.; Oppermann, W.; Okay, O. Tough and Self-Healing Hydrogels Formed via Hydrophobic Interactions. Macromolecules 2011, 44, 4997–5005. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chuo, T.-W. Self-healing polymers based on thermally reversible Diels–Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels-Alder reaction in total synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Kavitha, A.A.; Singha, N.K. Smart “all acrylate” ABA triblock copolymer bearing reactive functionality via atom transfer radical polymerization (ATRP): Demonstration of a “click reaction” in thermoreversible property. Macromolecules 2010, 43, 3193–3205. [Google Scholar] [CrossRef]
- Pramanik, N.B.; Nando, G.B.; Singha, N.K. Self-healing polymeric gel via RAFT polymerization and Diels–Alder click chemistry. Polymer 2015, 69, 349–356. [Google Scholar] [CrossRef]
- Nasresfahani, A.; Zelisko, P.M. Synthesis of a self-healing siloxane-based elastomer cross-linked via a furan-modified polyhedral oligomeric silsesquioxane: Investigation of a thermally reversible silicon-based cross-link. Polym. Chem. 2017, 8, 2942–2952. [Google Scholar] [CrossRef]
- De Bruycker, K.; Billiet, S.; Houck, H.A.; Chattopadhyay, S.; Winne, J.M.; Du Prez, F.E. Triazolinediones as highly enabling synthetic tools. Chem. Rev. 2016, 116, 3919–3974. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.L.; Bhattacharya, K.; Samanta, S.; Singha, N.K. Self-healable antifouling zwitterionic hydrogel based on synergistic phototriggered dynamic disulfide metathesis reaction and ionic interaction. ACS Appl. Mater. Interfaces 2018, 10, 27391–27406. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Zhao, K.; Zheng, J. A Highly Stretchable Self-Healing Poly(dimethylsiloxane) Elastomer with Reprocessability and Degradability. Macromol. Rapid Commun. 2018, 39, 201700686. [Google Scholar] [CrossRef]
- Michal, B.T.; Spencer, E.J.; Rowan, S.J. Stimuli-responsive reversible two-level adhesion from a structurally dynamic shape-memory polymer. ACS Appl. Mater. Interf. 2016, 8, 11041–11049. [Google Scholar] [CrossRef] [PubMed]
- Michal, B.T.; Jaye, C.A.; Spencer, E.J.; Rowan, S.J. Inherently Photohealable and Thermal Shape-Memory Polydisulfide Networks. ACS Macro Lett. 2013, 2, 694–699. [Google Scholar] [CrossRef]
- Kathan, M.; Kovaricek, P.; Jurissek, C.; Senf, A.; Dallmann, A.; Thuenemann, A.F.; Hecht, S. Control of Imine Exchange Kinetics with Photoswitches to Modulate Self-Healing in Polysiloxane Networks by Light Illumination. Ang. Chem. Inter. Ed. 2016, 55, 13882–13886. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Wang, C.; Keplinger, C.; Zuo, J.-L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, C.; Liu, W.; Li, D.; Zhang, J.; Zhao, N.; Xu, J. Recyclable Polydimethylsiloxane Network Crosslinked by Dynamic Transesterification Reaction. Sci. Rep. 2017, 7, 11833. [Google Scholar] [CrossRef]
- Lai, J.-C.; Mei, J.-F.; Jia, X.-Y.; Li, C.-H.; You, X.-Z.; Bao, Z. A Stiff and Healable Polymer Based on Dynamic-Covalent Boroxine Bonds. Adv. Mater. 2016, 28, 8277–8282. [Google Scholar] [CrossRef]
- Hua, J.; Liu, C.; Fei, B.; Liu, Z. Self-Healable and Super-Tough Double-Network Hydrogel Fibers from Dynamic Acylhydrazone Bonding and Supramolecular Interactions. Gels 2022, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Nevejans, S.; Ballard, N.; Miranda, J.I.; Reck, B.; Asua, J.M. The underlying mechanisms for self-healing of poly(disulfide)s. Phys. Chem. Chem. Phys. 2016, 18, 27577–27583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Huang, H.; Fielden, M.; Pan, J.; Ecco, L.; Schellbach, C.; Delmas, G.; Claesson, P.M. Towards the mechanism of electrochemical activity and self-healing of 1 wt% PTSA doped polyaniline in alkyd composite polymer coating: Combined AFM-based studies. RSC Adv. 2016, 6, 19111–19127. [Google Scholar] [CrossRef]
- Sanka, R.V.S.P.; Krishnakumar, B.; Leterrier, Y.; Pandey, S.; Rana, S.; Michaud, V. Soft Self-Healing Nanocomposites. Front. Mater. 2019, 6, 137. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, X.-H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W.; et al. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, S.; Khan, G.S.; Shah, S.M.; Hussain, Z.; Muhammad, S.; Siddiq, M.; Hussain, H. Water soluble polyhedral oligomeric silsesquioxane based amphiphilic hybrid polymers: Synthesis, self-assembly, and applications. Eur. Polym. J. 2016, 75, 67–92. [Google Scholar] [CrossRef]
- Kowalewska, A. Self-assembling polyhedral silsesquioxanes-Structure and properties. Curr. Org. Chem. 2017, 21, 1243–1264. [Google Scholar] [CrossRef]
- Kowalewska, A. Self-assembly of POSS-Containing Materials. In Polymer/POSS Nanocomposites and Hybrid Materials, Preparation, Properties, Applications; Kalia, S., Pielichowski, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 45–128. ISSN 2364-1878. [Google Scholar]
- Chi, H.; Wang, M.; Xiao, Y.; Wang, F.; Joshy, K.S. Self-Assembly and Applications of Amphiphilic Hybrid POSS Copolymers. Molecules 2018, 23, 2481. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Lin, F.; Zhang, Q.; Cai, R.; Wu, Y.; Ma, X. Polyhedral Oligomeric Silsesquioxane Hybrid Polymers: Well-Defined Architectural Design and Potential Functional Applications. Macromol. Rapid Commun. 2019, 40, 1900101. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, B.S. Polymeric nanocomposites-Polyhedral oligomeric silsesquioxanes (POSS) as hybrid nanofiller. J. Macromol. Sci.-Polym. Rev. 2004, 44, 389–410. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Chang, F.-C. POSS related polymer nanocomposites. Progr. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Zhang, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Progr. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Shi, H.; Yang, J.; You, M.; Li, Z.; He, C. Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Hybrid Soft Gels: Molecular Design, Material Advantages, and Emerging Application. ACS Mater. Lett. 2020, 2, 296–316. [Google Scholar] [CrossRef]
- Dong, F.; Lu, L.; Ha, C.-S. Silsesquioxane-Containing Hybrid Nanomaterials: Fascinating Platforms for Advanced Applications. Macromol. Chem. Phys. 2019, 220, 1800324. [Google Scholar] [CrossRef]
- Liu, S.; Guo, R.; Li, C.; Lu, C.; Yang, G.; Wang, F.; Nie, J.; Ma, C.; Gao, M. POSS hybrid hydrogels: A brief review of synthesis, properties and applications. Eur. Polym. J. 2021, 14315, 110180. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, L.; Du, Q.; Shan, T.; Zheng, K.; He, J.; He, H.; Chen, S.; Wang, X. Synthesis, properties and applications of well-designed hybrid polymers based on polyhedral oligomeric silsesquioxane. Polym. Int. 2022, 71, 379–392. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, B.; Adeel, M.; Zheng, S. Shape Memory and Self-Healing Properties of Poly(acrylate amide) Elastomers Reinforced with Polyhedral Oligomeric Silsesquioxanes. ACS Appl. Polym. Mater. 2019, 1, 359–368. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, S.; Zheng, S. Synthesis, self-assembly and self-healing properties of organic-inorganic ABA triblock copolymers with poly(POSS acrylate) endblocks. Polym. Chem. 2019, 10, 2424–2435. [Google Scholar] [CrossRef]
- Strachota, B.; Matějka, L.; Hodan, J.; Kobera, L.; Mahun, A.; Dybal, J.; Šlouf, M. Polyhedral oligomeric silsesquioxane (POSS)-based epoxy nanocomposite involving a reversible Diels–Alder-type network as a self-healing material. J. Adhes. Sci. Technol. 2021, 35, 2736–2757. [Google Scholar] [CrossRef]
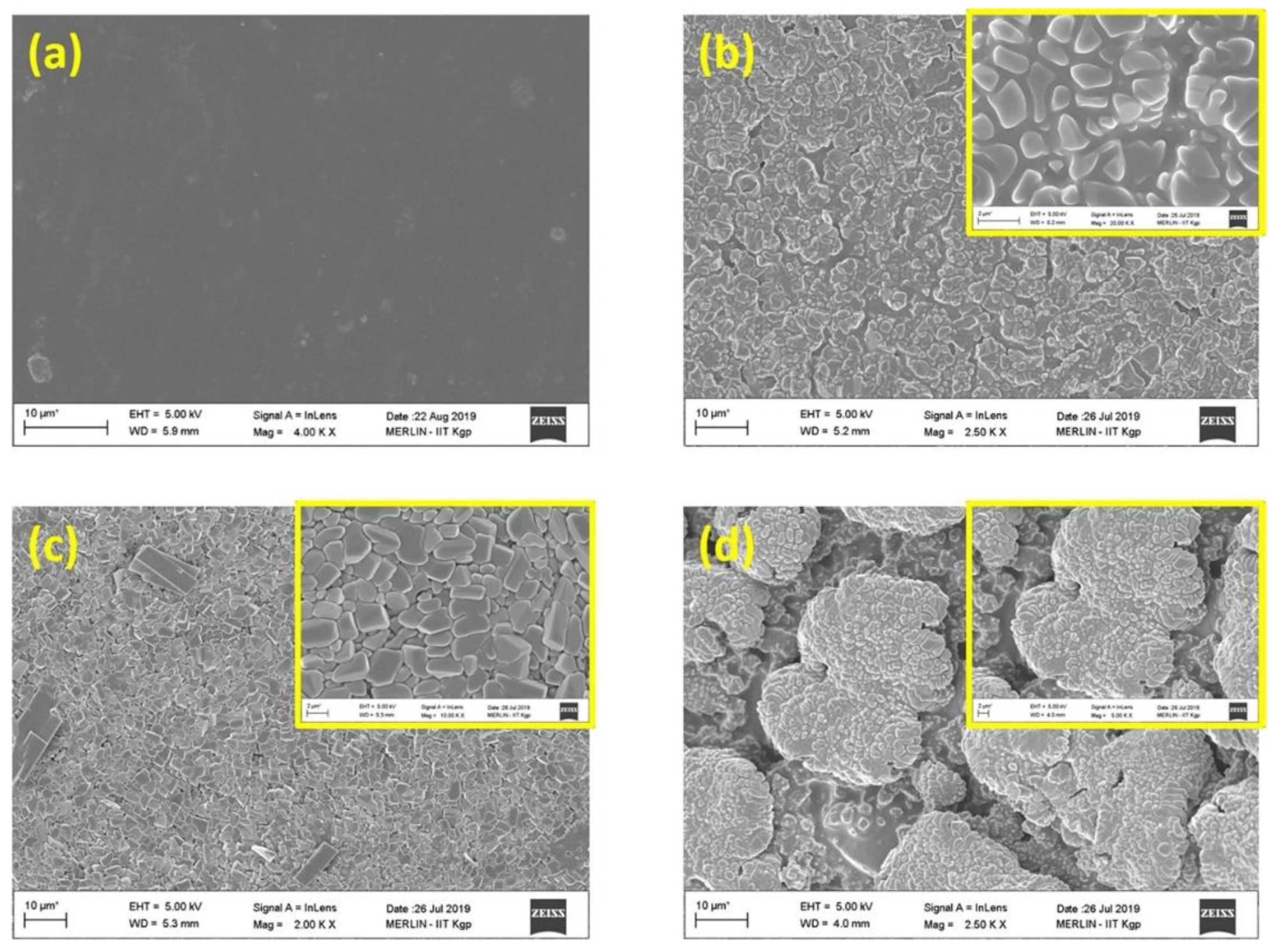
- Strąkowska, A.; Kosmalska, A.; Masłowski, M.; Szmechtyk, T.; Strzelec, K.; Zaborski, M. POSS as promoters of self-healing process in silicone composites. Polym. Bull. 2019, 76, 3387–3402. [Google Scholar] [CrossRef] [Green Version]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Barra, G.; Vertuccio, L.; Sorrentino, A.; Binder, W.H.; Kadlec, M. Development of self-healing multifunctional materials. Compos. Part B 2017, 128, 30–38. [Google Scholar] [CrossRef]
- Jing, Z.; Shi, X.; Zhang, G.; Gu, J. Synthesis and properties of poly(lactide)/poly(ε-caprolactone) multiblock supramolecular polymers bonded by the self-complementary quadruple hydrogen bonding. Polymer 2017, 121, 124–136. [Google Scholar] [CrossRef]
- Zhou, B.; He, D.; Hu, J.; Ye, Y.; Peng, H.; Zhou, X.; Xie, X.; Xue, Z. A Flexible, Self-healing and Highly Stretchable Polymer Electrolyte via Quadruple Hydrogen Bonding for Lithium-ion Batteries. J. Mater. Chem. A 2018, 6, 11725–11733. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Z.; Zhang, Q.; Lopez, J.; Wang, H.; Wu, H.-C.; Niu, S.; Yan, H.; Wang, S.; Lei, T.; et al. Quadruple H-Bonding Cross-Linked Supramolecular Polymeric Materials as Substrates for Stretchable, Antitearing, and Self-healable Thin Film Electrodes. J. Am. Chem. Soc. 2018, 140, 5280–5289. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Qi, T.; Li, G.L. Towards Dynamic but Supertough Healable Polymers through Biomimetic Hierarchical Hydrogen-Bonding Interactions. Angew. Chem. Int. Ed. 2018, 57, 13838–13842. [Google Scholar] [CrossRef]
- Hentschel, J.; Kushner, A.M.; Ziller, J.; Guan, Z. Self-healing Supramolecular Block Copolymers. Angew. Chem. Int. Ed. 2012, 51, 10561–10565. [Google Scholar] [CrossRef]
- Yari, H.; Mohseni, M.; Messori, M. A scratch resistant yet healable automotive clearcoat containing hyperbranched polymer and POSS nanostructures. RSC Adv. 2016, 6, 76028–76041. [Google Scholar] [CrossRef] [Green Version]
- Xiang, S.; Liu, W. Self-healing superhydrophobic surfaces: Healing principles and applications. Adv. Mater. Interfaces 2021, 8, 2100247. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Ding, J.; Feng, L.; Wang, X.; Lin, T. Durable, Self-healing superhydrophobic and superoleophobic surfaces from fluorinated-decyl polyhedral oligomeric silsesquioxane and hydrolyzed fluorinated alkyl silane. Angew. Chem. Int. Ed. 2011, 50, 11433–11436. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, H.; Gestos, A.; Fang, J.; Niu, H.; Ding, J.; Lin, T. Robust, electro-conductive, self-healing superamphiphobic fabric prepared by one-step vapour-phase polymerisation of poly(3,4-ethylenedioxythiophene) in the presence of fluorinated decyl polyhedral oligomeric silsesquioxane and fluorinated alkyl silane. Soft Matter 2013, 9, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhou, H.; Gestos, A.; Fang, J.; Lin, T. Robust, superamphiphobic fabric with multiple self-healing ability against both physical and chemical damages. ACS Appl. Mater. Interfaces 2013, 5, 10221–10226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, Y.; Liu, G.; Jiang, Y.; Chen, K. Fabrication of self-healing waterbased superhydrophobic coatings from POSS modified silica nanoparticles. Mater. Lett. 2018, 229, 281–285. [Google Scholar] [CrossRef]
- Golovin, K.; Boban, M.; Mabry, J.M.; Tuteja, A. Designing self-healing superhydrophobic surfaces with exceptional mechanical durability. ACS Appl. Mater. Interfaces 2017, 9, 11212–11223. [Google Scholar] [CrossRef] [PubMed]
- Yari, H.; Mohseni, M.; Messori, M.; Ranjbar, Z. Tribological properties and scratch healing of a typical automotive nano clearcoat modified by a polyhedral oligomeric silsesquioxane compound. Eur. Polym. J. 2014, 60, 79–91. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, J.; Ge, F.; Zhao, R.; Wang, C. Smart UV-curable fabric coatings with self-healing ability for durable self-cleaning and intelligent oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 86–96. [Google Scholar] [CrossRef]
- Li, H.; Miao, S.; Chen, W.; Yang, X.; Li, M.; Xing, T.; Zhao, Y.; Chen, G. Durable superhydrophobic and oleophobic cotton fabric based on the grafting of fluorinated POSS through silane coupling and thiol-ene click reaction. Colloids Surf. A Physicochem. Eng. Asp. 2021, 6305, 127566. [Google Scholar] [CrossRef]
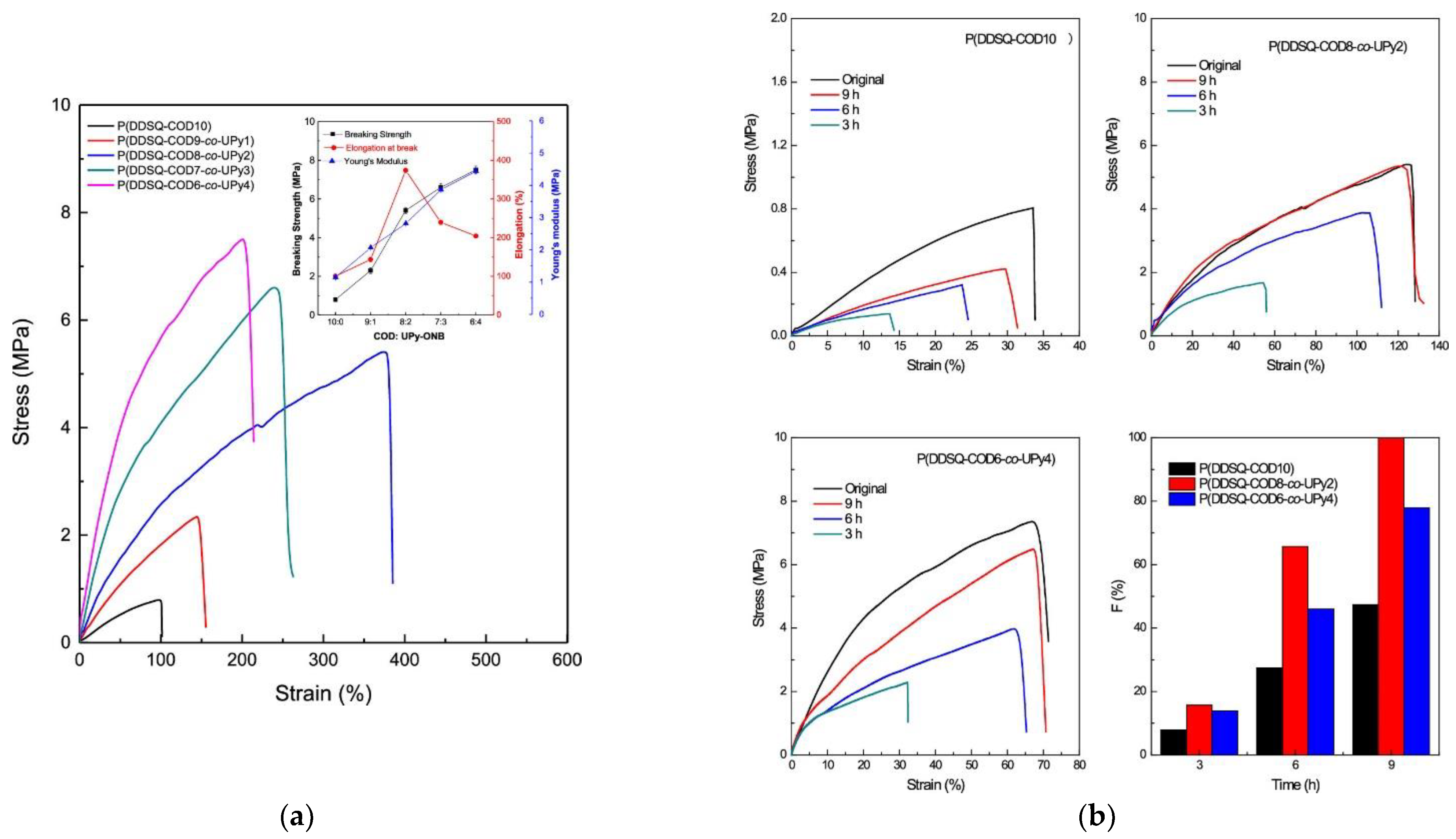
- Zhao, B.; Mei, H.; Liu, N.; Zheng, S. Organic−Inorganic Polycyclooctadienes with Double-Decker Silsesquioxanes in the Main Chains: Synthesis, Self-Healing, and Shape Memory Properties Regulated with Quadruple Hydrogen Bonds. Macromolecules 2020, 53, 7119–7131. [Google Scholar] [CrossRef]
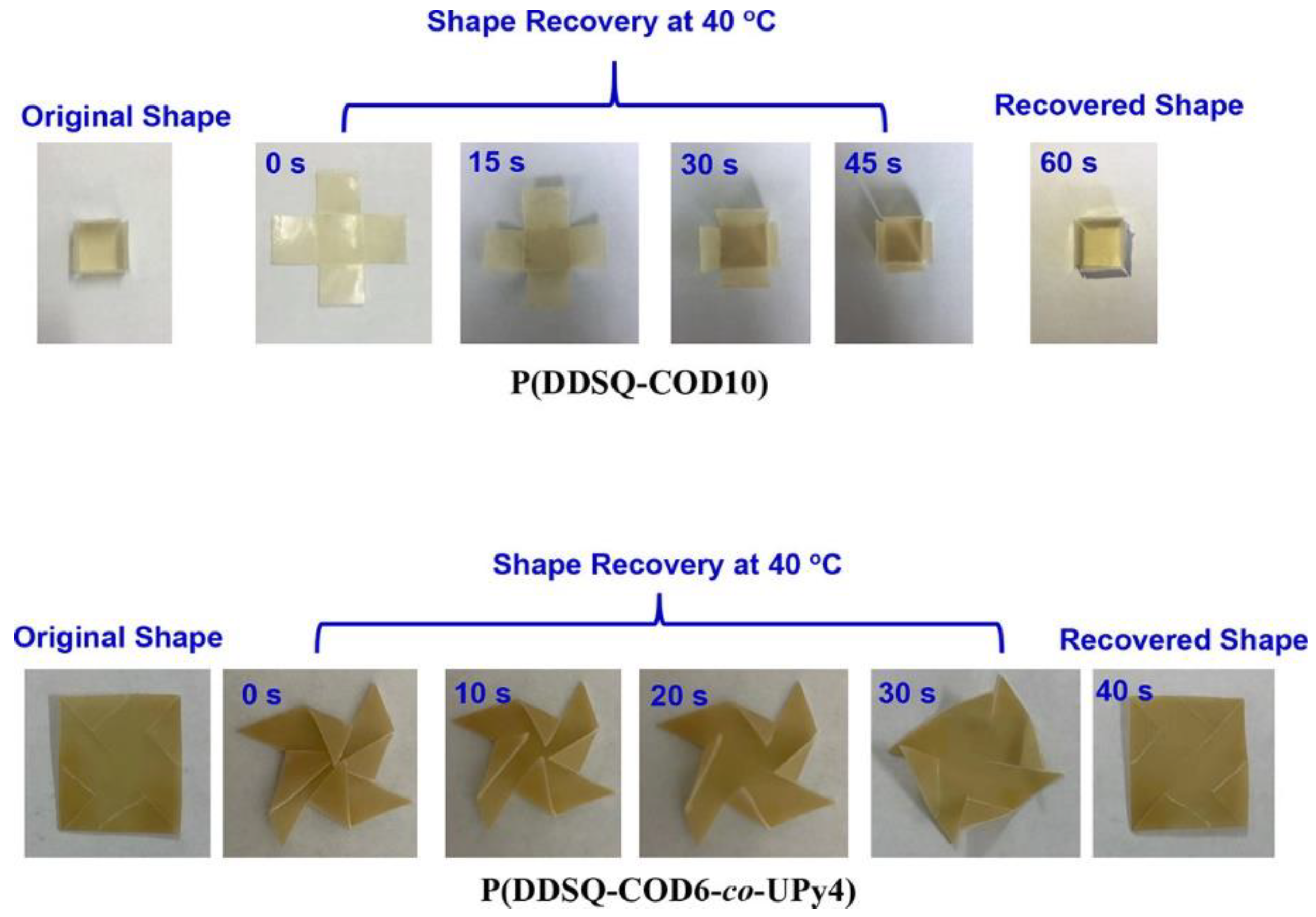
- Zhao, B.; Ding, H.; Xu, S.; Zheng, S. Organic-Inorganic Linear Segmented Polyurethanes Simultaneously Having Shape Recovery and Self-Healing Properties. ACS Appl. Polym. Mater. 2019, 1, 3174–3184. [Google Scholar] [CrossRef]
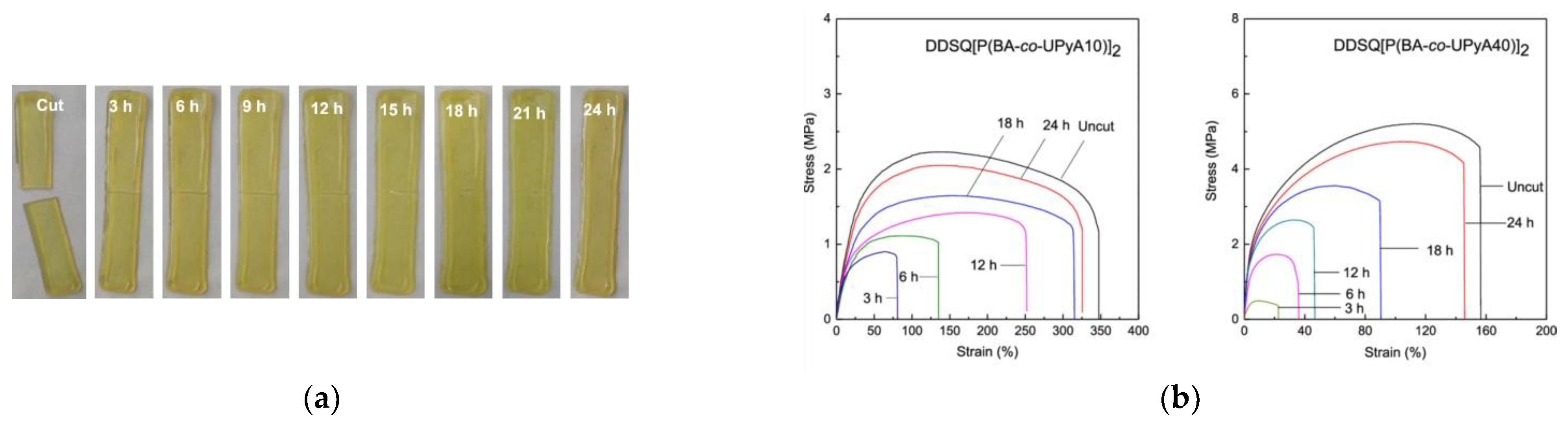
- Xu, S.; Zhao, B.; Raza, M.; Li, L.; Wang, H.; Zheng, S. Shape Memory and Self-Healing Nanocomposites with POSS-POSS Interactions and Quadruple Hydrogen Bonds. ACS Appl. Polym. Mater. 2020, 2, 3327–3338. [Google Scholar] [CrossRef]
- Zhao, B.; Mei, H.; Wang, H.; Li, L.; Zheng, S. Organic–Inorganic Polyureas with POSS Cages in the Main Chains via Polycondensation of Diamines with Carbon Dioxide. ACS Appl. Polym. Mater. 2022, 4, 509–520. [Google Scholar] [CrossRef]
- Behera, P.K.; Mondal, P.; Singha, N.K. Self-Healable and Ultrahydrophobic Polyurethane-POSS Hybrids by Diels-Alder "click" Reaction: A New Class of Coating Material. Macromolecules 2018, 51, 4770–4781. [Google Scholar] [CrossRef]
- Ponnupandian, S.; Mondal, P.; Becker, T.; Hoogenboom, R.; Lowe, A.B.; Singha, N.K. Self-healing hydrophobic POSS-functionalized fluorinated copolymers via RAFT polymerization and dynamic Diels–Alder reaction. Polym. Chem. 2021, 12, 876–884. [Google Scholar] [CrossRef]
- Raut, S.K.; Mondal, P.; Parameswaran, B.; Sarkar, S.; Dey, P.; Gilbert, R.; Bhadra, S.; Naskar, K.; Nair, S.; Singha, N.K. Self-healable ultrahydrophobic modified bio-based elastomer using Diels-Alder ‘click chemistry’. Eur. Polym. J. 2021, 146, 110204. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, K.; Tian, G.; Jiang, B.; Huang, Y. Stretchable Electronics Based on PDMS Substrates. Adv. Mater. 2021, 33, 2003155. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Wang, S.; Hou, C.; Huang, X.; Cui, J.; Yao, X. Dynamic siloxane materials: From molecular engineering to emerging applications. Chem. Eng. J. 2021, 405, 127023. [Google Scholar] [CrossRef]
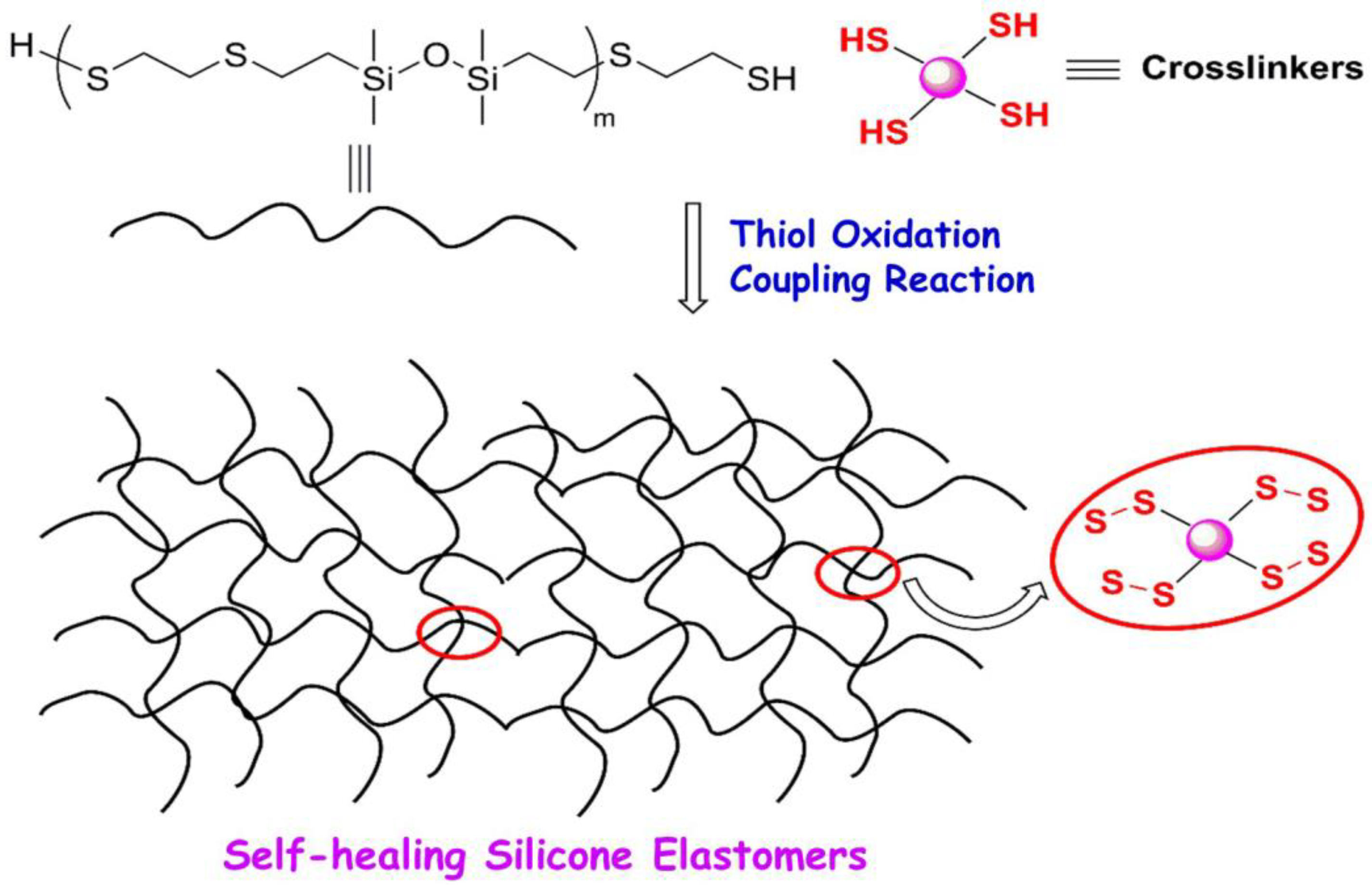
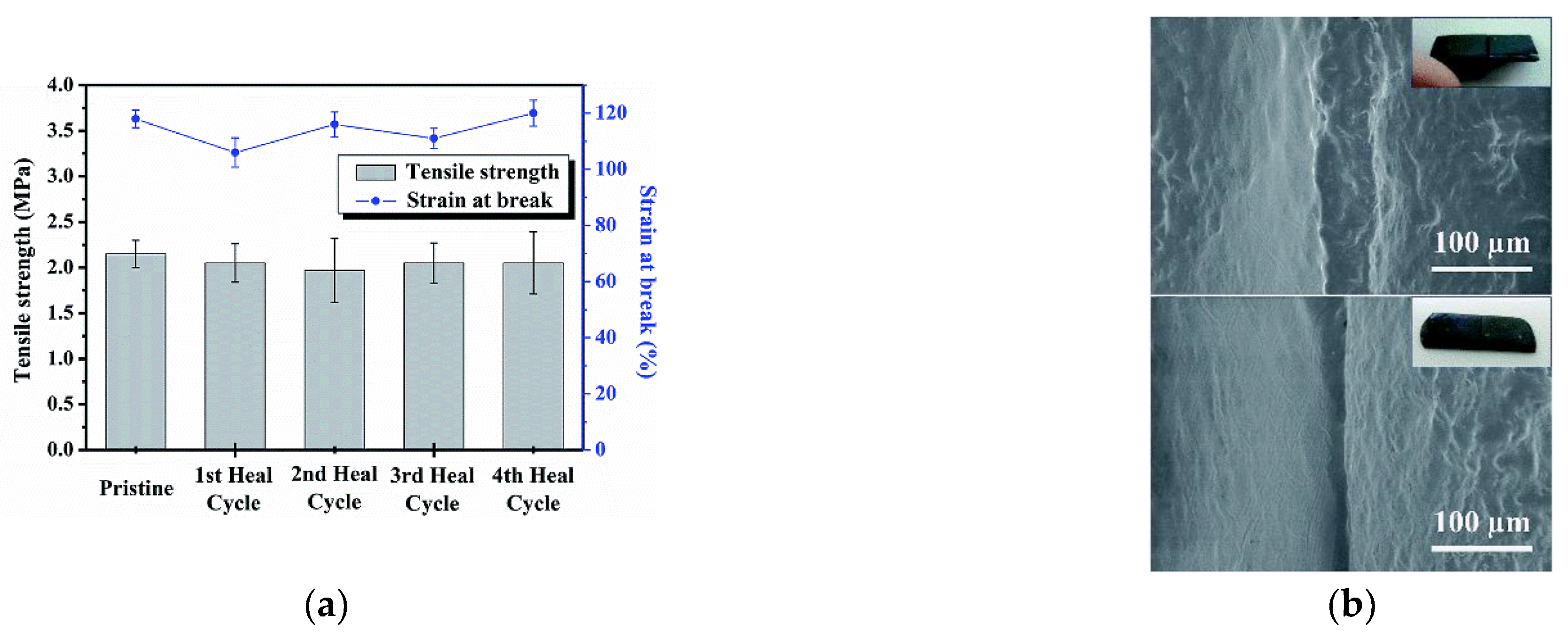
- Huang, Y.; Yan, J.; Wang, D.; Feng, S.; Zhou, C. Construction of Self-Healing Disulfide-Linked Silicone Elastomers by Thiol Oxidation Coupling Reaction. Polymers 2021, 13, 3729. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-C.; Chang, F.-C.; Dai, S.A.; Lin, Y.-L.; Lee, D.-J. Bio-complementary supramolecular polymers with effective self-healing functionality. RSC Adv. 2015, 5, 90466–90472. [Google Scholar] [CrossRef]
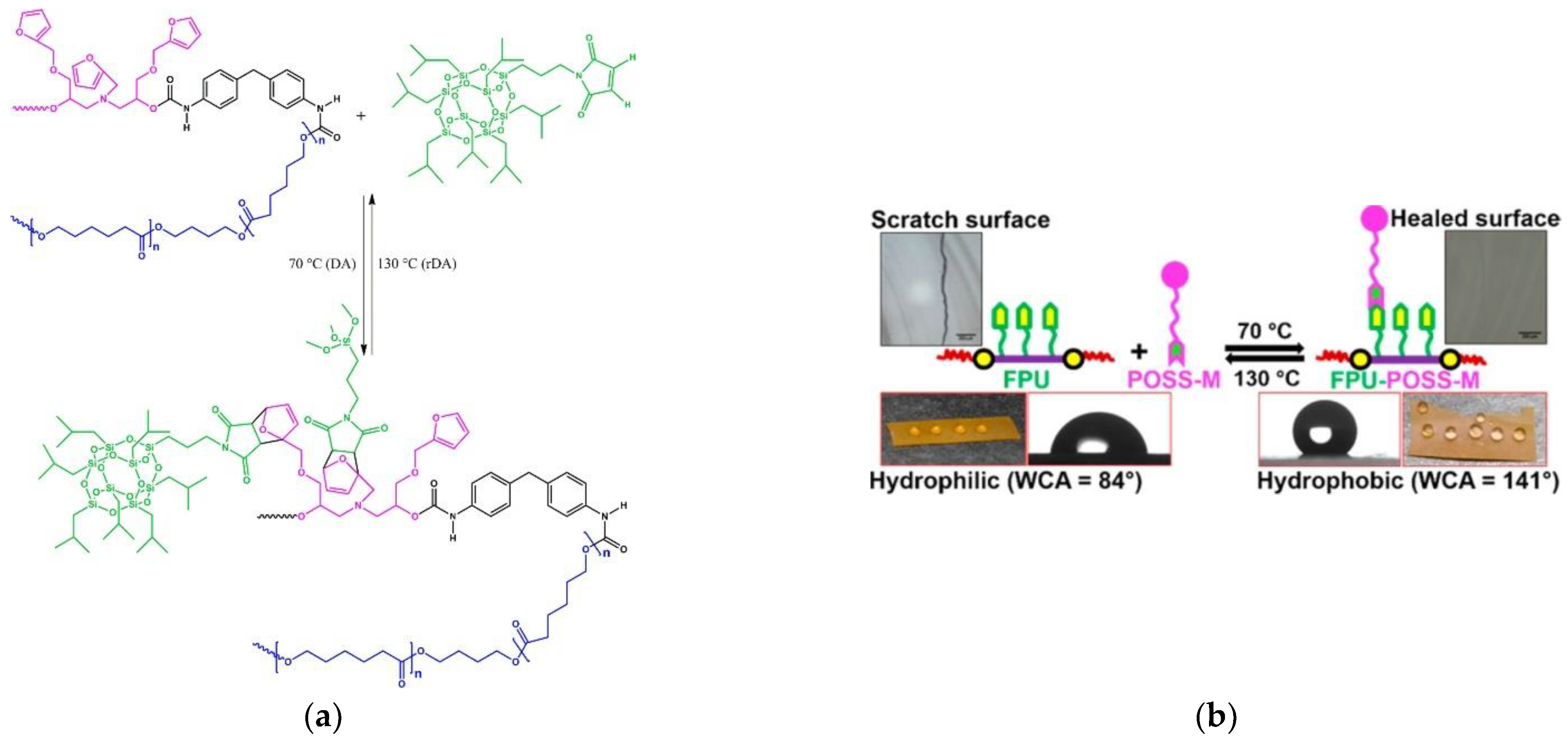
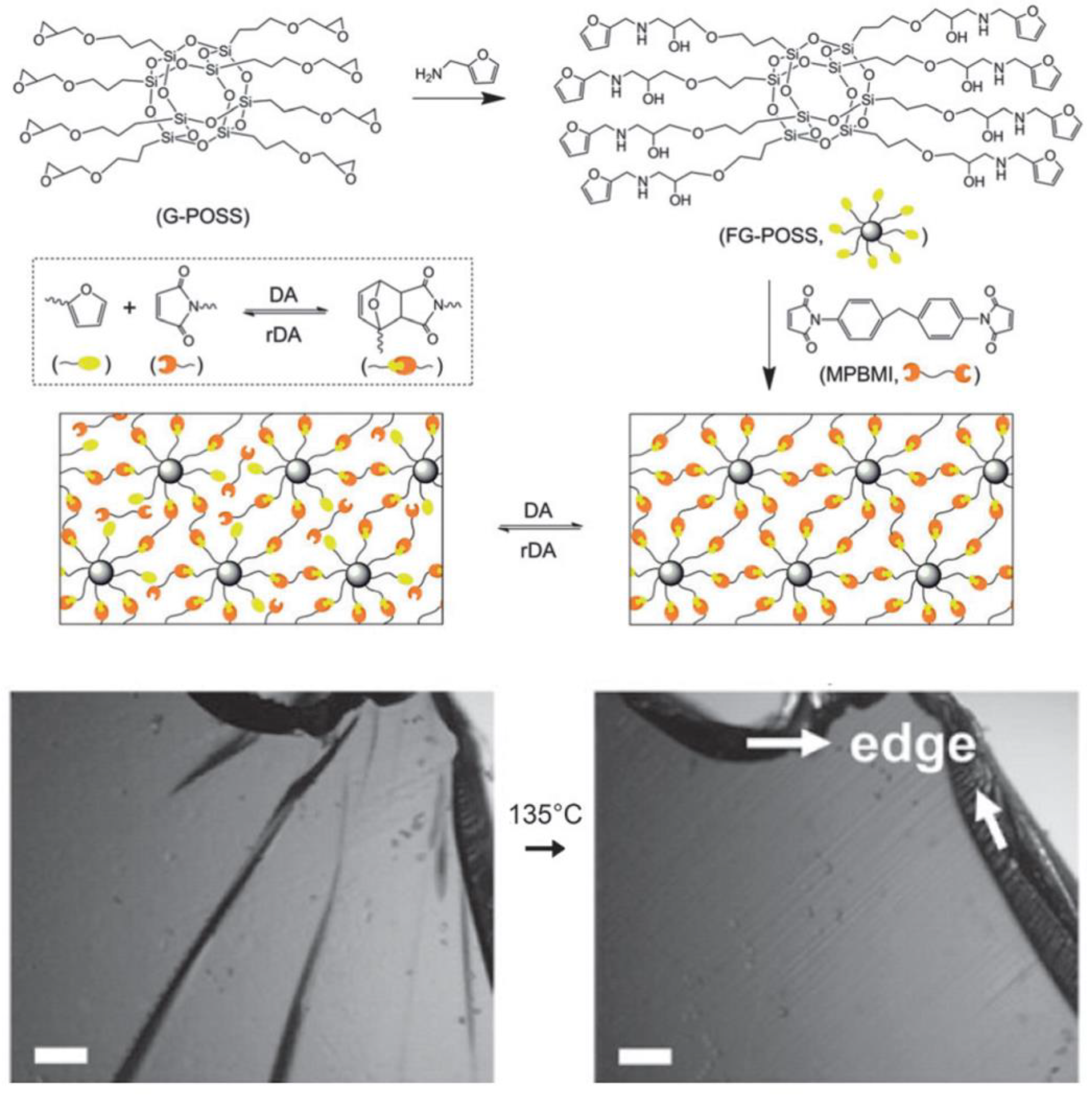
- Xu, Z.; Zhao, Y.; Wang, X.; Lin, T. A thermally healable polyhedral oligomeric silsesquioxane (POSS) nanocomposite based on Diels-Alder chemistry. Chem. Commun. 2013, 49, 6755–6757. [Google Scholar] [CrossRef] [Green Version]
- Goodman, S.H. Handbook of Thermoset Plastics, 2nd ed.; Noyes Publications: Westwood, NJ, USA, 1998. [Google Scholar]
- Tian, Q.; Yuan, Y.C.; Rong, M.Z.; Zhang, M.Q. A thermally remendable epoxy resin. J. Mater. Chem. 2009, 19, 1289–1296. [Google Scholar] [CrossRef]
- Chuo, T.-W.; Liu, Y.-L. Preparation of self-healing organic-inorganic nanocomposites with the reactions between methacrylated polyhedral oligomeric silsesquioxanes and furfurylamine. Compos. Sci. Technol. 2015, 118, 236–243. [Google Scholar] [CrossRef]
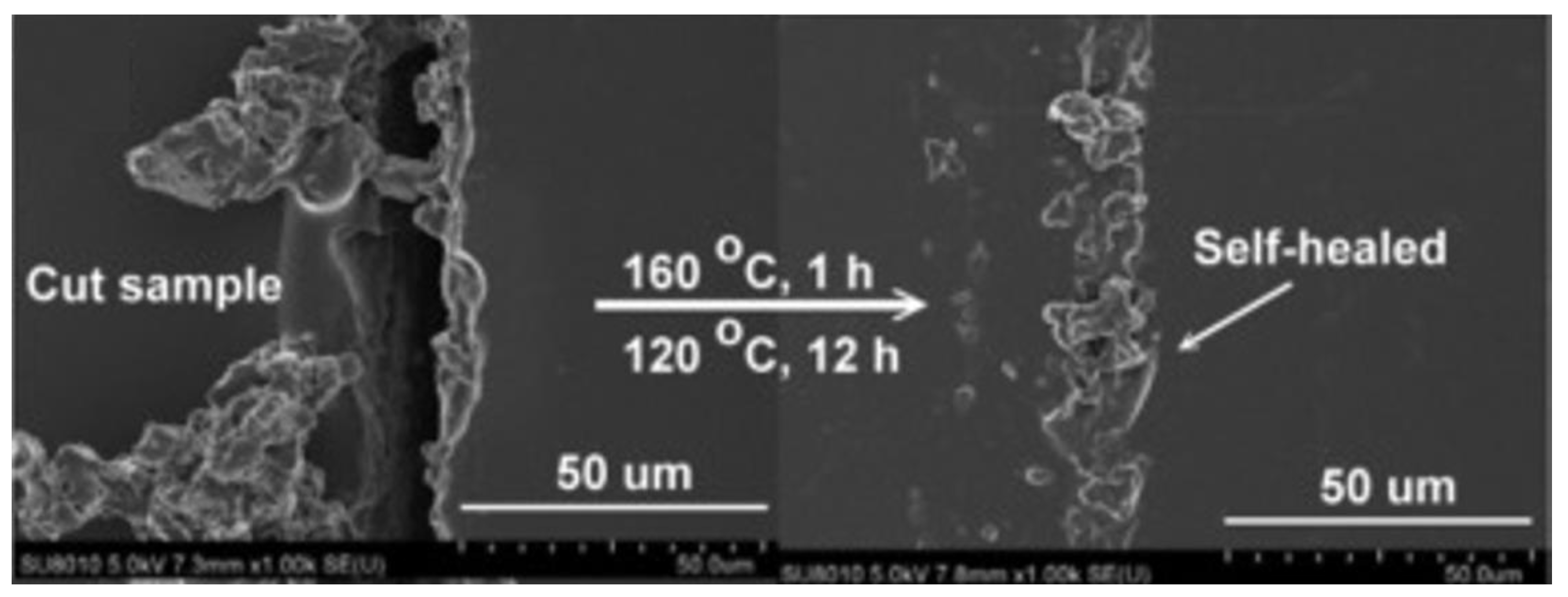
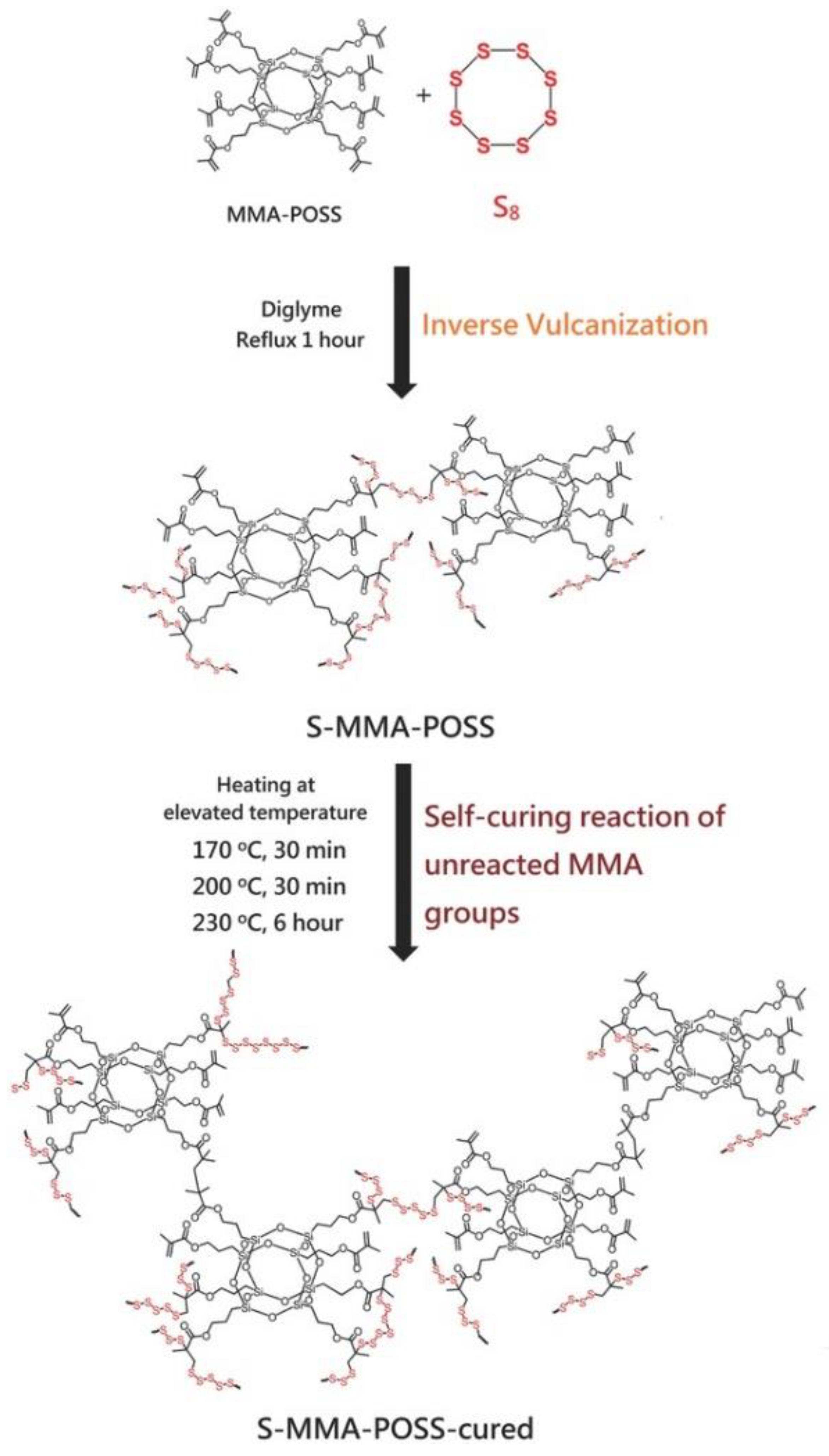
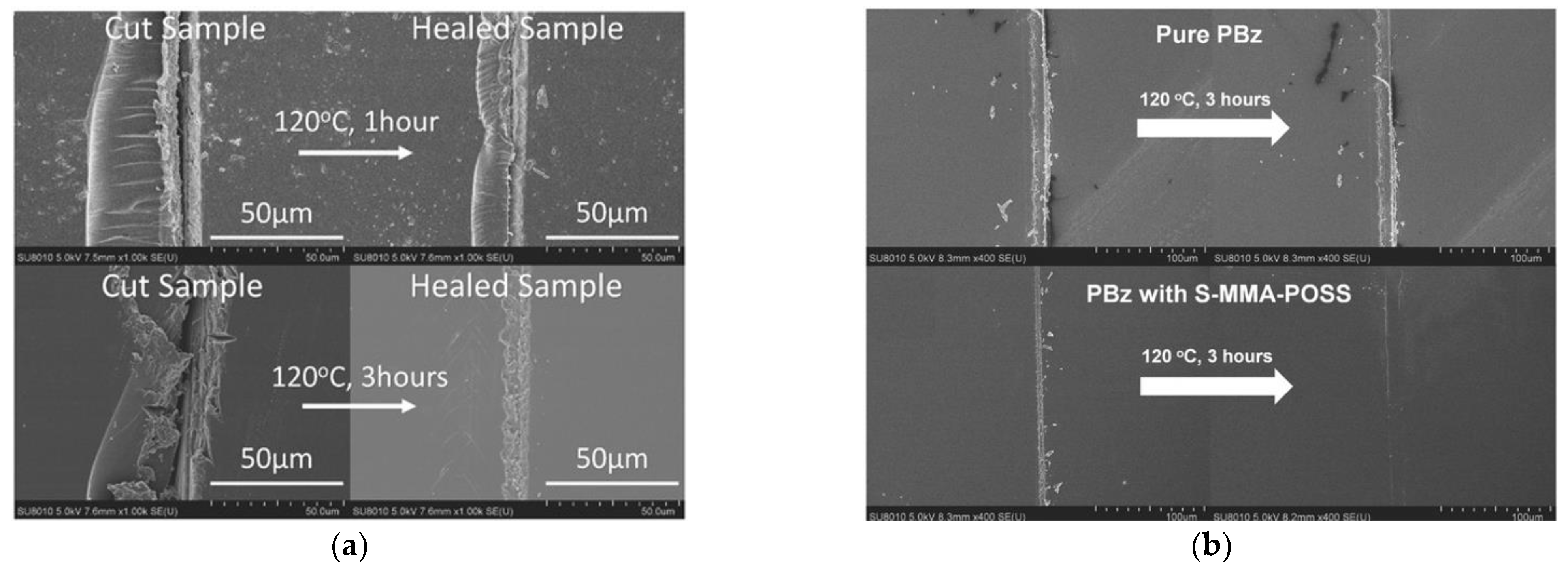
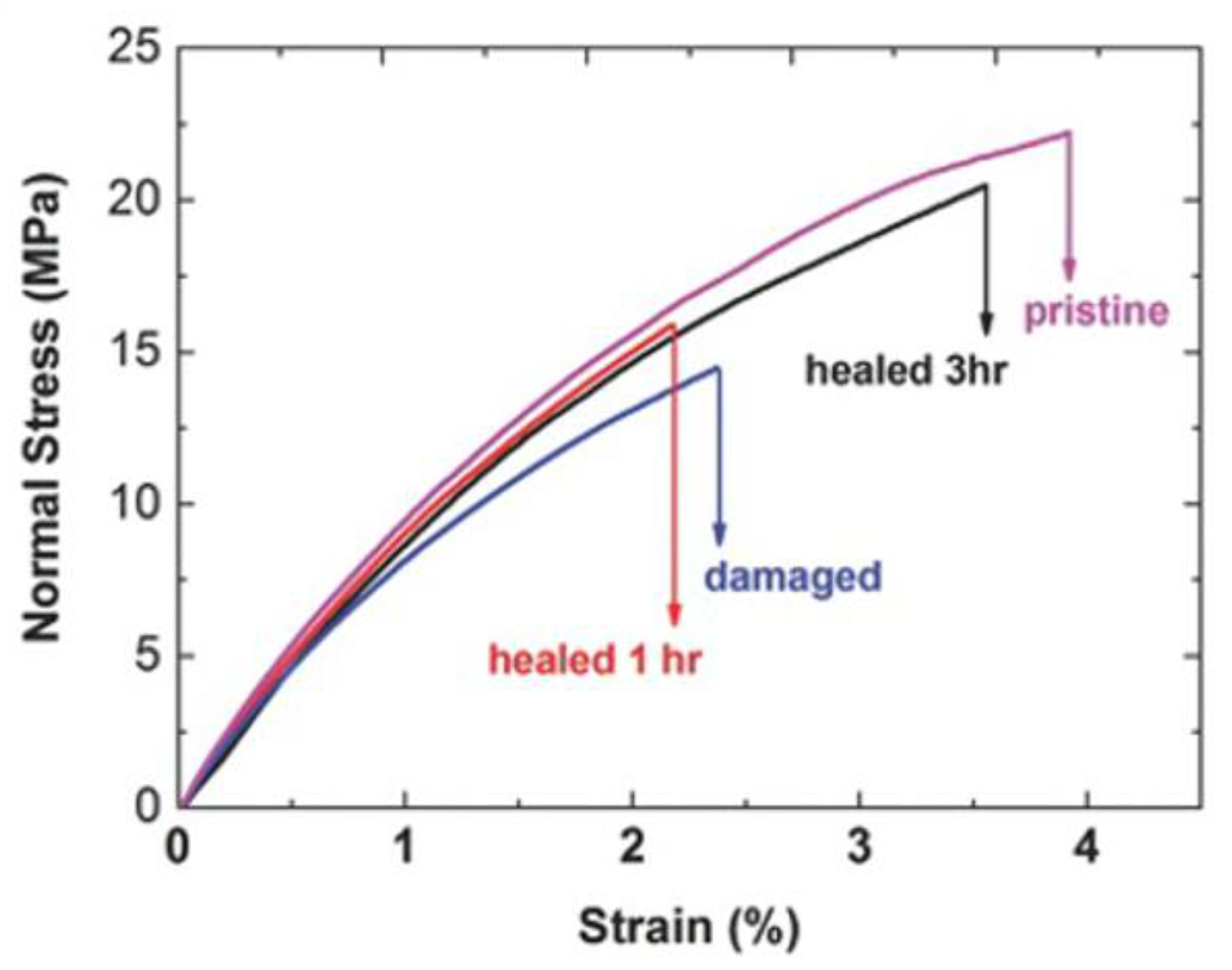
- Lin, H.-K.; Liu, Y.-L. Reactive Hybrid of Polyhedral Oligomeric Silsesquioxane (POSS) and Sulfur as a Building Block for Self-Healing Materials. Macromol. Rapid Commun. 2017, 38, 1700051. [Google Scholar] [CrossRef] [PubMed]
- Matxain, J.M.; Asuab, J.M.; Ruiperez, F. Design of new disulfide-based organic compounds for the improvement of self-healing materials. Phys. Chem. Chem. Phys. 2016, 18, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Whittell, G.R.; Hager, M.D.; Schubert, U.S.; Manners, I. Functional soft materials from metallopolymers and metallosupramolecular polymers. Nature Mater. 2011, 10, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Bode, S.; Zedler, L.; Schacher, F.H.; Dietzek, B.; Schmitt, M.; Popp, J.; Hager, M.D.; Schubert, U.S. Self-Healing Polymer Coatings Based on Crosslinked Metallosupramolecular Copolymers. Adv. Mater. 2013, 25, 1634–1638. [Google Scholar] [CrossRef]
- Burnworth, M.; Tang, L.; Kumpfer, J.R.; Duncan, A.J.; Beyer, F.L.; Fiore, G.L.; Rowan, S.J.; Weder, C. Optically healable supramolecular polymers. Nature 2011, 472, 334–337. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, Y.; Mori, H. Self-healing hybrids fabricated by metal complexation with imidazole-containing silsesquioxane nanoparticles. Mater. Chem. Front. 2020, 4, 2655–2664. [Google Scholar] [CrossRef]
- Enke, M.; Bode, S.; Vitz, J.; Schacher, F.H.; Harrington, M.J.; Hager, M.D.; Schubert, U.S. Self-healing response in supramolecular polymers based on reversible zinc–histidine interactions. Polymer 2015, 69, 274–282. [Google Scholar] [CrossRef]
- Sasaki, Y.; Yamamoto, T.; Mori, H. Mechanically robust, ion-conductive, self-healing glassy hybrid materials via tailored Zn/imidazole interaction. Mater. Today Chem. 2021, 22, 100611. [Google Scholar] [CrossRef]
- Wang, W.; Narain, R.; Zeng, H. Rational design of self-healing tough hydrogels: A mini review. Front. Chem. 2018, 6, 497. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and Hydrogel-Derived Materials for Energy and Water Sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, S.; Sprakel, J. Physical chemistry of supramolecular polymer networks. Chem. Soc. Rev. 2012, 41, 909–930. [Google Scholar] [CrossRef]
- Yang, B.; Wei, Z.; Chen, X.; Wei, K.; Bian, L. Manipulating the mechanical properties of biomimetic hydrogels with multivalent host–guest interactions. J. Mater. Chem. B 2019, 7, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Gradzinska, K.; Marcinkowska, M.; Klajnert-Maculewicz, B.; Stanczyk, W.A. In vitro studies of polyhedral oligo silsesquioxanes: Evidence for their low cytotoxicity. Materials 2015, 8, 6062–6070. [Google Scholar] [CrossRef] [Green Version]
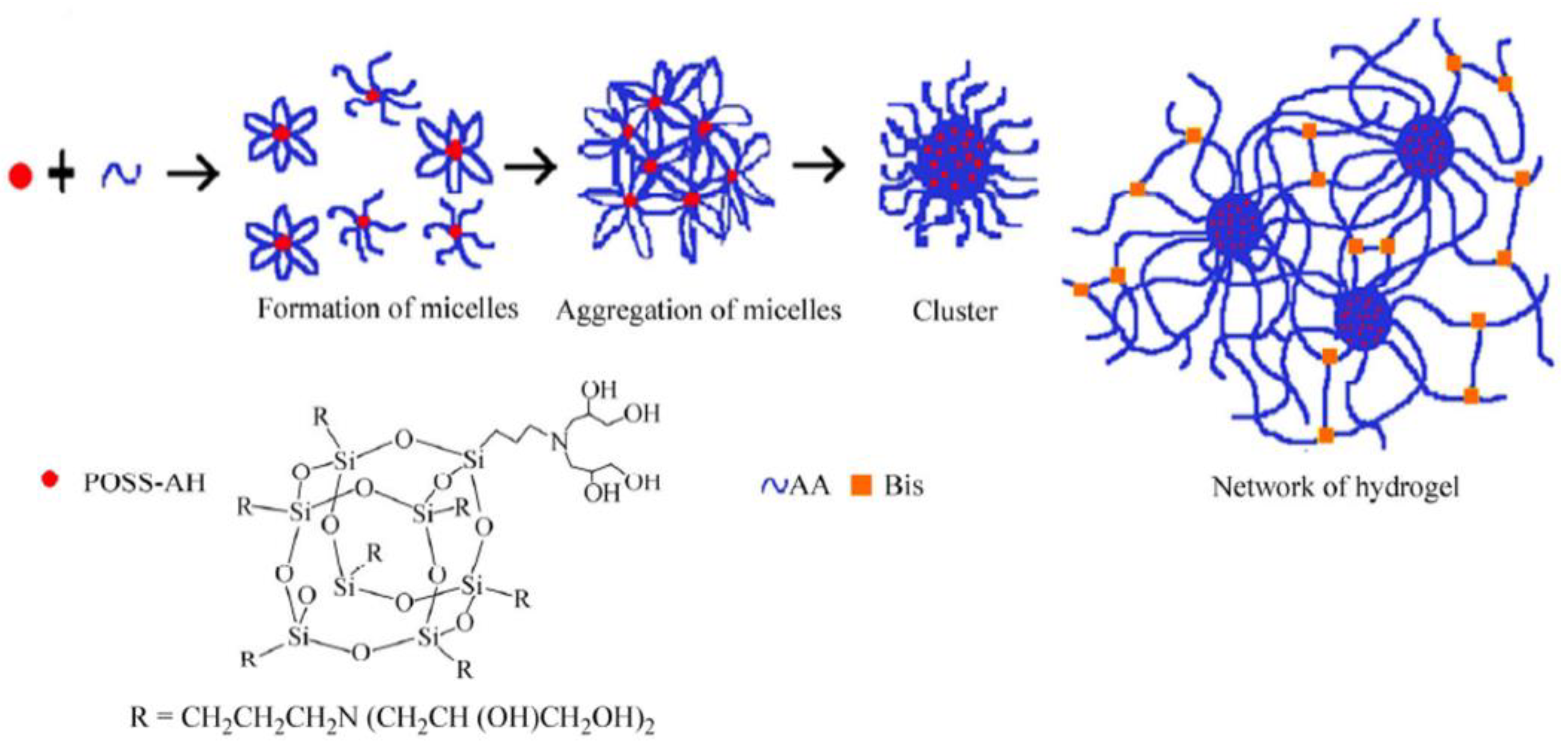
- Yang, L.-Q.; Lu, L.; Zhang, C.-W.; Zhou, C.-R. Highly Stretchable and Self-healing Hydrogels Based on Poly(acrylic acid) and Functional POSS. Chin. J. Polym. Sci. 2016, 34, 185–194. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, H.; Zhao, Y. Poly(vinyl alcohol) Hydrogel Can Autonomously Self-Heal. ACS Macro Lett. 2012, 1, 1233–1236. [Google Scholar] [CrossRef]
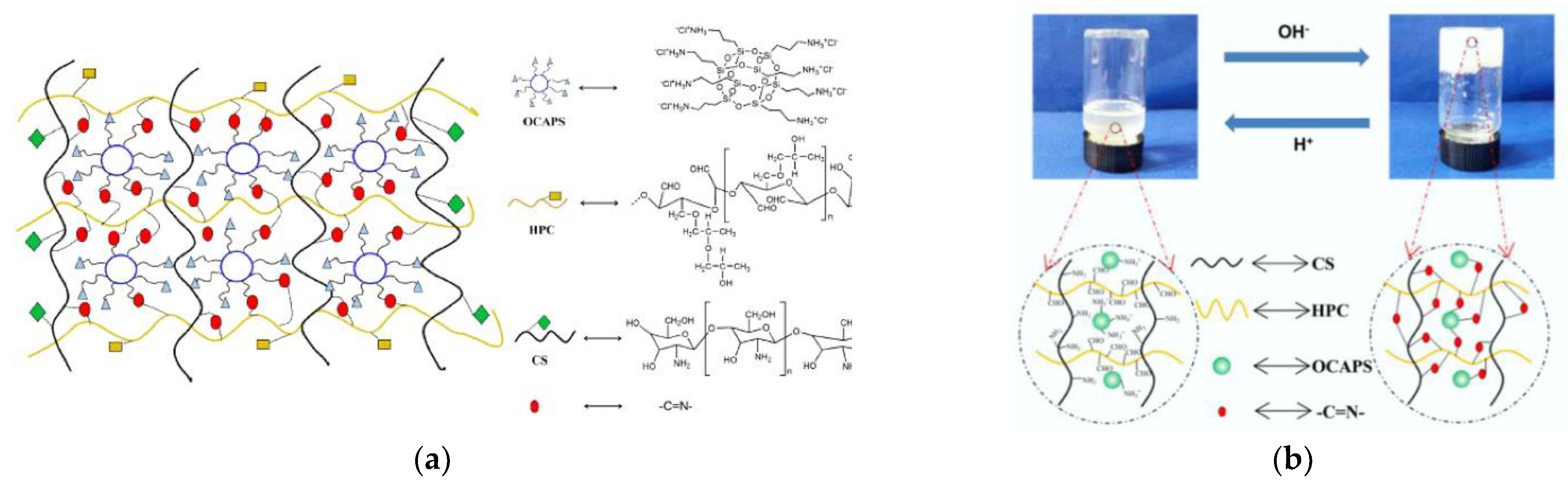
- Zhang, X.; Meng, Y.; Shen, W.; Dou, J.; Liu, R.; Jin, Q.; Fang, S. pH-responsive injectable polysaccharide hydrogels with self-healing, enhanced mechanical properties based on POSS. React. Funct. Polym. 2021, 158, 104773. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, W.; Dou, J.; Meng, Y.; Fang, S.; Liu, R. Enhanced mechanical properties and self-healing behavior of PNIPAM nanocomposite hydrogel by using POSS as a physical crosslinker. J. Appl. Polym. Sci. 2020, 137, 48486. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Albanil, L. POSS-Induced Dynamic Cross-Links Produced Self-Healing and Shape Memory Physical Hydrogels When Copolymerized with N-Isopropyl acrylamide. ACS Appl. Mater. Interf. 2019, 11, 24447–24458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Dai, Z.; Jiang, F.; Tian, J.; Zhang, W. A super-stretchable, self-healing and injectable supramolecular hydrogel constructed by a host–guest crosslinker. Biomater. Sci. 2020, 8, 3359–3369. [Google Scholar] [CrossRef] [PubMed]
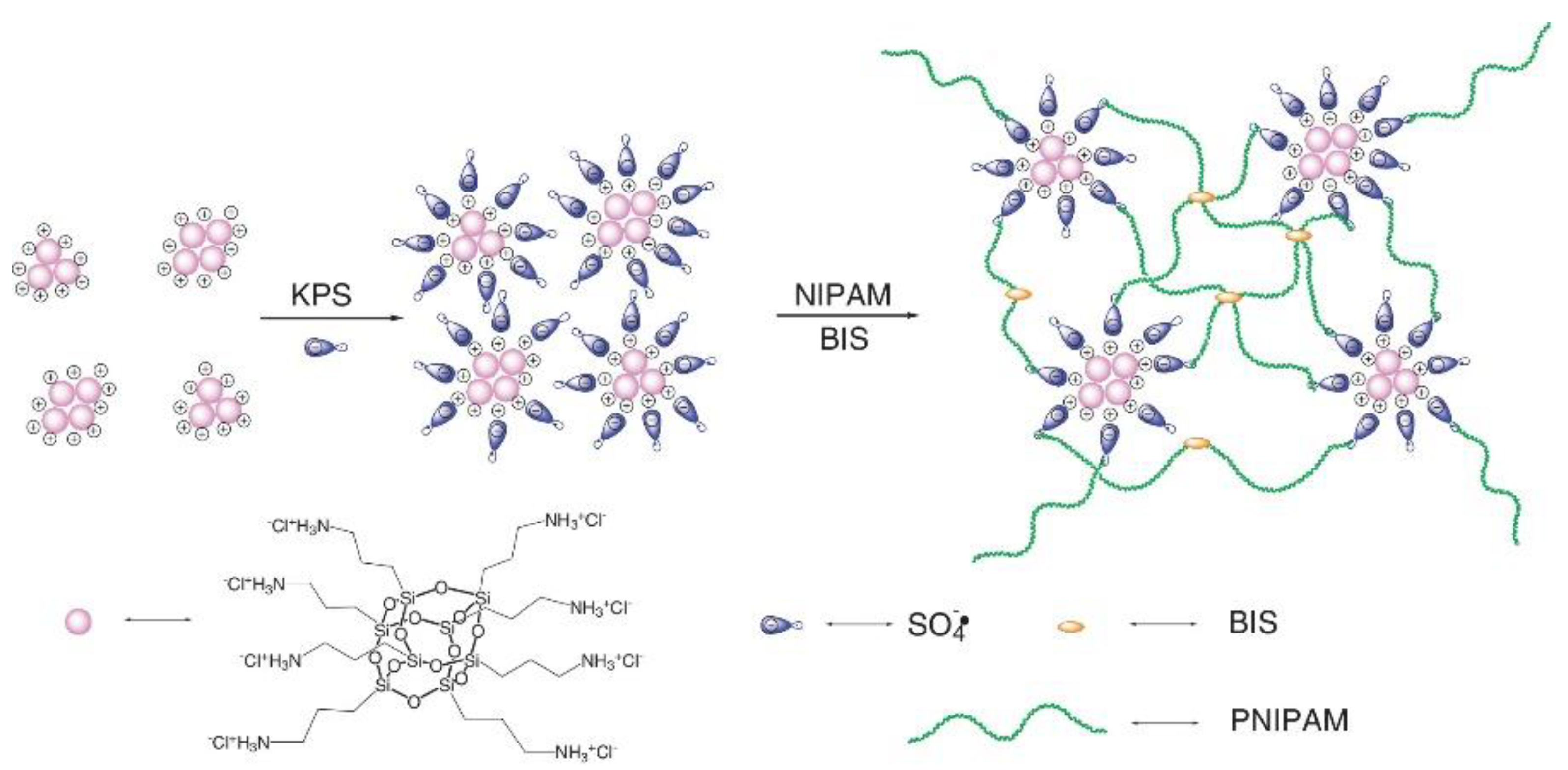
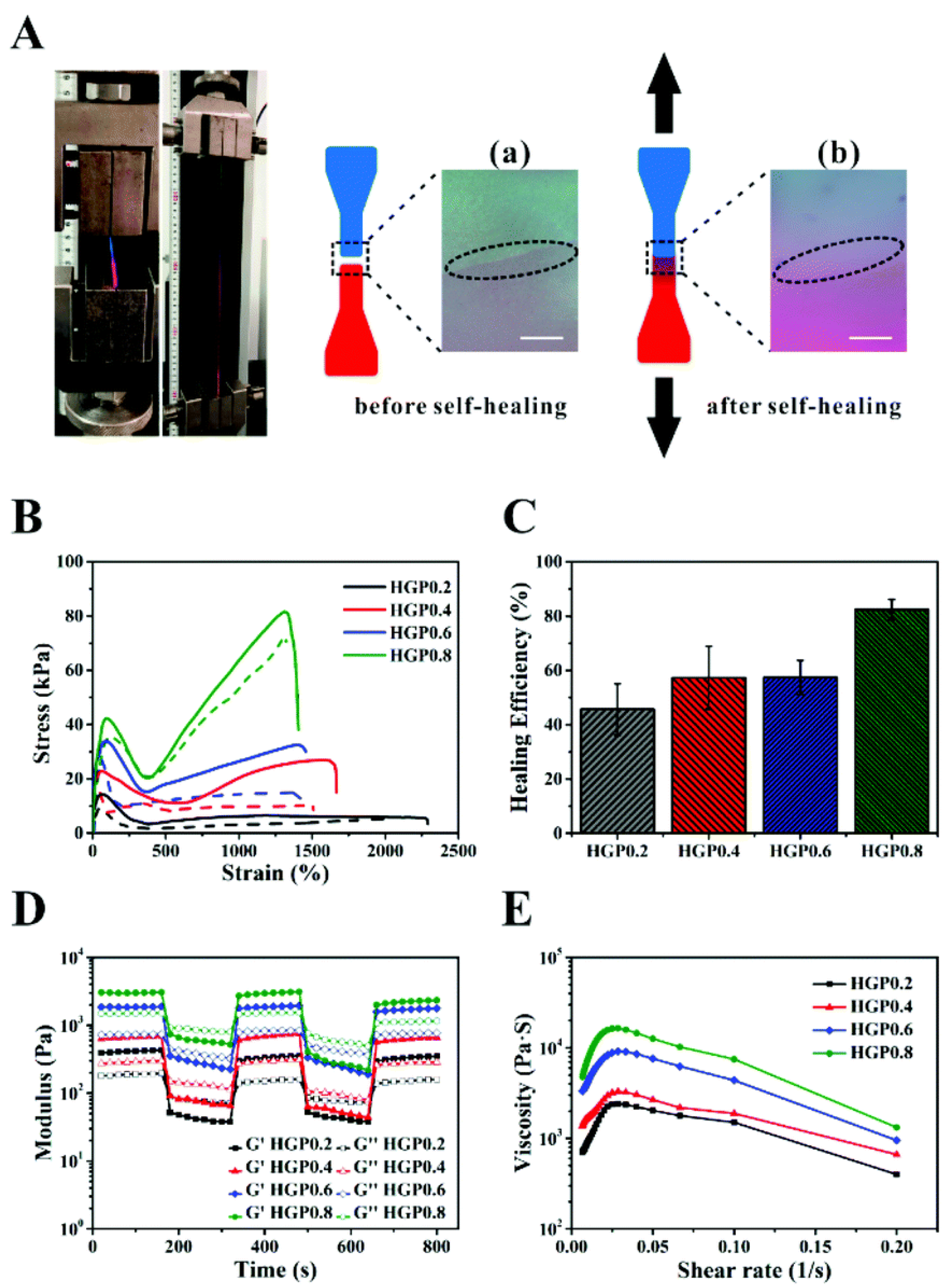
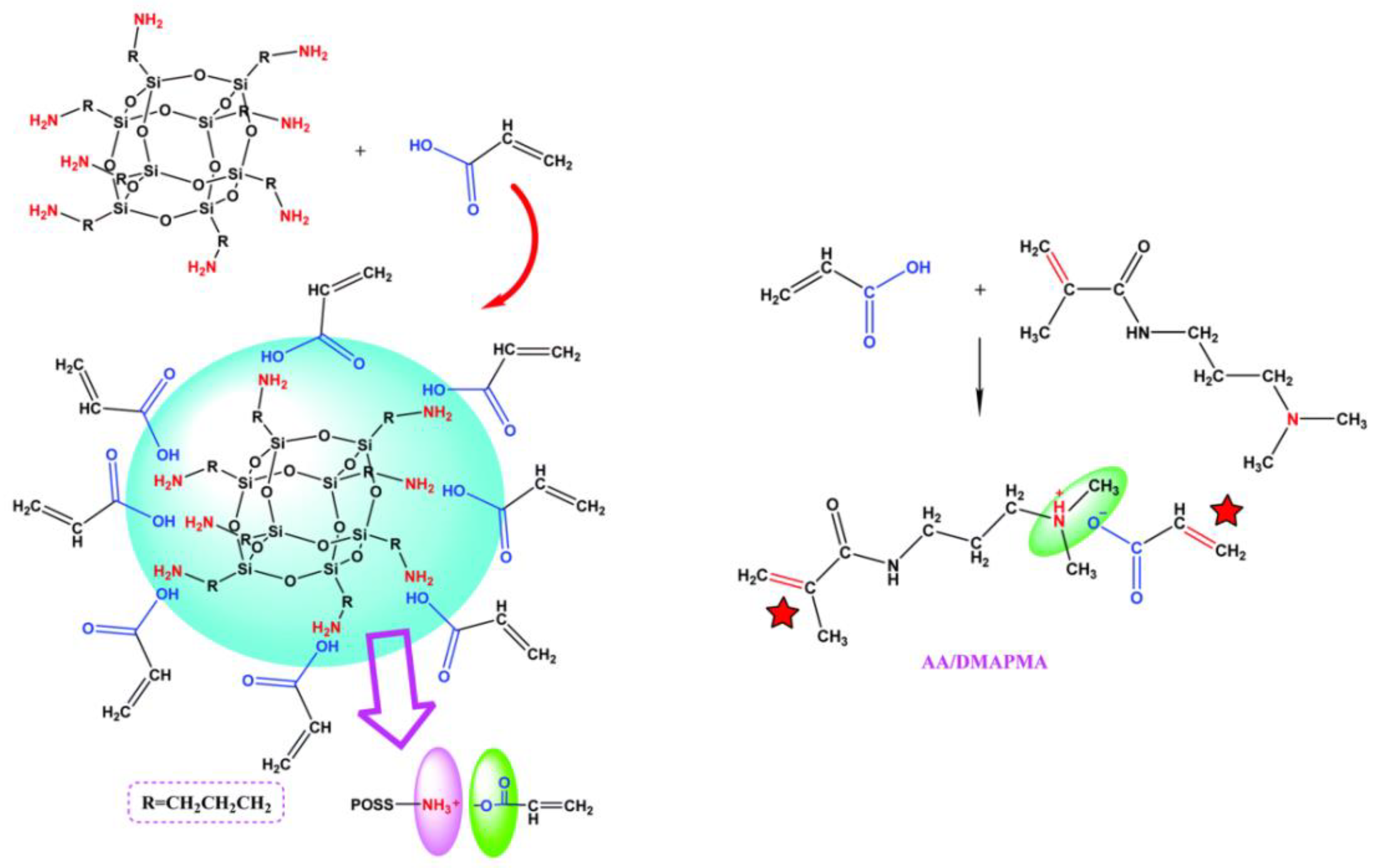
- Pu, W.; Jiang, F.; Chen, P.; Wei, B. A POSS based hydrogel with mechanical robustness, cohesiveness and a rapid self-healing ability by electrostatic interaction. Soft Matter 2017, 13, 5645–5648. [Google Scholar] [CrossRef] [PubMed]








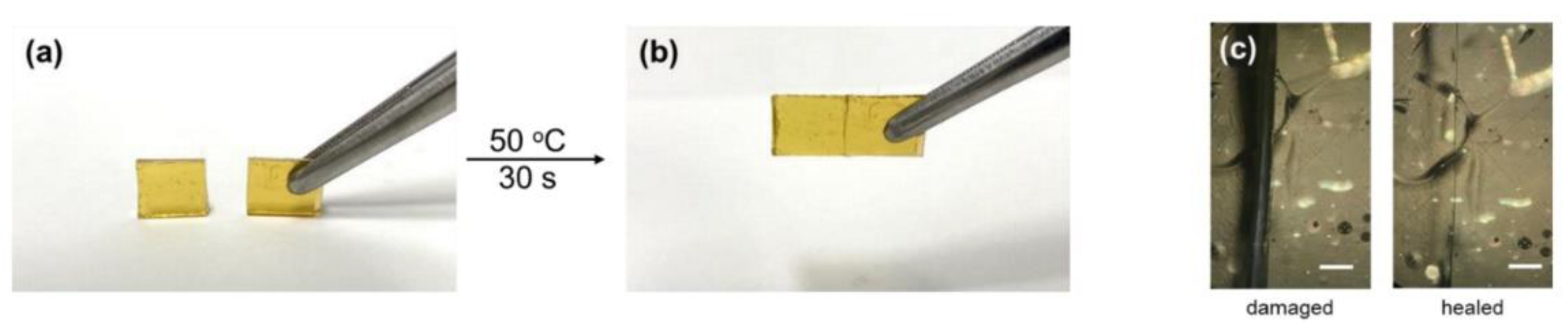




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka, M.; Kowalewska, A. Self-Healing Silsesquioxane-Based Materials. Polymers 2022, 14, 1869. https://doi.org/10.3390/polym14091869
Nowacka M, Kowalewska A. Self-Healing Silsesquioxane-Based Materials. Polymers. 2022; 14(9):1869. https://doi.org/10.3390/polym14091869
Chicago/Turabian StyleNowacka, Maria, and Anna Kowalewska. 2022. "Self-Healing Silsesquioxane-Based Materials" Polymers 14, no. 9: 1869. https://doi.org/10.3390/polym14091869
APA StyleNowacka, M., & Kowalewska, A. (2022). Self-Healing Silsesquioxane-Based Materials. Polymers, 14(9), 1869. https://doi.org/10.3390/polym14091869






