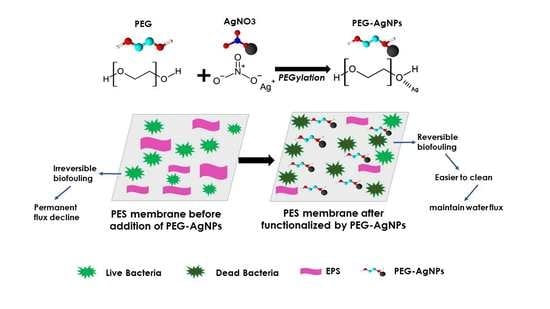
Functionalization of PEG-AgNPs Hybrid Material to Alleviate Biofouling Tendency of Polyethersulfone Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
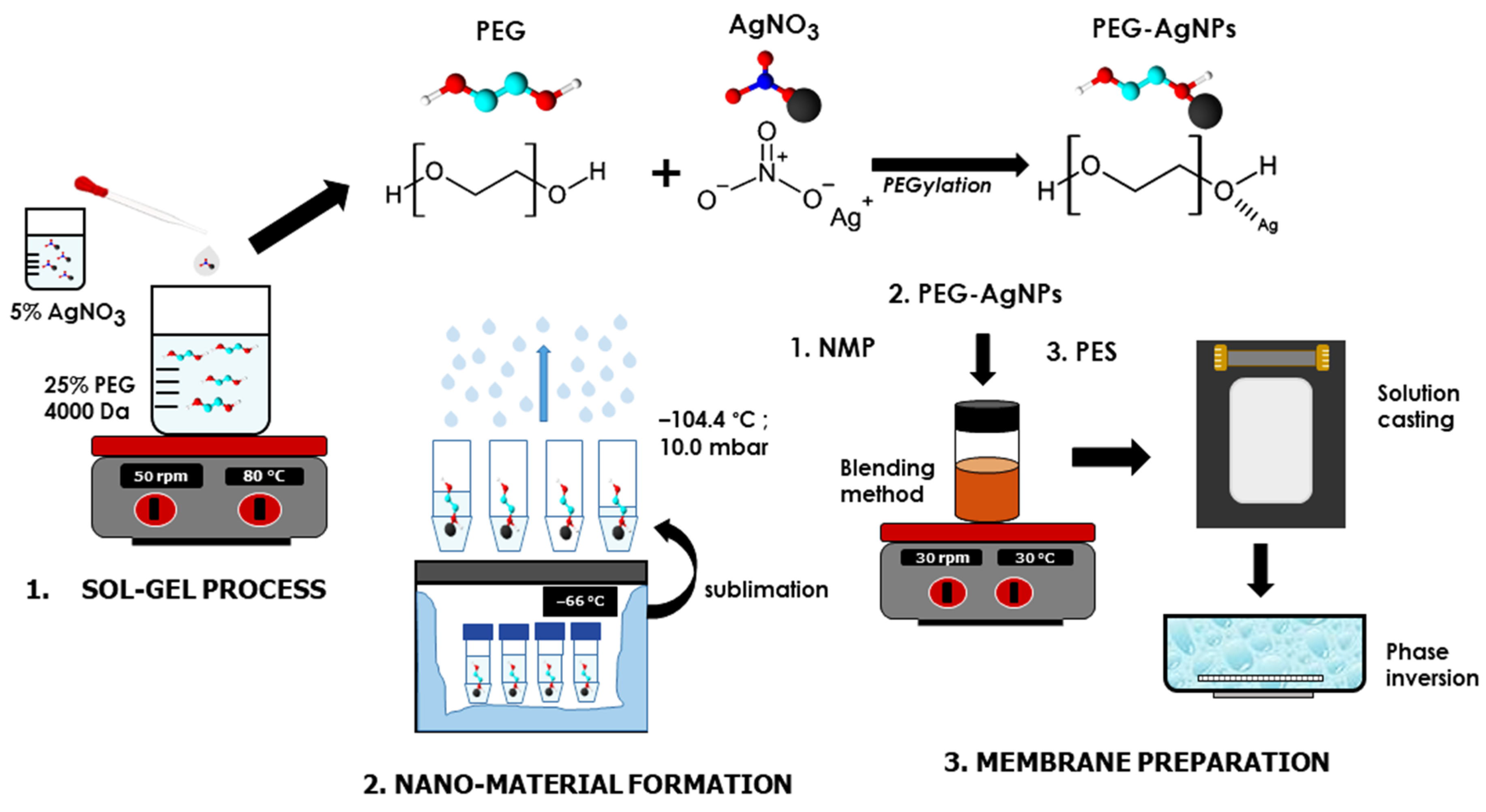
2.2. Synthesis of PEG-AgNPs Hybrid Materials
2.3. Preparation of PES/PEG-AgNPs Membrane
2.4. Characterization of the PEG-AgNPs Hybrid Material
2.5. Characterization and Selectivity Performance of PES/PEG-AgNPs Membranes
2.6. Evaluation of Membrane Anti-Bacterial and Anti-Biofouling Performance
3. Result and Discussions
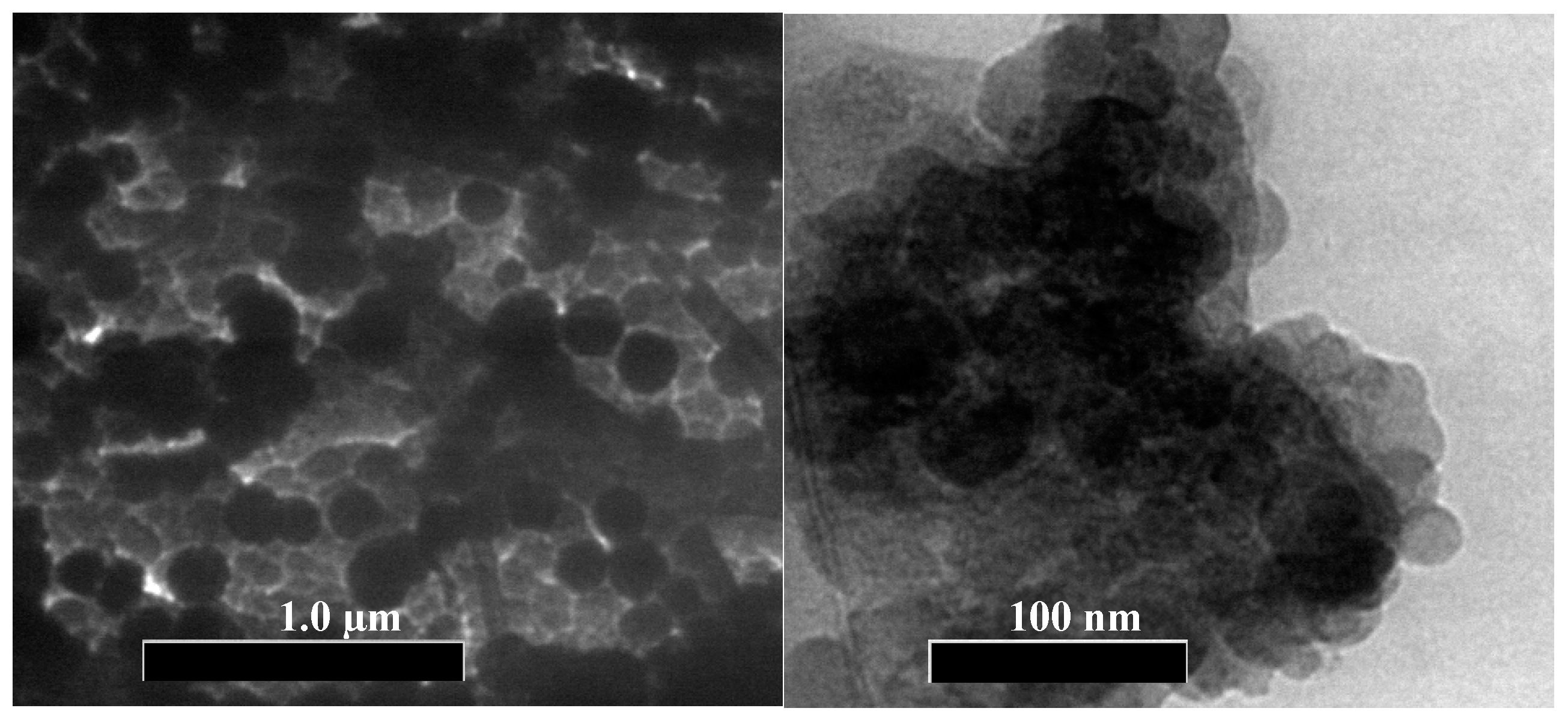
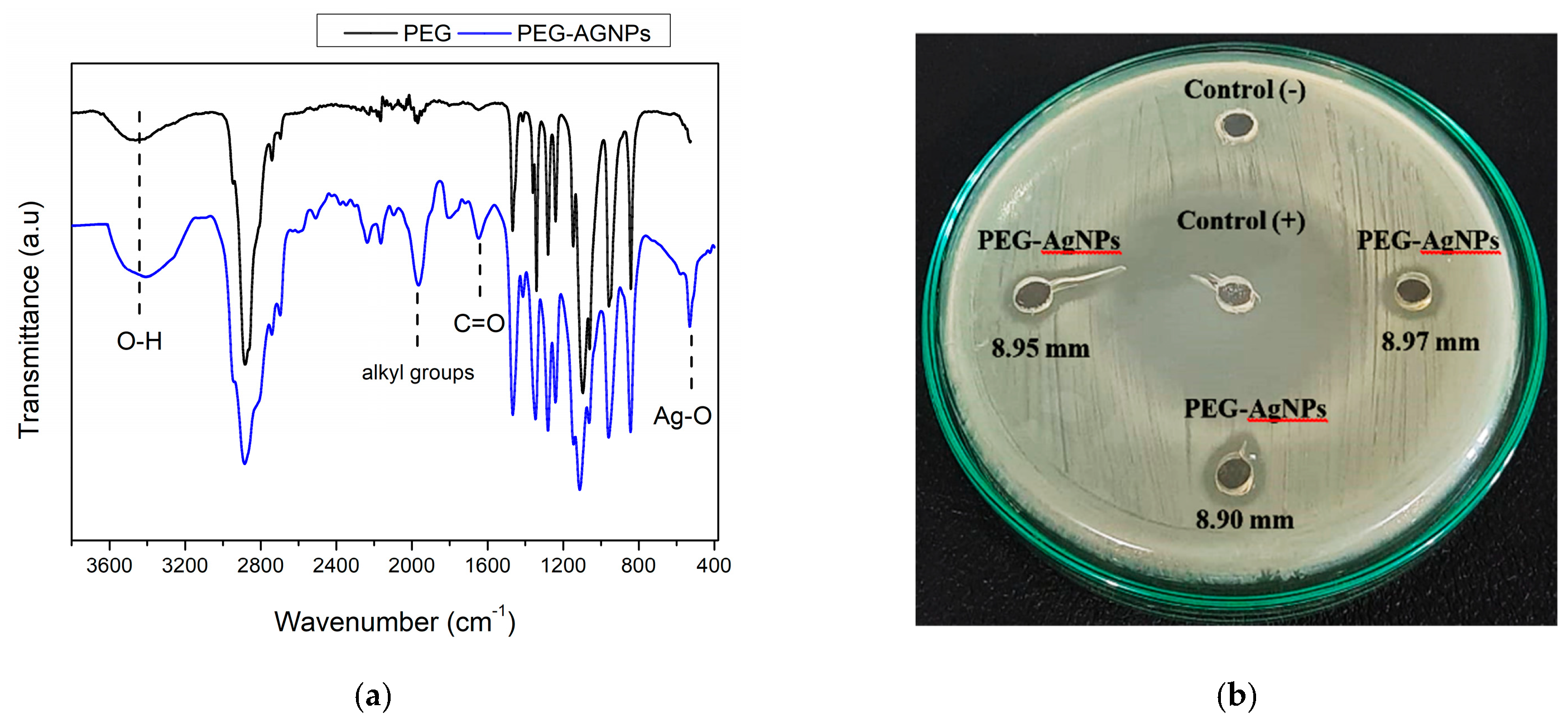
3.1. Characterization of PEG-AgNPs
3.2. Characteristics of the PES/PEG-AgNPs Membrane
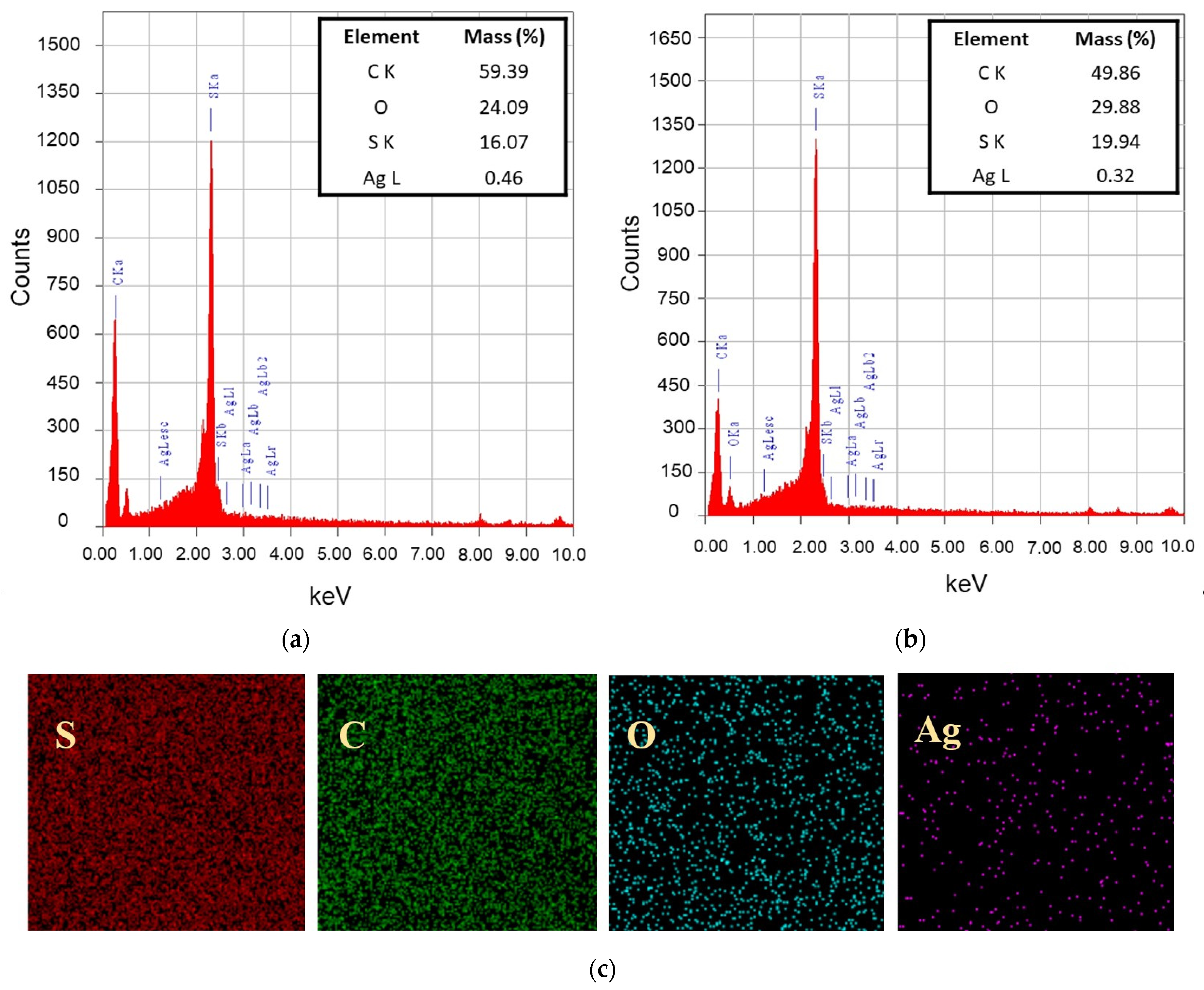
3.2.1. Chemical Groups and Elemental Composition
3.2.2. Membrane Morphological Structure
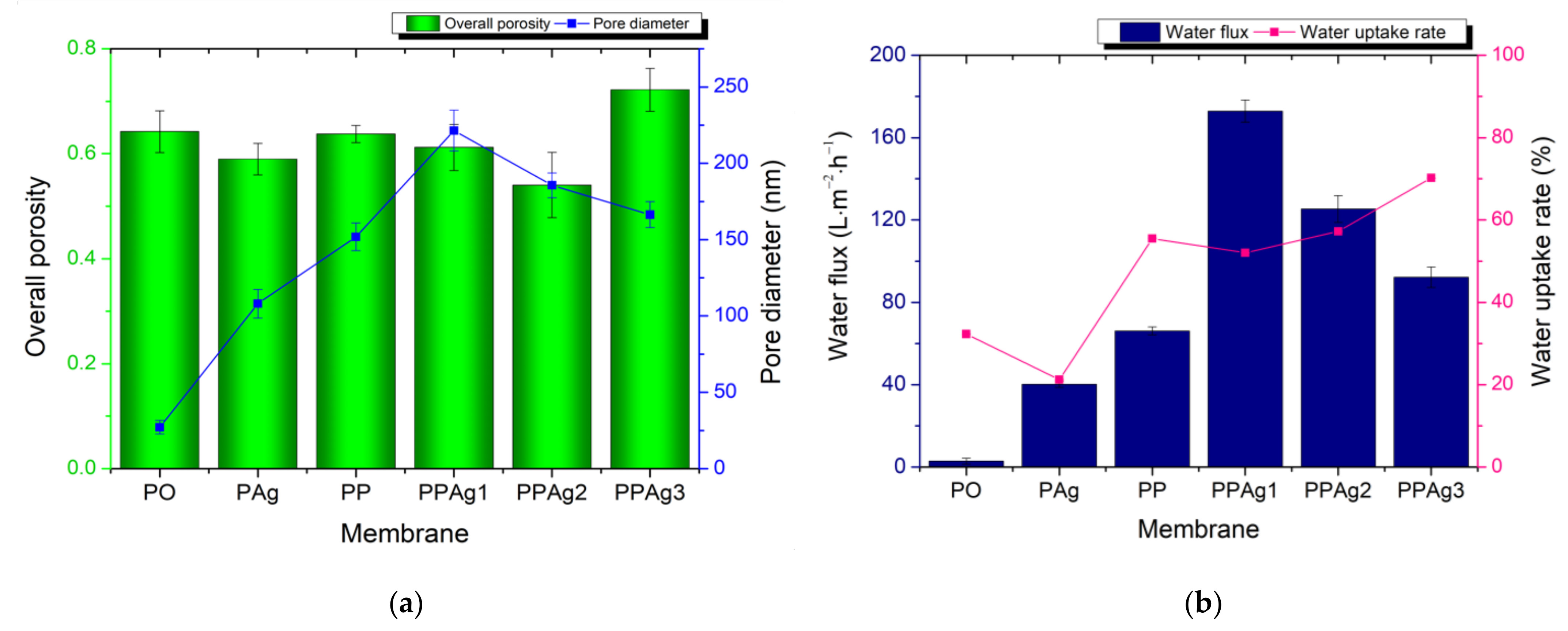
3.2.3. Membrane Porosity Analysis, Water Uptake Rate, and Water Flux
3.3. Membrane Selectivity Performance
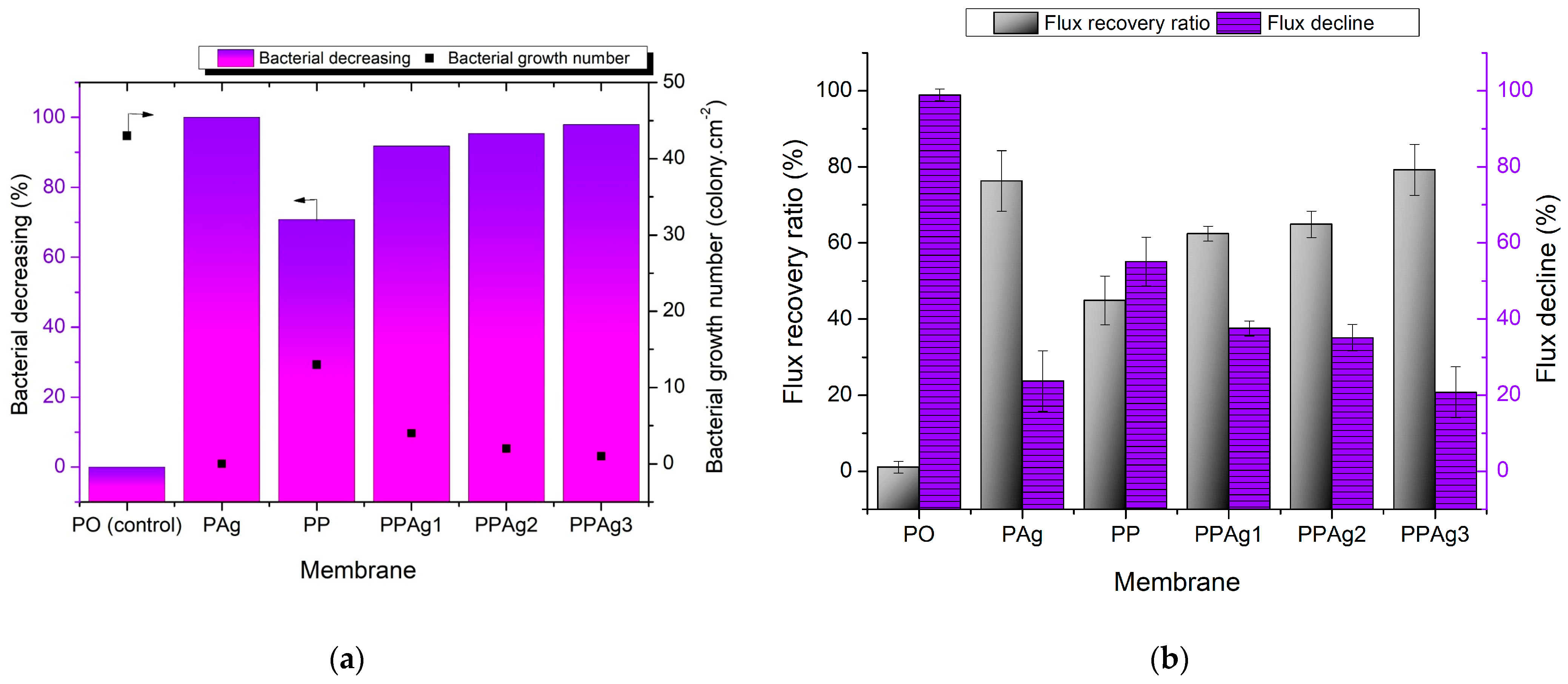
3.4. Anti-Bacterial Study and Anti-Biofouling Performance of the Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sali, S.; Mackey, H.R. Effect of Graphene Oxide Synthesis Method on Properties and Performance of Polysulfone-Graphene Oxide Mixed Matrix Membranes. Nanomaterials 2019, 9, 769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.S.A.; Lee, K.; Park, H.; Choo, K.H. Live membrane filters with immobilized quorum quenching bacterial strains for anti-biofouling. J. Memb. Sci. 2022, 641, 119895. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Gad-Allah, T.A.; El-Kalliny, A.S.; Ahmed, S.I.A.; Souaya, E.R.; Badawy, M.I.; Ulbricht, M. Fabrication of modified polyethersulfone membranes for wastewater treatment by submerged membrane bioreactor. Sep. Purif. Technol. 2017, 175, 36–46. [Google Scholar] [CrossRef]
- Nady, N.; Salem, N.; Amer, R.; El-Shazly, A.; Kandil, S.H.; Hassouna, M.S.E.D. Comparison between a conventional anti-biofouling compound and a novel modified low-fouling polyethersulfone ultrafiltration membrane: Bacterial anti-attachment, water quality and productivity. Membranes 2020, 10, 227. [Google Scholar] [CrossRef]
- Arahman, N.; Rosnelly, C.M.; Yusni, Y.; Fahrina, A.; Silmina, S.; Ambarita, A.C.; Bilad, M.R.; Gunawan, P.; Rajabzadeh, S.; Takagi, R.; et al. Ultrafiltration of α-lactalbumin protein: Acquaintance of the filtration performance by membrane structure and surface alteration. Polymers 2021, 13, 3632. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, L.; Shan, B.; Xie, C.; Liu, C.; Cui, F.; Li, G. Preparation and characterization of SLS-CNT/PES ultrafiltration membrane with antifouling and antibacterial properties. J. Memb. Sci. 2018, 548, 459–469. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, D.; Sun, X.; Jiang, S.; Liu, Y.; Zhang, D.; Zhang, L. Surface modified by green synthetic of Cu-MOF-74 to improve the anti-biofouling properties of PVDF membranes. Chem. Eng. J. 2021, 411, 128524. [Google Scholar] [CrossRef]
- Rahmawati, R.; Roil, M.; Izati, N.; Nawi, M.; Wibisono, Y.; Suhaimi, H.; Shamsuddin, N.; Arahman, N. Journal of Environmental Chemical Engineering Engineered spacers for fouling mitigation in pressure driven membrane processes: Progress and projection. J. Environ. Chem. Eng. 2021, 9, 106285. [Google Scholar] [CrossRef]
- Rahimi, Z.; Zinatizadeh, A.A.L.; Zinadini, S. Journal of Industrial and Engineering Chemistry Preparation of high antibiofouling amino functionalized MWCNTs/PES nanocomposite ultrafiltration membrane for application in membrane bioreactor. J. Ind. Eng. Chem. 2015, 29, 366–374. [Google Scholar] [CrossRef]
- Lan, Y.; Hiebner, D.W.; Casey, E. Self-assembly and regeneration strategy for mitigation of membrane biofouling by the exploitation of enzymatic nanoparticles. Chem. Eng. J. 2021, 412, 128666. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Boopathy, R.; Aamer, M. Bioresource Technology Reports Recent advances on bacterial quorum quenching as an effective strategy to control biofouling in membrane bioreactors. Bioresour. Technol. Rep. 2021, 15, 100745. [Google Scholar] [CrossRef]
- Song, Z.; Yang, S.; Li, P.; Sun, J.; Xing, D.; Peng, W.; Sun, F. Roles of initial bacterial attachment and growth in the biofouling development on the microfiltration membrane: From viewpoints of individual cell and interfacial interaction energy. J. Memb. Sci. 2021, 638, 119723. [Google Scholar] [CrossRef]
- Agrawal, A.; Sharma, A.; Awasthi, K.K.; Awasthi, A. Metal oxides nanocomposite membrane for biofouling mitigation in wastewater treatment. Mater. Today Chem. 2021, 21, 100532. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Wang, H.; Zhang, M.; Pu, J.; Hou, L. an Anti-biofouling behavior of quorum quenching for removal of pharmaceuticals by forward osmosis membrane based on pseudomonas quinolone signals. J. Memb. Sci. 2020, 612, 118475. [Google Scholar] [CrossRef]
- Armendáriz-Ontiveros, M.M.; Álvarez-Sánchez, J.; Dévora-Isiordia, G.E.; García, A.; Fimbres Weihs, G.A. Effect of seawater variability on endemic bacterial biofouling of a reverse osmosis membrane coated with iron nanoparticles (FeNPs). Chem. Eng. Sci. 2020, 223, 115753. [Google Scholar] [CrossRef]
- Piasecka, A.; Bernstein, R.; Ollevier, F.; Meersman, F.; Souffreau, C.; Bilad, R.M.; Cottenie, K.; Vanysacker, L.; Denis, C.; Vankelecom, I. Study of biofilms on PVDF membranes after chemical cleaning by sodium hypochlorite. Sep. Purif. Technol. 2015, 141, 314–321. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Tang, C.Y.; Kimura, K.; Wang, Q. Membrane cleaning in membrane bioreactors: A review. J. Membr. Sci. 2014, 468, 276–307. [Google Scholar] [CrossRef]
- Kumar, S.R.; Arthanareeswaran, G. Nano-curcumin incorporated polyethersulfone membranes for enhanced anti-biofouling in treatment of sewage plant effluent. Mater. Sci. Eng. C 2019, 94, 258–269. [Google Scholar] [CrossRef]
- Mohana Mukherjee, R.B. Silver nanoparticle impregnated polyethersulfone ultrafiltration membrane: Optimization of degree of grafting of acrylic acid for biofouling prevention and improved water permeability. J. Environ. Chem. Eng. 2020, 8, 103711. [Google Scholar] [CrossRef]
- Wang, S.Y.; Rolly Gonzales, R.; Zhang, P.; Istirokhatun, T.; Takagi, R.; Motoyama, A.; Fang, L.F.; Matsuyama, H. Surface charge control of poly(methyl methacrylate-co-dimethyl aminoethyl methacrylate)-based membrane for improved fouling resistance. Sep. Purif. Technol. 2021, 279, 119778. [Google Scholar] [CrossRef]
- Wang, S.; Fang, L.; Cheng, L.; Jeon, S.; Kato, N. Improved antifouling properties of membranes by simple introduction of zwitterionic copolymers via electrostatic adsorption. J. Memb. Sci. 2018, 564, 672–681. [Google Scholar] [CrossRef]
- Ham, S.Y.; Kim, H.S.; Jang, Y.; Sun, P.F.; Park, J.H.; Lee, J.S.; Byun, Y.; Park, H.D. Control of membrane biofouling by 6-gingerol analogs: Quorum sensing inhibition. Fuel 2019, 250, 79–87. [Google Scholar] [CrossRef]
- Biswas, P.; Bandyopadhyaya, R. Biofouling prevention using silver nanoparticle impregnated polyethersulfone (PES) membrane: E. coli cell-killing in a continuous cross-flow membrane module. J. Colloid Interface Sci. 2017, 491, 13–26. [Google Scholar] [CrossRef]
- Kakihana, Y.; Cheng, L.; Fang, L.; Wang, S.; Jeon, S.; Saeki, D. Preparation of positively charged PVDF membranes with improved antibacterial activity by blending modification: Effect of change in membrane surface material properties. Colloids Surf. A 2017, 533, 133–139. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Wang, Z.; Wang, L.; Wu, Z. QAC modified PVDF membranes: Antibiofouling performance, mechanisms, and effects on microbial communities in an MBR treating municipal wastewater. Water Res. 2017, 120, 256–264. [Google Scholar] [CrossRef]
- Ahsani, M.; Hazrati, H.; Javadi, M.; Ulbricht, M.; Yegani, R. Preparation of antibiofouling nanocomposite PVDF/Ag-SiO2 membrane and long-term performance evaluation in the MBR system fed by real pharmaceutical wastewater. Sep. Purif. Technol. 2020, 249, 116938. [Google Scholar] [CrossRef]
- Hu, M.; Cui, Z.; Li, J.; Zhang, L.; Mo, Y.; Dlamini, D.S.; Wang, H.; He, B.; Li, J.; Matsuyama, H. Ultra-low graphene oxide loading for water permeability, antifouling and antibacterial improvement of polyethersulfone/sulfonated polysulfone ultrafiltration membranes. J. Colloid Interface Sci. 2019, 552, 319–331. [Google Scholar] [CrossRef]
- Benavente, J.; García, M.E.; Urbano, N.; López-romero, J.M.; Contreras-cáceres, R.C.; Casado-rodríguez, M.A.; Moscoso, A.; Hierrezuelo, J. Inclusion of silver nanoparticles for improving regenerated cellulose membrane performance and reduction of biofouling. Int. J. Biol. Macromol. 2017, 103, 758–763. [Google Scholar] [CrossRef]
- Munnawar, I.; Iqbal, S.S.; Anwar, M.N.; Batool, M.; Tariq, S.; Faitma, N.; Khan, A.L.; Khan, A.U.; Nazar, U.; Jamil, T.; et al. Synergistic effect of Chitosan-Zinc Oxide Hybrid Nanoparticles on antibiofouling and water disinfection of mixed matrix polyethersulfone nanocomposite membranes. Carbohydr. Polym. 2017, 175, 661–670. [Google Scholar] [CrossRef]
- Ekambaram, K.; Doraisamy, M. Carboxymethylchitosan-Zinc oxide bionanocomposite for the removal of. Colloids Surf. A 2017, 525, 49–63. [Google Scholar] [CrossRef]
- Fahrina, A.; Yusuf, M.; Muchtar, S.; Fitriani, F.; Mulyati, S.; Aprilia, S.; Rosnelly, C.M.; Bilad, M.R.; Ismail, A.F.; Takagi, R.; et al. Development of anti-microbial polyvinylidene fluoride (PVDF) membrane using bio-based ginger extract-silica nanoparticles (GE-SiNPs) for bovine serum albumin (BSA) filtration. J. Taiwan Inst. Chem. Eng. 2021, 125, 323–331. [Google Scholar] [CrossRef]
- Kumar, R.S.; Arthanareeswaran, G.; Thuyavan, Y.L.; Ismail, A.F. Enhancement of permeability and antibiofouling properties of polyethersulfone (PES) membrane through incorporation of quorum sensing inhibition (QSI) compound. J. Taiwan Inst. Chem. Eng. 2017, 72, 200–212. [Google Scholar] [CrossRef]
- Arahman, N.; Rosnelly, C.M.; Windana, D.S.; Fahrina, A.; Silmina, S.; Maimun, T.; Mulyati, S.; Fathanah, U.; Aprilia, S.; Bilad, M.R.; et al. Antimicrobial hydrophilic membrane formed by incorporation of polymeric surfactant and patchouli oil. Polymers 2021, 13, 3872. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, X.; Gao, C.; Dong, M. Improved water flux and antifouling properties of cardo poly(aryl ether ketone) ultrafiltration membrane by novel sulfobetaine polyimides additive. Sep. Purif. Technol. 2020, 251, 117144. [Google Scholar] [CrossRef]
- Ilyas, H.; Shawuti, S.; Siddiq, M.; Niazi, J.H.; Qureshi, A. PEG functionalized graphene oxide-silver nano-additive for enhanced hydrophilicity, permeability and fouling resistance properties of PVDF-co-HFP membranes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123646. [Google Scholar] [CrossRef]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moacă, E.A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable PEG-coated silver nanoparticles–A comprehensive toxicological profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef]
- Peng, Z.; Ji, C.; Zhou, Y.; Zhao, T.; Leblanc, R.M. Polyethylene glycol (PEG) derived carbon dots: Preparation and applications. Appl. Mater. Today 2020, 20, 100677. [Google Scholar] [CrossRef]
- Mao, N.D.; Jeong, H.; Ngan Nguyen, T.K.; Loan Nguyen, T.M.; Vi Do, T.V.; Ha Thuc, C.N.; Perré, P.; Ko, S.C.; Kim, H.G.; Tran, D.T. Polyethylene glycol functionalized graphene oxide and its influences on properties of Poly(lactic acid) biohybrid materials. Compos. Part B Eng. 2019, 161, 651–658. [Google Scholar] [CrossRef]
- Jegatheeswaran, S.; Sundrarajan, M. PEGylation of novel hydroxyapatite/PEG/Ag nanocomposite particles to improve its antibacterial efficacy. Mater. Sci. Eng. C 2015, 51, 174–181. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, Y.; Zeng, X.; Zeng, Y.; Wang, Y. The role of poly(ethylene glycol) in the formation of silver nanoparticles. J. Colloid Interface Sci. 2005, 288, 444–448. [Google Scholar] [CrossRef]
- Popa, M.; Pradell, T.; Crespo, D.; Calderón-Moreno, J.M. Stable silver colloidal dispersions using short chain polyethylene glycol. Colloids Surf. A Physicochem. Eng. Asp. 2007, 303, 184–190. [Google Scholar] [CrossRef]
- Shameli, K.; Bin Ahmad, M.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Mahdavi, M.; Abdollahi, Y. Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method. Int. J. Mol. Sci. 2012, 13, 6639–6650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malathy, D.; Revathi, M.; Letticia, M. Microwave Synthesis of Silver Nanoparticles by Polyol Method and Testing Their Synergistic Antibacterial Activity in the Presence of Vancomycin. Asian J. Pharm. Clin. Res. 2018, 11, 288. [Google Scholar]
- Alfei, S.; Schito, A.M. Positively charged polymers as promising devices against multidrug resistant gram-negative bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Irfan, M.; Idris, A.; Nasiri, R.; Almaki, J.H. Fabrication and evaluation of polymeric membranes for blood dialysis treatments using functionalized MWCNT based nanocomposite and sulphonated-PES. RSC Adv. 2016, 6, 101513–101525. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Atta, R.R.; Zolotarev, A.A.; Plisko, T.V.; Mazur, A.S.; Solovyev, N.D.; Ermakov, S.S. The development and study of novel membrane materials based on polyphenylene isophthalamide-Pluronic F127 composite. Mater. Des. 2019, 165, 107596. [Google Scholar] [CrossRef]
- Mazinani, S.; Darvishmanesh, S.; Ehsanzadeh, A.; Van der Bruggen, B. Phase separation analysis of Extem/solvent/non-solvent systems and relation with membrane morphology. J. Memb. Sci. 2017, 526, 301–314. [Google Scholar] [CrossRef]
- Dehban, A.; Kargari, A.; Ashtiani, F.Z. Preparation and optimization of antifouling PPSU/PES/SiO2 nanocomposite ultrafiltration membranes by VIPS-NIPS technique. J. Ind. Eng. Chem. 2020, 88, 292–311. [Google Scholar] [CrossRef]
- Guo, J.; Khan, S.; Cho, S.H.; Kim, J. ZnS nanoparticles as new additive for polyethersulfone membrane in humic acid filtration. J. Ind. Eng. Chem. 2019, 79, 71–78. [Google Scholar] [CrossRef]
- Yu, R.; Zhu, R.; Jiang, J.; Liang, R.; Liu, X.; Liu, G. Mussel-inspired surface functionalization of polyamide microfiltration membrane with zwitterionic silver nanoparticles for efficient anti-biofouling water disinfection. J. Colloid Interface Sci. 2021, 598, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Jiang, T.; Liang, R.; Qin, W. Polymeric membrane ion-selective electrodes with anti-biofouling properties by surface modification of silver nanoparticles. Sens. Actuators B Chem. 2021, 328, 129014. [Google Scholar] [CrossRef]
- Kochkodan, V.; Johnson, D.J.; Hilal, N. Polymeric membranes: Surface modification for minimizing (bio)colloidal fouling. Adv. Colloid Interface Sci. 2014, 206, 116–140. [Google Scholar] [CrossRef] [PubMed]




| Labels | Membrane Composition (wt. %) | ||||
|---|---|---|---|---|---|
| PES | PEG | Ag | PEG-AgNPs | NMP | |
| PO | 18 | 0 | 0 | 0 | 82 |
| PAg | 18 | 0 | 5 | 0 | 77 |
| PP | 18 | 5 | 0 | 0 | 77 |
| PPAg1 | 18 | 0 | 0 | 3 | 79 |
| PPAg2 | 18 | 0 | 0 | 5 | 77 |
| PPAg3 | 18 | 0 | 0 | 7 | 75 |
| PEG-AgNPs Characteristics | Modus Value | Units |
|---|---|---|
| Particle size distribution | 16.4 | nm |
| Hydrodynamic diameter | 103.8 | nm |
| Zeta potential | −17.1 | mV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahrina, A.; Arahman, N.; Aprilia, S.; Bilad, M.R.; Silmina, S.; Sari, W.P.; Sari, I.M.; Gunawan, P.; Pasaoglu, M.E.; Vatanpour, V.; et al. Functionalization of PEG-AgNPs Hybrid Material to Alleviate Biofouling Tendency of Polyethersulfone Membrane. Polymers 2022, 14, 1908. https://doi.org/10.3390/polym14091908
Fahrina A, Arahman N, Aprilia S, Bilad MR, Silmina S, Sari WP, Sari IM, Gunawan P, Pasaoglu ME, Vatanpour V, et al. Functionalization of PEG-AgNPs Hybrid Material to Alleviate Biofouling Tendency of Polyethersulfone Membrane. Polymers. 2022; 14(9):1908. https://doi.org/10.3390/polym14091908
Chicago/Turabian StyleFahrina, Afrillia, Nasrul Arahman, Sri Aprilia, Muhammad Roil Bilad, Silmina Silmina, Widia Puspita Sari, Indah Maulana Sari, Poernomo Gunawan, Mehmet Emin Pasaoglu, Vahid Vatanpour, and et al. 2022. "Functionalization of PEG-AgNPs Hybrid Material to Alleviate Biofouling Tendency of Polyethersulfone Membrane" Polymers 14, no. 9: 1908. https://doi.org/10.3390/polym14091908
APA StyleFahrina, A., Arahman, N., Aprilia, S., Bilad, M. R., Silmina, S., Sari, W. P., Sari, I. M., Gunawan, P., Pasaoglu, M. E., Vatanpour, V., Koyuncu, I., & Rajabzadeh, S. (2022). Functionalization of PEG-AgNPs Hybrid Material to Alleviate Biofouling Tendency of Polyethersulfone Membrane. Polymers, 14(9), 1908. https://doi.org/10.3390/polym14091908