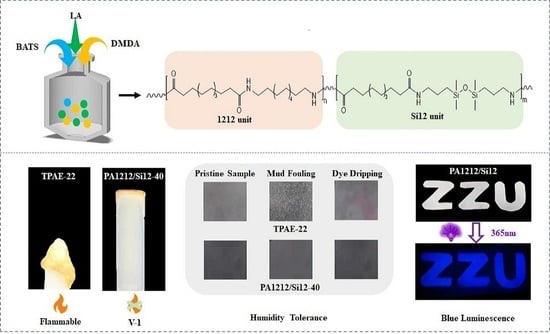
Inherent Flame-Retardant, Humid Environment Stable and Blue Luminescent Polyamide Elastomer Regulated by Siloxane Moiety
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Agents
2.2. Synthesis of PA1212/Si12 Copolymers
2.3. Characterization
3. Results and Discussion
3.1. Synthesis and Characterization of PA1212/Si12 Copolymers
3.2. Thermal Transition and Crystallization Behaviors
| Sample | Tg (°C) | Tm (°C) | Tc (°C) | ΔT (°C) | ΔH (J/g) | Xca (%) |
|---|---|---|---|---|---|---|
| PA1212/Si12–5 | 30 | 179 | 157 | 22 | 52.0 | 44.0 |
| PA1212/Si12–10 | 21 | 178 | 154 | 24 | 51.7 | 45.2 |
| PA1212/Si12–20 | 19 | 174 | 149 | 25 | 45.4 | 43.2 |
| PA1212/Si12–30 | 5.8 | 165 | 134 | 31 | 32.3 | 33.2 |
| PA1212/Si12–40 | 5.7 | 161 | 126 | 35 | 30.4 | 35.4 |
3.3. Mechanical Properties
3.4. Thermal Stability
3.5. Combustion Performances
3.6. Surface Properties and Anti-Fouling Test
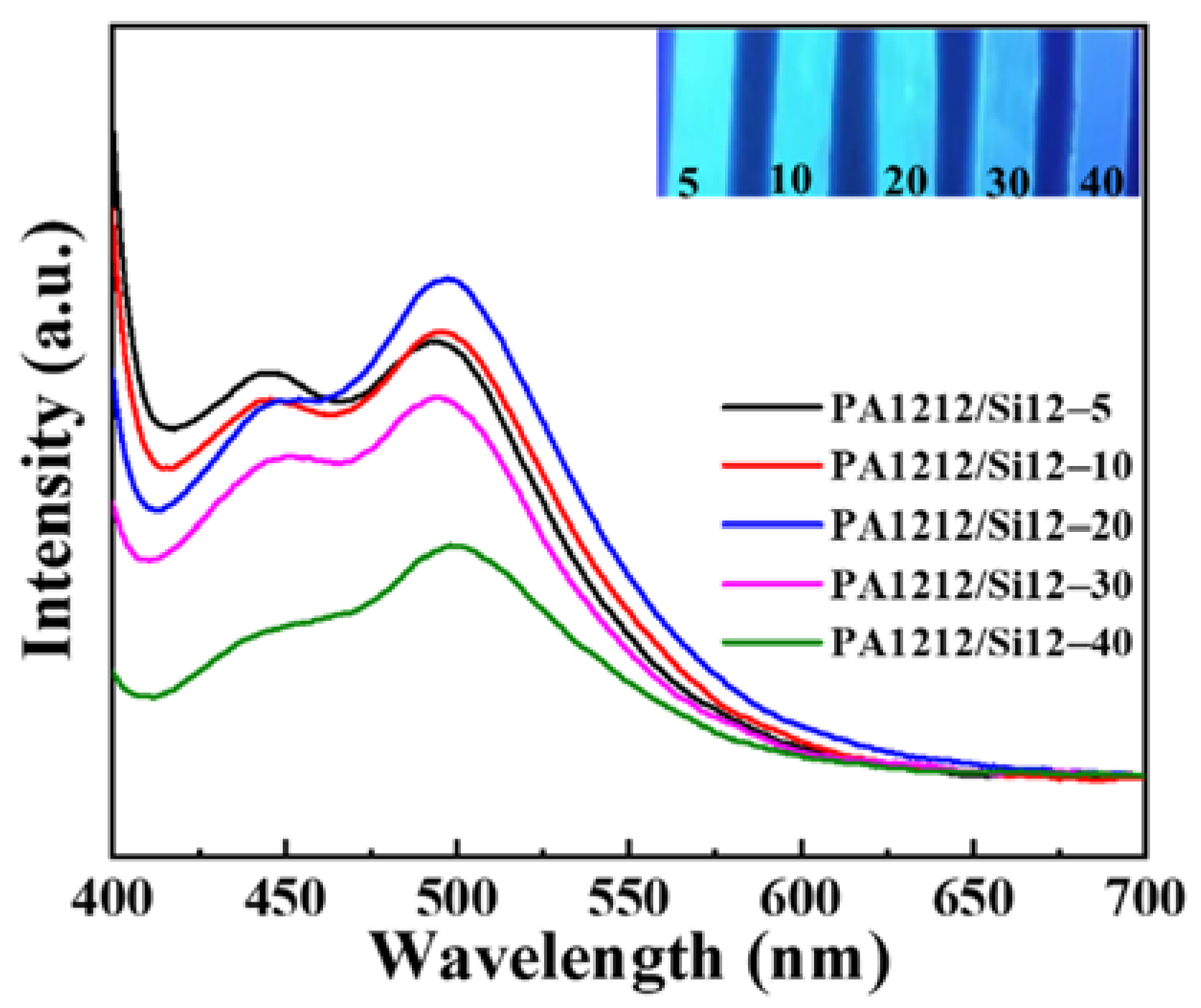
3.7. Fluorescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, S.; Zhao, S.; Chen, X.; Liu, H.; Deng, J.; Li, S.; Feng, X.; Li, Y.; Wu, X.; Pan, K. Thermoplastic polyamide elastomers: Synthesis, structures/properties, and applications. Macromol. Mater. Eng. 2021, 306, 2100568. [Google Scholar] [CrossRef]
- Buckwalter, D.J.; Dennis, J.M.; Long, T.E. Amide-containing segmented copolymers. Prog. Polym. Sci. 2015, 45, 1–22. [Google Scholar] [CrossRef]
- Kong, W.; Yang, Y.; Liu, Z.; Liang, J.; Zhou, C.; Lei, J. Structure-property relations of nylon-6 and polytetramethylene glycol based multiblock copolymers with microphase separation prepared through reactive processing. Polym. Int. 2017, 66, 436–442. [Google Scholar] [CrossRef]
- Barzegari, M.R.; Hossieny, N.; Jahani, D.; Park, C.B. Characterization of hard-segment crystalline phase of poly(ether-block-amide) (PEBAX (R)) thermoplastic elastomers in the presence of supercritical CO2 and its impact on foams. Polymer 2017, 114, 15–27. [Google Scholar] [CrossRef]
- Amirkhani, F.; Mosadegh, M.; Asghari, M.; Parnian, M.J. The beneficial impacts of functional groups of CNT on structure and gas separation properties of PEBA mixed matrix membranes. Polym. Test. 2020, 82, 106285. [Google Scholar] [CrossRef]
- Chen, Y.; Sijbesma, R.P. Dioxetanes as mechanoluminescent probes in thermoplastic elastomers. Macromolecules 2014, 47, 3797–3805. [Google Scholar] [CrossRef]
- Chen, J.; Gong, C.; Yang, C.; Yi, C. Flexible preparation of polyamide-6 based thermoplastic elastomers via amide exchange. J. Mater. Sci. 2021, 56, 12018–12029. [Google Scholar] [CrossRef]
- Kong, W.; Hu, K.; Fu, X.; Guo, D.; Lei, J. Preparation and characterization of thermoplastic elastomer based on amino-terminated polyamide-6 and diisocyanate-terminated polytetramethylene glycol. Polym. Plast. Technol. Eng. 2016, 55, 1–8. [Google Scholar] [CrossRef]
- Jeong, S.; Sohn, H.; Kang, S.W. Highly permeable PEBAX-1657 membranes to have long-term stability for facilitated olefin transport. Chem. Eng. J. 2018, 333, 276–279. [Google Scholar] [CrossRef]
- Chen, Z.X.; Ma, H.R.; Li, Y.X.; Meng, J.J.; Yao, Y.W.; Yao, C. Biomass polyamide elastomers based on hydrogen bonds with rapid self-healing properties. Eur. Polym. J. 2020, 133, 109802. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, J.H.; Zhou, T.; Zhang, A.M. Hydrogen bonding in micro-phase separation of poly (polyamide 12-block-polytetrahydrofuran) alternating block copolymer: Enthalpies and molecular movements. Vib. Spectrosc. 2016, 86, 160–172. [Google Scholar] [CrossRef]
- Yilgor, E.; Yilgor, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.B.; Su, Z.Z.; Wang, S.Q.; Zhang, B.L.; Zhang, H.P.; Zhang, Q.Y. Biomimetic brushlike slippery coatings with mechanically robust, self-cleaning, and icephobic properties. ACS Appl. Mater. Interfaces 2020, 12, 54041–54052. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-Cuesta, J.M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Ma, H.; Tong, L.; Xu, Z.; Fang, Z.; Jin, Y.; Lu, F. A novel intumescent flame retardant: Synthesis and application in ABS copolymer. Polym. Degrad. Stab. 2007, 92, 720–726. [Google Scholar] [CrossRef]
- Fan, S.; Zhu, C.; Wu, D.; Wang, X.; Yu, J.; Li, F. Silicon-containing inherent flame-retardant polyamide 6 with anti-dripping via introducing ethylene glycol as the chain-linker and charring agent. Polym. Degrad. Stab. 2020, 173, 109080. [Google Scholar] [CrossRef]
- Shi, Z. Preparation and characterization of crosslinked polyurethane-block-poly (trifluoropropylmethyl) siloxane elastomers with potential biomedical applications. Polym. Int. 2013, 62, 1351–1357. [Google Scholar] [CrossRef]
- Lin, Y.; He, D.; Hu, H.; Yi, P.; Liu, X.; Huang, J.; Wu, S.; Li, G. Preparation and properties of polydimethylsiloxane (PDMS)/polyethylene glycol (PEG)-based amphiphilic polyurethane elastomers. ACS Appl. Bio Mater. 2019, 2, 4377–4384. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, D.; Sun, J.; Wu, Y.; Gao, C. A new cross-linked system of silicone rubber based on silicone-polyurea block copolymer. Polym. Adv. Technol. 2018, 29, 2064–2071. [Google Scholar] [CrossRef]
- Yu, M.; Liu, M.; Fu, S. Slippery antifouling polysiloxane-polyurea surfaces with matrix self-healing and lubricant self-replenishing. ACS Appl. Mater. Interfaces 2021, 13, 32149–32160. [Google Scholar] [CrossRef]
- Kakimoto, M.A.; Kajiyama, M.; Imai, Y. Synthesis and properties of block copolymers based on amine-terminated polydimethylsiloxane and aromatic polyamides. Polym. J. 1986, 18, 935–940. [Google Scholar] [CrossRef][Green Version]
- Kajiyama, M.; Kakimoto, M.A.; Imai, Y. Synthesis and characterization of new multiblock copolymers based on poly (dimethylsiloxane) and aromatic polyamides. Macromolecules 1989, 22, 4143–4147. [Google Scholar] [CrossRef]
- Furuzono, T.; Kishida, A.; Yanagi, M.; Matsumoto, T.; Kanda, T.; Nakamura, T.; Aiko, T.; Maruyama, I.; Akashi, M. Novel functional polymers: Poly (dimethylsiloxane) polyamide multiblock copolymer. 5. The interaction between biomolecules and the surface of aramid-silicone resins. J. Biomater. Sci. Polym. Ed. 1996, 7, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Furuzono, T.; Seki, K.; Kishida, A.; Ohshige, T.A.; Waki, K.; Maruyama, I.; Akashi, M. Novel functional polymers: Poly (dimethylsiloxane) polyamide multiblock copolymer. 3. Synthesis and surface properties of disiloxane-aromatic polyamide multiblock copolymer. J. Appl. Polym. Sci. 1996, 59, 1059–1065. [Google Scholar] [CrossRef]
- Matsumoto, T.; Koinuma, Y.; Waki, K.; Kishida, A.; Furuzono, T.; Maruyama, I.; Akashi, M. Novel functional polymers: Poly (dimethylsiloxane) polyamide multiblock copolymer. 4. Gas permeability and thermomechanical properties of aramid-silicone resins. J. Appl. Polym. Sci. 1996, 59, 1067–1071. [Google Scholar] [CrossRef]
- Furuzono, T.; Senshu, K.; Kishida, A.; Matsumoto, T.; Akashi, M. Novel functional polymers: Poly (dimethylsiloxane)-polyamide multiblock copolymer. 6. A transmission electron microscopic study on microphase-separated structure in aramid-silicone resin. Polym. J. 1997, 29, 201–203. [Google Scholar] [CrossRef][Green Version]
- Matsumoto, T.; Uchida, T.; Kishida, A.; Furuzono, T.; Maruyama, I.; Akashi, M. Novel functional polymers: Poly (dimethylsiloxane)-polyamide multiblock copolymer. 7. Oxygen permeability of aramid-silicone membranes in a gas-membrane-liquid system. J. Appl. Polym. Sci. 1997, 64, 1153–1159. [Google Scholar] [CrossRef]
- Senshu, K.; Furuzono, T.; Koshizaki, N.; Yamashita, S.; Matsumoto, T.; Kishida, A.; Akashi, M. Novel functional polymers: Poly (dimethylsiloxane)-polyamide multiblock copolymer. 8. surface studies of aramid-silicone resin by means of XPS, static SIMS, and TEM. Macromolecules 1997, 30, 4421–4428. [Google Scholar] [CrossRef]
- Kishida, A.; Kanda, T.; Furuzono, T.; Maruyama, I.; Akashi, M. Novel functional polymers: Poly (dimethylsiloxane) polyamide multiblock copolymer. IX. Surface properties blend film of aramid-silicone resins with aramid. J. Appl. Polym. Sci. 2000, 78, 2198–2205. [Google Scholar] [CrossRef]
- Kang, E.C.; Akashi, M. Novel functional polymers. Poly (dimethylsiloxane)-polyamide multiblock copolymers X. 1H NMR analysis on fine structure of aramid-silicone resin. Polym. J. 2002, 34, 395–399. [Google Scholar] [CrossRef]
- Rached, R.; Hoppe, S.; Jonquieres, A.; Lochon, P.; Pla, F. A new macroinitiator for the synthesis of triblock copolymers PA12-b-MMS-b-PA12. J. Appl. Polym. Sci. 2006, 102, 2818–2831. [Google Scholar] [CrossRef]
- Rached, J.; Hoppe, S.; Meimaroglou, D.; Fonteix, C.; Pla, F. Modeling and simulation of activated anionic polymerization of lauryllactam in the presence of a macroactivator. Chem. Eng. Sci. 2014, 118, 20–31. [Google Scholar] [CrossRef]
- Poojari, Y.; Clarson, S.J. Lipase-catalyzed synthesis and properties of silicone aromatic polyesters and silicone aromatic polyamides. Macromolecules 2010, 43, 4616–4622. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, H.; Li, B. Synthesis and characterization of advance PA6-b-PDMS multiblock copolymers. J. Appl. Polym. Sci. 2014, 131, 41114. [Google Scholar] [CrossRef]
- Ji, F.; Fu, P.; Wang, S.; Liu, T.; Lv, L.; Guan, X.; Zhang, X.; Zhao, H.; Qiao, X.; Pang, X.; et al. Novel biocompatible multiblock Polydimethylsiloxane-PA1212 copolymers. React. Funct. Polym. 2020, 154, 104688. [Google Scholar] [CrossRef]
- Jung, D.; Bhattacharyya, D. Combined effect of silicate coating and phosphate loading on the performance improvement of a keratinous fiber-based flame retardant. Chem. Eng. J. 2021, 424, 130484. [Google Scholar] [CrossRef]
- Dubey, R.J.C.; Sasikumar, P.V.W.; Krumeich, F.; Blugan, G.; Kuebler, J.; Kravchyk, K.V.; Graule, T.; Kovalenko, M.V. Silicon oxycarbide-tin nanocomposite as a high-power-density anode for Li-ion batteries. Adv. Sci. 2019, 6, 1901220. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Salhi, S.; Abid, S.; El Gharbi, R.; Fradet, A. Random and quasi-alternating polyesteramides deriving from alpha-caprolactone and beta-alanine. Eur. Polym. J. 2014, 53, 160–170. [Google Scholar] [CrossRef]
- Telen, L.; Van Puyvelde, P.; Goderis, B. Random copolymers from polyamide 11 and polyamide 12 by reactive extrusion: Synthesis, eutectic phase behavior, and polymorphism. Macromolecules 2016, 49, 876–890. [Google Scholar] [CrossRef]
- Liu, M.Y.; Zhao, Q.X.; Wang, Y.; Zhang, C.G.; Mo, Z.S.; Cao, S.K. Melting behaviors, isothermal and non-isothermal crystallization kinetics of nylon 1212. Polymer 2003, 44, 2537–2545. [Google Scholar] [CrossRef]
- Lin, C.C. The rate of crystallization of poly (ethylene-terephthalate) by differential scanning calorimetry. Polym. Eng. Sci. 1983, 23, 113–116. [Google Scholar] [CrossRef]
- Molnar, A.; Eisenberg, A. Miscibility of polyamide-6 with lithium or sodium sulfonated polystyrene ionomers. Macromolecules 1992, 25, 5774–5782. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Liu, M.; Zhao, Q.; Ren, Y.; Guo, K.; Li, X. Study on the crystallization kinetics of flexible and toughness PFPA 1212 under the constant cooling rate conditions. Polym. Mat. Sci. Eng. 2002, 18, 158–161. [Google Scholar]
- Jones, N.A.; Atkins, E.; Hill, M.J. Comparison of structures and behavior on heating of solution-grown, chain-folded lamellar crystals of 31 even-even nylons. Macromolecules 2000, 33, 2642–2650. [Google Scholar] [CrossRef]
- Jones, N.A.; Sikorski, P.; Atkins, E.D.T.; Hill, M.J. Discovery in nylon-8 chain-folded lamellar crystals of new structure-lambda-structure-with progressive intersheet shear along the chain axis. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 3302–3308. [Google Scholar] [CrossRef]
- Zhu, P.; Dong, X.; Wang, D. Strain-induced crystallization of segmented copolymers: Deviation from the classic deformation mechanism. Macromolecules 2017, 50, 3911–3921. [Google Scholar] [CrossRef]
- Abbasi, A.; Sadeghi, G.M.M.; Ghasemi, I. Synthesis and characterization of novel environmentally friendly shape memory polyurethanes based on poly (epsilon-caprolactone) diol/castor oil mixtures. Polym. Sci. Ser. B 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Ren, M.; Mo, Z.; Chen, Q.; Song, J.; Wang, S.; Zhang, H.; Zhao, Q. Crystallization kinetics and morphology of nylon 1212. Polymer 2004, 45, 3511–3518. [Google Scholar] [CrossRef]
- Kretschmer, A.; Drake, R.; Neidhoefer, M.; Wilhelm, M. Quantification of composition and domain sizes of industrial poly (phthalamide)/poly (dimethylsiloxane) block copolymers using different 1H solid state NMR methods. Solid State Nucl. Magn. Reson. 2002, 22, 204–217. [Google Scholar] [CrossRef]
- Ge, H.; Wang, W.; Pan, Y.; Yu, X.; Hu, W.; Hu, Y. An inherently flame-retardant polyamide containing a phosphorus pendent group prepared by interfacial polymerization. RSC Adv. 2016, 6, 81802–81808. [Google Scholar] [CrossRef]
- Lu, P.; Zhao, Z.-Y.; Xu, B.-R.; Li, Y.-M.; Deng, C.; Wang, Y.-Z. A novel inherently flame-retardant thermoplastic polyamide elastomer. Chem. Eng. J. 2020, 379, 122278. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Li, S. A novel phosphorus-silicon containing epoxy resin with enhanced thermal stability, flame retardancy and mechanical properties. Polym. Degrad. Stab. 2019, 164, 36–45. [Google Scholar] [CrossRef]
- Cai, J.; Wirasaputra, A.; Zhu, Y.; Liu, S.; Zhou, Y.; Zhang, C.; Zhao, J. The flame retardancy and rheological properties of PA6/MCA modified by DOPO-based chain extender. Rsc Adv. 2017, 7, 19593–19603. [Google Scholar] [CrossRef]
- Si, G.; Li, D.; You, Y.; Hu, X. Investigation of the influence of red phosphorus, expansible graphite and zinc borate on flame retardancy and wear performance of glass fiber reinforced PA6 composites. Polym. Compos. 2017, 38, 2090–2097. [Google Scholar] [CrossRef]
- Zhou, Q.; Cao, B.Y.; Zhu, C.X.; Xu, S.; Gong, Y.Y.; Yuan, W.Z.; Zhang, Y.M. Clustering-triggered emission of nonconjugated polyacrylonitrile. Small 2016, 12, 6586–6592. [Google Scholar] [CrossRef]
- Song, L.; Zhu, T.; Yuan, L.; Zhou, J.; Zhang, Y.; Wang, Z.; Tang, C. Ultra-strong long-chain polyamide elastomers with programmable supramolecular interactions and oriented crystalline microstructures. Nat. Commun. 2019, 10, 1315. [Google Scholar] [CrossRef]
- Wang, Q.; Li, B.; Cao, H.; Jiang, X.; Kong, X.Z. Aliphatic amide salt, a new type of luminogen: Characterization, emission and biological applications. Chem. Eng. J. 2020, 388, 124182. [Google Scholar] [CrossRef]
- Zuo, Y.; Gou, Z.; Quan, W.; Lin, W. Silicon-assisted unconventional fluorescence from organosilicon materials. Coord. Chem. Rev. 2021, 438, 213887. [Google Scholar] [CrossRef]
- Wu, J.; Xin, W.; Wu, Y.; Zhan, Y.; Li, J.; Wang, J.; Huang, S.; Wang, X. Solid-state photoluminescent silicone-carbon dots/dendrimer composites for highly efficient luminescent solar concentrators. Chem. Eng. J. 2021, 422, 130158. [Google Scholar] [CrossRef]
- Lai, Y.; Zhu, T.; Geng, T.; Zheng, S.; Yang, T.; Zhao, Z.; Xiao, G.; Zou, B.; Yuan, W.Z. Effective internal and external modulation of nontraditional intrinsic luminescence. Small 2020, 16, 2005035. [Google Scholar] [CrossRef]
- Xu, L.F.; Liang, X.; Zhong, S.L.; Li, Z.F.; Gao, Y.; Cui, X.J. Natural silk fibroinbased on aggregation-induced emission with a clustering-triggered mechanism and its multiple applications. ACS Sustain. Chem. Eng. 2021, 9, 12043–12048. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, T.J.; Zhong, Z.H.; Kausar, F.; Wang, Z.Y.; Zhang, Y.M.; Yuan, W.Z. A clustering-triggered emission strategy for tunable multicolor persistent phosphorescence. Chem. Sci. 2020, 11, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Pan, Y.; Lei, Y.; Liu, M.; Peng, C.; Cai, Z.; Shen, G.; Wu, H.; Huang, X.; Dong, Y. Protic acids as third components improve the phosphorescence properties of the guest-host system through hydrogen bonds. Chem. Eng. J. 2022, 433, 133530. [Google Scholar] [CrossRef]
- Yan, J.; Wang, X.; Xiong, J.; Wang, L.; Pan, D.; Xu, Y.; Yang, M. Uncovering divergent fluorescence of aliphatic polyamides: Synthesis, dual polymerization-induced emissions, and organelle-specific imaging. Chem. Eng. J. 2022, 428, 132142. [Google Scholar] [CrossRef]






| Sample | Content of Si12 Unit (mol%) | Mnb (kg/mol) | Mwb (kg/mol) | PDI c | [ƞ] c (dL/g) | Residual Rate of Monomer (wt%) d | |
|---|---|---|---|---|---|---|---|
| Theoretical Value | Calculated Value a | ||||||
| PA1212/Si12–5 | 5 | 3.2 | 6.8 | 17 | 2.4 | 1.2 | 1.6 |
| PA1212/Si12–10 | 10 | 6.3 | 8.2 | 19 | 2.3 | 1.4 | 2.2 |
| PA1212/Si12–20 | 20 | 13.9 | 7.3 | 16 | 2.2 | 1.3 | 2.9 |
| PA1212/Si12–30 | 30 | 20.4 | 8.2 | 20 | 2.4 | 1.8 | 2.2 |
| PA1212/Si12–40 | 40 | 26.1 | 5.5 | 13 | 1.9 | 1.9 | 3.2 |
| Sample | N2 Atmosphere | Air Atmosphere | ||||||
|---|---|---|---|---|---|---|---|---|
| T5% (°C) | Tmax1 (°C) | Tmax2 (°C) | Residue (%) | T5% (°C) | Tmax1 (°C) | Tmax2 (°C) | Residue (%) | |
| PA1212/Si12–5 | 430 | 463 | 559 | 1.49 | 411 | 432 | 529 | 2.21 |
| PA1212/Si12–10 | 435 | 463 | 560 | 1.89 | 390 | 451 | 537 | 2.56 |
| PA1212/Si12–20 | 423 | 464 | 556 | 1.88 | 362 | 430 | 546 | 3.10 |
| PA1212/Si12–30 | 427 | 469 | 549 | 1.50 | 365 | 427 | 543 | 4.55 |
| PA1212/Si12–40 | 425 | 473 | 547 | 1.75 | 375 | 438 | 546 | 4.75 |
| Sample | Tp (°C) | PHRR (W/g) | THR (kJ/g) | UL–94 Rating |
|---|---|---|---|---|
| PA1212/Si12–5 | 482 | 1348 | 34.5 | V–2 |
| PA1212/Si12–10 | 464 | 1142 | 33.0 | V–2 |
| PA1212/Si12–20 | 474 | 1064 | 31.8 | V–1 |
| PA1212/Si12–30 | 474 | 1025 | 33.6 | V–1 |
| PA1212/Si12–40 | 473 | 827 | 31.5 | V–1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Q.; Xiao, Z.; Wang, Y.; Yan, X.; Fu, P.; Zhang, X.; Zhao, W.; Pang, X.; Liu, M.; Zhao, Q.; et al. Inherent Flame-Retardant, Humid Environment Stable and Blue Luminescent Polyamide Elastomer Regulated by Siloxane Moiety. Polymers 2022, 14, 1919. https://doi.org/10.3390/polym14091919
Qi Q, Xiao Z, Wang Y, Yan X, Fu P, Zhang X, Zhao W, Pang X, Liu M, Zhao Q, et al. Inherent Flame-Retardant, Humid Environment Stable and Blue Luminescent Polyamide Elastomer Regulated by Siloxane Moiety. Polymers. 2022; 14(9):1919. https://doi.org/10.3390/polym14091919
Chicago/Turabian StyleQi, Qianqian, Zhe Xiao, Yaowei Wang, Xinjin Yan, Peng Fu, Xiaomeng Zhang, Wei Zhao, Xinchang Pang, Minying Liu, Qingxiang Zhao, and et al. 2022. "Inherent Flame-Retardant, Humid Environment Stable and Blue Luminescent Polyamide Elastomer Regulated by Siloxane Moiety" Polymers 14, no. 9: 1919. https://doi.org/10.3390/polym14091919
APA StyleQi, Q., Xiao, Z., Wang, Y., Yan, X., Fu, P., Zhang, X., Zhao, W., Pang, X., Liu, M., Zhao, Q., & Cui, Z. (2022). Inherent Flame-Retardant, Humid Environment Stable and Blue Luminescent Polyamide Elastomer Regulated by Siloxane Moiety. Polymers, 14(9), 1919. https://doi.org/10.3390/polym14091919