Cadmium Sulfide—Reinforced Double-Shell Microencapsulated Phase Change Materials for Advanced Thermal Energy Storage
Abstract
:1. Introduction
2. Experiment
2.1. Materials
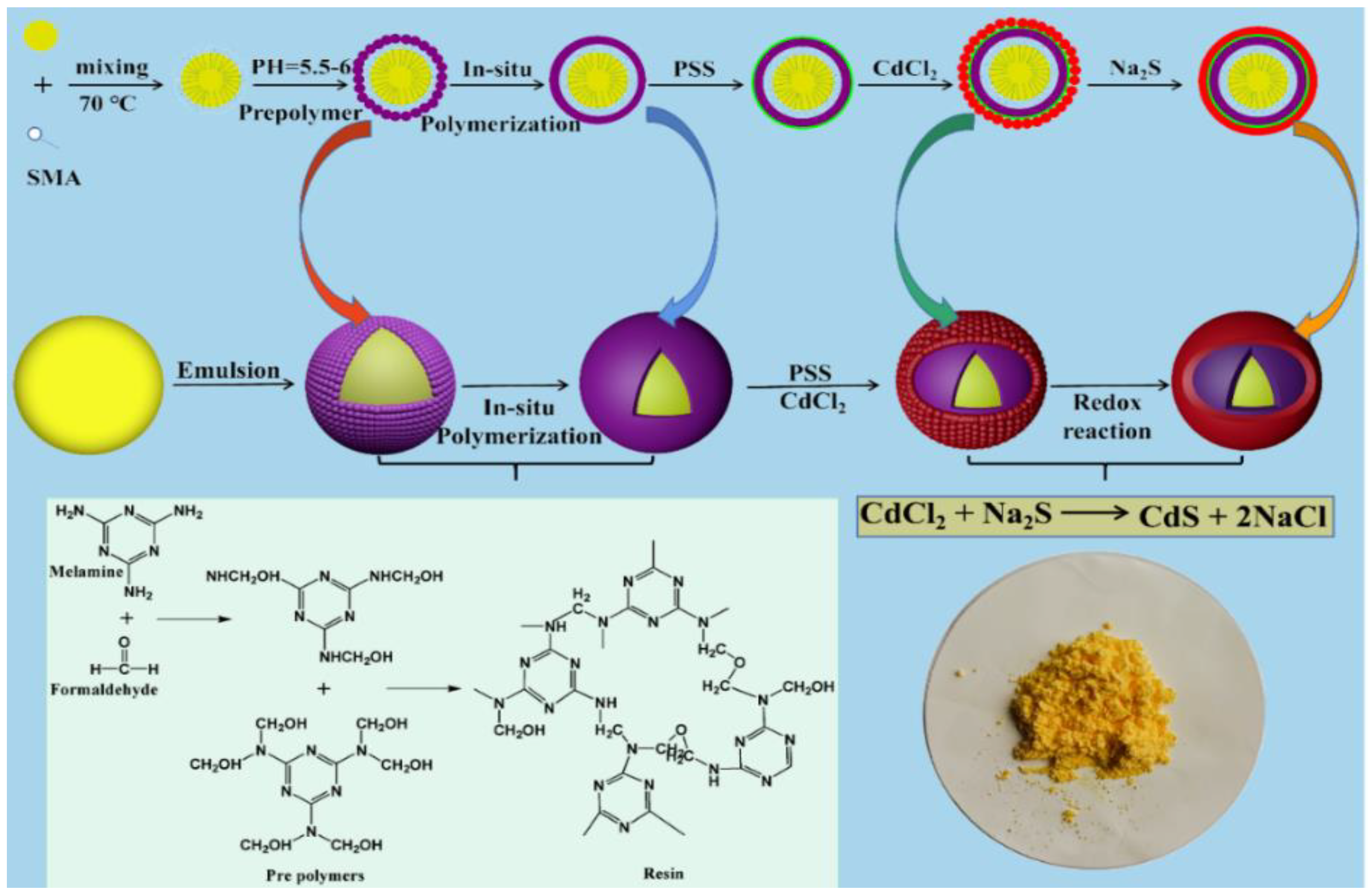
2.2. Preparation of the CdS-Reinforced Double-Shell Microencapsulated PCMs
2.2.1. Preparation of the MF Resin Microcapsules
2.2.2. Preparation of the CdS-Reinforced Double-Shell Microencapsulated PCMs
2.3. Characterization
3. Results and Discussion
3.1. Schematic Fabrication of the CdS-Reinforced Double-Shell Microencapsulated PCMs
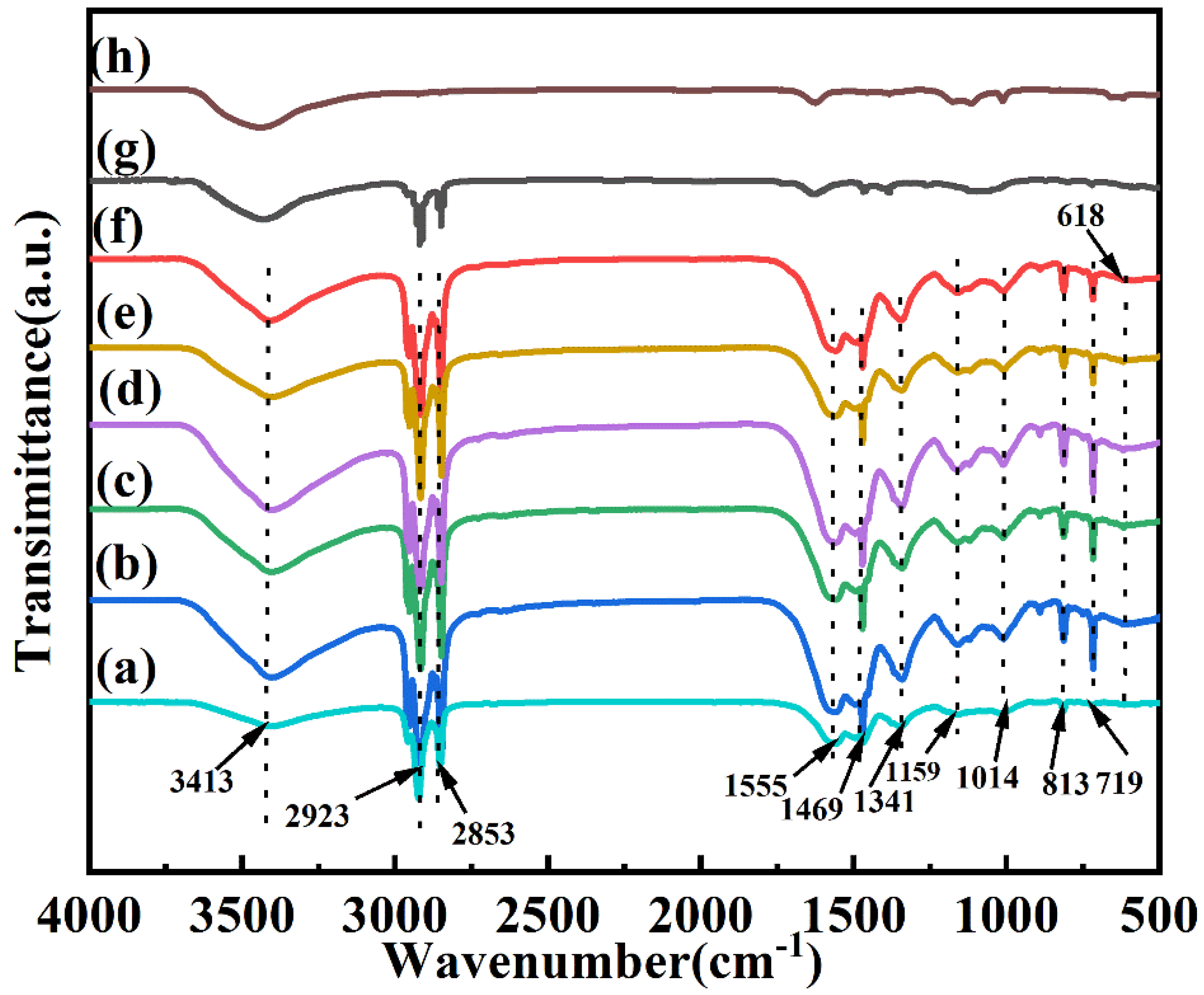
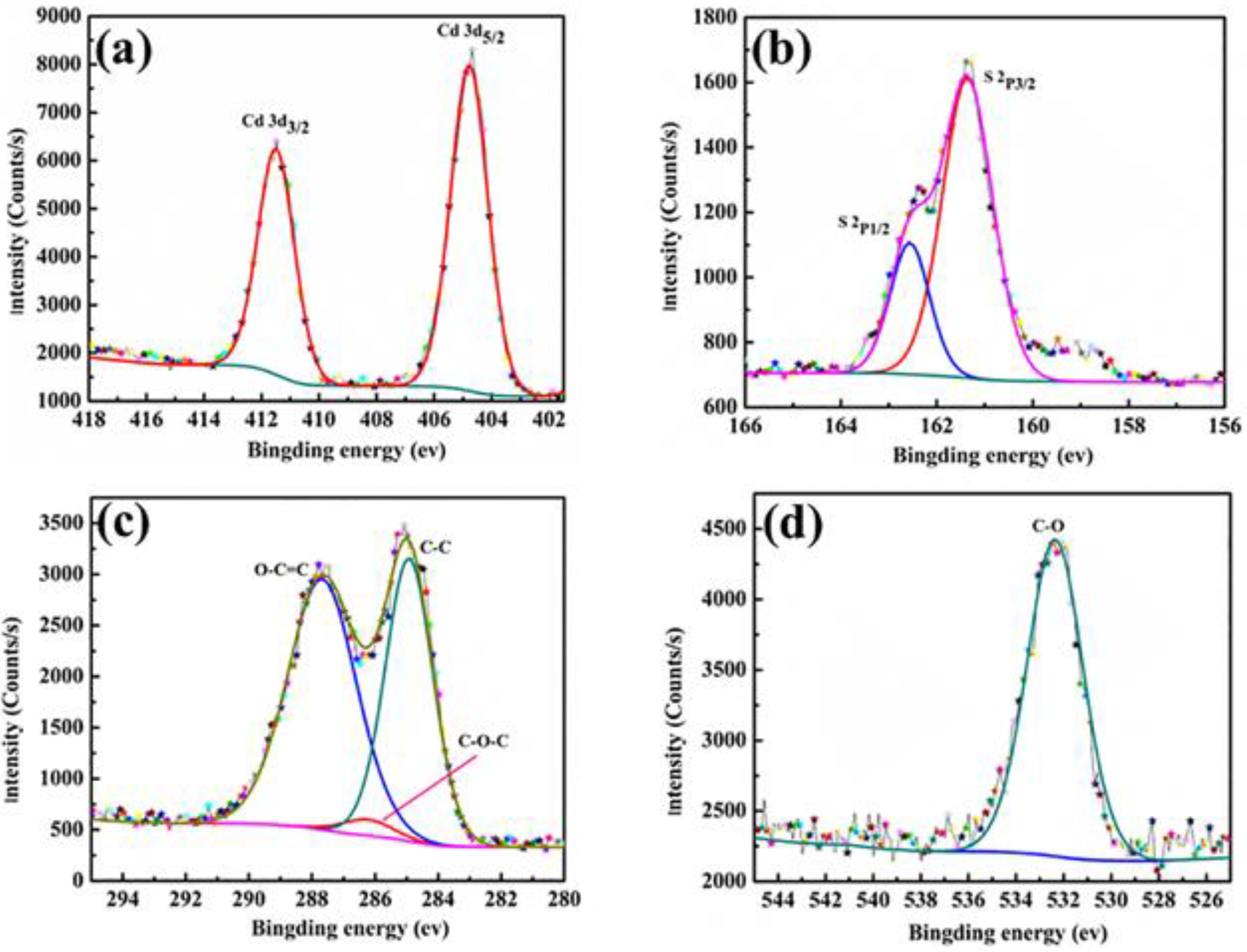
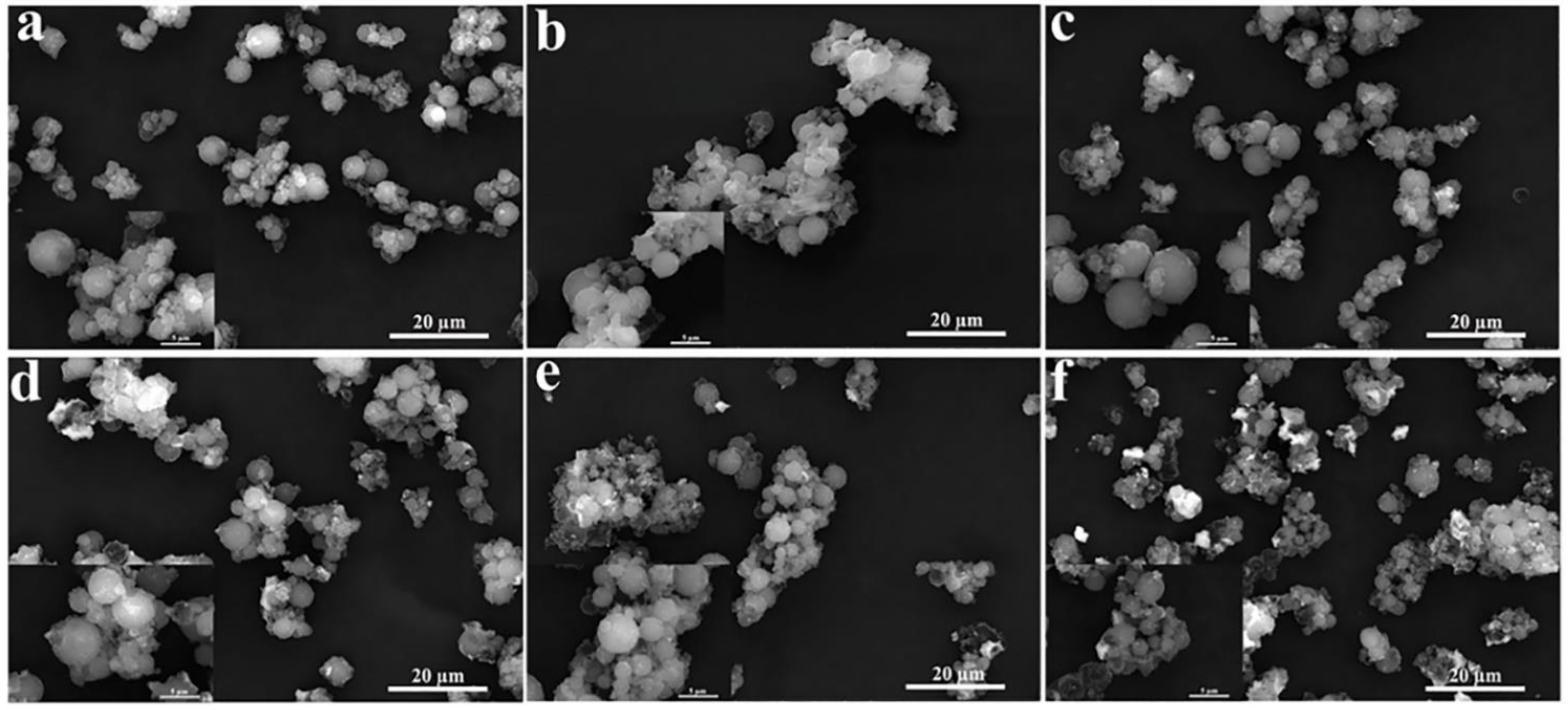
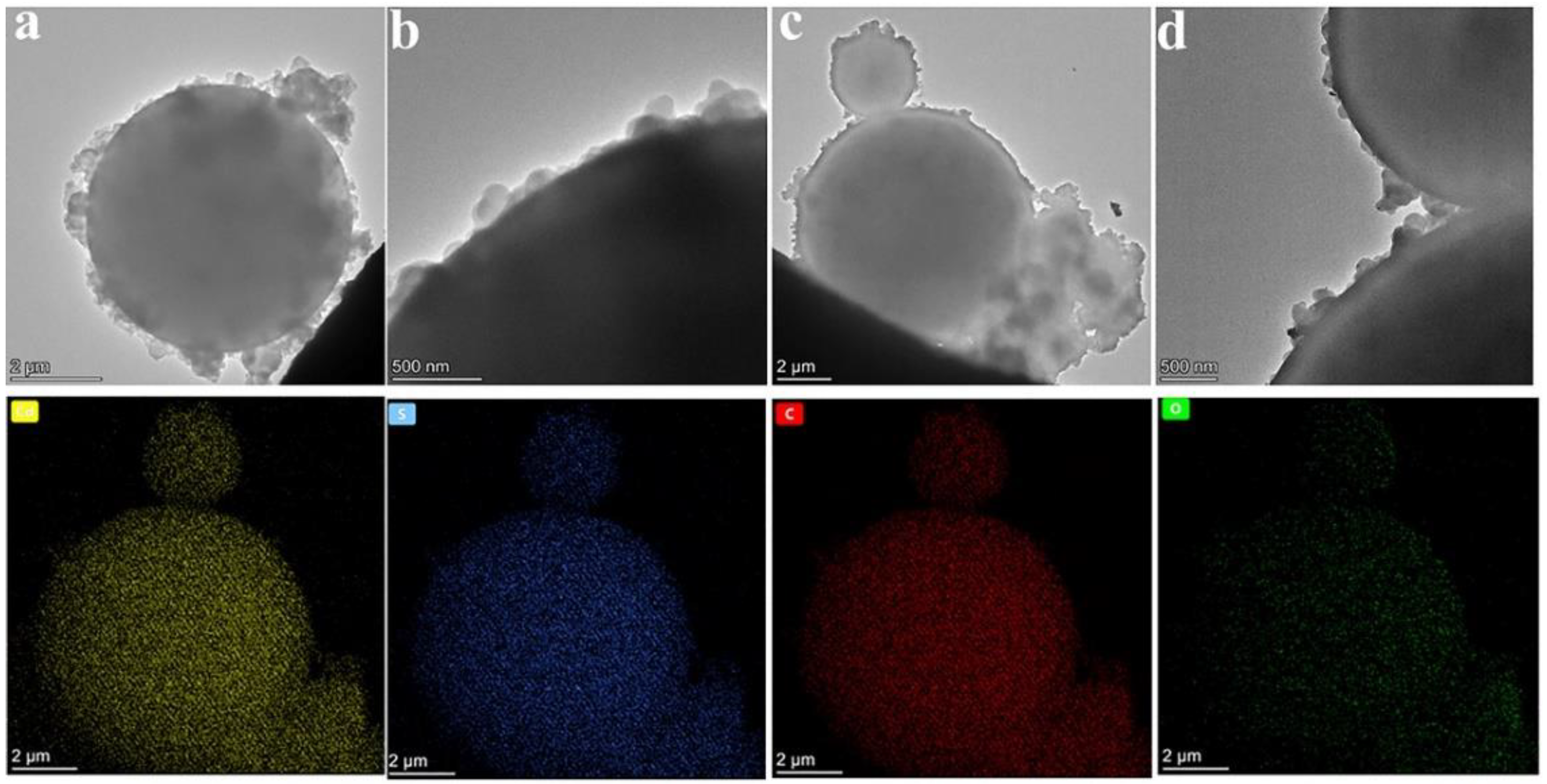
3.2. Microstructures of the CdS-Reinforced Double-Shell Microencapsulated PCMs
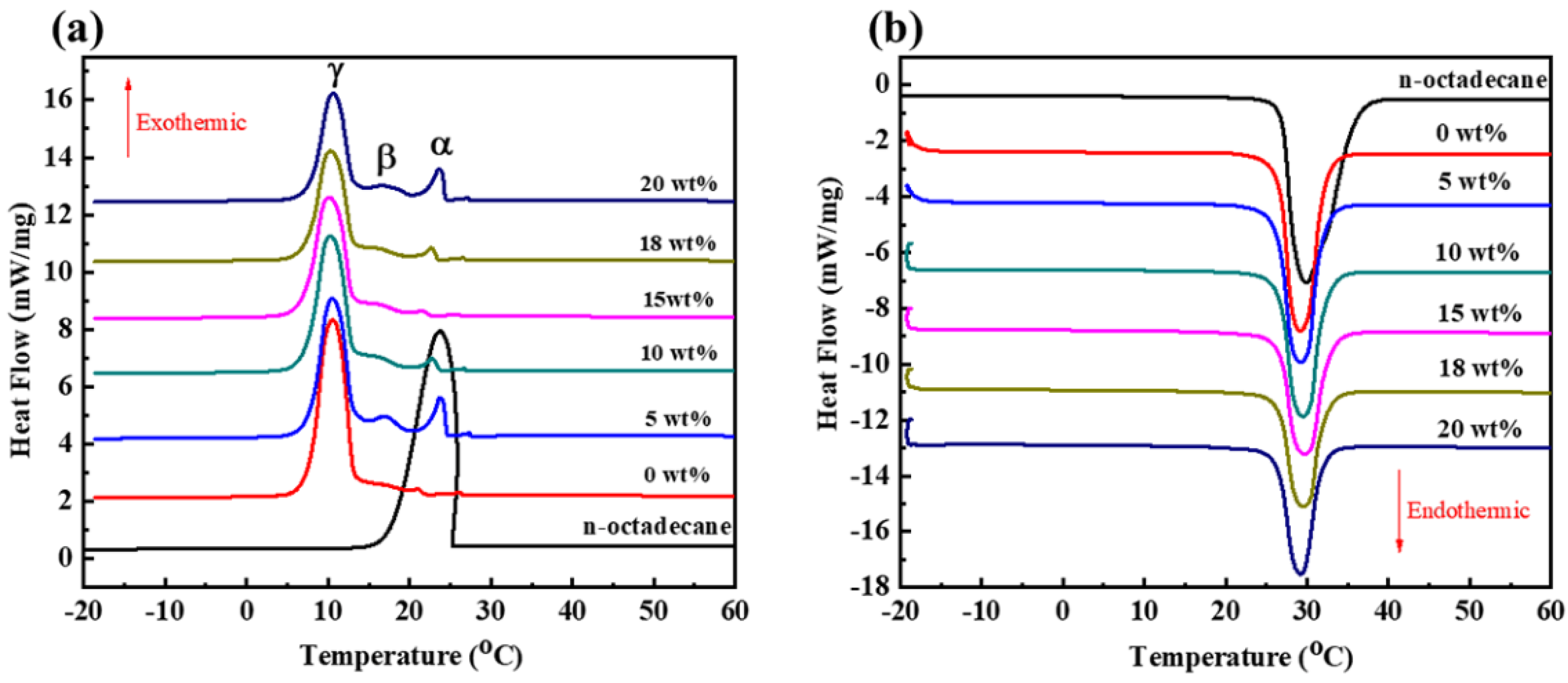
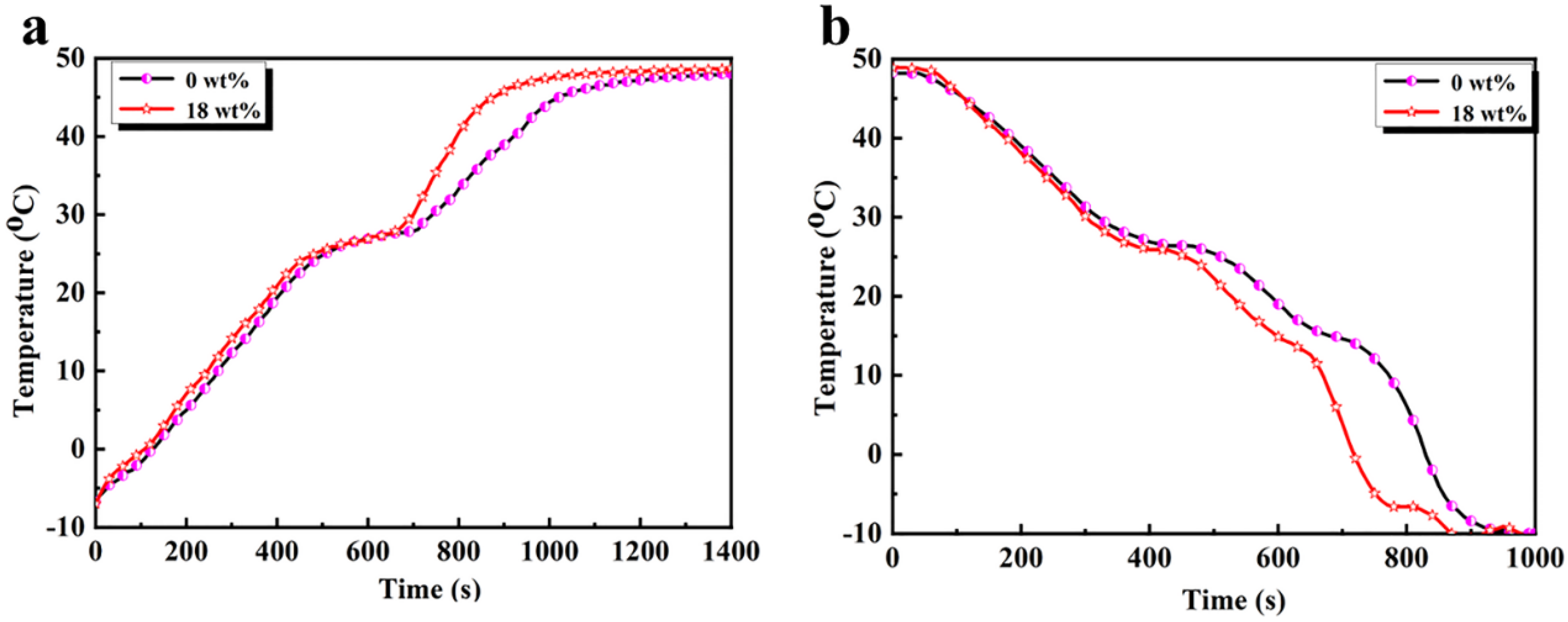
3.3. Thermal Properties of the CdS-Reinforced Double-Shell Microencapsulated PCMs
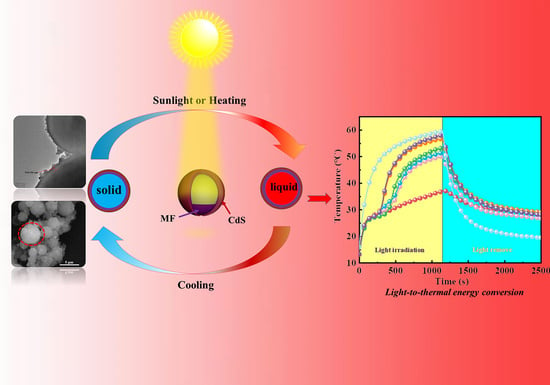
3.4. Photothermal Conversion Performance of the Microcapsules
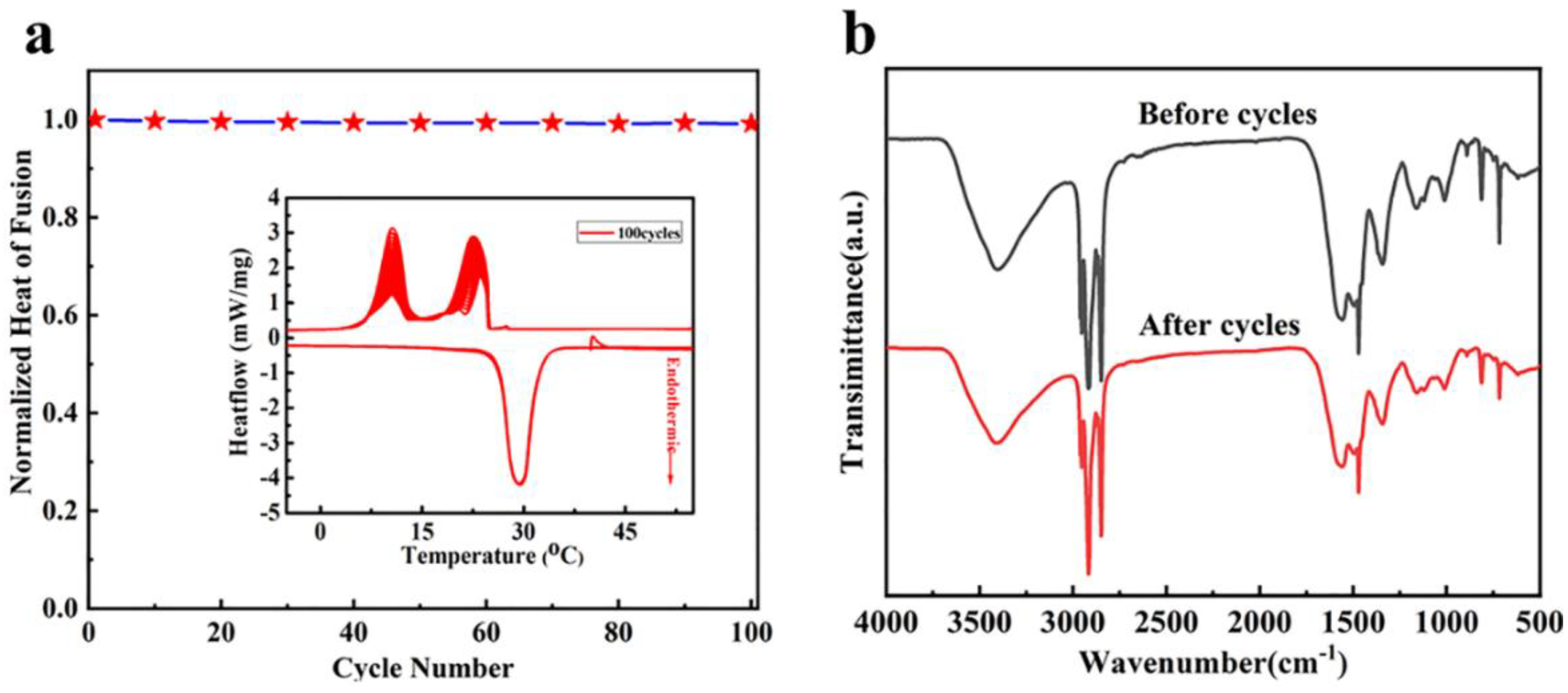
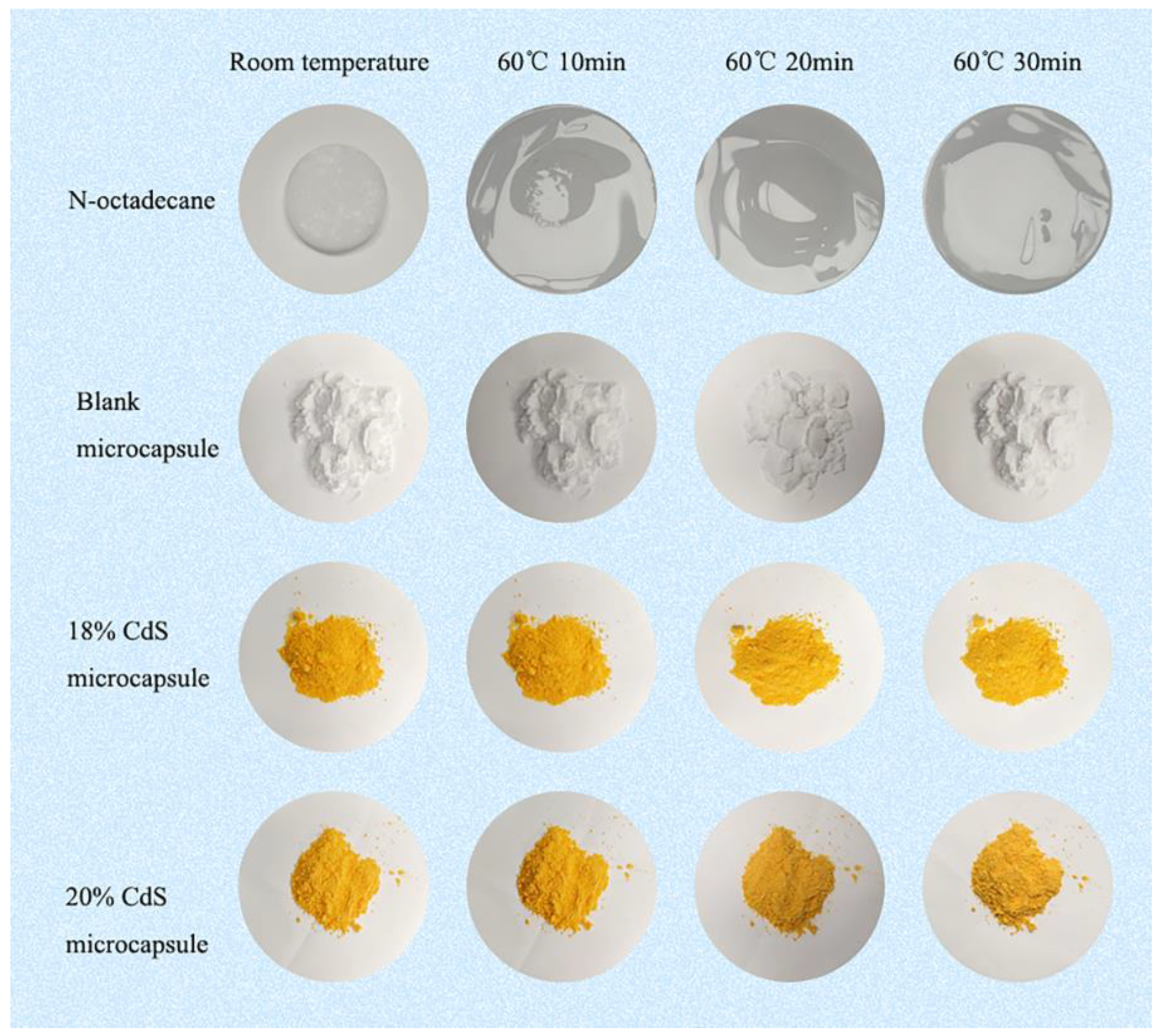
3.5. Shape Stability and Antileakage Properties of the Samples
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaqoob, A.A.; Ahmad, A.; Ibrahim, M.N.M.; Karri, R.R.; Rashid, M.; Ahamd, Z. Synthesis of metal oxide–based nanocomposites for energy storage application. Sustain. Nanotechnol. Environ. Remediat. 2022, 23, 611–635. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Ahmad, A.; Khatoon, A.; Setapar, S.H.M. Polyaniline-Based Materials for Supercapacitors. In Handbook of Supercapacitor Materials: Synthesis, Characterization, and Applications; WILEY-VCH GmbH: Weinheim, Germany, 2021; pp. 113–130. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, T.; Ma, A. Recent Advances in Design and Preparation of Polymer-Based Thermal Management Material. Polymers 2021, 13, 2797. [Google Scholar] [CrossRef]
- Kumar, P.M.; Mylsamy, K.; Saravanakumar, P. Experimental investigations on thermal properties of nano-SiO2/paraffin phase change material (PCM) for solar thermal energy storage applications. Energy Sour. Part A Recover. Util. Environ. Eff. 2020, 42, 2420–2433. [Google Scholar] [CrossRef]
- Karthick, A.; Athikesavan, M.M.; Pasupathi, M.; Kumar, N.M.; Chopra, S.; Ghosh, A. Investigation of Inorganic Phase Change Material for a Semi-Transparent Photovoltaic (STPV) Module. Energies 2020, 13, 3582. [Google Scholar] [CrossRef]
- Pasupathi, M.K.; Alagar, K.; Mm, M.; Aritra, G. Characterization of Hybrid-nano/Paraffin Organic Phase Change Material for Thermal Energy Storage Applications in Solar Thermal Systems. Energies 2020, 13, 5079. [Google Scholar] [CrossRef]
- Peng, G.; Hu, Y.; Dou, G.; Sun, Y.; Huan, Y.; Kang, S.H.; Piao, Z. Enhanced mechanical properties of epoxy composites embedded with MF/TiO2 hybrid shell microcapsules containing n-octadecane. J. Ind. Eng. Chem. 2022, 110, 414–423. [Google Scholar] [CrossRef]
- Motahar, S.; Alemrajabi, A.A.; Khodabandeh, R. Experimental study on solidification process of a phase change material containing TiO2 nanoparticles for thermal energy storage. Energy Convers. Manag. 2017, 138, 162–170. [Google Scholar] [CrossRef]
- Cheng, P.; Wei, K.; Shi, W.; Shi, J.; Wang, S.; Ma, B. Preparation and performance analysis of phase change microcapsule/epoxy resin composite phase change material. J. Energy Storage 2022, 47, 103581. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, S.-L.; Yu, S.-B.; Lin, C.-H.; Ning, Y.-H.; Li, Q.; Zhang, Y.-H.; Zhou, M.; Li, Y.-T.; Wang, S.-F.; et al. Effects of in-situ acid dopants on the latent heat storage properties and morphology of palmitic acid @ polyaniline microencapsulated phase change materials. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129207. [Google Scholar] [CrossRef]
- Hussain, S.I.; Roseline, A.A.; Kalaiselvam, S. Bifunctional nanoencapsulated eutectic phase change material core with SiO2/SnO2 nanosphere shell for thermal and electrical energy storage. Mater. Des. 2018, 154, 291–301. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Zhang, H.; Wan, X.-J.; Chen, D.-Z.; Chen, X.-F.; Ye, X.; Ouyang, X.; Qin, S.-Y.; Wen, H.-X.; Tang, J.-N. Carbon nanotube-enhanced double-walled phase-change microcapsules for thermal energy storage. J. Mater. Chem. A 2017, 5, 7482–7493. [Google Scholar] [CrossRef]
- Kim, I.; Lee, K.; Cho, G. Heat storage/release characteristics and mechanical properties of combat uniform fabrics treated with microcapsules containing octadecane as phase change materials. Fibers Polym. 2016, 17, 1726–1734. [Google Scholar] [CrossRef]
- Liu, C.; Cao, H.; Jin, S.; Bao, Y.; Cheng, Q.; Rao, Z. Synthesis and characterization of microencapsulated phase change material with phenol-formaldehyde resin shell for thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 243, 111789. [Google Scholar] [CrossRef]
- Hu, P.; Feng, Y.; Li, Q.; Lin, C.-H.; Ning, Y.-H.; Li, Y.-T.; Yu, L.-P.; Cao, Z.; Zeng, J.-L. Preparation and characterization of n-octadecane @ calcium fluoride microencapsulated phase change materials. Sol. Energy Mater. Sol. Cells 2022, 237, 111571. [Google Scholar] [CrossRef]
- Deng, H.; Yang, Y.; Tang, X.; Li, Y.; He, F.; Zhang, Q.; Huang, Z.; Yang, Z.; Yang, W. Phase-Change Composites Composed of Silicone Rubber and Pa@SiO2@PDA Double-Shelled Microcapsules with Low Leakage Rate and Improved Mechanical Strength. ACS Appl. Mater. Interfaces 2021, 13, 39394–39403. [Google Scholar] [CrossRef]
- Li, L.; Wu, X.; Zhu, X.; Li, Y.; Zou, L.; Wang, X.; Li, W.; Cheng, X. ZnO-Reinforced Thermal Regulating Microcapsules with Double-Layer Shell via Atomic Layer Deposition. Adv. Mater. Interfaces 2022, 9, 2200541. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wu, D. Design and synthesis of multifunctional microencapsulated phase change materials with silver/silica double-layered shell for thermal energy storage, electrical conduction and antimicrobial effectiveness. Energy 2016, 111, 498–512. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Fabrication and performances of microencapsulated phase change materials based on n-octadecane core and resorcinol-modified melamine–formaldehyde shell. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 129–138. [Google Scholar] [CrossRef]
- Gu, J.; Wang, W.; Yu, D. Temperature control and low infrared emissivity double-shell phase change microcapsules and their application in infrared stealth fabric. Prog. Org. Coat. 2021, 159, 106439. [Google Scholar] [CrossRef]
- Peng, G.; Dou, G.; Hu, Y.; Sun, Y.; Chen, Z. Phase Change Material (PCM) Microcapsules for Thermal Energy Storage. Adv. Polym. Technol. 2020, 2020, 9490873. [Google Scholar] [CrossRef]
- Slonopas, A.; Ryan, H.; Foley, B.; Sun, Z.; Sun, K.; Globus, T.; Norris, P. Growth mechanisms and their effects on the opto-electrical properties of CdS thin films prepared by chemical bath deposition. Mater. Sci. Semicond. Process. 2016, 52, 24–31. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, L.; Yang, T.; Su, L.; Chou, K.-C.; Hou, X. Cadmium sulfide with tunable morphologies: Preparation and visible-light driven photocatalytic performance. Phys. E Low Dimens. Syst. Nanostruct. 2017, 93, 116–123. [Google Scholar] [CrossRef]
- Koteswararao, J.; Satyanarayana, S.V.; Madhu, G.M.; Venkatesham, V. Estimation of structural and mechanical properties of Cadmium Sulfide/PVA nanocomposite films. Heliyon 2019, 5, e01851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuggili, S.B.; Kadiya, K.; Gaur, U.K.; Sharma, M. Synthesis of graphitic carbon nitride/cadmium sulfide core-shell nanofibers for enhanced photocatalysis. Environ. Sci. Pollut. Res. 2021, 28, 46377–46389. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Cui, W.; Ji, R.; Huang, C.; Huang, Y.; Zhang, H.; Xu, F.; Huang, P.; Li, B.; Sun, L. Design and synthesis of novel microencapsulated phase change materials with enhancement of thermal conductivity and thermal stability: Self-assembled boron nitride into shell materials. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124225. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Tailoring of bifunctional microencapsulated phase change materials with CdS/SiO2 double-layered shell for solar photocatalysis and solar thermal energy storage. Appl. Therm. Eng. 2018, 134, 603–614. [Google Scholar] [CrossRef]
- Biswas, A.; Sarkar, S.; Bhadra, K.; De, S. Novel synthesis of biologically active CdS nanoclusters in cell-mimicking vesicles. J. Exp. Nanosci. 2016, 11, 681–694. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, D.; Jiao, X.; Zhang, Y.; Shea, K.J.; Lu, X.; Qiu, G. Preparation, Properties, and Supercooling Prevention of Phase Change Material n-Octadecane Microcapsules with Peppermint Fragrance Scent. Ind. Eng. Chem. Res. 2015, 54, 8130–8136. [Google Scholar] [CrossRef]
- Hou, M.; Jiang, Z.; Chu, F.; Zhang, X.; Lai, N.-C. N-eicosane@TiO2/TiN composite phase change microcapsules: Efficient visible light-driven reversible solid-liquid phase transition. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129674. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, J.; Wang, B.; Li, Y.; Zhang, W.; Zhou, J.; Guo, H.; Liu, Y. Biodegradable wood plastic composites with phase change microcapsules of honeycomb-BN-layer for photothermal energy conversion and storage. Chem. Eng. J. 2022, 448, 137218. [Google Scholar] [CrossRef]
- Du, M.; Yu, X.; Zhang, Z.; Shao, M.; Zhou, L.; Zhu, G.; Militky, J.; Kremenakova, D.; Zhang, G. CuS Nanoparticle-Based Microcapsules for Solar-Induced Phase-Change Energy Storage. ACS Appl. Nano Mater. 2022, 5, 13009–13017. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Li, J.; Ma, C.; Guo, L.; Meng, X. Solar-driven phase change microencapsulation with efficient Ti4O7 nanoconverter for latent heat storage. Nano Energy 2018, 53, 579–586. [Google Scholar] [CrossRef]
- Sun, S.; Gao, Y.; Han, N.; Zhang, X.; Li, W. Reversible photochromic energy storage polyurea microcapsules via in-situ polymerization. Energy 2021, 219, 119630. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, J.; Xie, H.; Guo, Z. Enhanced photothermal conversion and thermal conductivity of phase change n-Octadecane microcapsules shelled with nano-SiC doped crosslinked polystyrene. Energy Storage Sav. 2022. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Wang, K.; Huang, Y.; Chen, Z. Highly efficient photothermal conversion capric acid phase change microcapsule: Silicon carbide modified melamine urea formaldehyde. J. Colloid Interface Sci. 2021, 582, 30–40. [Google Scholar] [CrossRef] [PubMed]

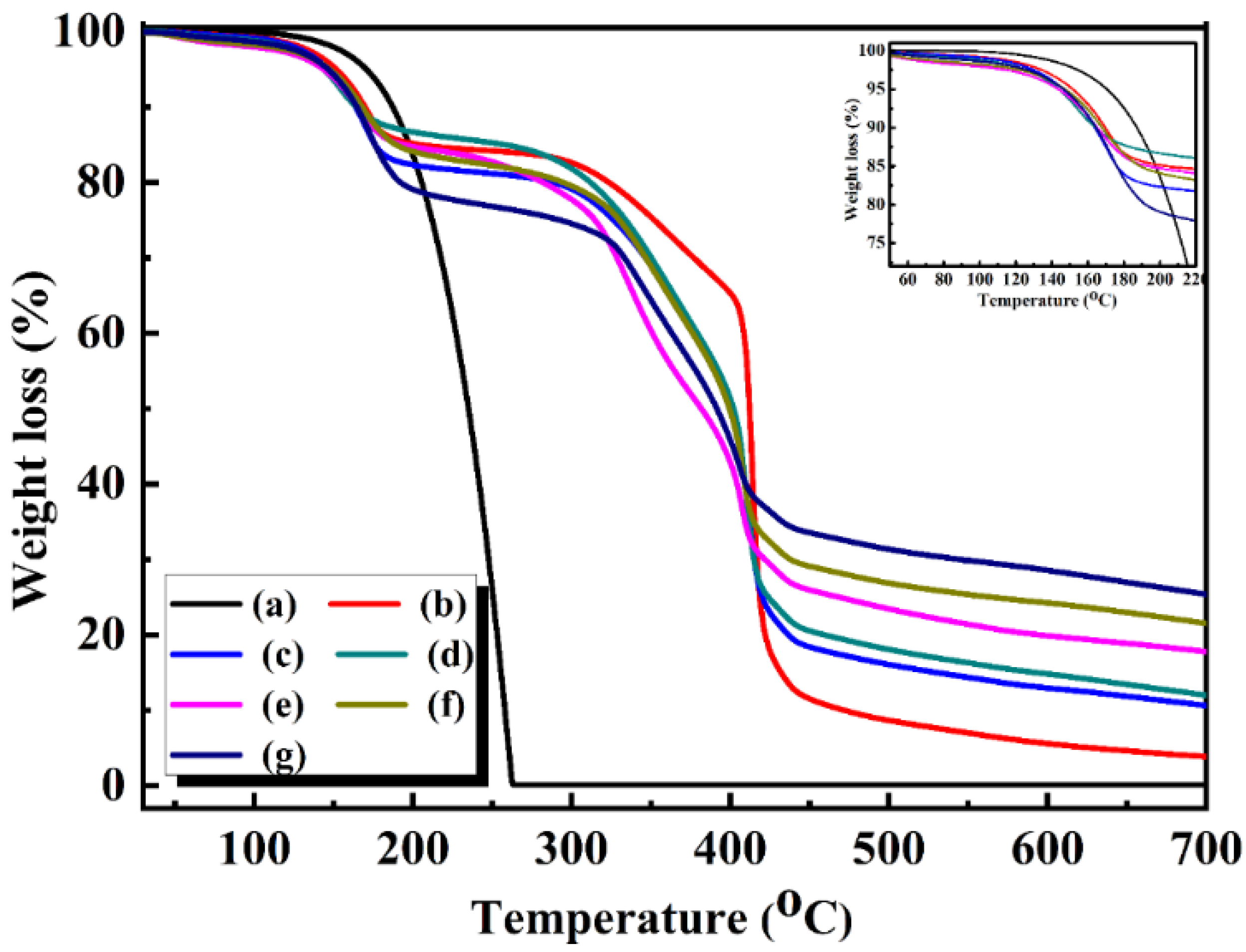

| Sample Code | Tm (°C) | ΔHm (J/g) | ΔHm t (J/g) | Tc (°C) | ΔHc (J/g) | ΔHc.t (J/g) | ||
|---|---|---|---|---|---|---|---|---|
| a | 29.9 | 236.40 | 236.40 | 23.91 | - | - | 235.4 | 235.4 |
| b | 29.14 | 169.06 | 169.35 | 23.10 | 15.20 | 10.56 | 168.55 | 168.35 |
| c | 29.17 | 154.94 | 154.88 | 23.76 | 16.97 | 10.53 | 153.23 | 153.76 |
| d | 29.49 | 143.13 | 143.04 | 22.79 | 15.86 | 10.24 | 141.63 | 142.44 |
| e | 29.73 | 129.26 | 129.23 | 21.84 | 15.85 | 10.15 | 129.12 | 128.69 |
| f | 29.48 | 114.58 | 114.61 | 22.79 | 15.89 | 10.28 | 114.17 | 114.07 |
| g | 29.20 | 113.73 | 113.80 | 23.44 | 16.74 | 10.61 | 113.40 | 113.32 |
| Microcapsules | Light-to-Thermal Conversion Efficiency | Latent Heat | Reference |
|---|---|---|---|
| N-eicosane@TiO2/TiN | 78.4% | 163 J/g | [30] |
| BN/SIO2@ n-octadecane | 69.54% | 140.6 J/g | [31] |
| MF/CuS@ dodecanol tetradecyl ester | 85.60% | 180.3 J/g | [32] |
| SiO2/Ti4O7@PA | 85.36% | 169 J/g | [33] |
| PU@butyl stearate containing | 50.7% | 72.14 J/g | [34] |
| nano-SiC/polystyrene@Octadecane | 54.91% | 105.7–106.3 J/g | [35] |
| nano-SiC/MUF@CA | 74.40% | 97.80 J/g | [36] |
| CdS/MF/n-octadecane | 85.2% | 114.58 J/g | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhu, Y.; Zhang, H.; Xu, F.; Sun, L.; Xia, Y.; Lin, X.; Peng, H.; Ma, L.; Li, B.; et al. Cadmium Sulfide—Reinforced Double-Shell Microencapsulated Phase Change Materials for Advanced Thermal Energy Storage. Polymers 2023, 15, 106. https://doi.org/10.3390/polym15010106
Zhang S, Zhu Y, Zhang H, Xu F, Sun L, Xia Y, Lin X, Peng H, Ma L, Li B, et al. Cadmium Sulfide—Reinforced Double-Shell Microencapsulated Phase Change Materials for Advanced Thermal Energy Storage. Polymers. 2023; 15(1):106. https://doi.org/10.3390/polym15010106
Chicago/Turabian StyleZhang, Shendao, Yucao Zhu, Huanzhi Zhang, Fen Xu, Lixian Sun, Yongpeng Xia, Xiangcheng Lin, Hongliang Peng, Lei Ma, Bin Li, and et al. 2023. "Cadmium Sulfide—Reinforced Double-Shell Microencapsulated Phase Change Materials for Advanced Thermal Energy Storage" Polymers 15, no. 1: 106. https://doi.org/10.3390/polym15010106