Nano Ag/Co3O4 Catalyzed Rapid Decomposition of Robinia pseudoacacia Bark for Production Biofuels and Biochemicals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
3. Results and Discussion
3.1. Behavior during Combustion of RPB via Catalysis
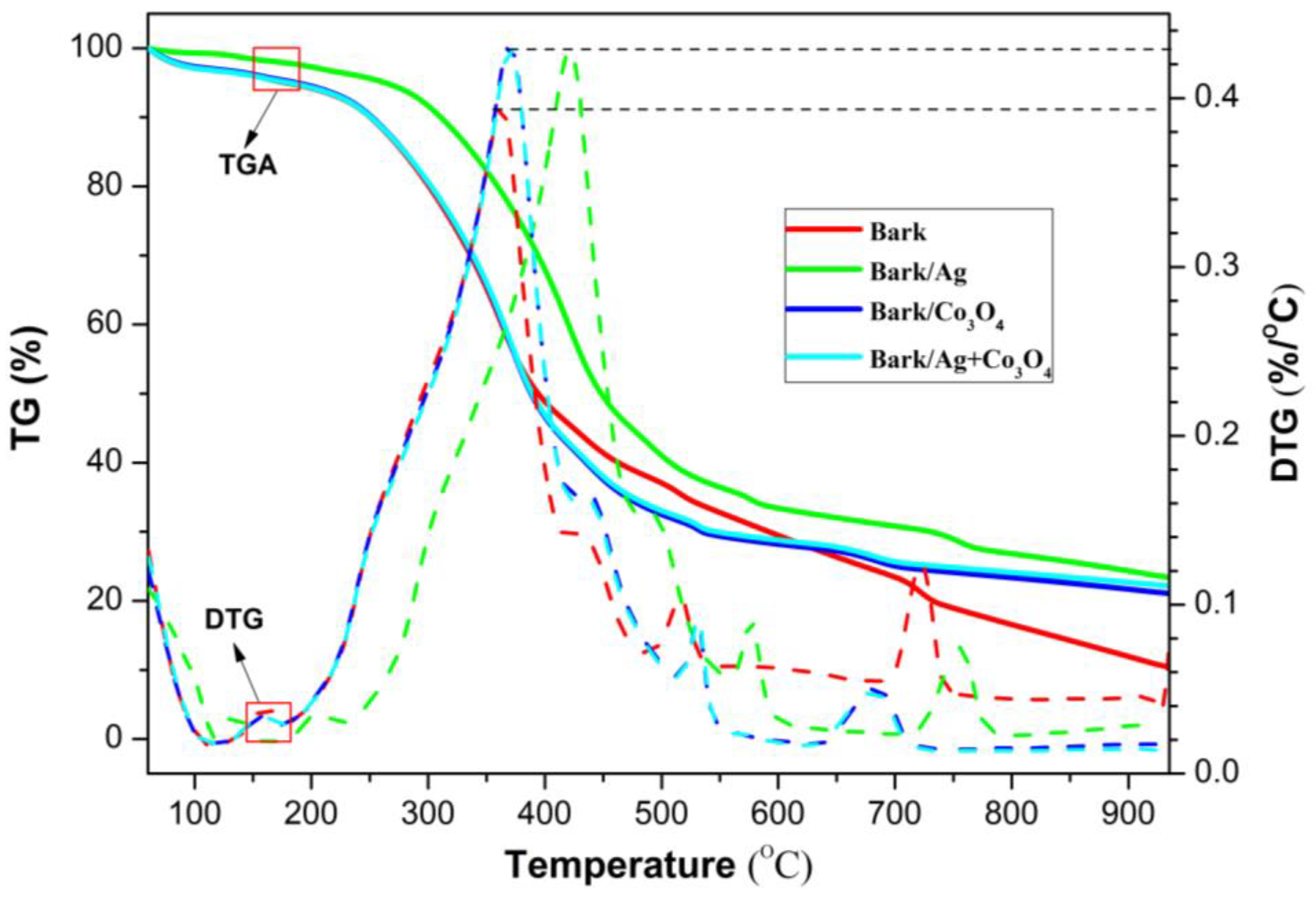
3.1.1. TG Analysis
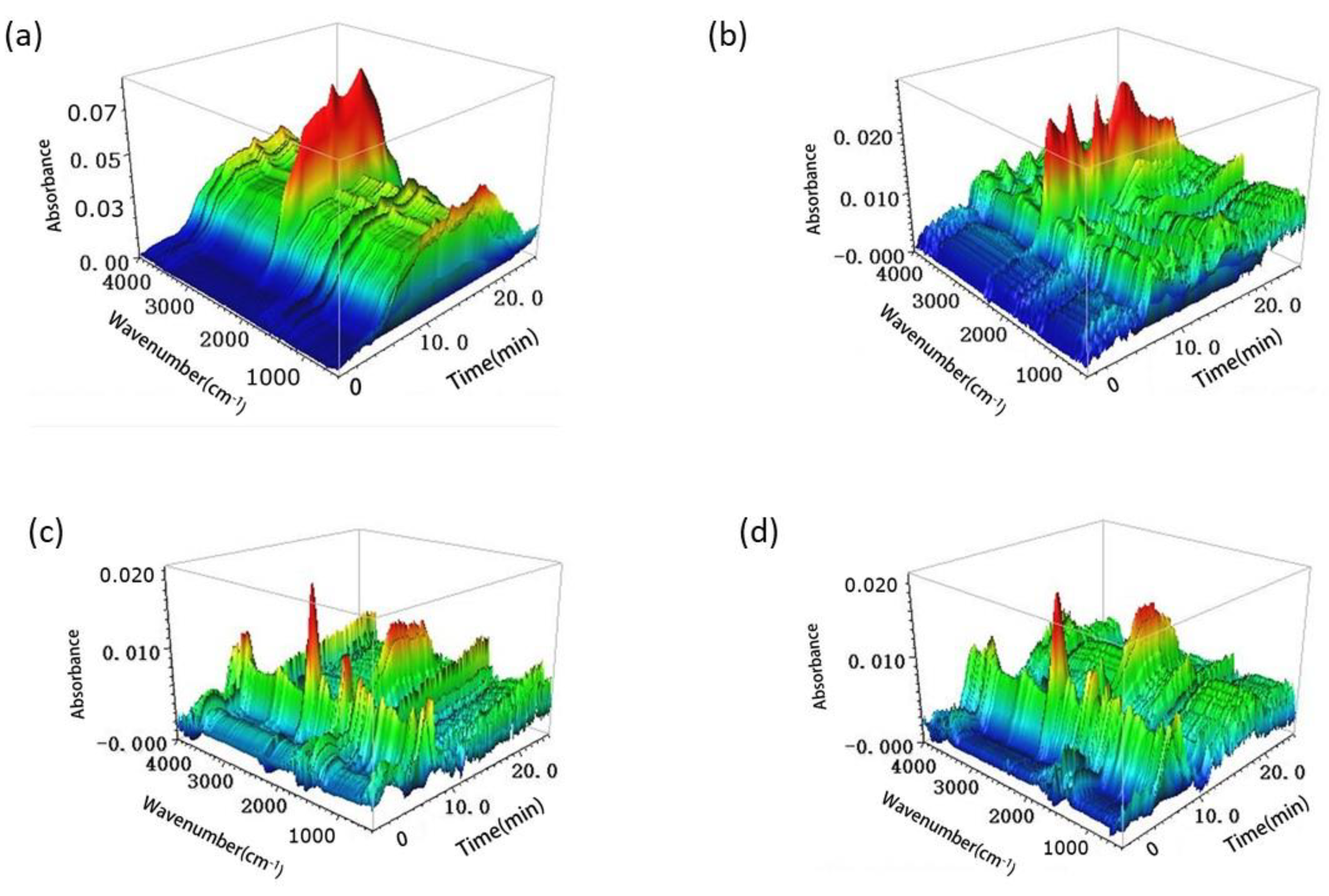
3.1.2. TG-FTIR Analysis
3.1.3. PY-GC-MS Analysis
3.2. Extractives of RPB for Added Energy
3.2.1. FT-IR Analysis
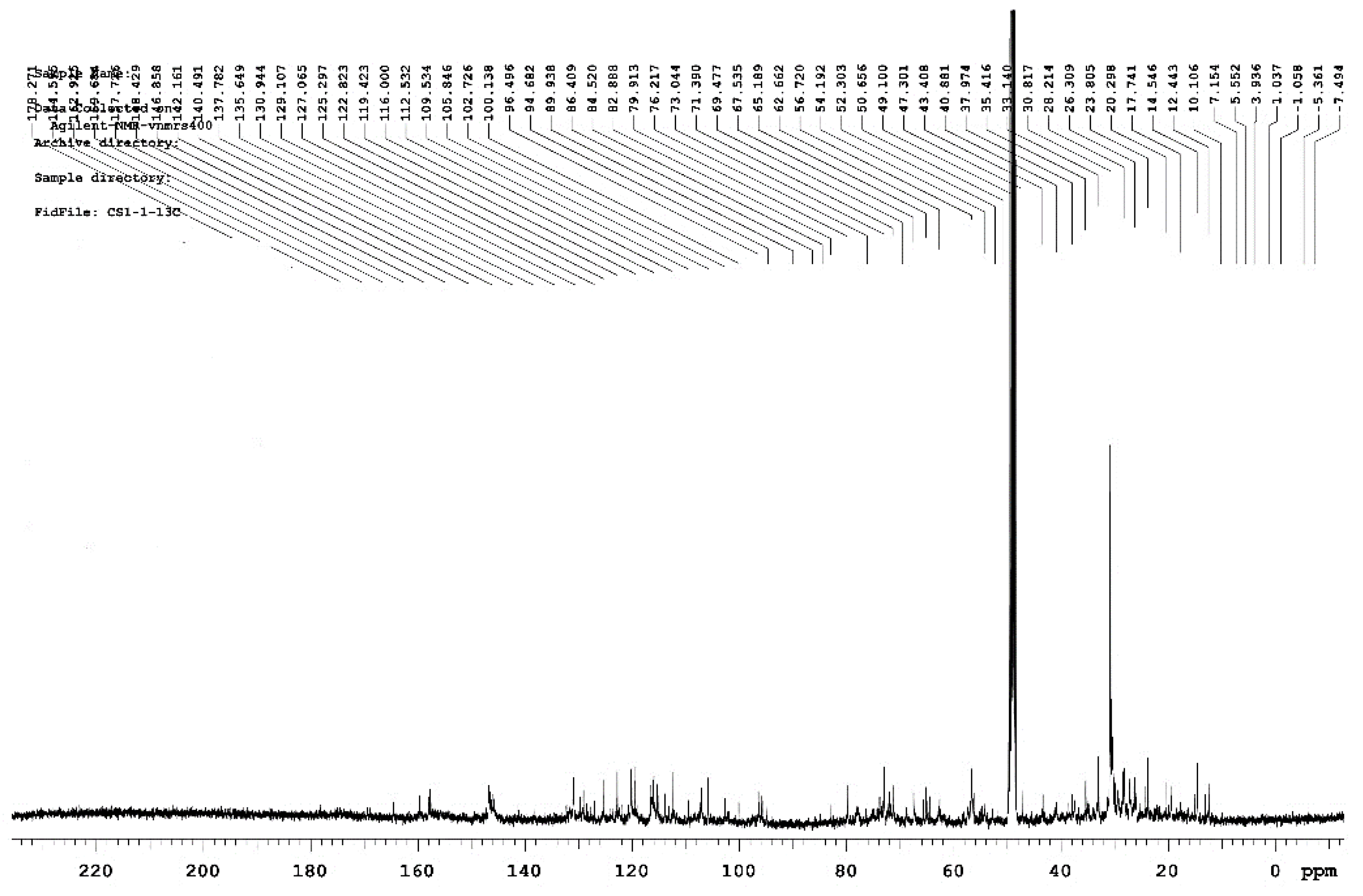
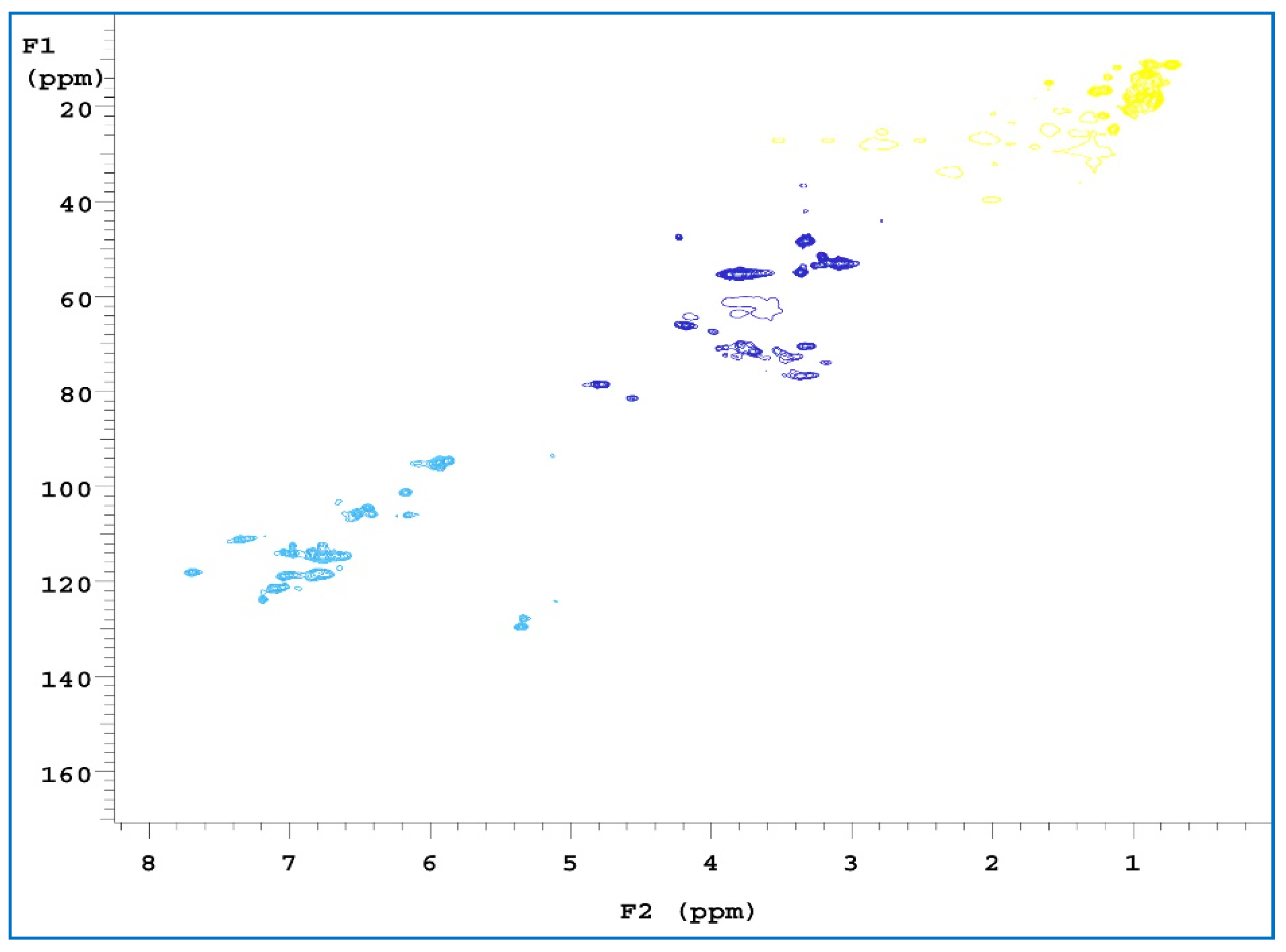
3.2.2. Analysis of NMR Spectra
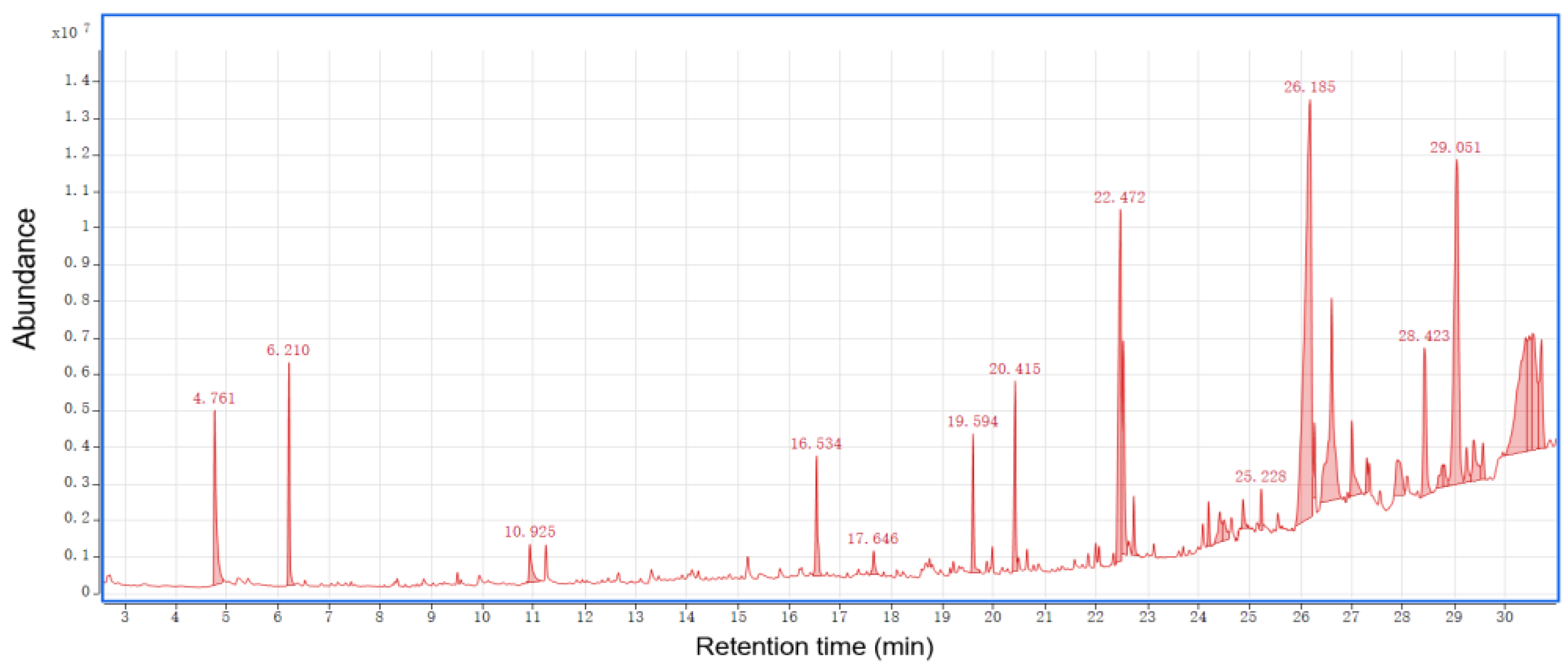
3.2.3. GC-MS Analysis
3.2.4. Analysis of LC-QTOF-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Bongers, F.; Aide, T.M.; Almeyda Zambrano, A.M.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; Broadbent, E.N.; Chazdon, R.L.; et al. Biomass resilience of Neotropical secondary forests. Nature 2016, 530, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Becidan, M.; Skreiberg, Ø.; Hustad, J.E. NOx and N2O Precursors (NH3 and HCN) in Pyrolysis of Biomass Residues. Energy Fuels 2007, 21, 1173–1180. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-oil production and upgrading research: A review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang; Xu, Y. Upgrading Bio-oil over Different Solid Catalysts. Energy Fuels 2006, 20, 2717–2720. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A.; Goldberg, N.M.; Lima, I.M.; Laird, D.A.; Hicks, K.B. Bio-oil and bio-char production from corn cobs and stover by fast pyrolysis. Biomass Bioenergy 2010, 34, 67–74. [Google Scholar] [CrossRef]
- Schnitzer, M.I.; Monreal, C.M.; Jandl, G. The conversion of chicken manure to bio-oil by fast pyrolysis. III. Analyses of chicken manure, bio-oils and char by Py-FIMS and Py-FDMS. J. Environ. Sci. Health B 2008, 43, 81–95. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, X.; Zhu, X. Analysis on chemical and physical properties of bio-oil pyrolyzed from rice husk. J. Anal. Appl. Pyrolysis 2008, 82, 191–198. [Google Scholar] [CrossRef]
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; von der Lippe, M.; Weber, E. Biological Flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Y.M.; Gao, J.M.; Stark, N.M. The effect of heat treatment on the chemical and color change of black locust (Robinia pseudoacacia) wood flour. Bioresources 2012, 7, 1157–1170. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef]
- Scala, F.; Salatino, P.; Chirone, R. Fluidized Bed Combustion of a Biomass Char (Robinia pseudoacacia). Energy Fuels 2000, 14, 781–790. [Google Scholar] [CrossRef]
- Gírio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Łukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef]
- Peng, F.; Peng, P.; Xu, F.; Sun, R.C. Fractional purification and bioconversion of hemicelluloses. Biotechnol. Adv. 2012, 30, 879–903. [Google Scholar] [CrossRef]
- Yang, H.P.; Yan, R.; Chen, H.P.; Lee, D.H.; Zheng, C.G. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Kang, D.M.; Park, W.S.; Kim, H.J.; Jeong, W.J.; Kang, K.K.; Ahn, M.J. Anti-inflammatory Constituents of Robinia pseudoacacia Root Bark. Korean J. Pharmacogn. 2022, 53, 8–15. [Google Scholar]
- Ge, S.B.; Brindhadevi, K.; Xia, C.L.; Khalifa, A.S.; Elfasakhany, A.; Unpaprom, Y.; Whangchai, K. Performance, combustion and emission characteristics of the CI engine fueled with Botryococcus braunii microalgae with addition of TiO2 nanoparticle. Fuel 2022, 317, 121898. [Google Scholar] [CrossRef]
- Ge, S.B.; Brindhadevi, K.; Xia, C.L.; Khalifa, A.S.; Elfasakhany, A.; Unpaprom, Y.; Doan, H.V. Enhancement of the combustion, performance and emission characteristics of spirulina microalgae biodiesel blends using nanoparticles. Fuel 2021, 308, 121822. [Google Scholar] [CrossRef]
- Deka, K.; Nath, N.; Saikia, B.K.; Deb, P. Kinetic analysis of ceria nanoparticle catalysed efficient biomass pyrolysis for obtaining high-quality bio-oil. J. Therm. Anal. Calorim. 2017, 130, 1875–1883. [Google Scholar] [CrossRef]
- Ge, S.B.; Ganesan, R.; Sekar, M.; Xia, C.L.; Shanmugam, S.; Alsehli, M.; Brindhadevi, K. Blending and emission characteristics of biogasoline produced using CaO/SBA-15 catalyst by cracking used cooking oil. Fuel 2022, 307, 121861. [Google Scholar] [CrossRef]
- Xie, J.C.; Sun, B.G.; Yu, M. Constituents of top fragrance from fresh flowers of Robinia pseudoacacia L. occurring in China. Flavour Fragr. J. 2006, 21, 798–800. [Google Scholar] [CrossRef]
- Spiteller, P.; Steglich, W. Biosynthesis of 2-aminobenzaldehyde in flowers of Robinia pseudoacacia and Philadelphus coronarius. Phytochemistry 2001, 57, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG–FTIR and Py–GC–FTIR. J. Anal. Appl. Pyrolysis 2010, 87, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Elbaz, A.M.; Gani, A.; Hourani, N.; Emwas, A.H.; Sarathy, S.M.; Roberts, W.L. TG/DTG, FT-ICR Mass Spectrometry, and NMR Spectroscopy Study of Heavy Fuel Oil. Energy Fuels 2015, 29, 7825–7835. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wang, S.; Zheng, Y.; Luo, Z.; Cen, K. Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J. Anal. Appl. Pyrolysis 2008, 82, 170–177. [Google Scholar] [CrossRef]
- Bassilakis, R.; Carangelo, R.M.; Wójtowicz, M.A. TG-FTIR analysis of biomass pyrolysis. Fuel 2001, 80, 1765–1786. [Google Scholar] [CrossRef]
- Subbalakshmi, Y.; Patti, A.F.; Lee, G.S.H.; Hooper, M.A. Structural characterisation of macromolecular organic material in air particulate matter using Py-GC-MS and solid state C-NMR. J. Environ. Monit. 2000, 2, 561–565. [Google Scholar] [CrossRef]
- Ge, S.B.; Liang, Y.Y.; Zhou, C.X.; Sheng, Y.Q.; Zhang, M.L.; Cai, L.P.; Zhou, Y.H.; Huang, Z.H.; Manzo, M.; Wu, C.Y.; et al. The potential of Pinus armandii Franch for high-grade resource utilization. Biomass Bioenergy 2022, 158, 106345. [Google Scholar] [CrossRef]
- Chen, J.; Ge, S.; Liu, Z.; Zhang, D.; Peng, W. GC-MS explores health care components in the extract of Pterocarpus Macarocarpus Kurz. Saudi J. Biol. Sci. 2018, 25, 1196–1201. [Google Scholar] [CrossRef]
- Ambili, K.U.; Sithambaresan, M.; Kurup, M.R.P. Interplay of bifurcated hydrogen bonds in making of inclusion/pseudo-inclusion complexes of Ni(II), Cu(II) and Zn(II) of a salophen type ligand: Crystal structures and spectral aspects. J. Mol. Struct. 2017, 1134, 687–696. [Google Scholar] [CrossRef]
- Naktiyok, J. Determination of the Self-Heating Temperature of Coal by Means of TGA Analysis. Energy Fuels 2018, 32, 2299–2305. [Google Scholar] [CrossRef]
- Yang, W.; Xiao, X.; Tan, J.; Cai, Q. In situ evaluation of breast cancer cell growth with 3D ATR-FTIR spectroscopy. Vib. Spectrosc. 2009, 49, 64–67. [Google Scholar] [CrossRef]
- Cen, K.; Chen, D.; Wang, J.; Cai, Y.; Wang, L. Effects of Water Washing and Torrefaction Pretreatments on Corn Stalk Pyrolysis: Combined Study Using TG-FTIR and a Fixed Bed Reactor. Energy Fuels 2016, 30, 10627–10634. [Google Scholar] [CrossRef]
- Song, H.; Liu, G.; Zhang, J.; Wu, J. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method. Fuel Process. Technol. 2017, 156, 454–460. [Google Scholar] [CrossRef]
- Stankovikj, F.; Garcia-Perez, M. TG-FTIR Method for the Characterization of Bio-oils in Chemical Families. Energy Fuels 2017, 31, 1689–1701. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Q.; Zhang, L.; Xiong, Y. Effects of water washing and torrefaction on the pyrolysis behavior and kinetics of rice husk through TGA and Py-GC/MS. Bioresour. Technol. 2016, 199, 352–361. [Google Scholar] [CrossRef]
- Siddiqui, M.T.H.; Baloch, H.A.; Nizamuddin, S.; Mubarak, N.M.; Mazari, S.A.; Grif, G.J.; Srinivasan, M. Dual-application of novel magnetic carbon nanocomposites as catalytic liquefaction for bio-oil synthesis and multi-heavy metal adsorption. Renew. Energy 2021, 172, 1103–1119. [Google Scholar] [CrossRef]
- Lu, J.W.; Zhang, Z.Z.; Fan, G.F.; Zhang, L.L.; Wu, Y.L.; Yang, M.D. Enhancement of microalgae bio-oil quality via hydrothermal liquefaction using functionalized carbon nanotubes. J. Clean. Prod. 2021, 285, 124835. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Li, Z.J.; Hou, Z.L.; Song, W.L.; Liu, X.D.; Cao, W.Q.; Shao, X.H.; Cao, M.S. Unusual continuous dual absorption peaks in Ca-doped BiFeO3 nanostructures for broadened microwave absorption. Nanoscale 2016, 8, 10415–10424. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Ma, J.; Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Brindle, J.T.; Antti, H.; Holmes, E.; Tranter, G.; Nicholson, J.K.; Bethell, H.W.L.; Clarke, S.; Schofield, P.M.; McKilligin, E.; Mosedale, D.E.; et al. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat. Med. 2002, 8, 1439–1444. [Google Scholar] [CrossRef]
- Weljie, A.M.; Newton, J.; Mercier, P.; Carlson, E.; Slupsky, C.M. Targeted Profiling: Quantitative Analysis of 1H NMR Metabolomics Data. Anal. Chem. 2006, 78, 4430–4442. [Google Scholar] [CrossRef]
- Miller, B.L. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed. 1991, 4, 47–52. [Google Scholar] [CrossRef]
- Zhang, Z.; Hancock, B.; Leen, S.; Ramaswamy, S.; Sollott, S.J.; Boheler, K.R.; Juhaszova, M.; Lakatta, E.G.; Spencer, R.G.; Fishbein, K.W. Compatibility of Superparamagnetic Iron Oxide Nanoparticle Labeling for H-1 MRI Cell Tracking with P-31 MRS for Bioenergetic Measurements. NMR Biomed. 2010, 23, 1166–1172. [Google Scholar] [CrossRef] [Green Version]
- Tkac, I.; Rao, R.; Georgieff, M.K.; Gruetter, R. Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo H-1 NMR spectroscopy. Magn. Reson. Med. 2003, 50, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Rothman, D.L.; Magnusson, I.; Katz, L.D.; Shulman, R.G.; Shulman, G.I. Quantitation of Hepatic Glycogenolysis and Gluconeogenesis in Fasting Humans with 13C NMR. Science 1991, 254, 573–576. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Sullivan, A.P.; Weber, R.J.; Ingall, E.D. Characterization of Water-Soluble Organic Carbon in Urban Atmospheric Aerosols Using Solid-State 13C NMR Spectroscopy. Environ. Sci. Technol. 2006, 40, 666–672. [Google Scholar] [CrossRef]
- Kamat, S.S.; Williams, H.J.; Raushel, F.M. Intermediates in the transformation of phosphonates to phosphate by bacteria. Nature 2011, 480, 570–573. [Google Scholar] [CrossRef]
- Lewis, I.A.; Schommer, S.C.; Hodis, B.; Robb, K.A.; Tonelli, M.; Westler, W.M.; Sussman, M.R.; Markley, J.L. Method for Determining Molar Concentrations of Metabolites in Complex Solutions from Two-Dimensional 1H−13C NMR Spectra. Anal. Chem. 2007, 79, 9385–9390. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.L.; Chen, W.; Yuan, S.; Kang, J.; Miyoshi, T. Chain Trajectory of Semicrystalline Polymers as Revealed by Solid-State NMR Spectroscopy. ACS Macro Lett. 2016, 5, 355–358. [Google Scholar] [CrossRef] [Green Version]
- Dutta Majumdar, R.; Gerken, M.; Mikula, R.; Hazendonk, P. Validation of the Yen–Mullins Model of Athabasca Oil-Sands Asphaltenes using Solution-State 1H NMR Relaxation and 2D HSQC Spectroscopy. Energy Fuels 2013, 27, 6528–6537. [Google Scholar] [CrossRef]
- Singh, S.K.; Dhepe, P.L. Ionic liquids catalyzed lignin liquefaction: Mechanistic studies using TPO-MS, FT-IR, RAMAN and 1D, 2D-HSQC/NOSEY NMR. Green Chem. 2016, 18, 4098–4108. [Google Scholar] [CrossRef]
- Kokkotou, K.; Loannou, E.; Nomikou, M.; Pitterl, F.; Vonaparti, A.; Siapi, E.; Zervou, M.; Roussis, V. An integrated approach using UHPLC-PDA-HRMS and 2D HSQC NMR for the metabolic profiling of the red alga Laurencia: Dereplication and tracing of natural products. Phytochemistry 2014, 108, 208–219. [Google Scholar] [CrossRef]
- Lee, I.A.; Kim, E.J.; Kim, D.H. Inhibitory Effect of beta-Sitosterol on TNBS-Induced Colitis in Mice. Planta Med. 2012, 78, 896–898. [Google Scholar]
- Baskar, A.A.; Al Numair, K.S.; Paulraj, M.G.; Alsaif, M.A.; Al Muamar, M.; Ignacimuthu, S. beta-Sitosterol Prevents Lipid Peroxidation and Improves Antioxidant Status and Histoarchitecture in Rats with 1,2-Dimethylhydrazine-Induced Colon Cancer. J. Med. Food 2012, 15, 335–343. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Hu, M.; Wei, P.; Zhu, W. BAMBI overexpression together with beta-sitosterol ameliorates NSCLC via inhibiting autophagy and inactivating TGF-beta/Smad2/3 pathway. Oncol. Rep. 2017, 37, 3046–3054. [Google Scholar] [CrossRef] [Green Version]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.D.; Sampson, M.B.; Neuffer, M.G.; Michalczuk, L.; Slovin, J.P.; Cohen, J.D. Indole-3-Acetic Acid Biosynthesis in the Mutant Maize orange pericarp, a Tryptophan Auxotroph. Science 1991, 254, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Kumari, T.; Sharma, C.; Bajpai, V.; Kumar, B.; Srivastava, M.; Arya, K.R. Qualitative determination of bioactive metabolites through Q-TOF LC/MS in different parts and undifferentiated cultures of Ulmus wallichiana Planchon. Plant Growth Regul. 2015, 75, 331–340. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Angulo, P.; Jorgensen, R.A.; Sylvestre, P.B.; Lindor, K.D. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: Results of a pilot study. Am. J. Gastroenterol. 2001, 96, 2711–2717. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Kaplowitz, N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 2003, 124, 1488–1499. [Google Scholar] [CrossRef]
- Baek, S.J.; Kim, J.S.; Jackson, F.R.; Eling, T.E.; McEntee, M.F.; Lee, S.H. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis 2004, 25, 2425–2432. [Google Scholar] [CrossRef] [Green Version]
- Spencer, J.P.E.; Schroeter, H.; Kuhnle, G.; Srai, S.K.S.; Tyrrell, R.M.; Hahn, U.; Rice-Evans, C. Epicatechin and its in vivo metabolite, 3′-O-methyl epicatechin, protect human fibroblasts from oxidative-stress-induced cell death involving caspase-3 activation. Biochem. J. 2001, 354, 493–500. [Google Scholar] [CrossRef]



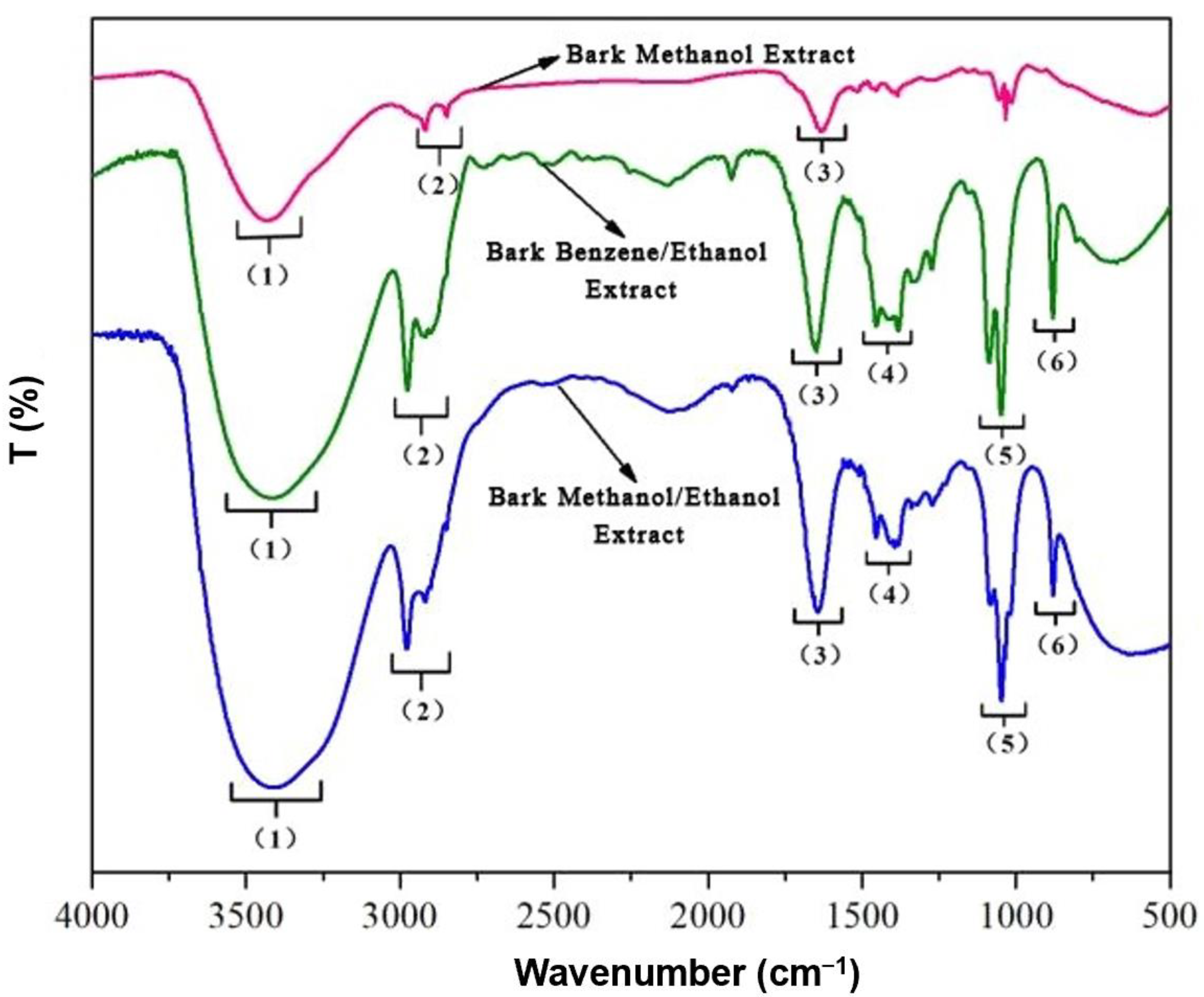





| Frequency Range (cm−1) | Frequency (cm−1) | Functional Group | Classification of Compounds | ||
|---|---|---|---|---|---|
| Methanol Extract | Benzene/Ethanol Extract | Methanol/Ethanol Extract | |||
| (1) 3500–3000 | 3434 | 3421 | 3413 | O-H stretching | Alcohol, carboxylic acids |
| (2) 3000–2800 | 2915 | 2975 | 2982 | C-H stretching | Alkane |
| (3) 1680–1610 | 1633 | 1653 | 1640 | C=C stretching | Alkenes |
| (4) 1470–1340 | - | 1457, 1384 | 1457, 1384 | C-H bending | Alkanes |
| (5) 1200–1000 | 1033 | 1087, 1047 | 1047 | C-O stretching | Alcohol, ether, carboxylic acids |
| (6) 900–690 | 878 | 878 | C-H out of plane bending | Aromatic rings | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, X.; Chen, X.; Li, H.; Ge, S.; Yang, Y.; Peng, W. Nano Ag/Co3O4 Catalyzed Rapid Decomposition of Robinia pseudoacacia Bark for Production Biofuels and Biochemicals. Polymers 2023, 15, 114. https://doi.org/10.3390/polym15010114
Yue X, Chen X, Li H, Ge S, Yang Y, Peng W. Nano Ag/Co3O4 Catalyzed Rapid Decomposition of Robinia pseudoacacia Bark for Production Biofuels and Biochemicals. Polymers. 2023; 15(1):114. https://doi.org/10.3390/polym15010114
Chicago/Turabian StyleYue, Xiaochen, Xiangmeng Chen, Hanyin Li, Shengbo Ge, Yafeng Yang, and Wanxi Peng. 2023. "Nano Ag/Co3O4 Catalyzed Rapid Decomposition of Robinia pseudoacacia Bark for Production Biofuels and Biochemicals" Polymers 15, no. 1: 114. https://doi.org/10.3390/polym15010114
APA StyleYue, X., Chen, X., Li, H., Ge, S., Yang, Y., & Peng, W. (2023). Nano Ag/Co3O4 Catalyzed Rapid Decomposition of Robinia pseudoacacia Bark for Production Biofuels and Biochemicals. Polymers, 15(1), 114. https://doi.org/10.3390/polym15010114








