Conversion of Free Fatty Acid in Calophyllum inophyllum Oil to Fatty Acid Ester as Precursor of Bio-Based Epoxy Plasticizer via SnCl2–Catalyzed Esterification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
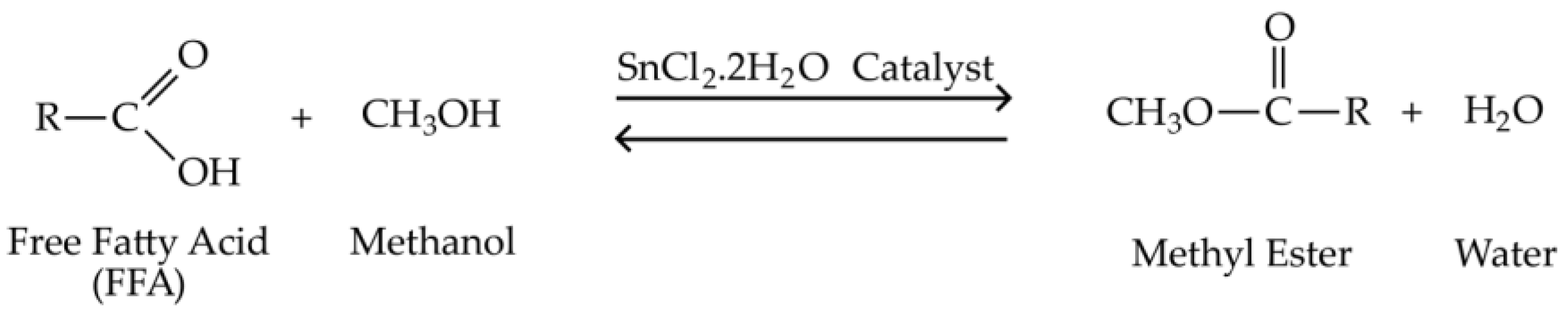
2.2.1. Esterification Reaction
2.2.2. Optimization Using Box–Behnken Design of Response Surface Methodology
3. Results and Discussion
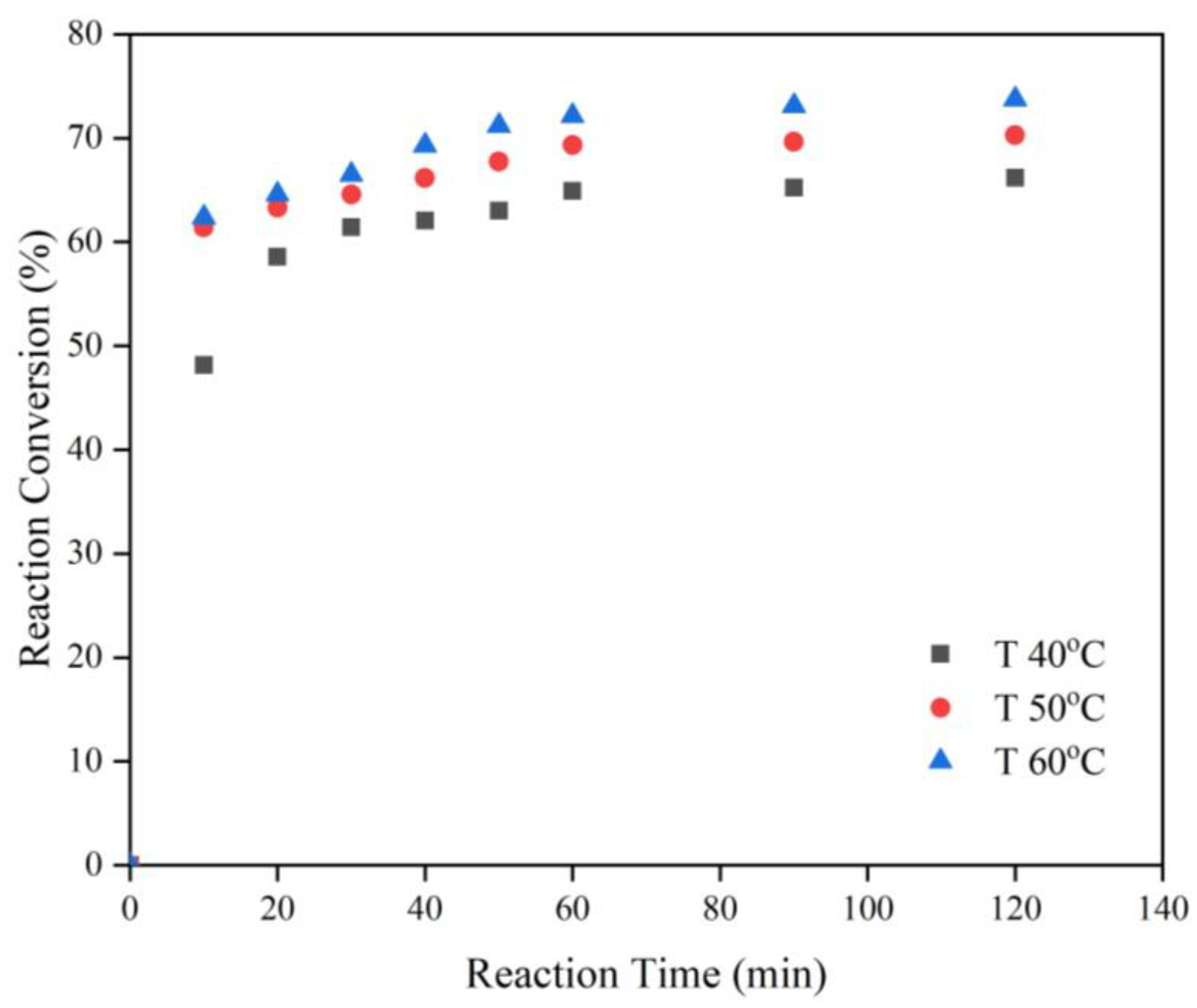
3.1. Effects of the Experimental Variables on the Reaction Conversion
3.2. Model Fitting in Box–Behnken Design (BBD)
3.3. Statistical Analysis Using ANOVA
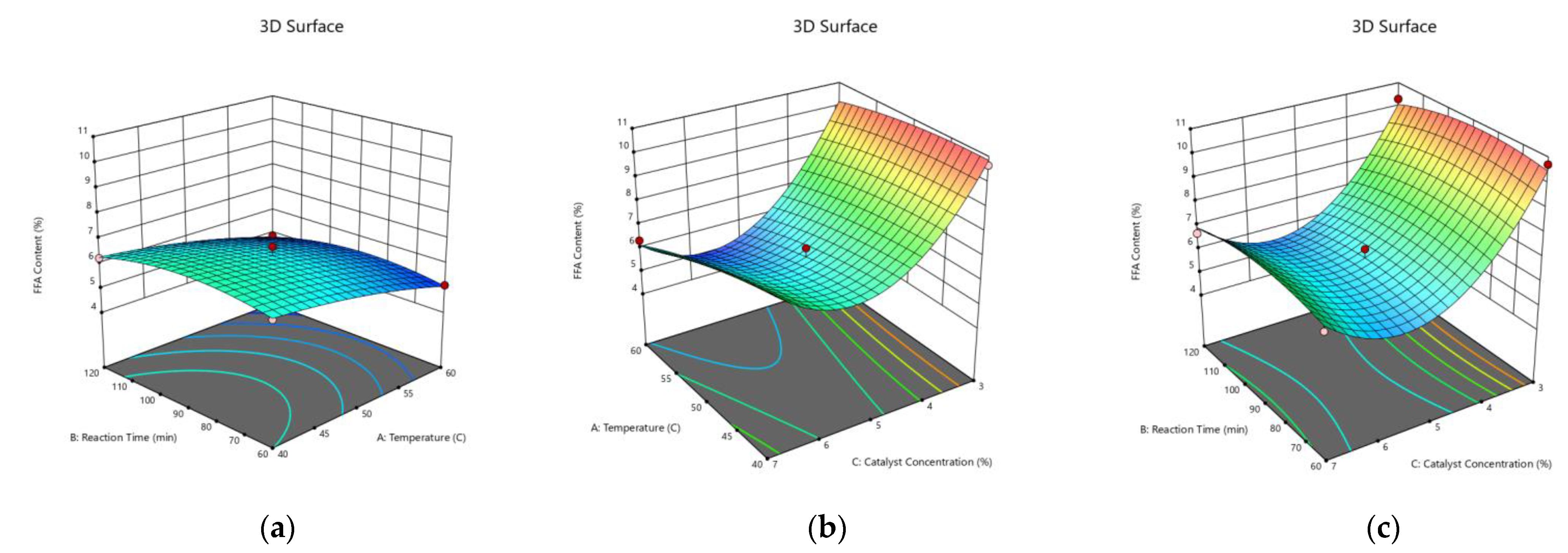
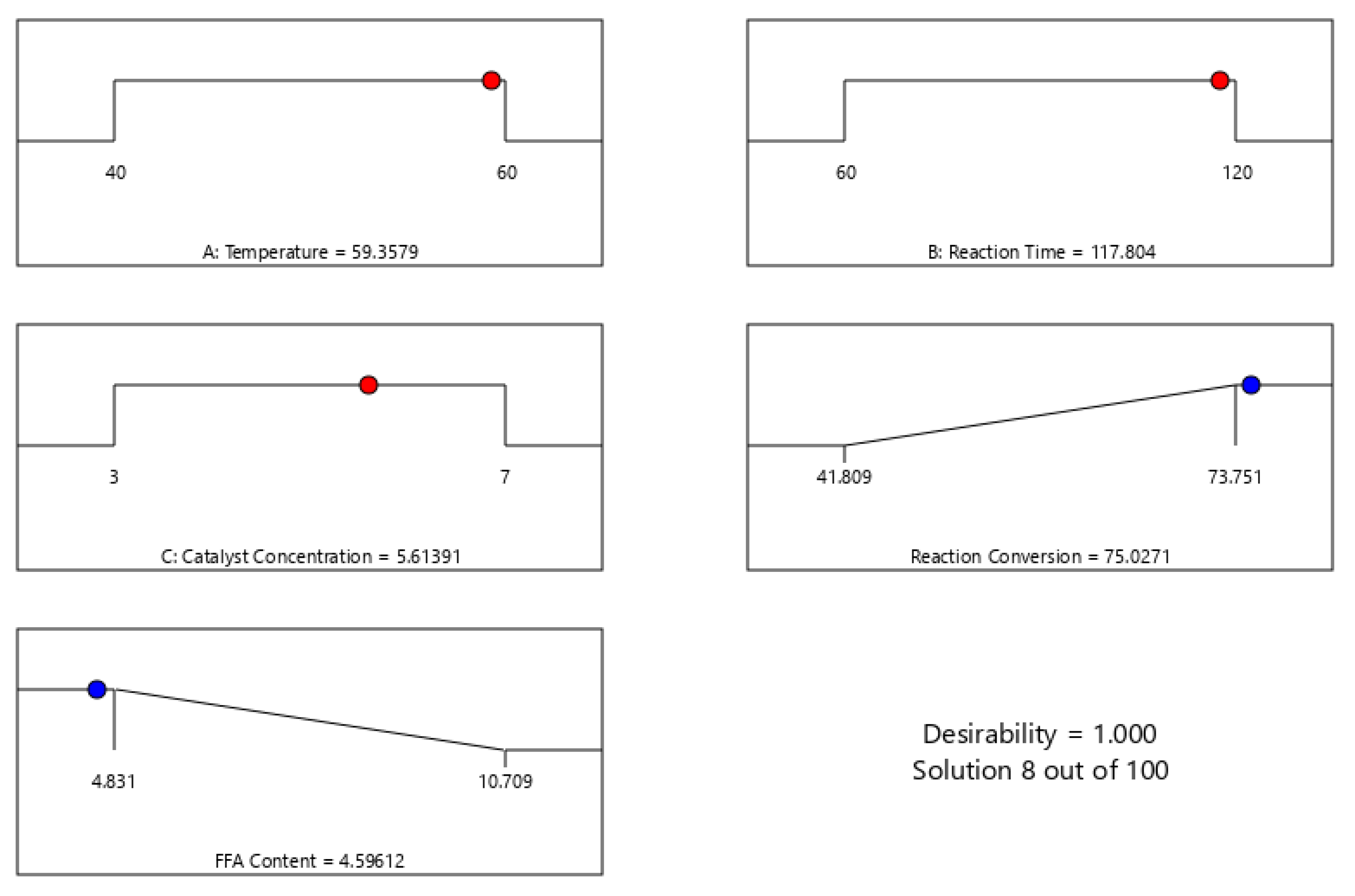
3.4. Optimization of the Process Variables Using BBD
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vieira, M.G.A.; Da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-Based Plasticizers and Biopolymer Films: A Review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Junthip, J.; Chaipalee, N.; Sangsorn, Y.; Maspornpat, C.; Jitcharoen, J.; Limrungruengrat, S.; Chotchuangchutchaval, T.; Martwong, E.; Sukhawipat, N. The Use of New Waste-Based Plasticizer Made from Modified Used Palm Oil for Non-Glutinous Thermoplastic Starch Foam. Polymers 2022, 14, 3997. [Google Scholar] [CrossRef] [PubMed]
- Janik, W.; Ledniowska, K.; Nowotarski, M.; Kudła, S.; Knapczyk-Korczak, J.; Stachewicz, U.; Nowakowska-Bogdan, E.; Sabura, E.; Nosal-Kovalenko, H.; Turczyn, R.; et al. Chitosan-Based Films with Alternative Eco-Friendly Plasticizers: Preparation, Physicochemical Properties and Stability. Carbohydr. Polym. 2023, 301, 120277. [Google Scholar] [CrossRef]
- Edwards, L.; McCray, N.L.; VanNoy, B.N.; Yau, A.; Geller, R.J.; Adamkiewicz, G.; Zota, A.R. Phthalate and Novel Plasticizer Concentrations in Food Items from U.S. Fast Food Chains: A Preliminary Analysis. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 366–373. [Google Scholar] [CrossRef]
- Kim, D.Y.; Chun, S.H.; Jung, Y.; Mohamed, D.F.M.S.; Kim, H.S.; Kang, D.Y.; An, J.W.; Park, S.Y.; Kwon, H.W.; Kwon, J.H. Phthalate Plasticizers in Children’s Products and Estimation of Exposure: Importance of Migration Rate. Int. J. Environ. Res. Public Health 2020, 17, 8582. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Xia, H.; Tang, K.; Zhou, Y. Plasticizers Derived from Biomass Resources: A Short Review. Polymers 2018, 10, 1303. [Google Scholar] [CrossRef] [Green Version]
- Hosney, H.; Nadiem, B.; Ashour, I.; Mustafa, I.; El-Shibiny, A. Epoxidized Vegetable Oil and Bio-Based Materials as PVC Plasticizer. J. Appl. Polym. Sci. 2018, 135, 46270. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Wang, J.; Liu, D.; Zhao, X. Conversion of Fatty Acid Methyl Ester to Epoxy Plasticizer by Auto-Catalyzed in Situ Formation of Performic Acid: Kinetic Modeling and Application of the Model. J. Clean. Prod. 2020, 259, 120791. [Google Scholar] [CrossRef]
- Pan, S.; Hou, D.; Yang, G.; Xie, Q.; Yan, S.; Zeng, Q.; Wang, Z.; Chen, Y.; Fan, H. Epoxidized Methyl Ricinoleate Bio-Plasticizer with a Pendant Acetate Ester for PVC Artificial Material: Circumventing Existing Limit on Achievable Migration Resistance. J. Leather Sci. Eng. 2019, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Galli, F.; Nucci, S.; Pirola, C.; Bianchi, C.L. Epoxy Methyl Soyate as Bio-Plasticizer: Two Different Preparation Strategies. Chem. Eng. Trans. 2014, 37, 601–606. [Google Scholar] [CrossRef]
- Ergashev, I.M.; Mamirzayev, M.A.; Sedalova, I.S.; Suyunov, S. Study of Triglycerides in Vegetable Oils by Gas Chromatography. Asian J. Phys. Chem. Math. 2022, 5, 93–96. [Google Scholar]
- Schuchardt, U.; Sercheli, R.; Matheus, R. Transesterification of Vegetable Oils: A Review. J. Braz. Chem. Soc. 1998, 9, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Simpen, I.; Winaya, I.S.; Subagia, I.G.A.; Suyasa, I.B. Solid Catalyst in Esterification and Transesterification Reactions for Biodiesel Production: A Review. Int. J. Eng. Emerg. Technol. 2020, 5, 168–174. [Google Scholar]
- Di Pietro, M.E.; Mannu, A.; Mele, A. NMR Determination of Free Fatty Acids in Vegetable Oils. Processes 2020, 8, 410. [Google Scholar] [CrossRef] [Green Version]
- Edyson, E.; Murgianto, F.; Ardiyanto, A.; Astuti, E.J.; Ahmad, M.P. Preprocessing Factors Affected Free Fatty Acid Content in Crude Palm Oil Quality. J. Ilmu Pertan. Indones. 2022, 27, 177–181. [Google Scholar] [CrossRef]
- Kusumaningtyas, R.D.; Ratrianti, N.; Purnamasari, I.; Budiman, A. Kinetics Study of Jatropha Oil Esterification with Ethanol in the Presence of Tin (II) Chloride Catalyst for Biodiesel Production. AIP Conf. Proc. 2017, 1788, 030086. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, P.; Álvarez, S.; Hunter, R.; Sánchez, A. Epoxidation of Fatty Acid Methyl Esters Derived from Algae Biomass to Develop Sustainable Bio-Based Epoxy Resins. Polymers 2020, 12, 2313. [Google Scholar] [CrossRef]
- Sustaita-Rodríguez, A.; Ramos-Sánchez, V.H.; Camacho-Dávila, A.A.; Zaragoza-Galán, G.; Espinoza-Hicks, J.C.; Chávez-Flores, D. Lipase Catalyzed Epoxidation of Fatty Acid Methyl Esters Derived from Unsaturated Vegetable Oils in Absence of Carboxylic Acid. Chem. Cent. J. 2018, 12, 39. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Taddeo, F.; Aguilera, A.F.; Cai, X.; Russo, V.; Tolvanen, P.; Leveneur, S. The Lord of the Chemical Rings: Catalytic Synthesis of Important Industrial Epoxide Compounds. Catalysts 2021, 11, 765. [Google Scholar] [CrossRef]
- Benaniba, M.T.; Benaniba, M.T.; Belhaneche-Bensemra, N.; Gelbard, G. Epoxidation of Sunflower Oil with Peroxoacetic Acid in Presence of Ion Exchange Resin by Various Processes Polymer Recycling View Project Miscible Polymer Blends View Project Epoxidation of Sunflower Oil with Peroxoacetic Acid in Presence of Ion Exchange. Energy Educ. Sci. Technol. 2008, 21, 71–82. [Google Scholar]
- Ifa, L.; Syarif, T.; Sabara, Z.; Nurjannah, N.; Munira, M.; Aryani, F. Study on the Kinetics of Epoxidation Reaction of RBD Palm Olein. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 012035. [Google Scholar] [CrossRef]
- Musik, M.; Janus, E.; Pełech, R.; Sałaciński, Ł. Effective Epoxidation of Fatty Acid Methyl Esters with Hydrogen Peroxide by the Catalytic System H3pw12o40/ Quaternary Phosphonium Salts. Catalysts 2021, 11, 1058. [Google Scholar] [CrossRef]
- Ginigini, J.; Lecellier, G.J.; Nicolas, M.; Nour, M.; Hnawia, E.; Lebouvier, N.; Herbette, G.; Lockhart, P.; Raharivelomanana, P. Chemodiversity of Calophyllum inophyllum L. Oil Bioactive Components Related to Their Specific Geographical Distribution in the South Pacific Region. PeerJ 2019, 2019, 6896. [Google Scholar] [CrossRef] [PubMed]
- Akram, F.; Haq, I.U.; Raja, S.I.; Mir, A.S.; Qureshi, S.S.; Aqeel, A.; Shah, F.I. Current Trends in Biodiesel Production Technologies and Future Progressions: A Possible Displacement of the Petro-Diesel. J. Clean. Prod. 2022, 370, 133479. [Google Scholar] [CrossRef]
- Kusumaningtyas, R.D.; Prasetiawan, H.; Putri, R.D.A.; Triwibowo, B.; Kurnita, S.C.F.; Anggraeni, N.D.; Veny, H.; Hamzah, F.; Rodhi, M.N.M. Optimisation of Free Fatty Acid Removal in Nyamplung Seed Oil (Callophyllum inophyllum L.) Using Response Surface Methodology Analysis. Pertanika J. Sci. Technol. 2021, 29, 2605–2623. [Google Scholar] [CrossRef]
- Ferreira, A.B.; Lemos Cardoso, A.; da Silva, M.J. Tin-Catalyzed Esterification and Transesterification Reactions: A Review. ISRN Renew. Energy 2012, 2012, 142857. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, M.J.; Cardoso, A.L. Assessing the Activity of Solid-Suported SnCl2 Catalysts on the Oleic Acid Esterification for Biodiesel Production. J. Thermodyn. Catal. 2016, 7, 1000173. [Google Scholar] [CrossRef]
- Sert, E.; Atalay, F.S. Application of Green Catalysts for the Esterification of Benzoic Acid with Different Alcohols. Celal Bayar Üniversitesi Fen Bilim. Derg. 2017, 13, 907–912. [Google Scholar] [CrossRef]
- Polat, S.; Sayan, P. Application of Response Surface Methodology with a Box–Behnken Design for Struvite Precipitation. Adv. Powder Technol. 2019, 30, 2396–2407. [Google Scholar] [CrossRef]
- Czyrski, A.; Jarzebski, H. Response Surface Methodology as a Useful Tool for Evaluation of the Recovery of the Fluoroquinolones from Plasma-the Study on Applicability of Box-Behnken Design, Central Composite Design and Doehlert Design. Processes 2020, 8, 473. [Google Scholar] [CrossRef]
- Riswanto, F.D.O.; Rohman, A.; Pramono, S.; Martono, S. Application of Response Surface Methodology as Mathematical and Statistical Tools in Natural Product Research. J. Appl. Pharm. Sci. 2019, 9, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Sharma, Y.C.; Yadav, M.; Upadhyay, S.N. Latest Advances in Degumming Feedstock Oils for Large-Scale Biodiesel Production. Biofuels Bioprod. Biorefining 2019, 13, 174–191. [Google Scholar] [CrossRef] [Green Version]
- Camacho, J.N.; Romero, R.; Galván Muciño, G.E.; Martínez-Vargas, S.L.; Pérez-Alonso, C.; Natividad, R. Kinetic Modeling of Canola Oil Transesterification Catalyzed by Quicklime. J. Appl. Res. Technol. 2018, 16, 446–454. [Google Scholar] [CrossRef]
- Yu, H.; Niu, S.; Lu, C.; Wei, W.; Zhang, X. Biodiesel Synthesis by Transesterification Using Coal-Based Solid Acid Catalyst. IOP Conf. Ser. Earth Environ. Sci. 2021, 634, 012041. [Google Scholar] [CrossRef]
- Ho, W.W.S.; Ng, H.K.; Gan, S. Biodiesel Synthesis from Refined Palm Oil Using a Calcium Oxide Impregnated Ash-Based Catalyst: Parametric, Kinetics, and Product Characterization Studies. Catalysts 2022, 12, 706. [Google Scholar] [CrossRef]
- Banchero, M.; Gozzelino, G. A Simple Pseudo-Homogeneous Reversible Kinetic Model for the Esterification of Different Fatty Acids with Methanol in the Presence of Amberlyst-15. Energies 2018, 11, 1843. [Google Scholar] [CrossRef] [Green Version]
- Intarat, N.; Somnuk, K.; Theppaya, T.; Prateepchaikul, G. Acid Value Reduction Process in Mixed Crude Palm Oil by Using Low-Grade Ethanol. Adv. Mater. Res. 2014, 1025–1026, 677–682. [Google Scholar] [CrossRef]
- Kurniati, S.; Soeparman, S.; Yuwono, S.S.; Hakim, L. Characteristics and Potential of Nyamplung (Calophyllum inophyllum L.) Seed Oil from Kebumen, Central Java, as a Biodiesel Feedstock. Int. Res. J. Adv. Eng. Sci. 2018, 3, 148–152. [Google Scholar]
- Kusumaningtyas, R.D.; Prasetiawan, H.; Pratama, B.R.; Prasetya, D.; Hisyam, A. Esterification of Non-Edible Oil Mixture in Reactive Distillation Column over Solid Acid Catalyst: Experimental and Simulation Study. J. Phys. Sci. 2018, 29, 212–226. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.K.; Viruthagiri, T.; Arunkumar, C. Statistical Optimization of Process Parameters for the Production of Tannase by Aspergillus Flavus under Submerged Fermentation. 3 Biotech 2014, 4, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Xia, Z.; Lu, J. Esterification and Deacidification of a Waste Cooking Oil (TAN 68.81 Mg KOH/g) for Biodiesel Production. Energies 2012, 5, 2683–2691. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current Developments in Esterification Reaction: A Review on Process and Parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Ngadi, N.; Ma, L.N.; Alias, H.; Johari, A.; Rahman, R.A.; Mohamad, M. Production of Biodiesel from Waste Cooking Oil via Ultrasonic-Assisted Catalytic System. Appl. Mech. Mater. 2014, 699, 552–557. [Google Scholar] [CrossRef]
- Khudsange, C.R.; Wasewar, K.L. Process Intensification of Esterification Reaction for the Production of Propyl Butyrate by Pervaporation. Resour. Technol. 2017, 3, 88–93. [Google Scholar] [CrossRef]
- Nindya, C.C.S.; Anggara, D.R.; Nuryoto; Teguh, K. Esterification Glycerol (by Product in Biodiesel Production) with Oleic Acid Using Mordenite Natural Zeolite as Catalyst: Study of Reaction Temperature and Catalyst Loading Effect. IOP Conf. Ser. Mater. Sci. Eng. 2020, 909, 012001. [Google Scholar] [CrossRef]
- Rani, K.N.P.; Neeharika, T.S.V.R.; Vardhan, G.H.; Kumar, T.P.; Devi, B.L.A.P. The Kinetics of the Esterification of Free Fatty Acids in Jatropha Oil Using Glycerol Based Solid Acid Catalyst. Eur. J. Sustain. Dev. Res. 2020, 4, em0116. [Google Scholar] [CrossRef] [Green Version]
- Handayani, P.A.; Wulansarie, R.; Husaen, P.; Ulfayanti, I.M. Esterification of Nyamplung (Calophyllum inophyllum) Oil With Ionic Liquid Catalyst of Bmimhso4 and Microwaves-Assisted. J. Bahan Alam Terbarukan 2018, 7, 59–63. [Google Scholar] [CrossRef]
- Ahmadian-Kouchaksaraie, Z.; Niazmand, R. Supercritical Carbon Dioxide Extraction of Antioxidants from Crocus Sativus Petals of Saffron Industry Residues: Optimization Using Response Surface Methodology. J. Supercrit. Fluids 2017, 121, 19–31. [Google Scholar] [CrossRef]
- Montaño Moreno, J.J.; Palmer Pol, A.; Sesé Abad, A.; Cajal Blasco, B. Using the R-MAPE Index as a Resistant Measure of Forecast Accuracy. Psicothema 2013, 25, 500–506. [Google Scholar] [CrossRef]
- Ahmad, A.; Rehman, M.U.; Wali, A.F.; El-Serehy, H.A.; Al-Misned, F.A.; Maodaa, S.N.; Aljawdah, H.M.; Mir, T.M.; Ahmad, P. Box–Behnken Response Surface Design of Polysaccharide Extraction from Rhododendron Arboreum and the Evaluation of Its Antioxidant Potential. Molecules 2020, 25, 3835. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Optimization of Esterification of Acrylic Acid and Ethanol by Box-Behnken Design of Response Surface Methodology. Indian J. Chem. Technol. 2019, 26, 89–94. [Google Scholar]
- Mia, M. Mathematical Modeling and Optimization of MQL Assisted End Milling Characteristics Based on RSM and Taguchi Method. Meas. J. Int. Meas. Confed. 2018, 121, 249–260. [Google Scholar] [CrossRef]
- Variyana, Y.; Muchammad, R.S.C.; Mahfud, M. Box-Behnken Design for the Optimization Using Solvent-Free Microwave Gravity Extraction of Garlic Oil from Allium sativum L. IOP Conf. Ser. Mater. Sci. Eng. 2019, 673, 012005. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Neves, S.C.G.; da Silva, M.J. Esterification of Oleic Acid for Biodiesel Production Catalyzed by SnCl2: A Kinetic Investigation. Energies 2008, 1, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, M.J.; Julio, A.A.; Dos Santos, K.T. Sn(II)-Catalyzed β-Citronellol Esterification: A Brønsted Acid-Free Process for Synthesis of Fragrances at Room Temperature. Catal. Sci. Technol. 2015, 5, 1261–1266. [Google Scholar] [CrossRef]
- Marso, T.M.M.; Kalpage, C.S.; Udugala-Ganehenege, M.Y. Metal Modified Graphene Oxide Composite Catalyst for the Production of Biodiesel via Pre-Esterification of Calophyllum Inophyllum Oil. Fuel 2017, 199, 47–64. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Park, S.H.; Park, J.O. Simultaneous Optimization of Multiple Responses Using. In Quality Improvement Through Statistical Methods; Abraham, B., Ed.; Springer Science: New York, NY, USA, 1998; pp. 299–300. [Google Scholar]
- Aziz, N.A.; AS, N.A.; Noraziman, S. Modified Desirability Function For Optimization of Multiple Responses Nasuhar. J. Math. Comput. Sci. 2018, 1, 39–54. [Google Scholar]
- Amdoun, R.; Khelifi, L.; Khelifi-Slaoui, M.; Amroune, S.; Asch, M.; Assaf-ducrocq, C.; Gontier, E. The Desirability Optimization Methodology; a Tool to Predict Two Antagonist Responses in Biotechnological Systems: Case of Biomass Growth and Hyoscyamine Content in Elicited Datura Starmonium Hairy Roots. Iran. J. Biotechnol. 2018, 16, 11–19. [Google Scholar] [CrossRef]


| Independent Variable | Factor | Coded Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Temperature (°C) | A | 40 | 50 | 60 |
| Reaction Time (min) | B | 60 | 90 | 120 |
| Catalyst Concentration (%) | C | 3 | 5 | 7 |
| Run | Factor A Temperature (°C) | Factor B Reaction Time (min) | Factor C Catalyst Concentration (%) |
|---|---|---|---|
| 1 | 40 | 120 | 5 |
| 2 | 40 | 60 | 5 |
| 3 | 60 | 90 | 3 |
| 4 | 40 | 90 | 7 |
| 5 | 60 | 90 | 7 |
| 6 | 50 | 120 | 3 |
| 7 | 60 | 120 | 5 |
| 8 | 50 | 60 | 7 |
| 9 | 50 | 90 | 5 |
| 10 | 40 | 90 | 3 |
| 11 | 60 | 60 | 5 |
| 12 | 50 | 60 | 3 |
| 13 | 50 | 90 | 5 |
| 14 | 50 | 120 | 7 |
| 15 | 50 | 90 | 5 |
| Run | Temperature (°C) A | Reaction Time (min) B | Catalyst Concentration (%) C | FFA Conversion % | Error (MAPE) % | FFA Content (%) | Error (MAPE) % | ||
|---|---|---|---|---|---|---|---|---|---|
| Experiment | Prediction | Experiment | Prediction | ||||||
| 1 | 40 | 120 | 5 | 66.161 | 65.963 | 0.2987 | 6.227 | 6.264 | 0.5862 |
| 2 | 40 | 60 | 5 | 64.896 | 64.619 | 0.4267 | 6.460 | 6.511 | 0.7895 |
| 3 | 60 | 90 | 3 | 46.237 | 44.695 | 3.3348 | 9.894 | 10.178 | 2.8704 |
| 4 | 40 | 90 | 7 | 52.878 | 54.420 | 2.9160 | 8.672 | 8.388 | 3.2749 |
| 5 | 60 | 90 | 7 | 65.528 | 66.595 | 1.6289 | 6.344 | 6.148 | 3.0974 |
| 6 | 50 | 120 | 3 | 44.023 | 45.288 | 2.8735 | 10.301 | 10.068 | 2.2619 |
| 7 | 60 | 120 | 5 | 73.751 | 74.028 | 0.3755 | 4.831 | 4.780 | 1.0557 |
| 8 | 50 | 60 | 7 | 62.682 | 61.417 | 2.0181 | 6.867 | 7.100 | 3.3930 |
| 9 | 50 | 90 | 5 | 63.631 | 65.634 | 2.0181 | 6.693 | 6.324 | 5.5132 |
| 10 | 40 | 90 | 3 | 42.125 | 41.058 | 2.5339 | 10.650 | 10.847 | 1.8451 |
| 11 | 60 | 60 | 5 | 72.170 | 72.368 | 0.2738 | 5.122 | 5.086 | 0.7126 |
| 12 | 50 | 60 | 3 | 41.809 | 43.153 | 3.2153 | 10.709 | 10.462 | 2.3111 |
| 13 | 50 | 90 | 5 | 69.640 | 65.634 | 5.7524 | 5.587 | 6.324 | 13.1967 |
| 14 | 50 | 120 | 7 | 63.631 | 62.287 | 2.1125 | 6.693 | 6.941 | 3.6979 |
| 15 | 50 | 90 | 5 | 63.631 | 65.634 | 3.1478 | 6.693 | 6.324 | 5.5087 |
| MAPE (%) | 2.2704 | 3.3410 | |||||||
| Component | Sum of Square | Degree of Freedom | Mean Square | F-Value | p-Value | Remarks |
|---|---|---|---|---|---|---|
| Sequential (Sum of Square) for the FFA Conversion | ||||||
| Mean | 53,138.62 | 1 | 53,138.62 | |||
| Linear | 751.26 | 3 | 250.42 | 2.87 | 0.09 | |
| 2FI | 18.65 | 3 | 6.22 | 0.05 | 0.98 | |
| Quadratic | 903.67 | 3 | 301.22 | 39.48 | 0.0007 | Suggested |
| Cubic | 14.08 | 3 | 4.69 | 0.39 | 0.7758 | Aliased |
| Residual | 24.07 | 2 | 12.04 | |||
| Total | 54,850.36 | 15 | 3656.69 | |||
| Sequential (Sum of Square) for the FFA Content | ||||||
| Mean | 832.43 | 1 | 832.43 | |||
| Linear | 25.44 | 3 | 8.48 | 2.87 | 0.09 | |
| 2FI | 0.63 | 3 | 0.21 | 0.05 | 0.98 | |
| Quadratic | 30.60 | 3 | 10.20 | 39.44 | 0.0007 | Suggested |
| Cubic | 0.48 | 3 | 0.16 | 0.39 | 0.7756 | Aliased |
| Residual | 0.82 | 2 | 0.41 | |||
| Total | 890.40 | 15 | 59.36 | |||
| Component | Standard Deviation | R2 | Adjusted R2 | Predicted R2 | Press | Remarks |
|---|---|---|---|---|---|---|
| Model Summary for the FFA Conversion | ||||||
| Linear | 9.34 | 0.44 | 0.29 | −0.12 | 1921.57 | |
| 2FI | 10.85 | 0.45 | 0.04 | −1.59 | 4446.52 | |
| Quadratic | 2.76 | 0.98 | 0.94 | 0.84 | 279.43 | Suggested |
| Cubic | 3.47 | 0.99 | 0.90 | * | Aliased | |
| Model Summary for the FFA Content | ||||||
| Linear | 1.72 | 0.44 | 0.29 | −0.12 | 65.07 | |
| 2FI | 2.00 | 0.45 | 0.04 | −1.59 | 150.57 | |
| Quadratic | 0.51 | 0.98 | 0.94 | 0.84 | 9.47 | Suggested |
| Cubic | 0.64 | 0.99 | 0.90 | * | Aliased | |
| Source | Sum of Square | DF | Mean Square | F Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 1673.58 | 9 | 185.95 | 24.37 | 0.00 | Significant |
| A Temperature (°C) | 125.03 | 1 | 125.03 | 16.39 | 0.01 | |
| B Reaction Time (min) | 4.51 | 1 | 4.51 | 0.59 | 0.48 | |
| C Catalyst Concentration (%) | 621.72 | 1 | 621.72 | 81.48 | 0.00 | |
| AB | 0.03 | 1 | 0.03 | 0.003 | 0.96 | |
| AC | 18.22 | 1 | 18.22 | 2.39 | 0.18 | |
| BC | 0.40 | 1 | 0.40 | 0.05 | 0.83 | |
| A2 | 4.74 | 1 | 4.74 | 0.62 | 0.47 | |
| B2 | 22.66 | 1 | 22.66 | 2.97 | 0.15 | |
| C2 | 839.11 | 1 | 839.11 | 109.97 | 0.00 | |
| Residual | 38.15 | 5 | 7.63 | |||
| Lack of Fit | 14.08 | 3 | 14.08 | 0.39 | 0.78 | Not Significant |
| Pure Error | 24.08 | 2 | 12.04 | |||
| Cor Total | 1711.73 | 14 | ||||
| Adeq Precision | 14.62 | |||||
| R2 | 0.98 |
| Source | Sum of Square | DF | Mean Square | F Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 56.67 | 9 | 6.30 | 24.35 | 0.00 | Significant |
| X1 | 4.23 | 1 | 4.23 | 16.36 | 0.01 | |
| X2 | 0.15 | 1 | 0.15 | 0.59 | 0.48 | |
| X3 | 21.05 | 1 | 21.05 | 81.41 | 0.00 | |
| X12 | 0.00 | 1 | 0.00 | 0.00 | 0.96 | |
| X13 | 0.62 | 1 | 0.62 | 2.39 | 0.18 | |
| X23 | 0.01 | 1 | 0.01 | 0.05 | 0.83 | |
| X12 | 0.16 | 1 | 0.16 | 0.62 | 0.47 | |
| X22 | 0.77 | 1 | 0.77 | 2.97 | 0.15 | |
| X32 | 28.41 | 1 | 28.41 | 109.88 | 0.00 | |
| Residual | 1.29 | 5 | 0.26 | |||
| Lack of Fit | 0.48 | 3 | 0.16 | 0.39 | 0.78 | Not Significant |
| Pure Error | 0.82 | 2 | 0.41 | |||
| Cor Total | 57.96 | 14 | ||||
| R2 | 0.98 | |||||
| Adeq Precision | 14.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusumaningtyas, R.D.; Prasetiawan, H.; Anggraeni, N.D.; Anisa, E.D.N.; Hartanto, D. Conversion of Free Fatty Acid in Calophyllum inophyllum Oil to Fatty Acid Ester as Precursor of Bio-Based Epoxy Plasticizer via SnCl2–Catalyzed Esterification. Polymers 2023, 15, 123. https://doi.org/10.3390/polym15010123
Kusumaningtyas RD, Prasetiawan H, Anggraeni ND, Anisa EDN, Hartanto D. Conversion of Free Fatty Acid in Calophyllum inophyllum Oil to Fatty Acid Ester as Precursor of Bio-Based Epoxy Plasticizer via SnCl2–Catalyzed Esterification. Polymers. 2023; 15(1):123. https://doi.org/10.3390/polym15010123
Chicago/Turabian StyleKusumaningtyas, Ratna Dewi, Haniif Prasetiawan, Nanda Dwi Anggraeni, Elva Dianis Novi Anisa, and Dhoni Hartanto. 2023. "Conversion of Free Fatty Acid in Calophyllum inophyllum Oil to Fatty Acid Ester as Precursor of Bio-Based Epoxy Plasticizer via SnCl2–Catalyzed Esterification" Polymers 15, no. 1: 123. https://doi.org/10.3390/polym15010123





