Fabrications of Electrospun Mesoporous TiO2 Nanofibers with Various Amounts of PVP and Photocatalytic Properties on Methylene Blue (MB) Photodegradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of TiO2 Nanofibers
2.3. Characterization
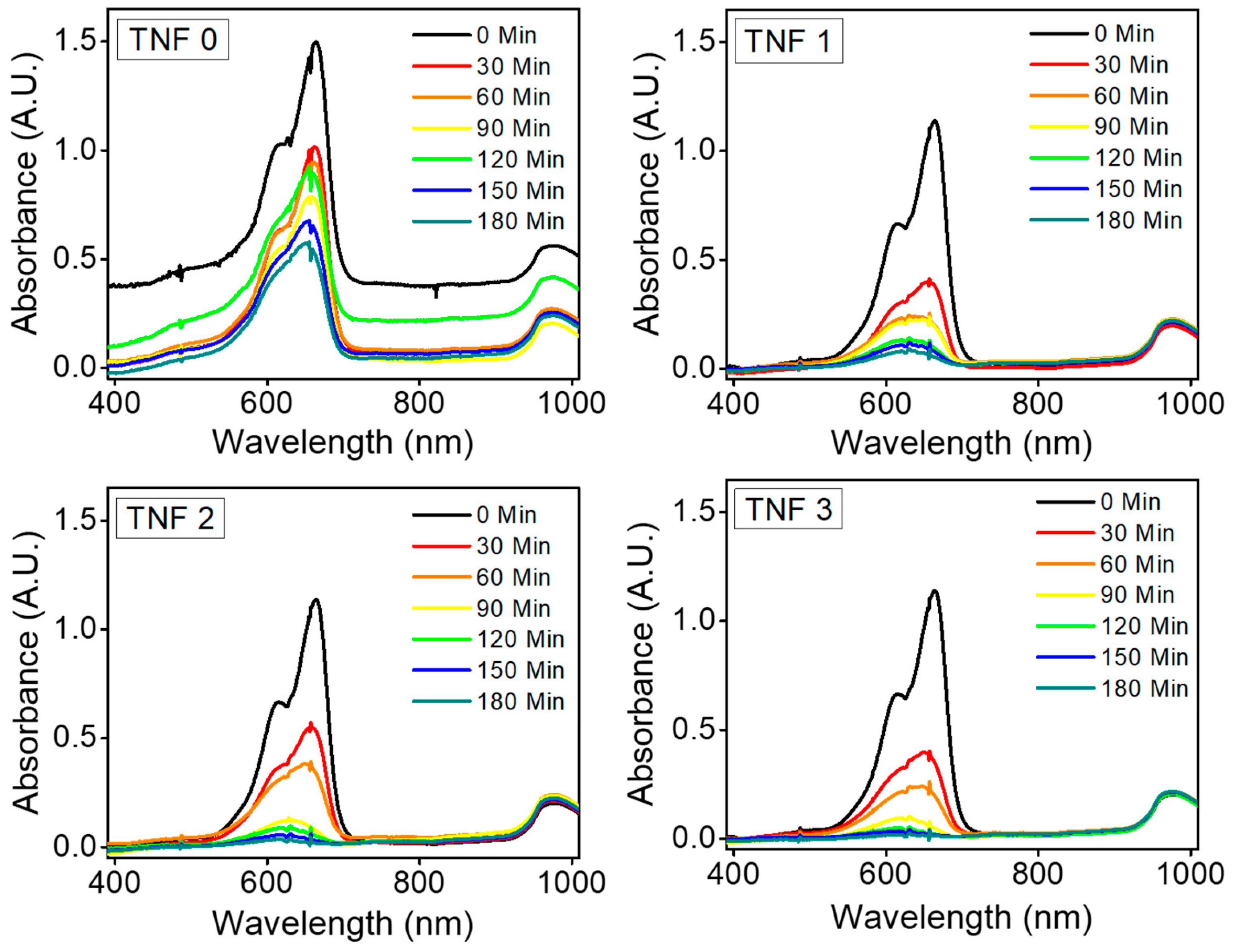
2.4. Photocatalytic MB Degradation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Appannagari, R.R. Environmental pollution causes and consequences: A study. North Asian Int. Res. J. Soc. Sci. Humanit. 2017, 3, 151–161. [Google Scholar]
- McMichael, A.J.; Campbell-Lendrum, D.; Kovats, S.; Edwards, S.; Wilkinson, P.; Wilson, T.; Nicholls, R.; Hales, S.; Tanser, F.; Le Sueur, D. Global climate change. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Due to Selected Major Risk Factors; Ezzati, M., Lopez, A., Rodgers, A., Murray, C., Eds.; WHO: Geneva, Switzerland, 2004; pp. 1543–1649. [Google Scholar]
- Balat, M. Status of fossil energy resources: A global perspective. Energy Sources Part B Econ. Plan. Policy 2007, 2, 31–47. [Google Scholar] [CrossRef]
- Baes, C.; Goeller, H.; Olson, J.; Rotty, R. Carbon Dioxide and Climate: The Uncontrolled Experiment: Possibly severe consequences of growing CO2 release from fossil fuels require a much better understanding of the carbon cycle, climate change, and the resulting impacts on the atmosphere. Am. Sci. 1977, 65, 310–320. [Google Scholar]
- Andres, R.J.; Gregg, J.S.; Losey, L.; Marland, G.; Boden, T.A. Monthly, global emissions of carbon dioxide from fossil fuel consumption. Tellus B Chem. Phys. Meteorol. 2011, 63, 309–327. [Google Scholar] [CrossRef] [Green Version]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Mukhopadhyay, K.; Forssell, O. An empirical investigation of air pollution from fossil fuel combustion and its impact on health in India during 1973–1974 to 1996–1997. Ecol. Econ. 2005, 55, 235–250. [Google Scholar] [CrossRef]
- Haseena, M.; Malik, M.F.; Javed, A.; Arshad, S.; Asif, N.; Zulfiqar, S.; Hanif, J. Water pollution and human health. Environ. Risk Assess. Remediat. 2017, 1, 2529–8046. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Rahman, F.A.; Aziz, M.M.A.; Saidur, R.; Bakar, W.A.W.A.; Hainin, M.R.; Putrajaya, R.; Hassan, N.A. Pollution to solution: Capture and sequestration of carbon dioxide (CO2) and its utilization as a renewable energy source for a sustainable future. Renew. Sustain. Energy Rev. 2017, 71, 112–126. [Google Scholar] [CrossRef]
- McLarnon, C.R.; Duncan, J.L. Testing of Ammonia Based CO2 Capture with Multi-Pollutant Control Technology. Energy Procedia 2009, 1, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Szulczewski, M.L.; MacMinn, C.W.; Herzog, H.J.; Juanes, R. Lifetime of carbon capture and storage as a climate-change mitigation technology. Proc. Natl. Acad. Sci. USA 2012, 109, 5185–5189. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Singh, P.; Raizada, P.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A.; Thakur, V.K. Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: A review. J. Clean. Prod. 2019, 228, 755–769. [Google Scholar] [CrossRef]
- Umar, M.; Aziz, H.A. Photocatalytic degradation of organic pollutants in water. Org. Pollut. Monit. Risk Treat. 2013, 8, 196–197. [Google Scholar]
- Opoku, F.; Govender, K.K.; van Sittert, C.G.C.E.; Govender, P.P. Recent progress in the development of semiconductor-based photocatalyst materials for applications in photocatalytic water splitting and degradation of pollutants. Adv. Sustain. Syst. 2017, 1, 1700006. [Google Scholar] [CrossRef]
- Hariharan, C. Photocatalytic degradation of organic contaminants in water by ZnO nanoparticles: Revisited. Appl. Catal. A Gen. 2006, 304, 55–61. [Google Scholar] [CrossRef]
- Moctezuma, E.; Leyva, E.; Aguilar, C.A.; Luna, R.A.; Montalvo, C. Photocatalytic degradation of paracetamol: Intermediates and total reaction mechanism. J. Hazard. Mater. 2012, 243, 130–138. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Al-Rasheed, R.A. Water treatment by heterogeneous photocatalysis an overview. In Proceedings of the 4th SWCC Acquired Experience Symposium, Jeddah, Saudi Arabia, 7 May 2005; pp. 1–14. [Google Scholar]
- Liu, H.; Wang, C.; Wang, G. Photocatalytic advanced oxidation processes for water treatment: Recent advances and perspective. Chem. Asian J. 2020, 15, 3239–3253. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.S.; Shah, M.; Muhammad, W.; Ahmad, A.; Ullah, M.A.; Nadeem, M.; Abbasi, B.H. Potentials of phyto-fabricated nanoparticles as ecofriendly agents for photocatalytic degradation of toxic dyes and waste water treatment, risk assessment and probable mechanism. J. Indian Chem. Soc. 2021, 98, 100019. [Google Scholar] [CrossRef]
- McIntyre, R.A. Common Nano-Materials and Their Use in Real World Applications. Sci. Prog. 2012, 95, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hamad, S.; Catlow, C.R.A.; Woodley, S.M.; Lago, S.; Mejías, J.A. Structure and Stability of Small TiO2 Nanoparticles. J. Phys. Chem. B 2005, 109, 15741–15748. [Google Scholar] [CrossRef]
- Kumar, A.; Jose, R.; Fujihara, K.; Wang, J.; Ramakrishna, S. Structural and Optical Properties of Electrospun TiO2 Nanofibers. Chem. Mater. 2007, 19, 6536–6542. [Google Scholar] [CrossRef]
- Sasaki, T.; Nakano, S.; Yamauchi, S.; Watanabe, M. Fabrication of Titanium Dioxide Thin Flakes and Their Porous Aggregate. Chem. Mater. 1997, 9, 602–608. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Yuhas, B.D.; Yang, P. ZnO−TiO2 Core−Shell Nanorod/P3HT Solar Cells. J. Phys. Chem. C 2007, 111, 18451–18456. [Google Scholar] [CrossRef]
- Pushpakanth, S.; Srinivasan, B.; Sreedhar, B.; Sastry, T.P. An in situ approach to prepare nanorods of titania–hydroxyapatite (TiO2–HAp) nanocomposite by microwave hydrothermal technique. Mater. Chem. Phys. 2008, 107, 492–498. [Google Scholar] [CrossRef]
- Suwarnkar, M.B.; Dhabbe, R.S.; Kadam, A.N.; Garadkar, K.M. Enhanced photocatalytic activity of Ag doped TiO2 nanoparticles synthesized by a microwave assisted method. Ceram. Int. 2014, 40, 5489–5496. [Google Scholar] [CrossRef]
- Kavan, L.; Kalbáč, M.; Zukalová, M.; Exnar, I.; Lorenzen, V.; Nesper, R.; Graetzel, M. Lithium Storage in Nanostructured TiO2 Made by Hydrothermal Growth. Chem. Mater. 2004, 16, 477–485. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Guo, D.; Yu, L.; Zhang, W. Anodization Fabrication of Highly Ordered TiO2 Nanotubes. J. Phys. Chem. C 2009, 113, 12759–12765. [Google Scholar] [CrossRef]
- Mills, A.; Elliott, N.; Parkin, I.P.; O’Neill, S.A.; Clark, R.J. Novel TiO2 CVD films for semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 2002, 151, 171–179. [Google Scholar] [CrossRef]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Taylor cone and jetting from liquid droplets in electrospinning of nanofibers. J. Appl. Phys. 2001, 90, 4836–4846. [Google Scholar] [CrossRef]
- Krishnappa, R.V.N. Morphological study of electrospunpolycarbonates as a function of the solventand processing voltage. J. Mater. Sci. 2003, 38, 2357–2365. [Google Scholar] [CrossRef]
- Albetran, H.; Dong, Y.; Low, I.M. Characterization and optimization of electrospun TiO2/PVP nanofibers using Taguchi design of experiment method. J. Asian Ceram. Soc. 2015, 3, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Motlak, M.; Hamza, A.M.; Hammed, M.G.; Barakat, N.A.M. Cd-doped TiO2 nanofibers as effective working electrode for the dye sensitized solar cells. Mater. Lett. 2019, 246, 206–209. [Google Scholar] [CrossRef]
- Song, J.; Guan, R.; Xie, M.; Dong, P.; Yang, X.; Zhang, J. Advances in electrospun TiO2 nanofibers: Design, construction, and applications. Chem. Eng. J. 2022, 431, 134343. [Google Scholar] [CrossRef]
- Secundino-Sánchez, O.; Díaz-Reyes, J.; Sánchez-Ramírez, J.F.; Arias-Cerón, J.S.; Galván-Arellano, M.; Vázquez-Cuchillo, O. Controlled synthesis of electrospun TiO2 nanofibers and their photocatalytic application in the decolouration of Remazol Black B azo dye. Catal. Today 2022, 392–393, 13–22. [Google Scholar] [CrossRef]
- Hwang, D.; Jo, S.M.; Kim, D.Y.; Armel, V.; MacFarlane, D.R.; Jang, S.-Y. High-Efficiency, Solid-State, Dye-Sensitized Solar Cells Using Hierarchically Structured TiO2 Nanofibers. ACS Appl. Mater. Interfaces 2011, 3, 1521–1527. [Google Scholar] [CrossRef]
- Bonincontro, D.; Fraschetti, F.; Squarzoni, C.; Mazzocchetti, L.; Maccaferri, E.; Giorgini, L.; Zucchelli, A.; Gualandi, C.; Focarete, M.L.; Albonetti, S. Pd/Au based catalyst immobilization in polymeric nanofibrous membranes via electrospinning for the selective oxidation of 5-hydroxymethylfurfural. Processes 2020, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Zhu, T.; Huang, Z.; Tang, Z.; Feng, L.; Zhang, H.; Li, D.; Xie, Y.; Zhu, C. Preparation of Nanofiber Bundles via Electrospinning Immiscible Polymer Blend for Oil/Water Separation and Air Filtration. Polymers 2022, 14, 4722. [Google Scholar] [CrossRef] [PubMed]
- Maccaferri, E.; Mazzocchetti, L.; Benelli, T.; Brugo, T.M.; Zucchelli, A.; Giorgini, L. Self-Assembled NBR/Nomex Nanofibers as Lightweight Rubbery Nonwovens for Hindering Delamination in Epoxy CFRPs. ACS Appl. Mater. Interfaces 2021, 14, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shi, Q.; Jin, S.; Shao, Y.; Huang, J. Self-assembled core-shell structured organic nanofibers fabricated by single-nozzle electrospinning for highly sensitive ammonia sensors. InfoMat 2019, 1, 525–532. [Google Scholar] [CrossRef]
- Yang, Y.; Wen, J.; Wei, J.; Xiong, R.; Shi, J.; Pan, C. Polypyrrole-Decorated Ag-TiO2 Nanofibers Exhibiting Enhanced Photocatalytic Activity under Visible-Light Illumination. ACS Appl. Mater. Interfaces 2013, 5, 6201–6207. [Google Scholar] [CrossRef]
- Zhang, M.; Shao, C.; Guo, Z.; Zhang, Z.; Mu, J.; Cao, T.; Liu, Y. Hierarchical Nanostructures of Copper(II) Phthalocyanine on Electrospun TiO2 Nanofibers: Controllable Solvothermal-Fabrication and Enhanced Visible Photocatalytic Properties. ACS Appl. Mater. Interfaces 2011, 3, 369–377. [Google Scholar] [CrossRef]
- Wang, H.; Lewis, J.P. Effects of dopant states on photoactivity in carbon-doped TiO2. J. Phys. Condens. Matter 2005, 17, L209–L213. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, J.-H.; Choi, D.-Y.; Hwang, C.-H.; Lee, J.-W. Influence of Fe doping on phase transformation and crystallite growth of electrospun TiO2 nanofibers for photocatalytic reaction. Mater. Lett. 2012, 88, 156–159. [Google Scholar] [CrossRef]
- Hamadanian, M.; Reisi-Vanani, A.; Majedi, A. Sol-gel preparation and characterization of Co/TiO2 nanoparticles: Application to the degradation of methyl orange. J. Iran. Chem. Soc. 2010, 7, S52–S58. [Google Scholar] [CrossRef]
- Zhao, Y.; Nie, L.; Yang, H.; Song, K.; Hou, H. Tailored fabrication of TiO2/In2O3 hybrid mesoporous nanofibers towards enhanced photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127455. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Cheng, B.; Zhao, X.; Zhang, Q. Preparation and photocatalytic activity of mesoporous anatase TiO2 nanofibers by a hydrothermal method. J. Photochem. Photobiol. A Chem. 2006, 182, 121–127. [Google Scholar] [CrossRef]
- Chun, H.-H.; Jo, W.-K. Polymer material-supported titania nanofibers with different polyvinylpyrrolidone to TiO2 ratios for degradation of vaporous trichloroethylene. J. Ind. Eng. Chem. 2014, 20, 1010–1015. [Google Scholar] [CrossRef]
- Nuansing, W.; Ninmuang, S.; Jarernboon, W.; Maensiri, S.; Seraphin, S. Structural characterization and morphology of electrospun TiO2 nanofibers. Mater. Sci. Eng. B 2006, 131, 147–155. [Google Scholar] [CrossRef]
- Jia, M.; Guo, S.; Gao, S.; Wang, Q.; Sun, J. Thermal decomposition mechanism of diisopropyl azodicarboxylate and its thermal hazard assessment. Thermochim. Acta 2020, 688, 178601. [Google Scholar] [CrossRef]
- Coste, G.; Negrell, C.; Caillol, S. From gas release to foam synthesis, the second breath of blowing agents. Eur. Polym. J. 2020, 140, 110029. [Google Scholar] [CrossRef]
- Lim, S.K.; Lee, S.I.; Jang, S.G.; Lee, K.H.; Choi, H.J.; Chin, I.J. Fabrication and physical characterization of biodegradable poly (butylene succinate)/carbon nanofiber nanocomposite foams. J. Macromol. Sci. Part B Phys. 2010, 50, 100–110. [Google Scholar] [CrossRef]
- Pan, W.; Ma, Z.; Liu, J.; Liu, Q.; Wang, J. Effect of heating rate on morphology and structure of CoFe2O4 nanofibers. Mater. Lett. 2011, 65, 3269–3271. [Google Scholar] [CrossRef]
- Thompson, C.J.; Chase, G.G.; Yarin, A.L.; Reneker, D.H. Effects of parameters on nanofiber diameter determined from electrospinning model. Polymer 2007, 48, 6913–6922. [Google Scholar] [CrossRef]
- Dehghan, R.; Barzin, J. Development of a polysulfone membrane with explicit characteristics for separation of low density lipoprotein from blood plasma. Polym. Test. 2020, 85, 106438. [Google Scholar] [CrossRef]
- Someswararao, M.V.; Dubey, R.S.; Subbarao, P.S.V.; Singh, S. Electrospinning process parameters dependent investigation of TiO2 nanofibers. Results Phys. 2018, 11, 223–231. [Google Scholar] [CrossRef]
- Surovčík, J.; Medvecká, V.; Greguš, J.; Gregor, M.; Roch, T.; Annušová, A.; Ďurina, P.; Vojteková, T. Characterization of TiO2 nanofibers with enhanced photocatalytic properties prepared by plasma assisted calcination. Ceram. Int. 2022, 48, 37322–37332. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, L.; He, X.; Li, J.; Wang, D. Controlled fabrication of Ag/TiO2 nanofibers with enhanced stability of photocatalytic activity. J. Mater. Sci. Mater. Electron. 2016, 27, 5190–5196. [Google Scholar] [CrossRef]
- Rahma, A.; Munir, M.M.; Prasetyo, A.; Suendo, V.; Rachmawati, H. Intermolecular interactions and the release pattern of electrospun curcumin-polyvinyl (pyrrolidone) fiber. Biol. Pharm. Bull. 2016, 39, 163–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Wang, G.; Cheng, B.; Zhou, M. Effects of hydrothermal temperature and time on the photocatalytic activity and microstructures of bimodal mesoporous TiO2 powders. Appl. Catal. B Environ. 2007, 69, 171–180. [Google Scholar] [CrossRef]


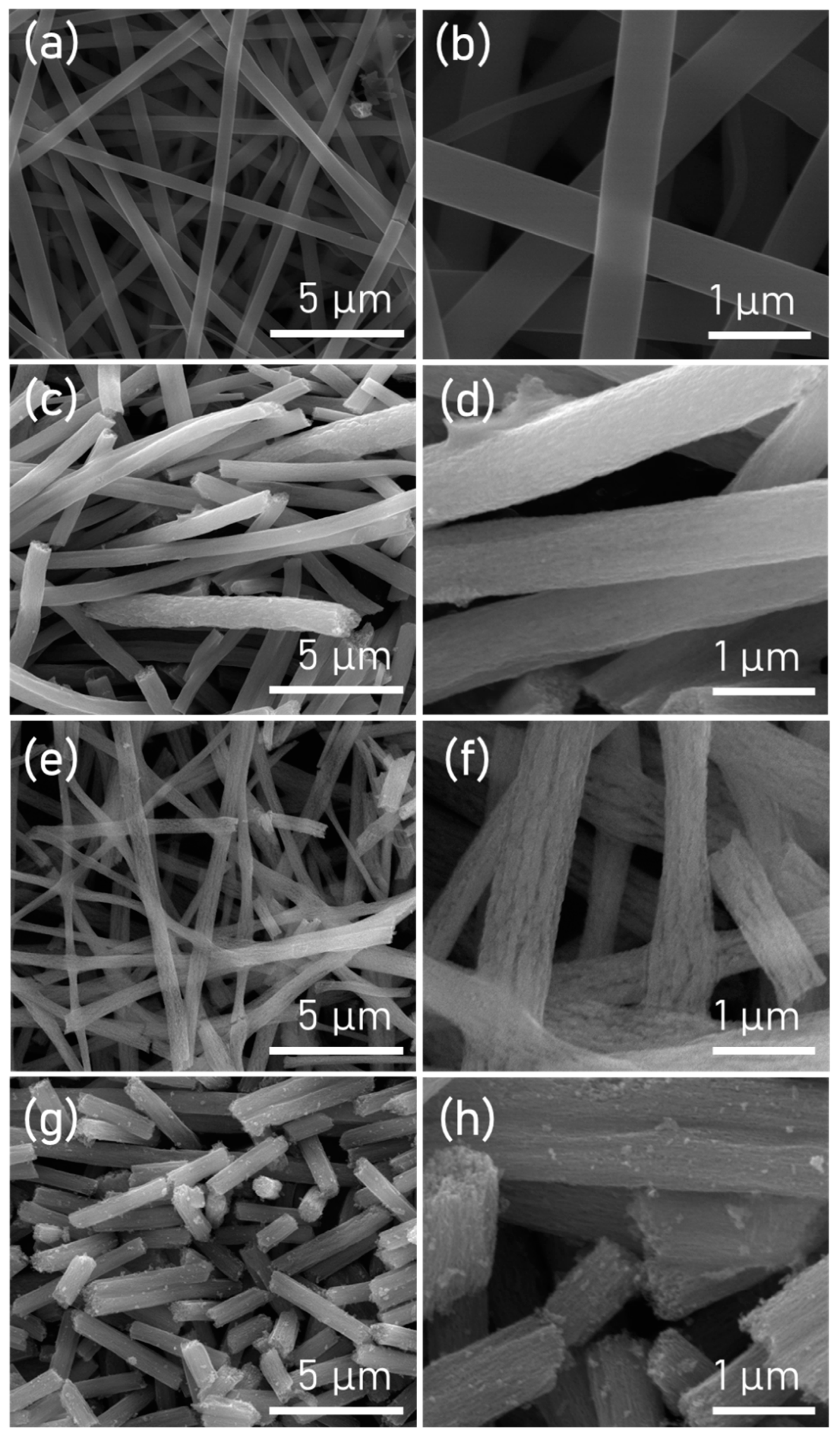

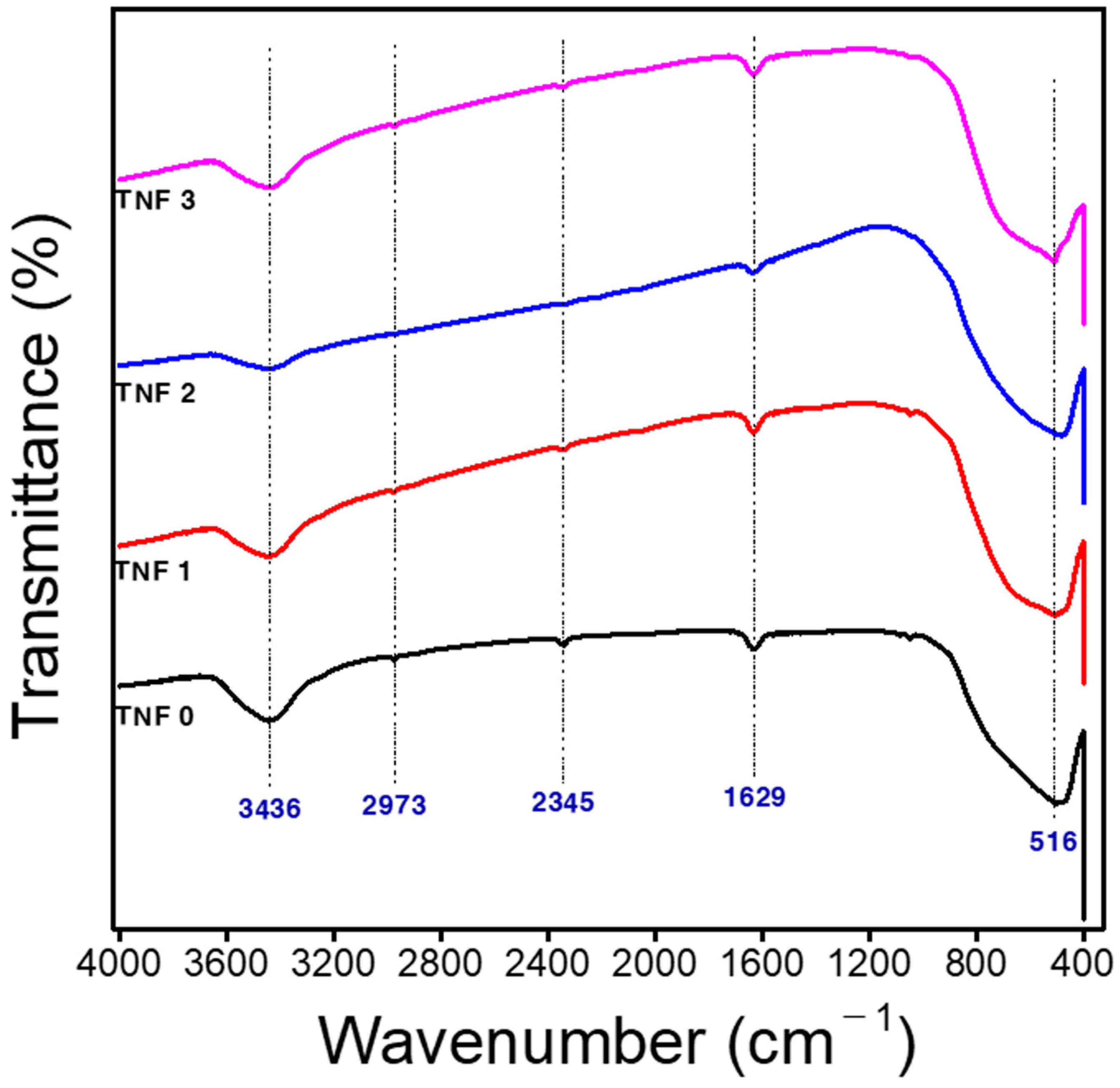
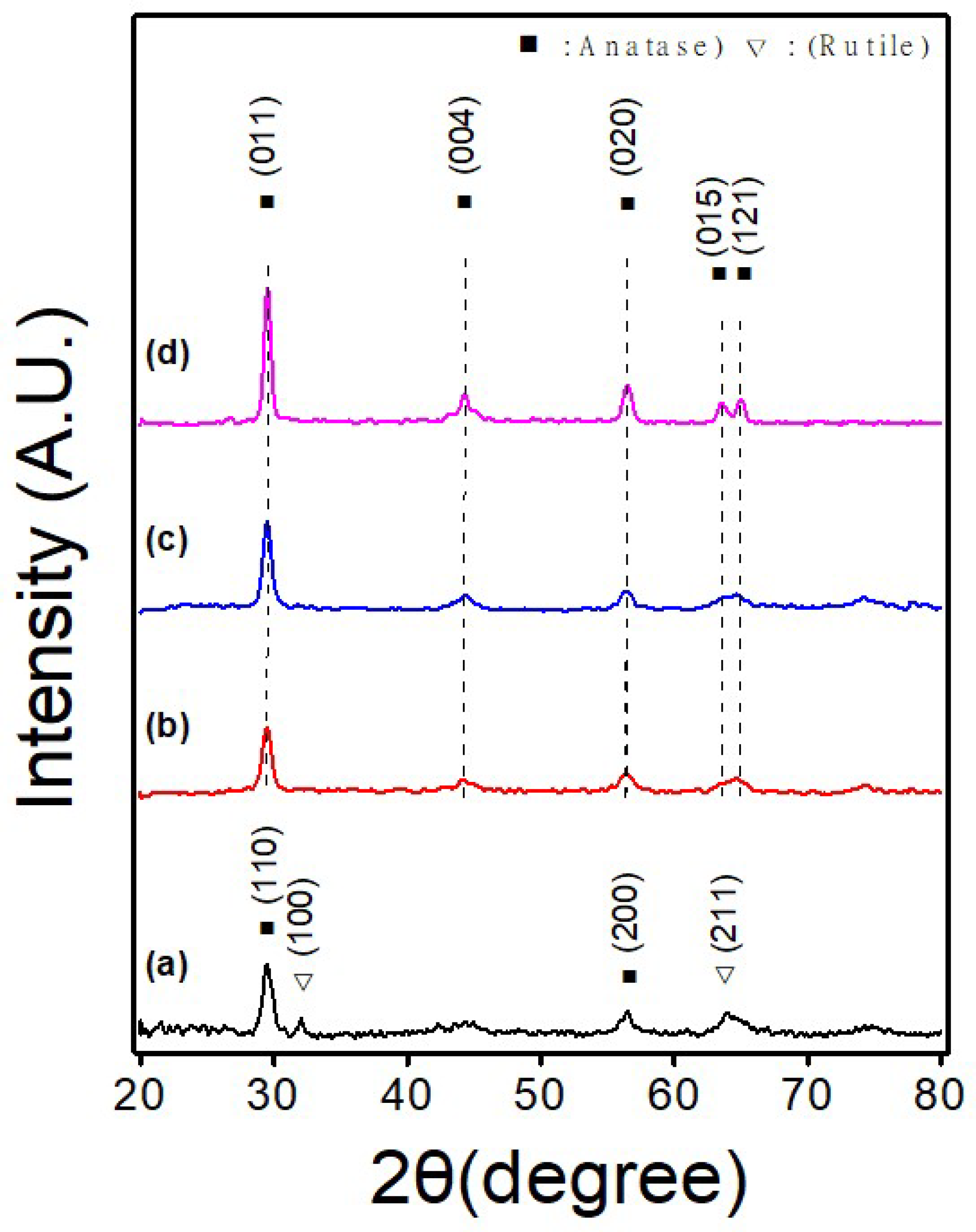


| TNF 0 | TNF 1 | TNF 2 | TNF 3 | |
|---|---|---|---|---|
| K-90: K-30 (g) | 10 g: 0 g (11.76wt%) | 5 g: 5 g (10.53wt%) | 5 g: 5 g (10.53wt%) | 5 g: 5 g (10.53wt%) |
| TTIP (g) | 15 g (17.65wt%) | 15 g (15.79wt%) | 15 g (15.79wt%) | 15 g (15.79wt%) |
| EtOH (g) | 50 g (58.82wt%) | 50 g (52.63wt%) | 50 g (52.63wt%) | 50 g (52.63wt%) |
| ACAC (g) | 10 g (11.76wt%) | 10 g (10.53wt%) | 10 g (10.53wt%) | 10 g (10.53wt%) |
| DIPA (g) | 0 g (0wt%) | 10 g (10.53wt%)) | 10 g (10.53wt%) | 10 g (10.53wt%) |
| Heating rate | 5 °C/min | 5 °C/min | 3 °C/min | 1 °C/min |
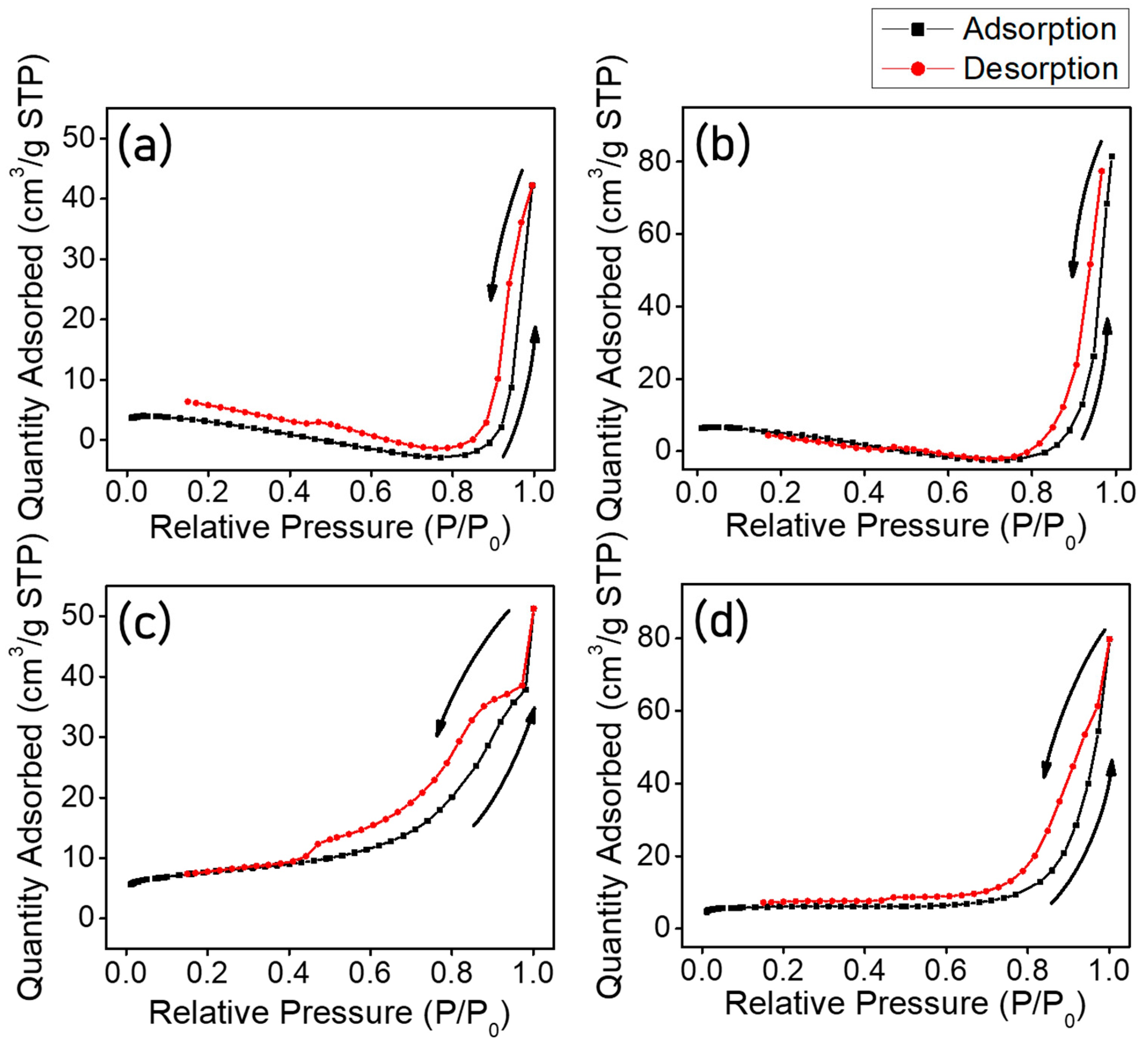
| Sample Name | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | BJH Desorption Average Pore Width (nm) | Micropore Area (m2/g) |
|---|---|---|---|---|
| TNF 0 | 17.2 | 0.05 | 13.37 | 11.4 |
| TNF 1 | 23.8 | 0.09 | 15.64 | 14.4 |
| TNF 2 | 27.4 | 0.06 | 8.7 | 14.0 |
| TNF 3 | 23.5 | 0.09 | 16.2 | 17.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, S.-H.; Yoon, H.-S.; Han, H.; Na, K.-H.; Choi, W.-Y. Fabrications of Electrospun Mesoporous TiO2 Nanofibers with Various Amounts of PVP and Photocatalytic Properties on Methylene Blue (MB) Photodegradation. Polymers 2023, 15, 134. https://doi.org/10.3390/polym15010134
Yoo S-H, Yoon H-S, Han H, Na K-H, Choi W-Y. Fabrications of Electrospun Mesoporous TiO2 Nanofibers with Various Amounts of PVP and Photocatalytic Properties on Methylene Blue (MB) Photodegradation. Polymers. 2023; 15(1):134. https://doi.org/10.3390/polym15010134
Chicago/Turabian StyleYoo, Sun-Ho, Han-Sol Yoon, HyukSu Han, Kyeong-Han Na, and Won-Youl Choi. 2023. "Fabrications of Electrospun Mesoporous TiO2 Nanofibers with Various Amounts of PVP and Photocatalytic Properties on Methylene Blue (MB) Photodegradation" Polymers 15, no. 1: 134. https://doi.org/10.3390/polym15010134







