Synthesis and Characterization of Cellulose Diacetate-Graft-Polylactide via Solvent-Free Melt Ring-Opening Graft Copolymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Synthesis and Purification of CDA−g−PLLA Copolymers
2.2.1. Synthesis of CDA−g−PLLA Copolymers
2.2.2. Purification of CDA−g−PLLA Copolymers
2.3. Characterization
2.3.1. Analysis of Grafting Rate
2.3.2. FTIR
2.3.3. 1H NMR
2.3.4. TGA/DTG
2.3.5. DSC
2.3.6. XRD
3. Results and Discussion
3.1. Influencing Factors of Graft
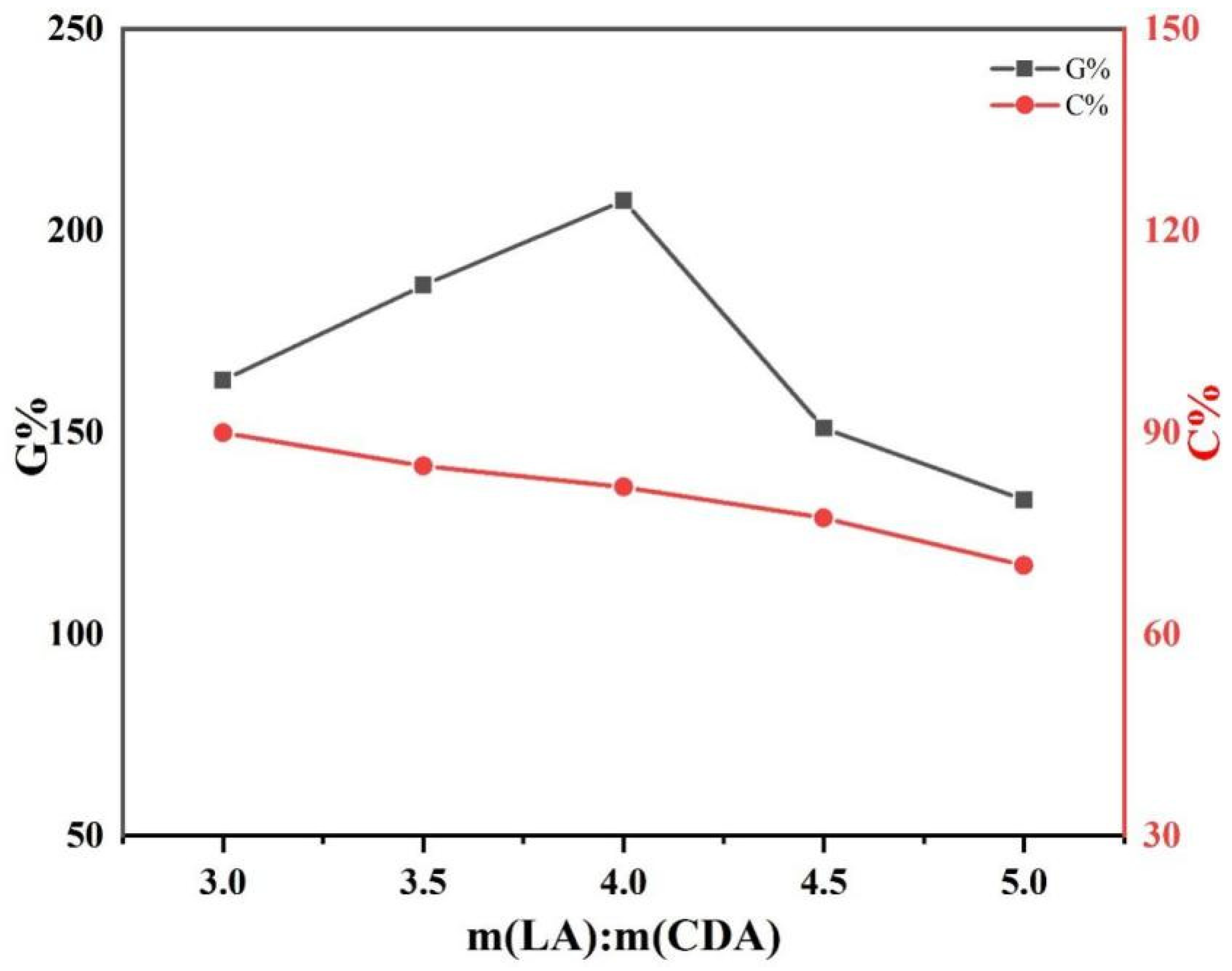
3.1.1. Effect of Feeding Mass Ratio of L-LA/CDA
3.1.2. Effect of Reaction Time
3.1.3. Effect of Reaction Temperature
3.2. FTIR Analysis
3.3. 1H NMR Analysis
3.4. TGA and DTG Analysis
3.5. DSC Analysis
3.6. XRD Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guiao, K.S.; Gupta, A.; Tzoganakis, C.; Mekonnen, T.H. Reactive extrusion as a sustainable alternative for the processing and valorization of biomass components. J. Clean. Prod. 2022, 355, 131840. [Google Scholar] [CrossRef]
- Curran, L.M.C.L.K.; Le Thanh Mai, P.; Sale, K.L.; Simmons, B.A. Review of advances in the development of laccases for the valorization of lignin to enable the production of lignocellulosic biofuels and bioproducts. Biotechnol. Adv. 2022, 54, 107809. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.; Akman, F.; Medimagh, M.; Issaoui, N.; Vasilieva, N.; Malyar, Y.N.; Sudakova, I.G.; Karacharov, A.; Miroshnikova, A.; Al-Dossary, O.M. Sulfation of Diethylaminoethyl-Cellulose: QTAIM Topological Analysis and Experimental and DFT Studies of the Properties. ACS Omega 2021, 6, 22603–22615. [Google Scholar] [CrossRef] [PubMed]
- Cindradewi, A.W.; Bandi, R.; Park, C.-W.; Park, J.-S.; Lee, E.-A.; Kim, J.-K.; Kwon, G.-J.; Han, S.-Y.; Lee, S.-H. Preparation and Characterization of Cellulose Acetate Film Reinforced with Cellulose Nanofibril. Polymers 2021, 13, 2990. [Google Scholar] [CrossRef] [PubMed]
- Wsoo, M.A.; Shahir, S.; Bohari, S.P.M.; Nayan, N.H.M.; Abd Razak, S.I. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohyd. Res. 2020, 491, 107978. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Deshmukh, N.A.; Pinjari, D.V. A critical review of manufacturing processes used in regenerated cellulosic fibres: Viscose, cellulose acetate, cuprammonium, LiCl/DMAc, ionic liquids, and NMMO based lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Qi, J.; Chen, Y.; Zhang, W.-T.; Li, L.; Huang, H.-D.; Lin, H.; Zhong, G.-J.; Li, Z.-M. Imparting Cellulose Acetate Films with Hydrophobicity, High Transparency, and Self-Cleaning Function by Constructing a Slippery Liquid-Infused Porous Surface. Ind. Eng. Chem. Res. 2022, 61, 7962–7970. [Google Scholar] [CrossRef]
- Luan, Y.; Wu, J.; Zhan, M.; Zhang, J.; Zhang, J.; He, J. “One pot” homogeneous synthesis of thermoplastic cellulose acetate-graft-poly(L-lactide) copolymers from unmodified cellulose. Cellulose 2013, 20, 327–337. [Google Scholar] [CrossRef]
- Babar, A.A.; Miao, D.; Ali, N.; Zhao, J.; Wang, X.; Yu, J.; Ding, B. Breathable and Colorful Cellulose Acetate-Based Nanofibrous Membranes for Directional Moisture Transport. ACS. Appl. Mater. Inter. 2018, 10, 22866–22875. [Google Scholar] [CrossRef]
- Du, L.-L.; Jiang, B.-L.; Chen, X.-H.; Wang, Y.-Z.; Zou, L.-M.; Liu, Y.-L.; Gong, Y.-Y.; Wei, C.; Yuan, W.-Z. Clustering-triggered Emission of Cellulose and Its Derivatives. Chin. J. Polym. Sci. 2019, 37, 409–415. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, M.; Liu, Z.; Shi, J.; Huang, F.; Luo, X. Constructing a Continuous Flow Bioreactor Based on a Hierarchically Porous Cellulose Monolith for Ultrafast and Nonstop Enzymatic Esterification/Transesterification. ACS. Sustain. Chem. Eng. 2019, 7, 2056–2063. [Google Scholar] [CrossRef]
- Soyama, M.; Kiuchi, Y.; Iji, M.; Tanaka, S.; Toyama, K. Improvement in Impact Strength of Modified Cardanol-Bonded Cellulose Thermoplastic Resin by Adding Modified Silicones. J. Appl. Polym. Sci. 2014, 131, 40366. [Google Scholar] [CrossRef]
- Hu, S.; Liu, X.; Zhang, M.; Wei, Y.; Qi, R.; Zhu, Y.; Chen, S. Interfacial effects of plasticizers on the properties of cellulose diacetate materials. Cellulose 2022, 29, 7849–7861. [Google Scholar] [CrossRef]
- Boulven, M.; Quintard, G.; Cottaz, A.; Joly, C.; Charlot, A.; Fleury, E. Homogeneous acylation of Cellulose diacetate: Towards bioplastics with tuneable thermal and water transport properties. Carbohyd. Polym. 2019, 206, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.Y.; Long, D.R.; Vergelati, C. Miscibility and dynamical properties of cellulose acetate/plasticizer systems. Carbohy. Polym. 2015, 116, 95–102. [Google Scholar] [CrossRef]
- Vu Thanh, P.; Verstichel, S.; Cinelli, P.; Anguillesi, I.; Coltelli, M.-B.; Lazzeri, A. Cellulose Acetate Blends—Effect of Plasticizers on Properties and Biodegradability. JRenew. Mater. 2014, 2, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-K.; Cho, M.S.; Kim, I.H.; Lee, Y.; Do Nam, J. Preparation and Physical Properties of the Biocomposite, Cellulose Diacetate/Kenaf Fiber Sized with Poly(vinyl alcohol). Macromo. Res. 2010, 18, 566–570. [Google Scholar] [CrossRef]
- Quintana, R.; Persenaire, O.; Bonnaud, L.; Dubois, P. Recent advances in (reactive) melt processing of cellulose acetate and related biodegradable bio-compositions. Polym. Chem. 2012, 3, 591–595. [Google Scholar] [CrossRef]
- Cortina-Puig, M.; Hurtado-Fernandez, E.; Lacorte, S. Plasticizers in Drinking Water and Beverages. Curr. Anal. Chem. 2018, 14, 344–357. [Google Scholar] [CrossRef]
- Ma, Y.; Liao, S.; Li, Q.; Guan, Q.; Jia, P.; Zhou, Y. Physical and chemical modifications of poly(vinyl chloride) materials to prevent plasticizer migration—Still on the run. Reac. Funct. Polym. 2020, 147, 104458. [Google Scholar] [CrossRef]
- Glova, A.D.; Melnikova, S.D.; Mercurieva, A.A.; Larin, S.V.; Lyulin, S.V. Grafting-Induced Structural Ordering of Lactide Chains. Polymers 2019, 11, 2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iji, M.; Toyama, K.; Tanaka, S. Mechanical and other characteristics of cellulose ester bonded with modified cardanol from cashew nut shells and additional aliphatic and aromatic components. Cellulose 2013, 20, 559–569. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Hou, J.; Fang, X.; Bai, F.; Zhu, T.; Gao, F.; Wei, C.; Mo, X.; Lang, M. Synthesis of cellulose diacetate based copolymer electrospun nanofibers for tissues scaffold. Appl. Surf. Sci. 2018, 443, 374–381. [Google Scholar] [CrossRef]
- Chaikeaw, C.; Srikulkit, K. Preparation and Properties of Poly(lactic Acid)/PLA-g-ABS Blends. Fiber. Polym. 2018, 19, 2016–2022. [Google Scholar] [CrossRef]
- Xue, G.L.; Sun, B.H.; Han, L.; Liu, B.C.; Liang, H.Y.; Pu, Y.F.; Tang, H.M.; Ma, F.W. Triblock Copolymer Compatibilizers for Enhancing the Mechanical Properties of a Renewable Bio-Polymer. Polymers 2022, 14, 2734. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, H.; Wang, K.; Xu, H.; He, Y.; Wang, X. Influence of scPLA microsphere on the crystallization behavior of PLLA/PDLA composites. Compos. Commun. 2020, 21, 100380. [Google Scholar] [CrossRef]
- Alisir, S.H.; Ozdemir, N.; Burgaz, E.; Dege, N.; Canavar, Y.E. Fabrication and Antimicrobial Activity of Poly(lactic acid) Nanofibers Containing Firstly Synthesized Silver Diclofenac Complex with (2-methylimidazole) for Wound Dressing Applications. Fiber. Polym. 2021, 22, 2738–2749. [Google Scholar] [CrossRef]
- Chanklom, P.; Kreetachat, T.; Chotigawin, R.; Suwannahong, K. Photocatalytic Oxidation of PLA/TiO2-Composite Films for Indoor Air Purification. ACS. Omega. 2021, 6, 10629–10636. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Aytac, Z.; Pricope, G.M.; Uyar, T.; Vasile, C. Polylactic acid (PLA)/Silver-NP/VitaminE bionanocomposite electrospun nanofibers with antibacterial and antioxidant activity. J. Nanopart. Res. 2014, 16, 2643. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Wang, X.; Ferraris, E.; Zhang, J. Melt crystallization of PLA/Talc in fused filament fabrication. Mater. Design. 2019, 182, 108013. [Google Scholar] [CrossRef]
- Kuo, C.C.; Chen, J.Y.; Chang, Y.H. Optimization of Process Parameters for Fabricating Polylactic Acid Filaments Using Design of Experiments Approach. Polymers 2021, 13, 1222. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.-M.; Yang, T.-T.; Vidovic, E.; Jia, R.-N.; Zhang, J.-M.; Mi, Q.-Y.; Zhang, J. Cellulose Acetate Thermoplastics with High Modulus, Dimensional Stability and Anti-migration Properties by Using CA-g-PLA as Macromolecular Plasticizer. Chinese. J. Polym. Sci. 2020, 38, 1141–1148. [Google Scholar] [CrossRef]
- Bao, J.; Han, L.; Shan, G.; Bao, Y.; Pan, P. Preferential Stereocomplex Crystallization in Enantiomeric Blends of Cellulose Acetate-g-Poly(lactic acid)s with Comb like Topology. J. Phys. Chem. B 2015, 119, 12689–12698. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.-W.; Lai, X.-L.; Jiang, Y.-P.; Yan, C.; Liu, Z.-Y.; Yang, W.; Yang, M.-B. Synthesis of Inorganic Silica Grafted Three-arm PLLA and Their Behaviors for PLA Matrix. Chinese. J. Polym. Sci. 2019, 37, 216–226. [Google Scholar] [CrossRef]
- Teramoto, Y.; Nishio, Y. Cellulose diacetate-graft-poly(lactic acid)s: Synthesis of wide-ranging compositions and their thermal and mechanical properties. Polymer 2003, 44, 2701–2709. [Google Scholar] [CrossRef]







| Sample | MS a | Lactyl DS a | DPs a |
|---|---|---|---|
| CDA−g−PLLA−130 | 13.61 | 0.229 | 59.42 |
| CDA−g−PLLA−140 | 10.12 | 0.234 | 43.24 |
| CDA−g−PLLA−150 | 19.38 | 0.413 | 47.27 |
| CDA−g−PLLA−160 | 13.78 | 0.317 | 43.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Li, J.; Wu, L.; Hua, M.; Jiang, C.; Pan, Y.; Yao, L.; Xu, S.; Ge, J.; Pan, G. Synthesis and Characterization of Cellulose Diacetate-Graft-Polylactide via Solvent-Free Melt Ring-Opening Graft Copolymerization. Polymers 2023, 15, 143. https://doi.org/10.3390/polym15010143
Zhao S, Li J, Wu L, Hua M, Jiang C, Pan Y, Yao L, Xu S, Ge J, Pan G. Synthesis and Characterization of Cellulose Diacetate-Graft-Polylactide via Solvent-Free Melt Ring-Opening Graft Copolymerization. Polymers. 2023; 15(1):143. https://doi.org/10.3390/polym15010143
Chicago/Turabian StyleZhao, Shiyou, Jin Li, Lifeng Wu, Ming Hua, Changmei Jiang, Ying Pan, Lirong Yao, Sijun Xu, Jianlong Ge, and Gangwei Pan. 2023. "Synthesis and Characterization of Cellulose Diacetate-Graft-Polylactide via Solvent-Free Melt Ring-Opening Graft Copolymerization" Polymers 15, no. 1: 143. https://doi.org/10.3390/polym15010143
APA StyleZhao, S., Li, J., Wu, L., Hua, M., Jiang, C., Pan, Y., Yao, L., Xu, S., Ge, J., & Pan, G. (2023). Synthesis and Characterization of Cellulose Diacetate-Graft-Polylactide via Solvent-Free Melt Ring-Opening Graft Copolymerization. Polymers, 15(1), 143. https://doi.org/10.3390/polym15010143






