Recycling Carbon Fiber from Carbon Fiber-Reinforced Polymer and Its Reuse in Photocatalysis: A Review
Abstract
1. Introduction
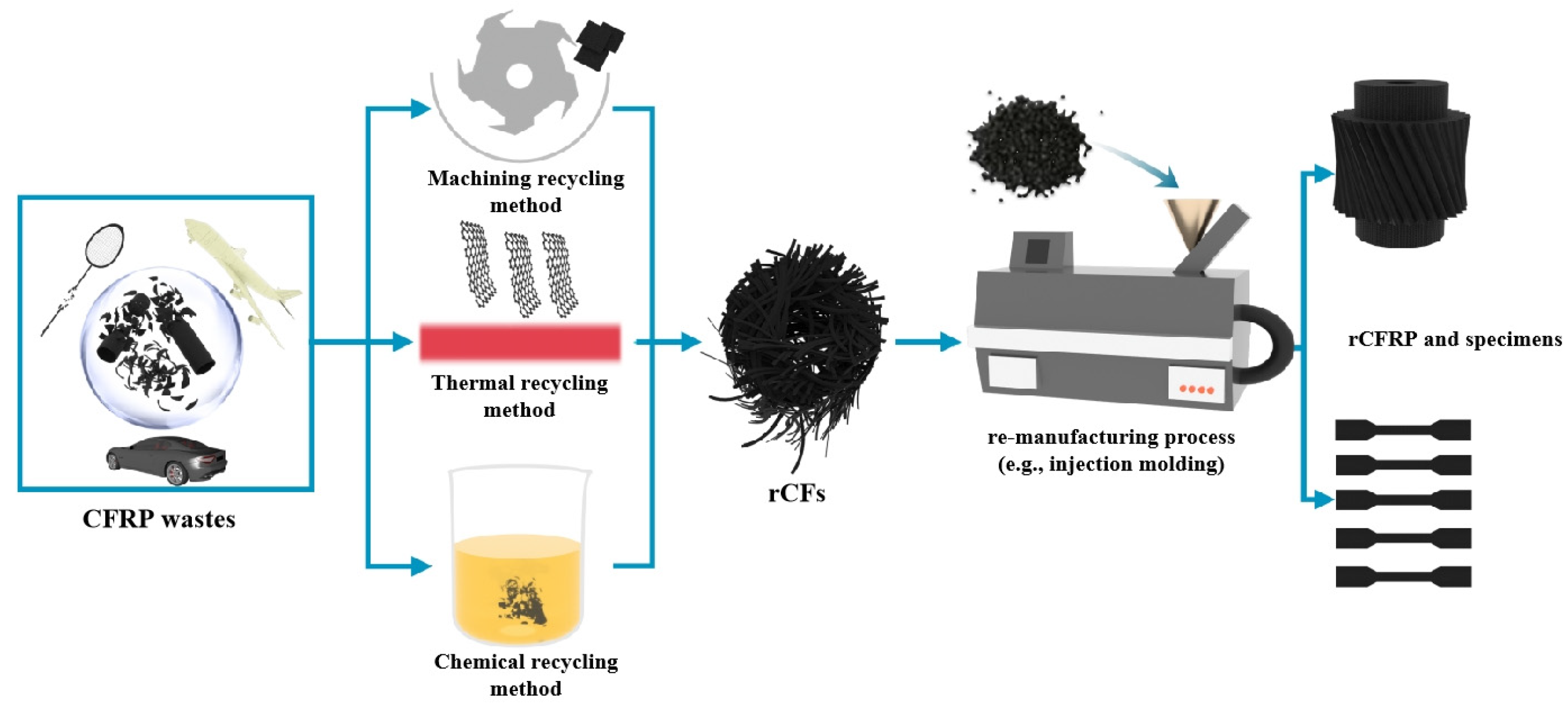
2. Methods of Recycling rCFs from CFRPs
2.1. Machining Recycling Method
2.2. Chemical Recycling Method
2.3. Thermal Recycling Method
2.3.1. Pyrolysis Method
2.3.2. Fluidized Bed Method
3. Recent Optimizations in Recycling Methods
3.1. Some Optimizations in the Machining Recycling Method
3.2. Pretreatment in a Chemical Recycling Method
3.3. Some Optimizations in the Pyrolysis Recycling Method
3.3.1. Superheated Steam Method
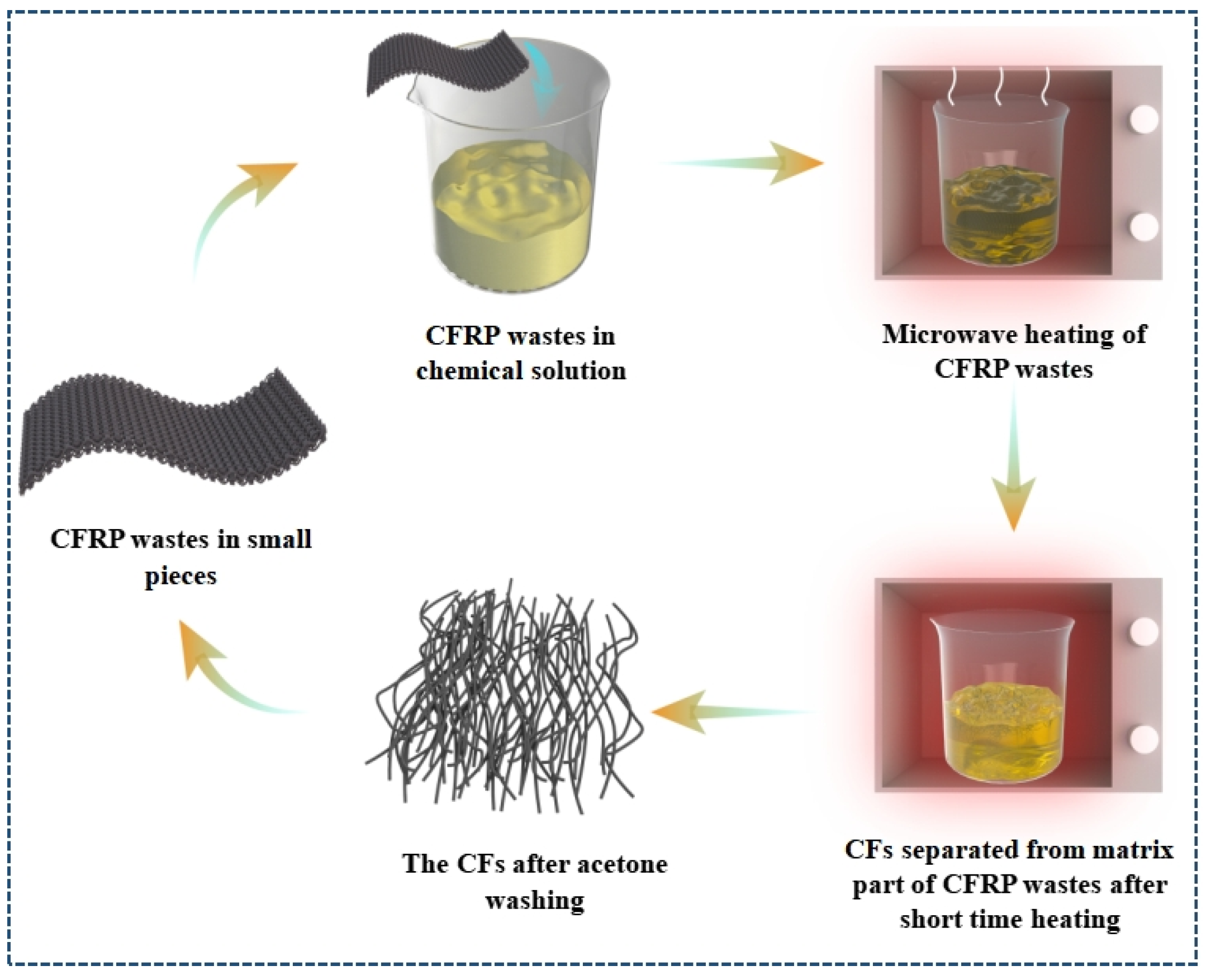
3.3.2. Microwave-Assisted Pyrolysis
3.4. Other Optimizations in the Recycling Methods
4. Reuse of rCFs in Photocatalysis
4.1. Degradation of Dyes
4.2. Degradation of Emerging Pollutants
4.3. Antimicrobial Application
4.4. Degradation of Volatile Pollutants
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Giorgini, L.; Benelli, T.; Brancolini, G.; Mazzocchetti, L. Recycling of carbon fiber reinforced composite waste to close their life cycle in a cradle-to-cradle approach. Curr. Opin. Green Sustain. Chem. 2020, 26, 100368. [Google Scholar] [CrossRef]
- Galvez, P.; Abenojar, J.; Martinez, M.A. Effect of moisture and temperature on the thermal and mechanical properties of a ductile epoxy adhesive for use in steel structures reinforced with CFRP. Compos. Part B 2019, 176, 107194. [Google Scholar] [CrossRef]
- Koumoulos, E.P.; Trompeta, A.F.; Santos, R.M.; Martins, M.; Santos, C.M.D.; Iglesias, V.; Böhm, R.; Gong, G.; Chiminelli, A.; Verpoest, I. Research and development in carbon fibers and advanced high-performance composites supply chain in Europe: A roadmap for challenges and the industrial uptake. J. Compos. Sci. 2019, 3, 86. [Google Scholar] [CrossRef]
- Xiong, Z.; Wei, W.; Liu, F.; Cui, C.; Li, L.; Zou, R.; Zeng, Y. Bond behaviou of recycled aggregate concrete with basalt fibre-reinforced polymer bars. Compos. Struct. 2021, 256, 113078. [Google Scholar] [CrossRef]
- He, D.; Soo, V.K.; Kim, H.C.; Compston, P.; Doolan, M. Comparative life cycle energy analysis of carbon fibre pre-processing, processing and post-processing recycling methods. Resour. Conserv. Recycl. 2020, 158, 104794. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.; Nguyen, T.T.; Wu, J.; Guo, M.; Liu, W.; Du, C. Green and low-cost natural lignocellulosic biomass-based carbon fibers-Processing, properties, and applications in sports equipment: A review. Polymers 2022, 14, 2591. [Google Scholar] [CrossRef] [PubMed]
- Soltani, A.; Noroozi, R.; Bodaghi, M.; Zolfagharian, A.; Hedayati, R. 3D printing on-water sports boards with bio-inspired core designs. Polymers 2020, 12, 250. [Google Scholar] [CrossRef]
- Al Rashid, A.; Khalid, M.Y.; Imran, R.; Ali, U.; Koc, M. Utilization of banana fiber-reinforced hybrid composites in the sports industry. Materials 2020, 13, 3167. [Google Scholar] [CrossRef]
- Wang, F. Application of new carbon fiber material in sports equipment. Carpathian J. Earth Environ. Sci. 2021, 14, 032064. [Google Scholar] [CrossRef]
- Tang, D.Z. The application of carbon fiber materials in sports equipment. Mater. Phys. Mech. 2013, 443, 613–616. [Google Scholar] [CrossRef]
- Kai, Y. Study of biosafety of nanomaterials in sports engineering. Mater. Phys. Mech. 2013, 12, 348–352. [Google Scholar] [CrossRef]
- Naqvi, S.; Prabhakara, H.M.; Bramer, E.; Dierkes, W.; Akkerman, R.; Brem, G. A critical review on recycling of end-of-life carbon fibre/glass fibre reinforced composites waste using pyrolysis towards a circular economy. Resour. Conserv. Recycl. 2018, 136, 118–129. [Google Scholar] [CrossRef]
- Meng, F.; Olivetti, E.A.; Zhao, Y.; Chang, J.C.; Pickering, S.J.; McKechnie, J. Comparing life cycle energy and global warming potential of carbon fiber composite recycling technologies and waste management options. ACS Sustain. Chem. Eng. 2018, 6, 9854–9865. [Google Scholar] [CrossRef]
- Zhang, J.; Chevali, V.S.; Wang, H.; Wang, C.H. Current status of carbon fibre and carbon fibre composites recycling. Compos. Part B 2020, 193, 108053. [Google Scholar] [CrossRef]
- Vo Dong, P.A.; Azzaro-Pantel, C.; Cadene, A.L. Economic and environmental assessment of recovery and disposal pathways for CFRP waste management. Resour. Conserv. Recycl. 2018, 133, 63–75. [Google Scholar] [CrossRef]
- Pakdel, E.; Kashi, S.; Varley, R.; Wang, X. Recent progress in recycling carbon fibre reinforced composites and dry carbon fibre wastes. Resour. Conserv. Recycl. 2021, 166, 105340. [Google Scholar] [CrossRef]
- Pimenta, S.; Pinho, S.T. Recycling carbon fibre reinforced polymers for structural applications: Technology review and market outlook. Waste Manag. 2011, 31, 378–392. [Google Scholar] [CrossRef]
- Saburow, O.; Huether, J.; Märtens, R.; Trauth, A.; Kechaou, Y.; Henning, F.; Weidenmann, K.A. A direct process to reuse dry fiber production waste for recycled carbon fiber bulk molding compounds. Procedia CIRP 2017, 66, 265–270. [Google Scholar] [CrossRef]
- Pickering, S.J. Recycling technologies for thermoset composite materials-current status. Compos. Part A 2006, 37, 1206–1215. [Google Scholar] [CrossRef]
- Meng, F.; McKechnie, J.; Turner, T.; Wong, K.H.; Pickering, S.J. Environmental aspects of use of recycled carbon fiber composites in automotive applications. Environ. Sci. Technol. 2017, 51, 12727–12736. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current status of recycling of fibre reinforced polymers: Review of technologies, reuse and resulting properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef]
- Khalil, Y. Comparative environmental and human health evaluations of thermolysis and solvolysis recycling technologies of carbon fiber reinforced polymer waste. Waste Manag. 2018, 76, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Howarth, J.; Mareddy, S.S.; Mativenga, P.T. Energy intensity and environmental analysis of mechanical recycling of carbon fibre composite. J. Clean. Prod. 2014, 81, 46–50. [Google Scholar] [CrossRef]
- Duflou, J.R.; Deng, Y.; Van Acker, K.; Dewulf, W. Do fiber-reinforced polymer composites provide environmentally benign alternatives? A life-cycle-assessment-based study. MRS Bull. 2012, 37, 374–382. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Ge, H.; Yang, Y.; Wang, Y.; Zhang, C.; Li, J.; Deng, T.; Qin, Z.; Hou, X. Chemical recycling of carbon fiber reinforced epoxy resin composites via selective cleavage of the carbon-nitrogen bond. ACS Sustain. Chem. Eng. 2015, 3, 3332–3337. [Google Scholar] [CrossRef]
- Hadi, P.; Ning, C.; Ouyang, W.; Xu, M.; Lin, C.S.; McKay, G. Toward environmentally-benign utilization of nonmetallic fraction of waste printed circuit boards as modifier and precursor. Waste Manag. 2015, 35, 236–246. [Google Scholar] [CrossRef]
- Pillain, B.; Loubet, P.; Pestalozzi, F.; Woidasky, J.; Erriguible, A.; Aymonier, C.; Sonnemann, G. Positioning supercritical solvolysis among innovative recycling and current waste management scenarios for carbon fiber reinforced plastics thanks to comparative life cycle assessment. J. Supercrit. Fluids 2019, 154, 104607. [Google Scholar] [CrossRef]
- Nunes, A.O.; Viana, L.R.; Guineheuc, P.M.; Da Silva Moris, V.A.; De Paiva, J.M.F.; Barna, R.; Soudais, Y. Life cycle assessment of a steam thermolysis process to recover carbon fibers from carbon fiber-reinforced polymer waste. Int. J. Life Cycle Assess. 2018, 23, 1825–1838. [Google Scholar] [CrossRef]
- Gentil, E.C.; Damgaard, A.; Hauschild, M.; Finnveden, G.; Eriksson, O.; Thorneloe, S.; Kaplan, P.O.; Barlaz, M.; Muller, O.; Matsui, Y. Models for waste life cycle assessment: Review of technical assumptions. Waste Manag. 2010, 30, 2636–2648. [Google Scholar] [CrossRef]
- Yu, K.; Shi, Q.; Dunn, M.L.; Wang, T.; Qi, H.J. Carbon fiber reinforced thermoset composite with near 100% recyclability. Adv. Funct. Mater. 2016, 26, 6098–6106. [Google Scholar] [CrossRef]
- Scott, T.F.; Schneider, A.D.; Cook, W.D.; Bowman, C.N. Photoinduced plasticity in cross-linked polymers. Science 2005, 308, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-catalyzed transesterification for healing and assembling of thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Taynton, P.; Zhang, W.; Dunn, M.L.; Qi, H.J. Influence of stoichiometry on the glass transition and bond exchange reactions in epoxy thermoset polymers. RSC Adv. 2014, 4, 48682–48690. [Google Scholar] [CrossRef]
- Ogi, K.; Nishikawa, T.; Okano, Y.; Taketa, I. Mechanical properties of ABS resin reinforced with recycled CFRP. Adv. Compos. Mater. 2007, 16, 181–194. [Google Scholar] [CrossRef]
- Kouparitsas, C.; Kartalis, C.; Varelidis, P.; Tsenoglou, C.; Papaspyrides, C. Recycling of the fibrous fraction of reinforced thermoset composites. Polym. Compos. 2002, 23, 682–689. [Google Scholar] [CrossRef]
- Palmer, J.; Ghita, O.R.; Savage, L.; Evans, K.E. Successful closed-loop recycling of thermoset composites. Compos. Part A 2009, 40, 490–498. [Google Scholar] [CrossRef]
- Conroy, A.; Halliwell, S.; Reynolds, T. Composite recycling in the construction industry. Compos. Part A 2006, 37, 1216–1222. [Google Scholar] [CrossRef]
- Butenegro, J.A.; Bahrami, M.; Abenojar, J.; Martínez, M.Á. Recent progress in carbon fiber reinforced polymers recycling: A review of recycling methods and reuse of carbon fibers. Materials 2021, 14, 6401. [Google Scholar] [CrossRef]
- Roux, M.; Eguémann, N.; Dransfeld, C.; Thiébaud, F.; Perreux, D. Thermoplastic carbon fibre-reinforced polymer recycling with electrodynamical fragmentation: From cradle to cradle. J. Thermoplast. Compos. 2017, 30, 381–403. [Google Scholar] [CrossRef]
- Yamamoto, T.; Makino, Y.; Uematsu, K. Improved mechanical properties of PMMA composites: Dispersion, diffusion and surface adhesion of recycled carbon fiber fillers from CFRP with adsorbed particulate PMMA. Adv. Powder Technol. 2017, 28, 2774–2778. [Google Scholar] [CrossRef]
- Okayasu, M.; Yamazaki, T.; Ota, K.; Ogi, K.; Shiraishi, T. Mechanical properties and failure characteristics of a recycled CFRP under tensile and cyclic loading. Int. J. Fatigue 2013, 55, 257–267. [Google Scholar] [CrossRef]
- Piñero-Hernanz, R.; García-Serna, J.; Dodds, C.; Hyde, J.; Poliakoff, M.; Cocero, M.J.; Kingman, S.; Pickering, S.; Lester, E. Chemical recycling of carbon fibre composites using alcohols under subcritical and supercritical conditions. J. Supercrit. Fluids 2008, 46, 83–92. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Jiang, Z.; Tang, T. Chemical recycling of carbon fibre reinforced epoxy resin composites in subcritical water: Synergistic effect of phenol and KOH on the decomposition efficiency. Polym. Degrad. Stab. 2012, 97, 214–220. [Google Scholar] [CrossRef]
- Kim, Y.N.; Kim, Y.O.; Kim, S.Y.; Park, M.; Yang, B.; Kim, J.; Jung, Y.C. Application of supercritical water for green recycling of epoxy-based carbon fiber reinforced plastic. Compos. Sci. Technol. 2019, 173, 66–72. [Google Scholar] [CrossRef]
- Okajima, I.; Hiramatsu, M.; Sako, T. Recycling of carbon fiber reinforced plastics using subcritical water. Adv. Mater. Res. 2011, 34, 243–246. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.; Feng, L. Chemical recycling of carbon fibers reinforced epoxy resin composites in oxygen in supercritical water. Mater. Design 2010, 31, 999–1002. [Google Scholar] [CrossRef]
- Das, M.; Chacko, R.; Varughese, S. An efficient method of recycling of CFRP waste using peracetic acid. ACS Sustain. Chem. Eng. 2018, 6, 1564–1571. [Google Scholar] [CrossRef]
- Nahil, M.A.; Williams, P.T. Recycling of carbon fibre reinforced polymeric waste for the production of activated carbon fibres. J. Anal. Appl. Pyrolysis 2011, 91, 67–75. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Liu, W.; Wang, J.; Tang, T. Recycling of carbon fibre reinforced epoxy resin composites under various oxygen concentrations in nitrogen–oxygen atmosphere. J. Anal. Appl. Pyrolysis. 2015, 112, 253–261. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Ding, J. Chemical recycling of carbon fibre/epoxy composites in a mixed solution of peroxide hydrogen and N,N-dimethylformamide. Compos. Sci. Technol. 2013, 82, 54–59. [Google Scholar] [CrossRef]
- Lester, E.; Kingman, S.; Wong, K.H.; Rudd, C.; Pickering, S.; Hilal, N. Microwave heating as a means for carbon fibre recovery from polymer composites: A technical feasibility study. Mater. Res. Bull. 2004, 39, 1549–1556. [Google Scholar] [CrossRef]
- Jiang, L.; Ulven, C.A.; Gutschmidt, D.; Anderson, M.; Balo, S.; Lee, M.; Vigness, J. Recycling carbon fiber composites using microwave irradiation: Reinforcement study of the recycled fiber in new composites. J. Appl. Polym. Sci. 2015, 132, 41. [Google Scholar] [CrossRef]
- Job, S. Recycling glass fibre reinforced composites–history and progress. Reinf. Plast. 2013, 57, 19–23. [Google Scholar] [CrossRef]
- Kao, C.; Ghita, O.; Hallam, K.; Heard, P.; Evans, K. Mechanical studies of single glass fibres recycled from hydrolysis process using sub-critical water. Compos. Part A 2012, 43, 398–406. [Google Scholar] [CrossRef]
- Yan, H.; Lu, C.X.; Jing, D.Q.; Chang, C.B.; Liu, N.X.; Hou, X.L. Recycling of carbon fibers in epoxy resin composites using supercritical 1-propanol. New Carbon Mater. 2016, 31, 46–54. [Google Scholar] [CrossRef]
- Mazzocchetti, L.; Benelli, T.; D’Angelo, E.; Leonardi, C.; Zattini, G.; Giorgini, L. Validation of carbon fibers recycling by pyro-gasification: The influence of oxidation conditions to obtain clean fibers and promote fiber/matrix adhesion in epoxy composites. Compos. Part A 2018, 112, 504–514. [Google Scholar] [CrossRef]
- Badrul, H.M.M.; Nitsche, S.; Abdkader, A.; Cherif, C. Influence of process parameters on the tensile properties of DREF-3000 friction spun hybrid yarns consisting of waste staple carbon fiber for thermoplastic composites. Text. Res. J. 2019, 89, 32–42. [Google Scholar] [CrossRef]
- Zhang, M.; Qi, Y.; Zhang, Z. AgBr/BiOBr nano-heterostructure-decorated polyacrylonitrile nanofibers: A recyclable high-performance photocatalyst for dye degradation under visible-light irradiation. Polymers 2019, 11, 1718. [Google Scholar] [CrossRef]
- Zhou, T.T.; Zhao, F.H.; Cui, Y.Q.; Chen, L.X.; Yan, J.S.; Wang, X.X.; Long, Y.Z. Flexible TiO2/PVDF/g-C3N4 nanocomposite with excellent light photocatalytic performance. Polymers 2019, 12, 55. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Ma, L.; Xiang, C.; Li, L. TiO2-doped chitosan microspheres supported on cellulose acetate fibers for adsorption and photocatalytic degradation of methyl orange. Polymers 2019, 11, 1293. [Google Scholar] [CrossRef] [PubMed]
- Witik, R.A.; Teuscher, R.; Michaud, V.; Ludwig, C.; Månson, J.A.E. Carbon fibre reinforced composite waste: An environmental assessment of recycling, energy recovery and landfilling. Compos. Part A 2013, 49, 89–99. [Google Scholar] [CrossRef]
- Hagnell, M.; Åkermo, M. The economic and mechanical potential of closed loop material usage and recycling of fibre-reinforced composite materials. J. Clean. Prod. 2019, 223, 957–968. [Google Scholar] [CrossRef]
- Liu, Y.; Farnsworth, M.; Tiwari, A. A review of optimisation techniques used in the composite recycling area: State-of-the-art and steps towards a research agenda. J. Clean. Prod. 2017, 140, 1775–1781. [Google Scholar] [CrossRef]
- Wang, S.; Xing, X.; Zhang, X.; Wang, X.; Jing, X. Room-temperature fully recyclable carbon fibre reinforced phenolic composites through dynamic covalent boronic ester bonds. J. Mater. Chem. A 2018, 6, 10868–10878. [Google Scholar] [CrossRef]
- Thomas, C.; Borges, P.; Panzera, T.; Cimentada, A.; Lombillo, I. Epoxy composites containing CFRP powder wastes. Compos. Part B 2014, 59, 260–268. [Google Scholar] [CrossRef]
- Colucci, G.; Ostrovskaya, O.; Frache, A.; Martorana, B.; Badini, C. The effect of mechanical recycling on the microstructure and properties of PA66 composites reinforced with carbon fibers. J. Appl. Polym. Sci. 2015, 132, 29. [Google Scholar] [CrossRef]
- Rajak, D.K.; Pagar, D.D.; Menezes, P.L.; Linul, E. Fiber-reinforced polymer composites: Manufacturing, properties, and applications. Polymers 2019, 11, 1667. [Google Scholar] [CrossRef]
- Rani, M.; Choudhary, P.; Krishnan, V.; Zafar, S. A review on recycling and reuse methods for carbon fiber/glass fiber composites waste from wind turbine blades. Compos. Part B 2021, 215, 108768. [Google Scholar] [CrossRef]
- Oliveux, G.; Bailleul, J.L.; La Salle, E.L.G. Chemical recycling of glass fibre reinforced composites using subcritical water. Compos. Part A 2012, 43, 1809–1818. [Google Scholar] [CrossRef]
- Asmatulu, E.; Twomey, J.; Overcash, M. Recycling of fiber-reinforced composites and direct structural composite recycling concept. J. Compos. Mater. 2014, 48, 593–608. [Google Scholar] [CrossRef]
- Bledzki, A.; Spaude, R.; Ehrenstein, G. Corrosion phenomena in glass fibers and glass fiber reinforced thermosetting resins. Sustain. Prod. Consum. 1985, 23, 263–285. [Google Scholar] [CrossRef]
- Khalil, Y. Sustainability assessment of solvolysis using supercritical fluids for carbon fiber reinforced polymers waste management. Sustain. Prod. Consum. 2019, 17, 74–84. [Google Scholar] [CrossRef]
- Henry, L.; Schneller, A.; Doerfler, J.; Mueller, W.M.; Aymonier, C.; Horn, S. Semi-continuous flow recycling method for carbon fibre reinforced thermoset polymers by near-and supercritical solvolysis. Polym. Degrad. Stab. 2016, 133, 264–274. [Google Scholar] [CrossRef]
- Ferrari, F.; Carallo, G.A.; Greco, A. Innovative closed-loop recyclable bio-based composites from epoxidized waste flour and recycled carbon fibers. Polymers 2022, 14, 3878. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Jones, N.; Williams, P.T. Recycling of fibre-reinforced polymeric waste by pyrolysis: Thermo-gravimetric and bench-scale investigations. J. Anal. Appl. Pyrolysis 2003, 70, 315–338. [Google Scholar] [CrossRef]
- Jiang, G.; Pickering, S.; Walker, G.; Bowering, N.; Wong, K.; Rudd, C. Soft ionisation analysis of evolved gas for oxidative decomposition of an epoxy resin/carbon fibre composite. Thermochim. Acta 2007, 454, 109–115. [Google Scholar] [CrossRef]
- Yip, H.; Pickering, S.; Rudd, C. Characterisation of carbon fibres recycled from scrap composites using fluidised bed process. Plast. Rubber Compos. 2002, 31, 278–282. [Google Scholar] [CrossRef]
- Giorgini, L.; Benelli, T.; Leonardi, C.; Mazzocchetti, L.; Zattini, G.; Cavazzoni, M.; Montanari, I.; Tosi, C. Efficient recovery of non-shredded tires via pyrolysis in an innovative pilot plant. Environ. Eng. Manag. J. 2015, 14, 1611–1622. [Google Scholar] [CrossRef]
- Neri, E.; Passarini, F.; Vassura, I.; Berti, B.; Giorgini, L.; Zattini, G.; Tosi, C.; Cavazzoni, M. Application of LCA methodology in the assessment of a pyrolysis process for tyres recycling. Environ. Eng. Manag. J. 2018, 17, 2437–2445. [Google Scholar]
- Mazzocchetti, L.; Benelli, T.; Zattini, G.; Maccaferri, E.; Brancolini, G.; Giorgini, L. Evaluation of carbon fiber structure and morphology after their recycling via pyro-gassification of CFRPs. AIP Conf. Proc. 2019, 2196, 020036. [Google Scholar]
- Meyer, L.O.; Schulte, K.; Grove-Nielsen, E. CFRP-recycling following a pyrolysis route: Process optimization and potentials. J. Compos. Mater. 2009, 43, 1121–1132. [Google Scholar] [CrossRef]
- Meng, F.; McKechnie, J.; Pickering, S.J. An assessment of financial viability of recycled carbon fibre in automotive applications. J. Compos. Part A 2018, 109, 207–220. [Google Scholar] [CrossRef]
- Pickering, S.; Kelly, R.; Kennerley, J.; Rudd, C.; Fenwick, N. A fluidised-bed process for the recovery of glass fibres from scrap thermoset composites. Compos. Sci. Technol. 2000, 60, 509–523. [Google Scholar] [CrossRef]
- Pickering, S.J.; Turner, T.A.; Meng, F.; Morris, C.N.; Heil, J.P.; Wong, K.H.; Melendi, S. Developments in the fluidised bed process for fibre recovery from thermoset composites. In Proceedings of the 2nd Annual Composites and Advanced Materials Expo, Dallas, TX, USA, 27–29 October 2015; pp. 2384–2394. [Google Scholar]
- Shuaib, N.A.; Mativenga, P.T. Effect of process parameters on mechanical recycling of glass fibre thermoset composites. Procedia CIRP 2016, 48, 134–139. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Guo, X.; Liu, C.; Liu, T.; Xin, J.; Zhang, J. Mild chemical recycling of aerospace fiber/epoxy composite wastes and utilization of the decomposed resin. Polym. Degrad. Stab. 2017, 139, 20–27. [Google Scholar] [CrossRef]
- Jiang, J.; Deng, G.; Chen, X.; Gao, X.; Guo, Q.; Xu, C.; Zhou, L. On the successful chemical recycling of carbon fiber/epoxy resin composites under the mild condition. Compos. Sci. Technol. 2017, 151, 243–251. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, H.M.; An, J.H.; Chung, D.C.; An, K.H.; Kim, B.J. Recycling and characterization of carbon fibers from carbon fiber reinforced epoxy matrix composites by a novel super-heated-steam method. J. Environ. Manag. 2017, 203, 872–879. [Google Scholar] [CrossRef]
- Limburg, M.; Stockschläder, J.; Quicker, P. Thermal treatment of carbon fibre reinforced polymers (Part 1: Recycling). Waste Manag. Res. 2019, 37, 73–82. [Google Scholar] [CrossRef]
- Wada, M.; Kawai, K.; Suzuki, T.; Hira, H.; Kitaoka, S. Effect of superheated steam treatment of carbon fiber on interfacial adhesion to epoxy resin. Compos. Part A 2016, 85, 156–162. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, K.W.; An, K.H.; Kim, B.J. Fast recovery process of carbon fibers from waste carbon fibers-reinforced thermoset plastics. J. Environ. Manag. 2019, 247, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sánchez-Rodríguez, D.; Kamo, T. Influence of thermal treatment on the properties of carbon fiber reinforced plastics under various conditions. Polym. Degrad. Stab. 2020, 178, 109199. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Chen, F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [PubMed]
- Deng, J.; Xu, L.; Zhang, L.; Peng, J.; Guo, S.; Liu, J.; Koppala, S. Recycling of carbon fibers from CFRP waste by microwave thermolysis. Processes 2019, 7, 207. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Liu, C.; Mahmoodi, R.; Li, Q.; Naebe, M. Development of a low cost and green microwave assisted approach towards the circular carbon fibre composites. Compos. Part B 2020, 184, 107750. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.L.; Tian, F.; An, W.L.; Xu, S.; Wang, Y.Z. A fast and mild closed-loop recycling of anhydride-cured epoxy through microwave-assisted catalytic degradation by trifunctional amine and subsequent reuse without separation. Green Chem. 2019, 21, 2487–2493. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yabushita, S.; Irisawa, T.; Tanabe, Y. Enhancement of bending strength, thermal stability and recyclability of carbon-fiber-reinforced thermoplastics by using silica colloids. Compos. Sci. Technol. 2019, 181, 107665. [Google Scholar] [CrossRef]
- Wu, J.; Li, C.; Hailatihan, B.; Mi, L.; Baheti, Y.; Yan, Y. Effect of the addition of thermoplastic resin and composite on mechanical and thermal properties of epoxy resin. Polymers 2022, 14, 1087. [Google Scholar] [CrossRef]
- Butenegro, J.A.; Bahrami, M.; Swolfs, Y.; Ivens, J.; Martínez, M.Á.; Abenojar, J. Novel thermoplastic composites strengthened with carbon fiber-reinforced epoxy composite waste rods: Development and characterization. Polymers 2022, 14, 3951. [Google Scholar] [CrossRef]
- Das, M.; Varughese, S. A novel sonochemical approach for enhanced recovery of carbon fiber from CFRP waste using mild acid-peroxide mixture. ACS Sustain. Chem. Eng. 2016, 4, 2080–2087. [Google Scholar] [CrossRef]
- Sun, H.; Guo, G.; Memon, S.A.; Xu, W.; Zhang, Q.; Zhu, J.H.; Xing, F. Recycling of carbon fibers from carbon fiber reinforced polymer using electrochemical method. Compos. Part A 2015, 78, 10–17. [Google Scholar] [CrossRef]
- Zhu, J.H.; Chen, P.Y.; Su, M.N.; Pei, C.; Xing, F. Recycling of carbon fibre reinforced plastics by electrically driven heterogeneous catalytic degradation of epoxy resin. Green Chem. 2019, 21, 1635–1647. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, W.; Jin, X.; Liang, X.; Sui, G.; Yang, X. Efficient reclamation of carbon fibers from epoxy composite waste through catalytic pyrolysis in molten ZnCl2. RSC Adv. 2019, 9, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Van de Werken, N.; Reese, M.S.; Taha, M.R.; Tehrani, M. Investigating the effects of fiber surface treatment and alignment on mechanical properties of recycled carbon fiber composites. Compos. Part A 2019, 119, 38–47. [Google Scholar] [CrossRef]
- Jensen, J.P.; Skelton, K. Wind turbine blade recycling: Experiences, challenges and possibilities in a circular economy. Renew. Sustain. Energ. Rev. 2018, 97, 165–176. [Google Scholar] [CrossRef]
- Mamanpush, S.H.; Li, H.; Englund, K.; Tabatabaei, A.T. Recycled wind turbine blades as a feedstock for second generation composites. Waste. Manag. 2018, 76, 708–714. [Google Scholar] [CrossRef]
- Jagadish, P.R.; Khalid, M.; Li, L.P.; Hajibeigy, M.T.; Amin, N.; Walvekar, R.; Chan, A. Cost effective thermoelectric composites from recycled carbon fibre: From waste to energy. J. Clean. Prod. 2018, 195, 1015–1025. [Google Scholar] [CrossRef]
- Liu, W.H.; Huang, H.H.; Zhu, L.B.; Liu, Z.F. Integrating carbon fiber reclamation and additive manufacturing for recycling CFRP waste. Compos. Part B 2021, 215, 108808. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Jiang, Y.; Zhao, Z.; Jiang, G.; Sun, Z. Synthesis of Fe2O3 loaded porous g-C3N4 photocatalyst for photocatalytic reduction of dinitrogen to ammonia. Chem. Eng. J. 2019, 373, 572–579. [Google Scholar] [CrossRef]
- Zhu, Z.; Huo, P.; Lu, Z.; Yan, Y.; Liu, Z.; Shi, W.; Li, C.; Dong, H. Fabrication of magnetically recoverable photocatalysts using g-C3N4 for effective separation of charge carriers through like-Z-scheme mechanism with Fe3O4 mediator. Chem. Eng. J. 2018, 331, 615–625. [Google Scholar] [CrossRef]
- Alghamdi, Y.G.; Krishnakumar, B.; Malik, M.A.; Alhayyani, S. Design and preparation of biomass-derived activated carbon loaded TiO2 photocatalyst for photocatalytic degradation of reactive red 120 and ofloxacin. Polymers 2022, 14, 880. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Lee, J.W.; Chang, C.Y.; Chang, Y.J.; Lee, Y.C.; Hwa, M.Y. Novel TiO2 thin films/glass fiber photocatalytic reactors in the removal of bioaerosols. Surf. Coat. Technol. 2010, 205, 341–344. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Amal, R. TiO2-coated natural zeolite: Rapid humic acid adsorption and effective photocatalytic regeneration. Chem. Eng. Sci. 2014, 105, 46–52. [Google Scholar] [CrossRef]
- Guo, H.; Kemell, M.; Heikkilä, M.; Leskelä, M. Noble metal-modified TiO2 thin film photocatalyst on porous steel fiber support. Appl. Catal. B 2010, 95, 358–364. [Google Scholar] [CrossRef]
- Dey, N.K.; Kim, M.J.; Kim, K.D. Adsorption and photocatalytic degradation of methylene blue over TiO2 films on carbon fiber prepared by atomic layer deposition. J. Mol. Catal. A 2011, 337, 33–38. [Google Scholar] [CrossRef]
- Fu, P.F.; Yong, L.; Dai, X.G. Interposition fixing structure of TiO2 film deposited on activated carbon fibers. Trans. Nonferr. Metal. Soc. 2006, 16, 965–969. [Google Scholar] [CrossRef]
- Jiang, G.; Li, X.; Wei, Z.; Jiang, T.; Du, X.; Chen, W. Growth of N-doped BiOBr nanosheets on carbon fibers for photocatalytic degradation of organic pollutants under visible light irradiation. Powder Technol. 2014, 260, 84–89. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Y.; Xiao, Z.; Li, W.; Li, B.; Huang, X.; Liu, X.; Hu, J. Heterostructures of CuS nanoparticle/ZnO nanorod arrays on carbon fibers with improved visible and solar light photocatalytic properties. J. Mater. Chem. A 2015, 3, 7304–7313. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Xu, P.; Tang, S.; Liu, C. Efficient photocatalytic degradation of acid orange 7 over N-doped ordered mesoporous titania on carbon fibers under visible-light irradiation based on three synergistic effects. Appl. Catal. A 2016, 524, 163–172. [Google Scholar] [CrossRef]
- Ding, J.; Bu, Y.; Ou, M.; Yu, Y.; Zhong, Q.; Fan, M. Facile decoration of carbon fibers with Ag nanoparticles for adsorption and photocatalytic reduction of CO2. Catal. B Environ. 2017, 202, 314–325. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Shen, X.; Duoerkun, G.; Zhu, B.; Zhang, L.; Li, M.; Chen, Z. Fabrication of g-C3N4/BiOBr heterojunctions on carbon fibers as weaveable photocatalyst for degrading tetracycline hydrochloride under visible light. Chem. Eng. J. 2020, 386, 124010. [Google Scholar] [CrossRef]
- Demircivi, P.; Gulen, B.; Simsek, E.B.; Berek, D. Enhanced photocatalytic degradation of tetracycline using hydrothermally synthesized carbon fiber decorated BaTiO3. Mater. Chem. Phys. 2020, 241, 122236. [Google Scholar] [CrossRef]
- Lv, B.; Xia, L.; Yang, Y.; Wang, X.; Yin, C.; Li, Y.; Zhang, F. Synthesis of nanostructured TiC/TiO2 with controllable morphology on carbon fibers as photocatalyst for degrading RhB and reducing Cr (VI) under visible light. J. Mater. Sci. 2020, 55, 14953–14964. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, M.; Sun, A.; Shi, Z.; Zhu, B.; Macharia, D.K.; Li, F.; Chen, Z.; Liu, J.; Zhang, L. MIL-101 (Fe) nanodot-induced improvement of adsorption and photocatalytic activity of carbon fiber/TiO2-based weavable photocatalyst for removing pharmaceutical pollutants. J. Clean. Prod. 2021, 290, 125782. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, T.; Xu, P.; Zhang, L.; Liu, J.; Chen, Z. Growth of C3N4 nanosheets on carbon-fiber cloth as flexible and macroscale filter-membrane-shaped photocatalyst for degrading the flowing wastewater. Appl. Catal. B Environ. 2017, 219, 425–431. [Google Scholar] [CrossRef]
- Shen, X.; Song, L.; Luo, L.; Zhang, Y.; Zhu, B.; Liu, J.; Chen, Z.; Zhang, L. Preparation of TiO2/C3N4 heterojunctions on carbon-fiber cloth as efficient filter-membrane-shaped photocatalyst for removing various pollutants from the flowing wastewater. J. Colloid Interface Sci. 2018, 532, 798–807. [Google Scholar] [CrossRef]
- Zhang, Y.; Duoerkun, G.; Shi, Z.; Cao, W.; Liu, T.; Liu, J.; Zhang, L.; Li, M.; Chen, Z. Construction of TiO2/Ag3PO4 nanojunctions on carbon fiber cloth for photocatalytically removing various organic pollutants in static or flowing wastewater. J. Colloid Interface Sci. 2020, 571, 213–221. [Google Scholar] [CrossRef]
- Qian, T.; Zhang, Y.; Cai, J.; Cao, W.; Liu, T.; Chen, Z.; Liu, J.; Li, F.; Zhang, L. Decoration of amine functionalized zirconium metal organic framework/silver iodide heterojunction on carbon fiber cloth as a filter-membrane-shaped photocatalyst for degrading antibiotics. J. Colloid Interface Sci. 2021, 603, 582–593. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Czyżewski, A.; Wanag, A.; Dubicki, M.; Sadłowski, M.; Wróbel, R.J.; Morawski, A.W. Photocatalytic oxidation of nitric oxide over AgNPs/TiO2-loaded carbon fiber cloths. J. Environ. Manag. 2020, 262, 110343. [Google Scholar] [CrossRef]
- Kadirova, Z.C.; Hojamberdiev, M.; Katsumata, K.I.; Isobe, T.; Matsushita, N.; Nakajima, A.; Okada, K. Fe2O3-loaded activated carbon fiber/polymer materials and their photocatalytic activity for methylene blue mineralization by combined heterogeneous-homogeneous photocatalytic processes. Appl. Surf. Sci. 2017, 402, 444–455. [Google Scholar] [CrossRef]
- Li, H.; Li, N.; Chen, D.; Xu, Q.; Lu, J. Polyethylene imine-grafted ACF@BiOI0.5CI0.5 as a recyclable photocatalyst for high-efficient dye removal by adsorption-combined degradation. Appl. Surf. Sci. 2017, 403, 80–88. [Google Scholar] [CrossRef]
- Luo, S.; Liu, C.; Wan, Y.; Li, W.; Ma, C.; Liu, S.; Heeres, H.J.; Zheng, W.; Seshan, K.; He, S. Self-assembly of single-crystal ZnO nanorod arrays on flexible activated carbon fibers substrates and the superior photocatalytic degradation activity. Appl. Surf. Sci. 2020, 513, 145878. [Google Scholar] [CrossRef]
- Kar, P.; Shukla, K.; Jain, P.; Gupta, R.K. An activated carbon fiber supported Fe2O3@bismuth carbonate heterojunction for enhanced visible light degradation of emerging pharmaceutical pollutants. React. Chem. Eng. 2021, 6, 2029–2041. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Ma, Y.K.; Ke, Q.F.; Chu, L.F.; Guo, C.X.; Guo, Y.P. Hydrothermal deposition of CoFe2O4 nanoparticles on activated carbon fibers promotes atrazine removal via physical adsorption and photo-Fenton degradation. J. Environ. Chem. Eng. 2021, 9, 105940. [Google Scholar] [CrossRef]
- Dang, C.; Sun, F.; Jiang, H.; Huang, T.; Liu, W.; Chen, X.; Ji, H. Pre-accumulation and in-situ destruction of diclofenac by a photo-regenerable activated carbon fiber supported titanate nanotubes composite material: Intermediates, DFT calculation, and ecotoxicity. J. Hazard. Mater. 2020, 400, 123225. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Ahmed, T.; Ammar, M.; Zhang, H.L.; Xu, H.B.; Tabassum, R. Direct growth of m-BiVO4@ carbon fibers for highly efficient and recyclable photocatalytic and antibacterial applications. J. Photochem. Photobiol. B 2020, 213, 112070. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, B.; Ke, Q.F.; Guo, Y.J.; Guo, Y.P. Synergetic effect between adsorption and photodegradation on nanostructured TiO2/activated carbon fiber felt porous composites for toluene removal. J. Hazard. Mater. 2017, 333, 88–98. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, B.K. Photocatalytic reduction of carbon dioxide to methanol using nickel-loaded TiO2 supported on activated carbon fiber. Catal. Today 2017, 298, 158–167. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Shi, Z.; Shan, S.; Liu, J.; Zhang, L. Construction of C3N4/CdS nanojunctions on carbon fiber cloth as a filter-membrane-shaped photocatalyst for degrading flowing wastewater. J. Alloys Compd. 2021, 851, 156743. [Google Scholar] [CrossRef]
- Behpour, M.; Shirazi, P.; Rahbar, M. Immobilization of the Fe2O3/TiO2 photocatalyst on carbon fiber cloth for the degradation of a textile dye under visible light irradiation. React. Kinet. Mech. Catal. 2019, 127, 1073–1085. [Google Scholar] [CrossRef]
- Tian, H.; Sun, M.; Zai, J.; Chen, M.; Li, W.; Hu, J.; Ali, N.; He, K.; Xin, Z.; Qian, X. Interlocked 3D active carbon fibers and monolithic I-doped Bi2O2CO3 structure built by 2D face-to-face interaction; endowed with cycling stability and photocatalytic activity. Cryst. Eng. Comm. 2021, 23, 3204–3211. [Google Scholar] [CrossRef]
- Nahyoon, N.A.; Liu, L.F.; Rabé, K.; Yuan, L.X.; Nahyoon, S.A.; Yang, F.L. An ideal visible nanocomposite (Fe/GTiP) photoanode catalyst for treatment of antibiotics in water and simultaneous electricity generation in the photocatalytic fuel cell. Int. J. Hydrogen Energy 2019, 44, 21703–21715. [Google Scholar] [CrossRef]
- Shi, J.; Chen, J.; Li, G.; An, T.; Yamashita, H. Fabrication of Au/TiO2 nanowires@ carbon fiber paper ternary composite for visible-light photocatalytic degradation of gaseous styrene. Catal. Today 2017, 281, 621–629. [Google Scholar] [CrossRef]
- Yao, C.; Yuan, A.; Zhang, H.; Li, B.; Liu, J.; Xi, F.; Dong, X. Facile surface modification of textiles with photocatalytic carbon nitride nanosheets and the excellent performance for self-cleaning and degradation of gaseous formaldehyde. J. Colloid Interface Sci. 2019, 533, 144–153. [Google Scholar] [CrossRef]




| Recycling Method | Principal Advantaged and Critical Issue | Current Recycling Company | The Properties of rCFs | Recycling Material/Chemical Agents | Experimental Condition | Output | Ref. |
|---|---|---|---|---|---|---|---|
| Landfilling or incineration | Energy recovery but kTons of CFs are lost; Unfriendly environment | - | - | - | - | - | [1] |
| Mechanical method | Fast processing speed; High energy consumption and rCFs with poor mechanical properties | Procotex (Belgium); University of Manchester (UK) | rCFs with resin residues and poor mechanical properties (such as shortened lengths and uneven surfaces) | Carbon fiber reinforced (CFR) polyether ether ketone | Electronic equipment + sieving | rCFs with 2–10 mm length and 0.16–2 mm thickness | [40] |
| CFR epoxy | Microfine mill | rCFs with 20–100 µm diameter | [41] | ||||
| CFR epoxy | Rotating blade with a sieve/ball mill | rCFs with 1–10 mm length and 1–10 µm diameter | [42] | ||||
| Chemical method | rCFs with good quality; Not highly eco-friendly | Hitachi Chemical; V-Carbon (US) | rCFs with almost unaffected mechanical and physical properties | Subcritical and supercritical alcohols (methanol, ethanol 1-propanol, and acetone) | Alkali catalysts were used as reactive-extraction media, 200–450 °C | Clean rCFs retaining 85–99% of strength compared to vCFs | [43] |
| Supercritical methanol, 1-propanol, 2-propanol, 1-butanol, 2-butanol, tert-butanol, acetone, and methyl ethyl ketone | Water as reaction medium, 250 °C | Clean, defect or crack-free rCFs attained a tensile strength of about 98.2% of vCFs | [44] | ||||
| Supercritical water | Without any catalysts after optimization, 120 min | 99.5% resin removal efficiency | [45] | ||||
| Supercritical water | Potassium carbonate as catalysts, 400 °C, 20 MPa, 45 min | 70.9% phenolic monomer, 85% strength of clean rCFs compared to vCFs | [46] | ||||
| Supercritical water | 29 MPa–31 MPa, 430–450 °C, 25 min–35 min | Clean rCFs were almost equal to vCFs | [47] | ||||
| Peracetic acid (acetic acid + H2O2) | 65 °C, 4 h | Similar to vCFs | [48] | ||||
| Thermal method | rCFs with good mechanical and chemical properties; High energy recovery and recovery efficiency of products | Alpha Recyclage Composites (France); Carbon Conversions Inc. (Toyota Tsushon America, US); CFK Valley Stade Recycling GmbH & Co. KG (Germany); Curti SpA (Italy); ELG Carbon Fibre (UK); SGL Automotive Carbon Fibres (US) | rCFs can retain at least 50–75% of mechanical properties, and 90–95% after optimization; When the temperature is too low, the rCF are stiff with poor mechanical properties; When the temperature is too high, the rCF with reduced diameters and mechanical properties | The composites made of woven CFs (55–60%) and polybenzoxazine resin (40–45%) | 500 °C for 1 h in a static bed reactor; The post-oxidation process was carried out at 500 °C | 93% and 96% of the tensile strength and Young’s modulus were maintained | [49] |
| Composite made of 4,4-diaminodiphenylmethane cured epoxy resin | 650 °C, 5% oxygen, 45 min | rCF showed 80% retention in tensile strength | [50] | ||||
| Waste composite panels | Different ratios of H2O2/TA (1 to 3), 1–3 min | The matrix decomposition yield of up to 95%, the tensile strength retention of 92%, and negligible reduction in the modulus | [51] | ||||
| CF epoxy composites | Multimode microwave applicator with power 3 kW and heating time 8 s | Relatively clean rCFs with better tensile strength and modulus | [52] | ||||
| CFRPCs | 400 °C, 500 °C and 600 °C | Intermediate temperature was selected as the optimized temperature | [53] |
| Support | Composite Photocatalytic System | Performance Advantage | Target Pollutant | Photocatalytic Effect | Ref. |
|---|---|---|---|---|---|
| CFs | CuS/ZnO/CF heterostructures | Easily separated and recycled with little loss in the photocatalytic activity | Methylene blue (MB) | Degraded up to 98.62% after 120 min | [119] |
| NOMT/CFs | The CFs serve to concentrate the pollutant around the active sites, favor electron transfer, and facilitate convenient recycling | Acid orange 7 (AO7) solution | KR and KS were 0.015 mg L−1 min−1 and 4.26 mg L−1 min−1, respectively | [120] | |
| Ag NPs/CFs | The increase of CO2 adsorption and the efficient electron transfer to CO2 as well as the active site splitting of CO2 reduction and H2O decomposition | CO2 photocatalytic reduction | CH3OH production is 0.475 μg/mg h | [121] | |
| CFs/g-C3N4/BiOBr bundles | Serve as a flexible, wearable and recyclable photocatalyst | Tetracycline hydrochloride (TC-HCl) | Degraded 86.1% in 120 min | [122] | |
| BaTiO3/CF | CFs were used to decrease the band gap energy of BaTiO3 | Tetracycline (TC) | Degraded 96% under UV light | [123] | |
| CFs@TiC/TiO2 composite | Easily recycled and reused with good reactivity | RhB and Cr(VI) | Degradation of RhB and reduction of Cr(VI) were about 90% and 80%, respectively | [124] | |
| CFs/TiO2/MIL-101(Fe) cloth | Filter-membrane-shaped photocatalyst with efficient, low-cost and recyclable | Pharmaceutical pollutants | Efficiently adsorbed 46.9% 17b-estradiol (E2) and 40.2% TC after 60 min in the dark | [125] | |
| CFCs | CF/C3N4 cloth (4 × 4 cm2) | Excellent flexibility and strong visible-light absorption at ~450 nm | RhB and parachlorophenol (4-CP) | Degraded 98% RhB in 60 min and 99.3% colorless 4-CP after 120 min of visible-light irradiation | [126] |
| CF/TiO2/C3N4 cloth (4 × 4 cm2) | Excellent visible photoabsorption (edge: ~450 nm) and improved photocurrent | Various pollutants | Degraded 98% MB after 60 min, 93% AO7 in 100 min, 97% 4-CP in 80 min, 82% TC in 60 min, and 97% Cr(VI) after 90 min | [127] | |
| CFC/TiO2/Ag3PO4 (4 × 4 cm2) | Flexible filter-membrane with high photocatalytic activity | Organic pollutants | Under Vis or UV-Vis light illumination, efficiently degraded phenol (80.6%/89.4%), TC (91.7%/94.2%), RhB (98.4%/99.5%) and AO7 (97.6%/98.3%) | [128] | |
| CFC/UiO-66-NH2/AgI (4 × 4 cm2) | Recyclable, high adsorption and photocatalytic capacity | Antibiotics | Degraded 19.0% levofloxacin (LVFX) or 18.4% ciprofloxacin (CIP) in 60 min in the dark and degrade 84.5% LVFX or 79.6% CIP in 120 min under visible light irradiation | [129] | |
| AgNPs/TiO2-loaded CFC composite | Good thermal and photocatalytic stability | Nitric oxide (NO) | The minimal and maximal NO removal rates reached about 80% and 95%, respectively | [130] | |
| ACFs | Fe-ACFTs | The combination of heterogeneous and homogeneous photocatalysis | MB | Almost complete decolorization of MB (96–98%) and more than 91% total organic carbon (TOC) removal were achieved | [131] |
| ACF@BiOI0.5Cl0.5 | ACF, as flexible, conductive, and corrosion-resistant supports, were beneficial to the photocatalytic degradation process | Anionic dyes | The maximum adsorption efficiency was about 80% in 70 min | [132] | |
| ZnO NRAs/ACFs | High surface area and intensive blue, green, and yellow emissions, robust recyclability | MB | Degraded 77.5% MB in 2 h | [133] | |
| Fe2O3@BC/ACF | Interfacial tuning of the heterojunction and overall charge carrier separation | Emerging pharmaceutical pollutants | Degradation of antipyrine (60%) | [134] | |
| ACF/CoFe2O4 composite | Excellent adsorption ability due to the synergistic effect between CoFe2O4 nanoparticles and ACF felts | Atrazine (ATZ) | Degradation efficiency was 96% in 240 min | [135] | |
| TNTs@ACF | Well-defined hybrid structure | Pharmaceuticals and personal care products (PPCPs) | Photodegraded 98.8% diclofenac (DCF) under solar light within 2 h | [136] | |
| BiVO4@ACF | Enhanced photocatalytic and antibacterial activity, chemical stability and good recyclability | (RhB) and pathogenic microbes (Escherichia coli and Staphylococcus aureus) | Degraded 86% RhB and inhibited the growth of both bacteria | [137] | |
| TiO2/activated carbon fiber felt (TiO2/ACFF) porous composite | Excellent adsorption and photodegradation properties due to the synergetic effects between the nanostructured TiO2 and ACFF | Toluene | At the toluene concentrations of 230 ppm and 460 ppm, the photocatalytic oxidation efficiency of toluene into CO2 arrives at 100% and 81.5%, respectively | [138] | |
| NiO-TiO2/ ACF | The ACF support decreased the recombination of photo-generated electron-hole pairs | Photocatalytic reduction of CO2 to methanol fuel | The methanol yield in 2 h was 755.1 μmol g−1 and 986.3 μmol g−1 under UV and visible light irradiation, respectively | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Gao, X.; Wu, Y.; Wang, Y.; Nguyen, T.T.; Guo, M. Recycling Carbon Fiber from Carbon Fiber-Reinforced Polymer and Its Reuse in Photocatalysis: A Review. Polymers 2023, 15, 170. https://doi.org/10.3390/polym15010170
Wu J, Gao X, Wu Y, Wang Y, Nguyen TT, Guo M. Recycling Carbon Fiber from Carbon Fiber-Reinforced Polymer and Its Reuse in Photocatalysis: A Review. Polymers. 2023; 15(1):170. https://doi.org/10.3390/polym15010170
Chicago/Turabian StyleWu, Jie, Xing Gao, Yueting Wu, Yutong Wang, Tat Thang Nguyen, and Minghui Guo. 2023. "Recycling Carbon Fiber from Carbon Fiber-Reinforced Polymer and Its Reuse in Photocatalysis: A Review" Polymers 15, no. 1: 170. https://doi.org/10.3390/polym15010170
APA StyleWu, J., Gao, X., Wu, Y., Wang, Y., Nguyen, T. T., & Guo, M. (2023). Recycling Carbon Fiber from Carbon Fiber-Reinforced Polymer and Its Reuse in Photocatalysis: A Review. Polymers, 15(1), 170. https://doi.org/10.3390/polym15010170








