Multifunctional Nanoparticles as High-Efficient Targeted Hypericin System for Theranostic Melanoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Instruments
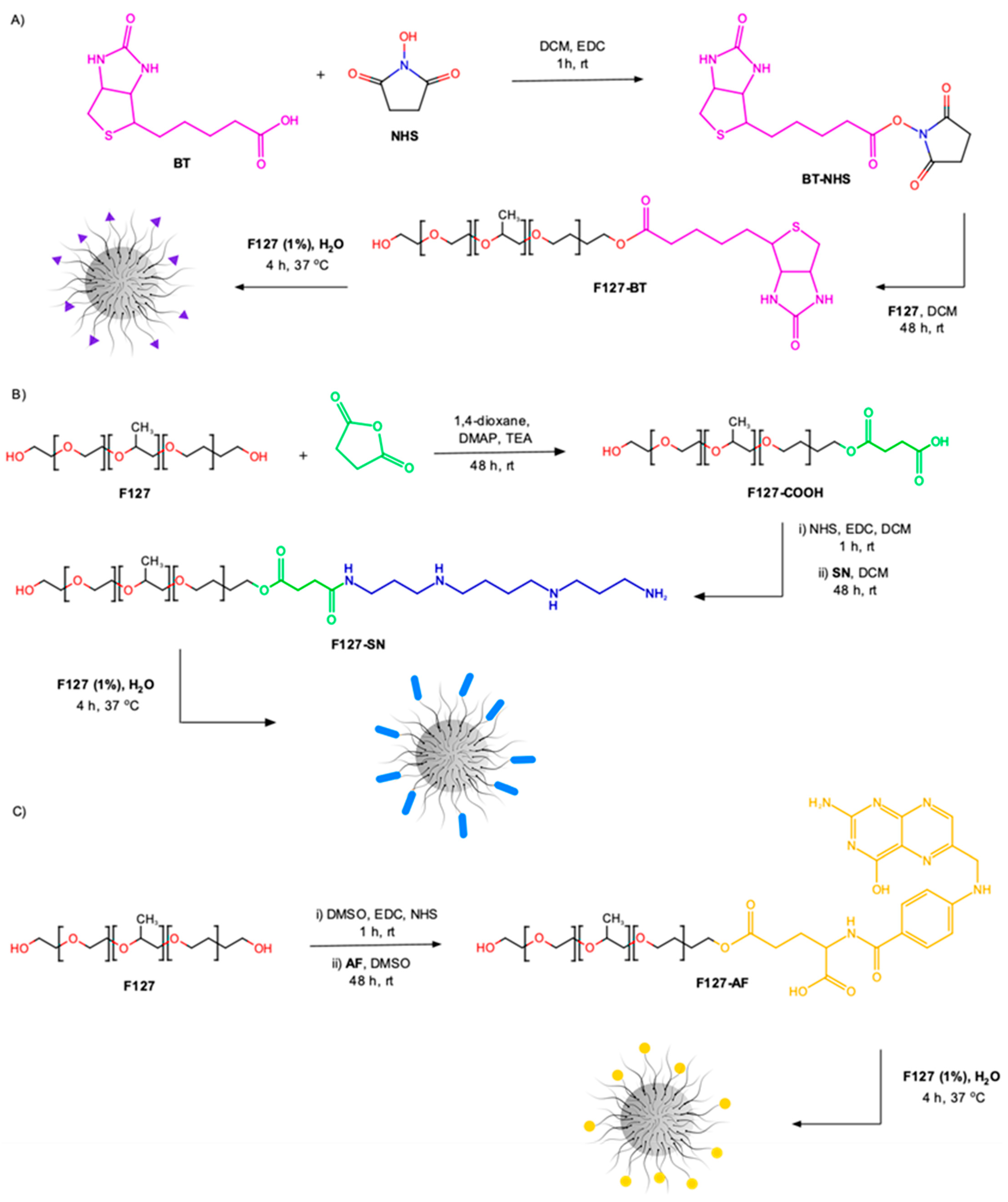
2.3. Synthesis of BT-Conjugated F127 Pluronic® (F127-BT)
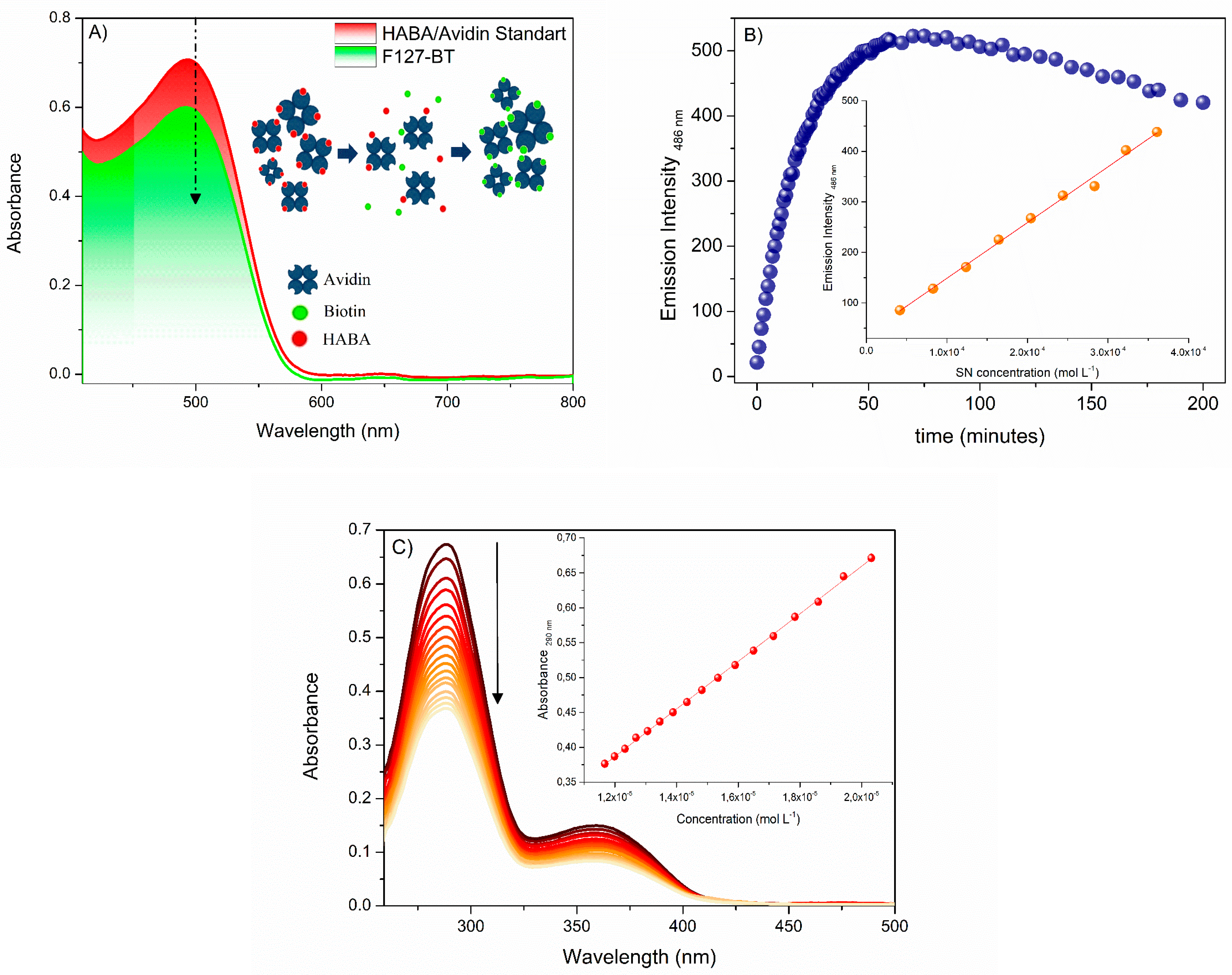
2.4. HABA/Avidin Assay
2.5. Synthesis of SN-Conjugated F127 Pluronic® (F127-SN)
2.6. Fluorescamine Assay
2.7. Synthesis of FA-Conjugated F127 Pluronic® (F127-AF)
2.8. Folate Assay
2.9. Critical Micelle Concentration (CMC)
2.10. Micelle Preparation
2.11. Critical Micelle Temperature (CMT)
2.12. MTT Assay
3. Results and Discussions
3.1. Synthesis
3.2. 1H NMR Characterization
3.3. Percentage of FA, BT, and SN on F127
3.4. Micelle Characterization
3.5. MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moura, N.M.; Castro, K.A.; Biazzotto, J.C.; Prandini, J.A.; Lodeiro, C.; Faustino, M.A.; Simões, M.M.; da Silva, R.S.; Neves, M.G. Ruthenium and iridium complexes bearing porphyrin moieties: PDT efficacy against resistant melanoma cells. Dye. Pigment. 2022, 205, 110501. [Google Scholar] [CrossRef]
- Montaseri, H.; Nkune, N.W.; Abrahamse, H. Active targeted photodynamic therapeutic effect of silver-based nanohybrids on melanoma cancer cells. J. Photochem. Photobiol. 2022, 11, 100136. [Google Scholar] [CrossRef]
- Wagstaff, W.; Mwamba, R.N.; Grullon, K.; Armstrong, M.; Zhao, P.; Hendren-Santiago, B.; Qin, K.H.; Li, A.J.; Hu, D.A.; Youssef, A.; et al. Melanoma: Molecular genetics, metastasis, targeted therapies, immunotherapies, and therapeutic resistance. Genes Dis. 2022, 9, 1608–1623. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Venturini, M.; Sala, R.; Calzavara-Pinton, P. Photodynamic therapy: Update 2006 Part 1: Photochemistry and photobiology. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Zhang, Y.; Zhao, Z.; Liu, C.; Li, M.; Liu, J.; Wang, S.; Yang, D.; Luo, F.; et al. LS-HB-Mediated Photodynamic Therapy Inhibits Proliferation and Induces Cell Apoptosis in Melanoma. Mol. Pharm. 2022, 19, 2607–2619. [Google Scholar] [CrossRef]
- Nakajima, N.; Kawashima, N. A basic study on Hypericin-PDT in vitro. Photodiagn. Photodyn. Ther. 2012, 9, 196–203. [Google Scholar] [CrossRef]
- Karioti, A.; Bilia, A.R. Hypericins as Potential Leads for New Therapeutics. Int. J. Mol. Sci. 2010, 11, 562–594. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cona, M.M.; Chen, F.; Feng, Y.; Zhou, L.; Yu, J.; Nuyts, J.; de Witte, P.; Zhang, J.; Himmelreich, U.; et al. Exploring Theranostic Potentials of Radioiodinated Hypericin in Rodent Necrosis Models. Theranostics 2012, 2, 1010–1019. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Wang, J.; Ni, Y.; Chen, F. Necrosis Avidity: A Newly Discovered Feature of Hypericin and its Preclinical Applications in Necrosis Imaging. Theranostics 2013, 3, 667–676. [Google Scholar] [CrossRef]
- D’Hallewin, M.-A.; Kamuhabwa, A.; Roskams, T.; de Witte, P.; Baert, L. Hypericin-based fluorescence diagnosis of bladder carcinoma. Br. J. Urol. 2002, 89, 760–763. [Google Scholar] [CrossRef]
- Olivo, M.; Fu, C.Y.; Raghavan, V.; Lau, W.K.O. New Frontier in Hypericin-Mediated Diagnosis of Cancer with Current Optical Technologies. Ann. Biomed. Eng. 2011, 40, 460–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Morais, F.A.P.; Gonçalves, R.S.; Braga, G.; Calori, I.R.; Pereira, P.C.D.S.; Batistela, V.R.; Caetano, W.; Hioka, N. Stable Dipalmitoylphosphatidylcholine Liposomes Coated with an F127 Copolymer for Hypericin Loading and Delivery. ACS Appl. Nano Mater. 2020, 3, 4530–4541. [Google Scholar] [CrossRef]
- de Morais, F.A.P.; Gonçalves, R.S.; Campanholi, K.S.; de França, B.M.; Capeloto, O.A.; Lazarin-Bidoia, D.; Balbinot, R.B.; Nakamura, C.V.; Malacarne, L.C.; Caetano, W.; et al. Photophysical characterization of Hypericin-loaded in micellar, liposomal and copolymer-lipid nanostructures based F127 and DPPC liposomes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 248, 119173. [Google Scholar] [CrossRef]
- Gonçalves, R.S.; Braga, G.; Oliveira, A.C.V.; Cesar, G.B.; Tominaga, T.T.; Zampiere, E.H.; Calori, I.R.; de Morais, F.A.P.; Basso, E.A.; Pontes, R.M.; et al. Hypericin Delivery System Based on P84 Copolymeric Micelles Linked with N-(3-Aminopropyl)-2-pyrrolidone for Melanoma-Targeted Photodynamic Therapy. ACS Appl. Polym. Mater. 2020, 2, 1692–1701. [Google Scholar] [CrossRef]
- Barras, A.; Boussekey, L.; Courtade, E.; Boukherroub, R. Hypericin-loaded lipid nanocapsules for photodynamic cancer therapy in vitro. Nanoscale 2013, 5, 10562–10572. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, A.; Zheng, G. Improving accessibility of EPR-insensitive tumor phenotypes using EPR-adaptive strategies: Designing a new perspective in nanomedicine delivery. Theranostics 2019, 9, 8091–8108. [Google Scholar] [CrossRef]
- Ikeda-Imafuku, M.; Wang, L.L.-W.; Rodrigues, D.; Shaha, S.; Zhao, Z.; Mitragotri, S. Strategies to improve the EPR effect: A mechanistic perspective and clinical translation. J. Control. Release 2022, 345, 512–536. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Greish, K.; Fang, J. The EPR Effect and Polymeric Drugs: A Paradigm Shift for Cancer Chemotherapy in the 21st Century. Adv. Polym. Sci. 2005, 193, 103–121. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2012, 65, 71–79. [Google Scholar] [CrossRef]
- Singla, P.; Garg, S.; McClements, J.; Jamieson, O.; Peeters, M.; Mahajan, R.K. Advances in the therapeutic delivery and applications of functionalized Pluronics: A critical review. Adv. Colloid Interface Sci. 2021, 299, 102563. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.D.; Hochberg, J.D.; Pokorski, J.K.; Steinmetz, N.F. Bioconjugation of Active Ingredients to Plant Viral Nanoparticles Is Enhanced by Preincubation with a Pluronic F127 Polymer Scaffold. ACS Appl. Mater. Interfaces 2021, 13, 59618–59632. [Google Scholar] [CrossRef] [PubMed]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef]
- Gohy, J. Block copolymer micelles. Adv. Polym. Sci. 2005, 190, 65–136. [Google Scholar]
- Le Garrec, D.; Ranger, M.; Leroux, J.-C. Micelles in Anticancer Drug Delivery. Am. J. Drug Deliv. 2004, 2, 15–42. [Google Scholar] [CrossRef]
- Basalious, E.B.; Shamma, R.N. Novel self-assembled nano-tubular mixed micelles of Pluronics P123, Pluronic F127 and phosphatidylcholine for oral delivery of nimodipine: In vitro characterization, ex vivo transport and in vivo pharmacokinetic studies. Int. J. Pharm. 2015, 493, 347–356. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef]
- Managa, M.; Ngoy, B.P.; Mafukidze, D.; Nyokong, T. Incorporation of metal free and Ga 5,10,15,20-tetrakis(4-bromophenyl) porphyrin into Pluronic F127-folic acid micelles. J. Lumin. 2018, 194, 739–746. [Google Scholar] [CrossRef]
- Shaki, H.; Vasheghani-Farahani, E.; Ganji, F.; Jafarzadeh-Holagh, S.; Taebnia, N.; Dolatshahi-Pirouz, A. A self assembled dextran-stearic acid-spermine nanocarrier for delivery of rapamycin as a hydrophobic drug. J. Drug Deliv. Sci. Technol. 2021, 66, 102768. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Luo, Y.; Zhao, H. Bioconjugation of Biotin to the Interfaces of Polymeric Micelles via In Situ Click Chemistry. Langmuir 2008, 25, 744–750. [Google Scholar] [CrossRef]
- Cavallaro, S.G.; Scirè, M.; Licciardi, M.; Ogris, E.; Wagner, G. Giammona, Polyhydroxyethylaspartamide-spermine copolymers: Efficient vectors for gene delivery. J. Control. Release 2008, 131, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Giorgini, M.; De Medici, C.; Palaia, I.; Iadarola, R.; Vertechy, L.; Muzii, L.; Panici, P.B.; Domenici, L.; Di Donato, V.; et al. Targeted drug delivery via folate receptors in recurrent ovarian cancer: A review. OncoTargets Ther. 2014, 7, 1223–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu-Quang, H.; Vinding, M.S.; Nielsen, T.; Ullisch, M.G.; Nielsen, N.C.; Nguyen, D.-T.; Kjems, J. Pluronic F127-Folate Coated Super Paramagenic Iron Oxide Nanoparticles as Contrast Agent for Cancer Diagnosis in Magnetic Resonance Imaging. Polymers 2019, 11, 743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maitani, Y.; Kaneko, M.; Watanabe, K. Functional coating of liposomes using a folate–polymer conjugate to target folate receptors. Int. J. Nanomed. 2012, 7, 3679–3688. [Google Scholar] [CrossRef] [Green Version]
- Pawar, A.; Singh, S.; Rajalakshmi, S.; Shaikh, K.; Bothiraja, C. Development of fisetin-loaded folate functionalized pluronic micelles for breast cancer targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 347–361. [Google Scholar] [CrossRef] [Green Version]
- Maiti, S.; Paira, P. Biotin conjugated organic molecules and proteins for cancer therapy: A review. Eur. J. Med. Chem. 2018, 145, 206–223. [Google Scholar] [CrossRef]
- Wang, Y.; van Steenbergen, M.J.; Beztsinna, N.; Shi, Y.; Lammers, T.; van Nostrum, C.F.; Hennink, W.E. Biotin-decorated all-HPMA polymeric micelles for paclitaxel delivery. J. Control. Release 2020, 328, 970–984. [Google Scholar] [CrossRef]
- Popova, T.V.; Khan, H.; Chubarov, A.S.; Lisitskiy, V.A.; Antonova, N.M.; Akulov, A.E.; Shevelev, O.B.; Zavjalov, E.L.; Silnikov, V.N.; Ahmad, S.; et al. Biotin-decorated anti-cancer nucleotide theranostic conjugate of human serum albumin: Where the seed meets the soil? Bioorganic. Med. Chem. Lett. 2017, 28, 260–264. [Google Scholar] [CrossRef]
- Russo, D.S.A.; Pellosi, V.; Pagliara, M.R.; Milone, B.; Pucci, W.; Caetano, N.; Hioka, A.; Budillon, F.; Ungaro, G.; Russo, F. Quaglia, Biotin-targeted Pluronic ® P123/F127 mixed micelles delivering niclosamide: A repositioning strategy to treat drug-resistant lung cancer cells. Int. J. Pharm. 2016, 511, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Zhou, L.; Yue, Q.; Liu, Q.; Cai, X.; Xiao, W.; Hai, L.; Guo, L.; Wu, Y. Liposomes modified with double-branched biotin: A novel and effective way to promote breast cancer targeting. Bioorganic. Med. Chem. 2019, 27, 3115–3127. [Google Scholar] [CrossRef] [PubMed]
- Rompicharla, S.V.K.; Kumari, P.; Bhatt, H.; Ghosh, B.; Biswas, S. Biotin functionalized PEGylated poly(amidoamine) dendrimer conjugate for active targeting of paclitaxel in cancer. Int. J. Pharm. 2018, 557, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Vinothini, K.; Rajendran, N.K.; Munusamy, M.A.; Alarfaj, A.A.; Rajan, M. Development of biotin molecule targeted cancer cell drug delivery of doxorubicin loaded κ-carrageenan grafted graphene oxide nanocarrier. Mater. Sci. Eng. C 2019, 100, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Darmostuk, M.; Jurášek, M.; Lengyel, K.; Zelenka, J.; Rumlová, M.; Drašar, P.; Ruml, T. Conjugation of chlorins with spermine enhances phototoxicity to cancer cells in vitro. J. Photochem. Photobiol. B Biol. 2017, 168, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, X.; Zhang, Q.; Li, W. Constitutive activity of a spermine receptor is maintained by a single site in the C-terminal. Biochem. Biophys. Res. Commun. 2020, 526, 389–395. [Google Scholar] [CrossRef]
- Gonçalves, R.S.; César, G.B.; Barbosa, P.M.; Hioka, N.; Nakamura, C.V.; Bruschi, M.L.; Caetano, W. Optimized protocol for multigram preparation of emodin anthrone, a precursor in the hypericin synthesis. Nat. Prod. Res. 2018, 33, 1196–1199. [Google Scholar] [CrossRef]
- Bian, Q.; Xiao, Y.; Lang, M. Thermoresponsive biotinylated star amphiphilic block copolymer: Synthesis, self-assembly, and specific target recognition. Polymer 2012, 53, 1684–1693. [Google Scholar] [CrossRef]
- Lin, H.J.; Kirsch, J.F. A sensitive fluorometric assay for avidin and biotin. Anal. Biochem. 1977, 81, 442–446. [Google Scholar] [CrossRef]
- Böhlen, P.; Stein, S.; Dairman, W.; Udenfriend, S. Fluorometric assay of proteins in the nanogram range. Arch. Biochem. Biophys. 1973, 155, 213–220. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Caetano, W.; Tabak, M. Interaction of Chlorpromazine and Trifluoperazine with Anionic Sodium Dodecyl Sulfate (SDS) Micelles: Electronic Absorption and Fluorescence Studies. J. Colloid Interface Sci. 2000, 225, 69–81. [Google Scholar] [CrossRef]
- Ray, G.B.; Chakraborty, I.; Moulik, S.P. Pyrene absorption can be a convenient method for probing critical micellar concentration (cmc) and indexing micellar polarity. J. Colloid Interface Sci. 2005, 294, 248–254. [Google Scholar] [CrossRef]
- Alexandridis, P.; Athanassiou, V.; Hatton, T.A. Pluronic-P105 PEO-PPO-PEO Block Copolymer in Aqueous Urea Solutions: Micelle Formation, Structure, and Microenvironment. Langmuir 1995, 11, 2442–2450. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Copolymers in Aqueous Solutions: Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Derayea, S.M.; Samir, E. A review on the use of fluorescamine as versatile and convenient analytical probe. Microchem. J. 2020, 156, 104835. [Google Scholar] [CrossRef]
- Derayea, S.M.; Ahmed, A.B.; Omar, M.A.; Abdelwahab, N.S.; Abdelrahman, M.M. The convenient use of fluorescamine for spectrofluorimetric quantitation of pramipexole in pure form and pharmaceutical formulation; application to content uniformity testing. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 238, 118414. [Google Scholar] [CrossRef]
- Lin, J.-J.; Chen, J.-S.; Huang, S.-J.; Ko, J.-H.; Wang, Y.-M.; Chen, T.-L.; Wang, L.-F. Folic acid–Pluronic F127 magnetic nanoparticle clusters for combined targeting, diagnosis, and therapy applications. Biomaterials 2009, 30, 5114–5124. [Google Scholar] [CrossRef]
- Bains, G.; Patel, A.B.; Narayanaswami, V. Pyrene: A Probe to Study Protein Conformation and Conformational Changes. Molecules 2011, 16, 7909–7935. [Google Scholar] [CrossRef]
- Repáková, J.; Holopainen, J.M.; Karttunen, M.; Vattulainen, I. Influence of Pyrene-Labeling on Fluid Lipid Membranes. J. Phys. Chem. B 2006, 110, 15403–15410. [Google Scholar] [CrossRef]
| Sample | CMC (mol L−1) | CMT (°C) | Dh (nm) | PDI | ζ (mV) |
|---|---|---|---|---|---|
| F127-SN | 5.2 × 10−5 | 23.4 | 19.9 ± 1.9 | 0.37 ± 0.12 | −7.2 ± 0.2 |
| F127-BT | 3.6 × 10−4 | 21.2 | 13.2 ± 0.5 | 0.15 ± 0.01 | −14.9 ± 0.9 |
| F127-AF | 6.6 × 10−4 | 20.4 | 15.7 ± 0.9 | 0.27 ± 0.02 | −13.7 ± 1.1 |
| CMCF127 = 9.02 × 10−5 mol/L DhF127 = 14.6 ± 0.53 at 37.0 °C CMTF127 = 25.9 °C ζ F127 = −2.32 ± 0.58 at 37.0 °C | |||||
| Formulations without HY | B16F10 Cells CC50 (µmol L−1) with Illumination | B16F10 Cells CC50 (µmol L−1) without Illumination |
|---|---|---|
| F127 | >5 | >5 |
| F127-SN | >5 | >5 |
| F127-FA | >5 | >5 |
| F127-BT | >5 | >5 |
| Formulations with HY | B16F10 cells CC50 (µmol L−1) with illumination | B16F10 cells CC50 (µmol L−1) without illumination |
| F127 | 1.28 ± 0.11 | >5 |
| F127-SN | 0.77 ± 0.03 | >5 |
| F127-FA | 0.47 ± 0.02 | >5 |
| F127-BT | 0.24 ± 0.02 | >5 |
| CC50-cytotoxic concentration for 50% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Morais, F.A.P.; De Oliveira, A.C.V.; Balbinot, R.B.; Lazarin-Bidóia, D.; Ueda-Nakamura, T.; de Oliveira Silva, S.; da Silva Souza Campanholi, K.; da Silva Junior, R.C.; Gonçalves, R.S.; Caetano, W.; et al. Multifunctional Nanoparticles as High-Efficient Targeted Hypericin System for Theranostic Melanoma. Polymers 2023, 15, 179. https://doi.org/10.3390/polym15010179
de Morais FAP, De Oliveira ACV, Balbinot RB, Lazarin-Bidóia D, Ueda-Nakamura T, de Oliveira Silva S, da Silva Souza Campanholi K, da Silva Junior RC, Gonçalves RS, Caetano W, et al. Multifunctional Nanoparticles as High-Efficient Targeted Hypericin System for Theranostic Melanoma. Polymers. 2023; 15(1):179. https://doi.org/10.3390/polym15010179
Chicago/Turabian Stylede Morais, Flávia Amanda Pedroso, Ana Carolina Vieira De Oliveira, Rodolfo Bento Balbinot, Danielle Lazarin-Bidóia, Tânia Ueda-Nakamura, Sueli de Oliveira Silva, Katieli da Silva Souza Campanholi, Ranulfo Combuca da Silva Junior, Renato Sonchini Gonçalves, Wilker Caetano, and et al. 2023. "Multifunctional Nanoparticles as High-Efficient Targeted Hypericin System for Theranostic Melanoma" Polymers 15, no. 1: 179. https://doi.org/10.3390/polym15010179






