Polymeric Encapsulate of Streptomyces Mycelium Resistant to Dehydration with Air Flow at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.1.1. Preinoculum
2.1.2. Spore Stock
2.2. Biomass Production
2.2.1. Vegetative Cell Production
2.2.2. Spore Production
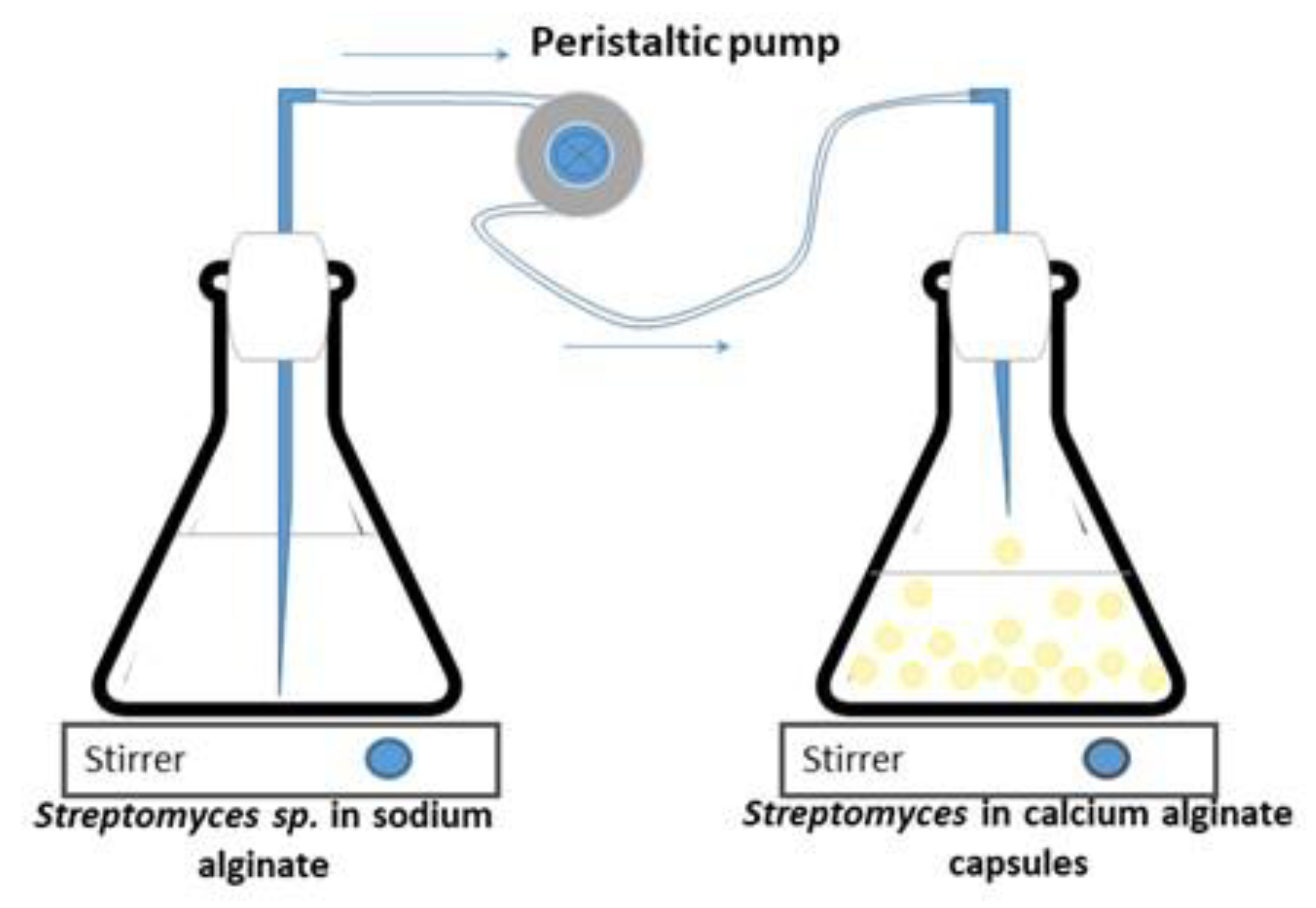
2.3. Immobilization of Streptomyces CDBB1232 on Calcium Alginate Capsules
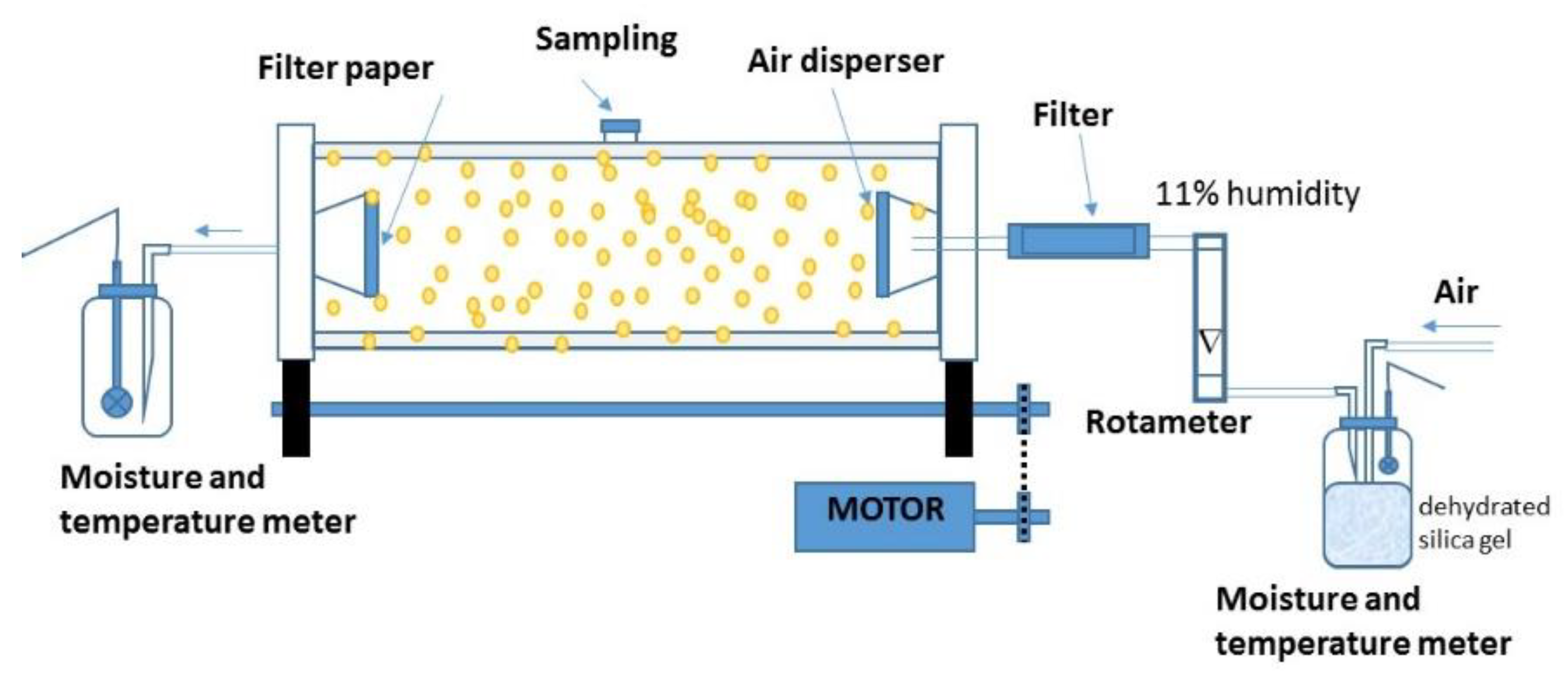
2.4. Dehydration Process of Encapsulated Biomass
2.5. Additional Protectors
2.6. Control Experiments
2.7. Analysis of the Drying Process
2.8. Statistical Analysis
3. Results
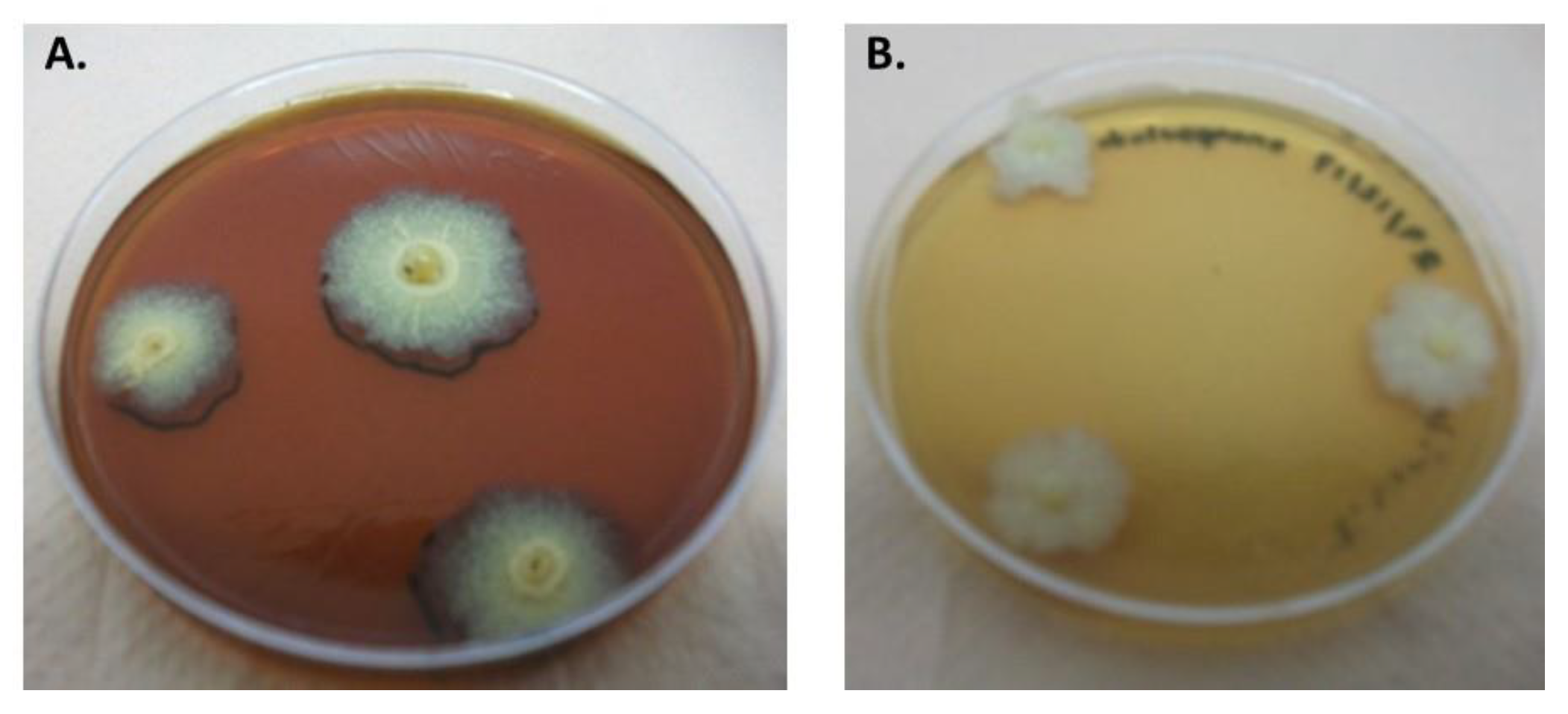
3.1. Biomass Production
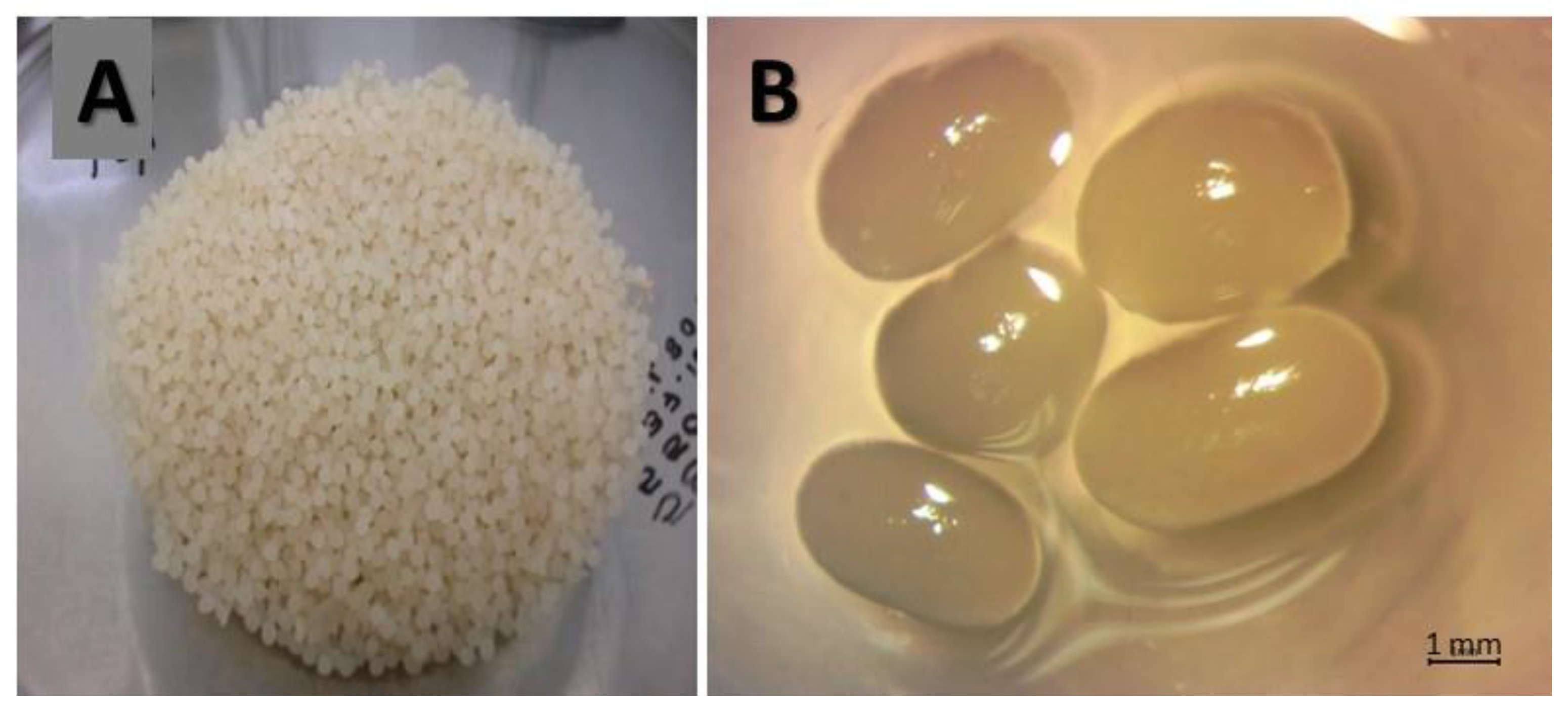
3.2. Immobilization of Streptomyces CDBB1232 in a Calcium Alginate Matrix
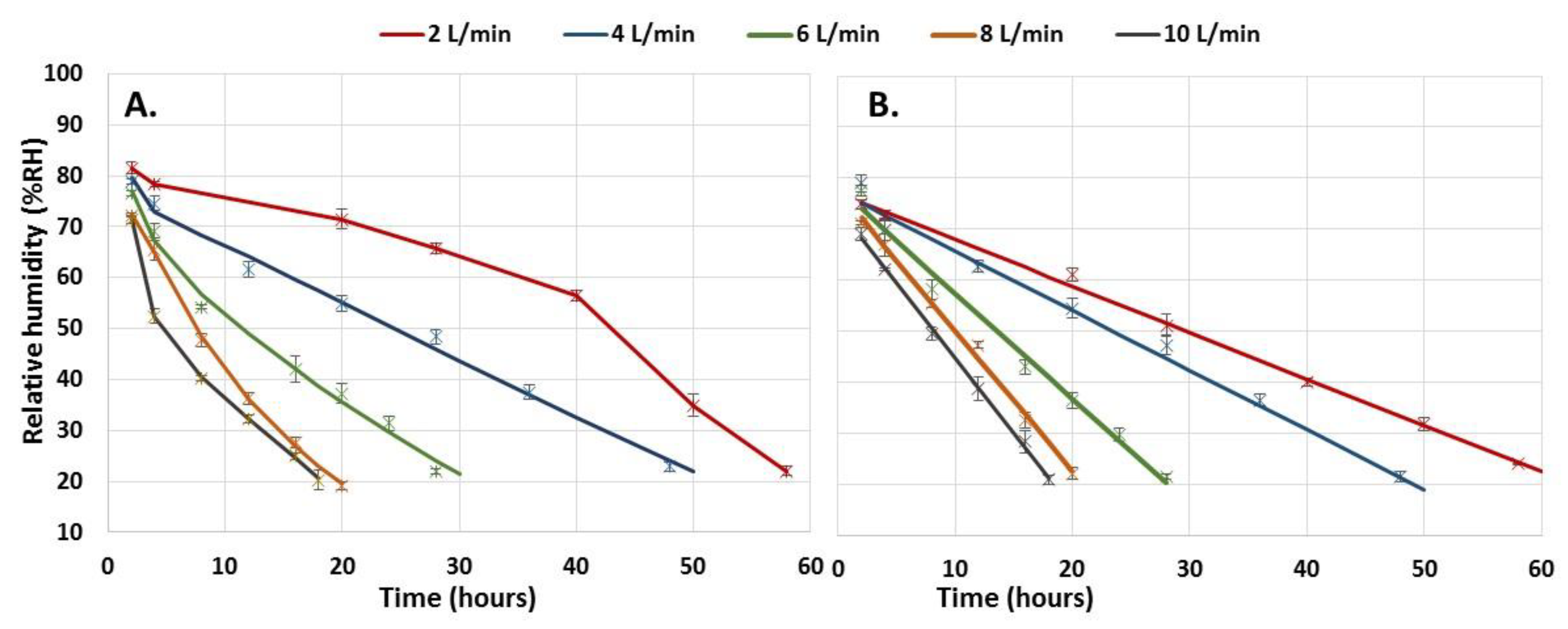
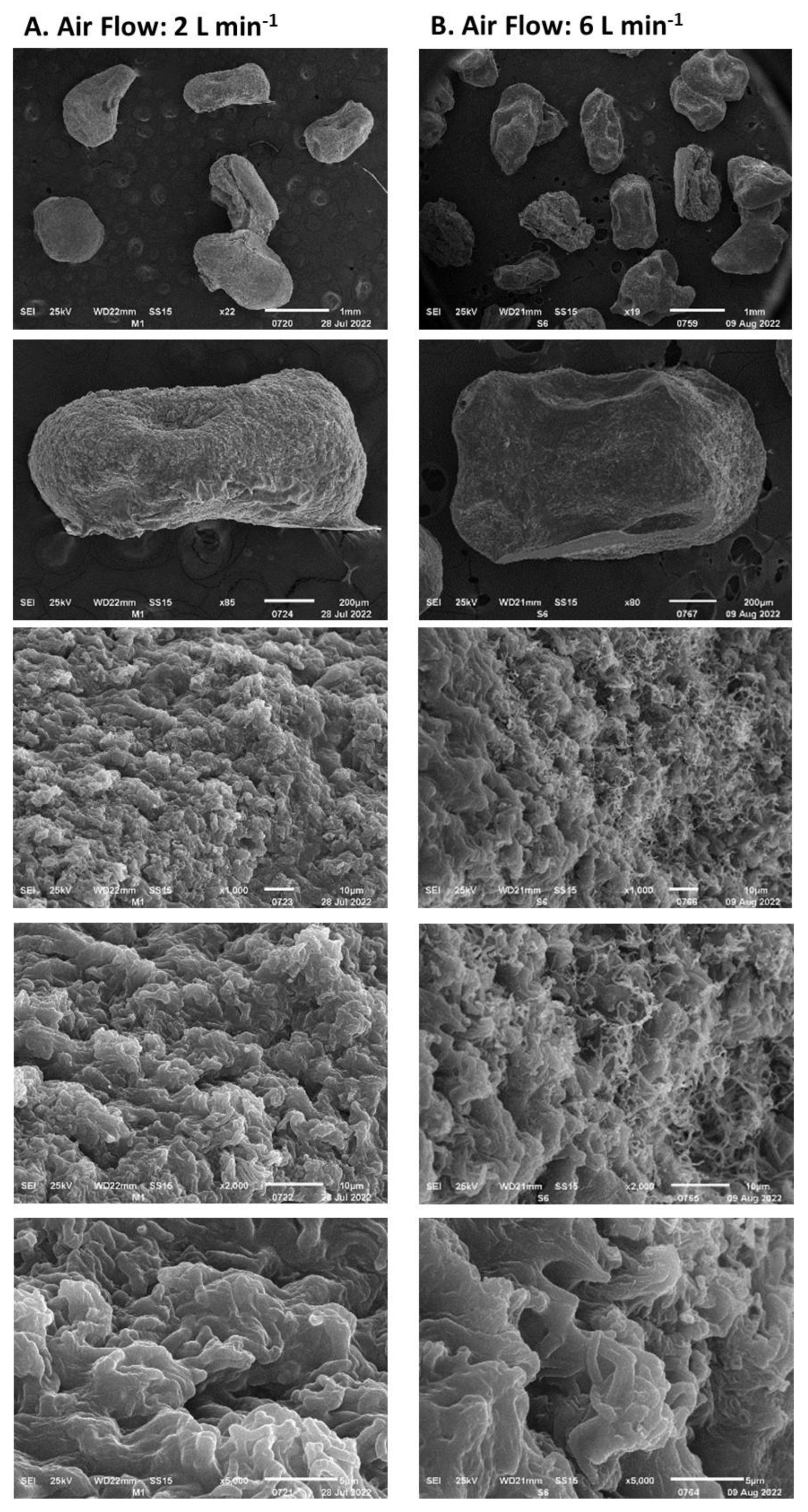
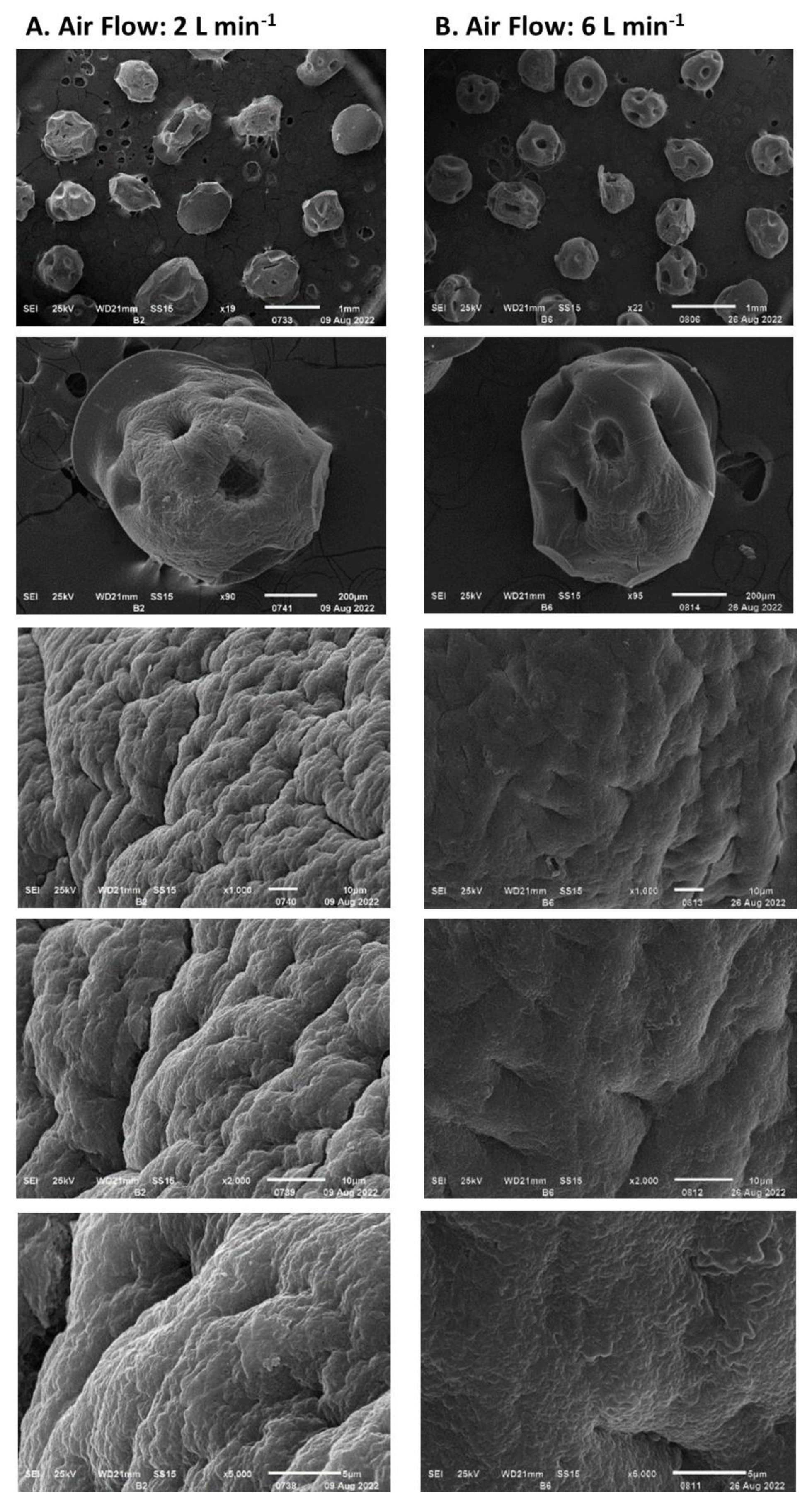
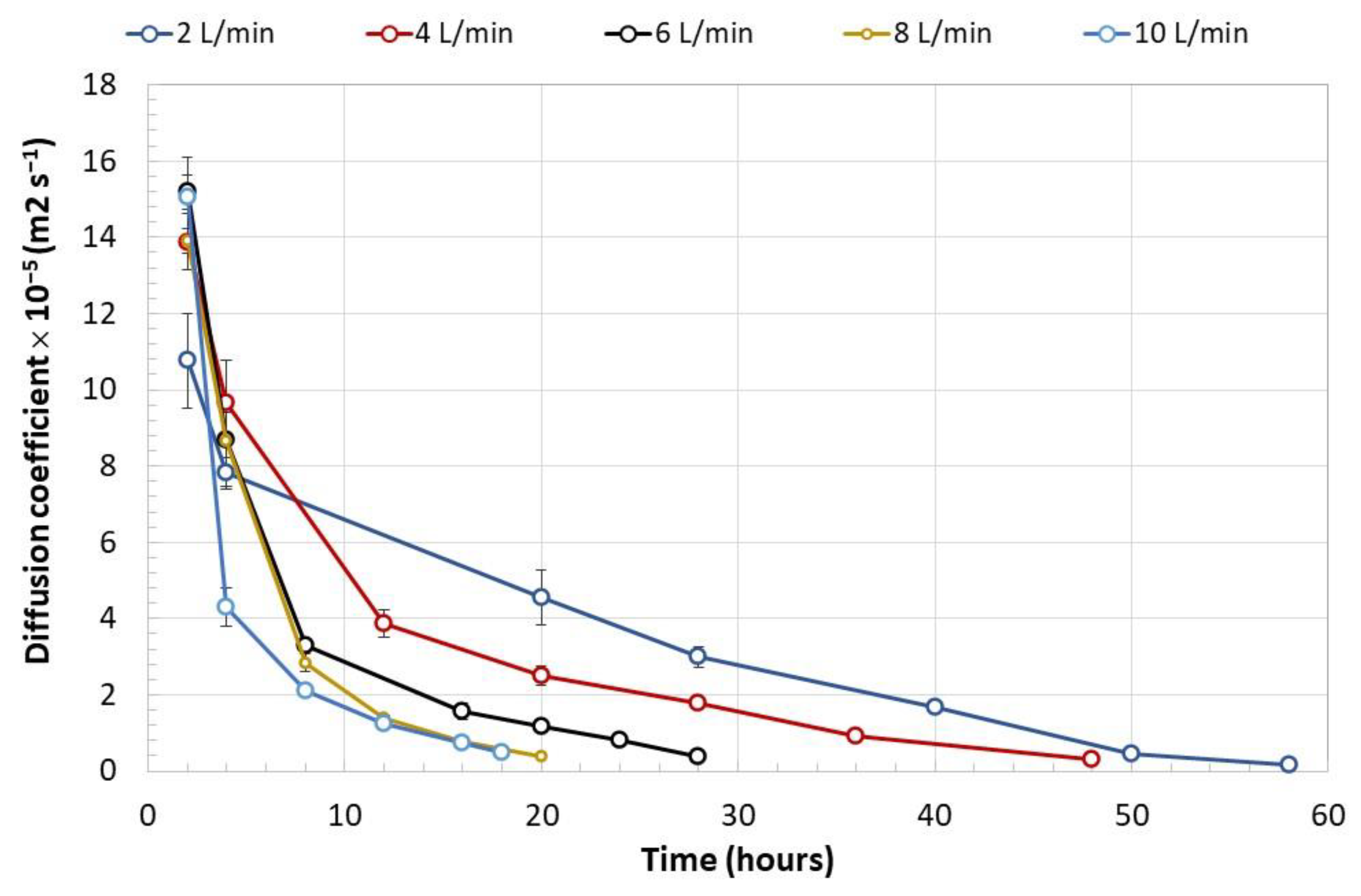
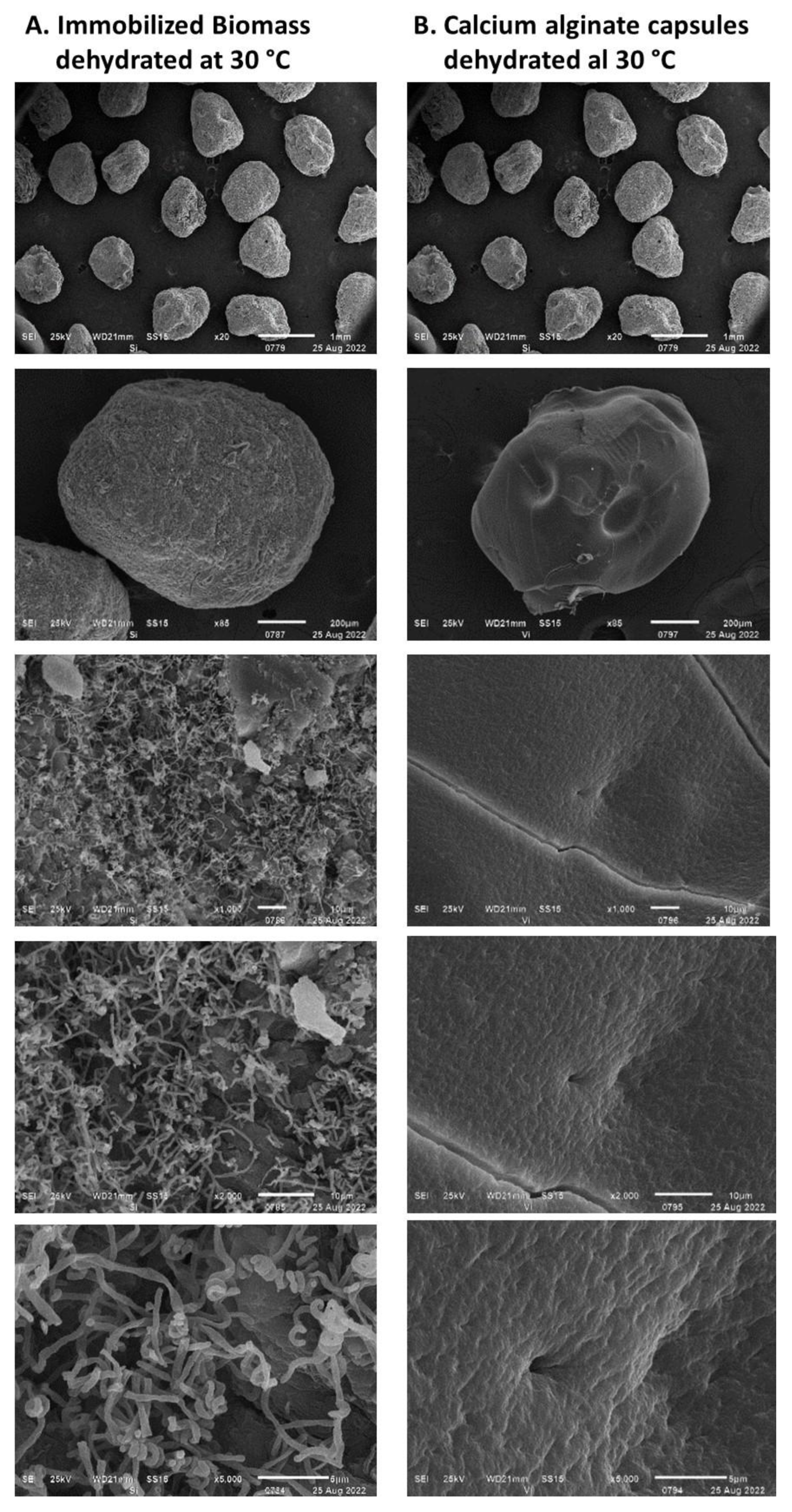
3.3. Dehydration of Immobilized Biomass in Alginate Capsules
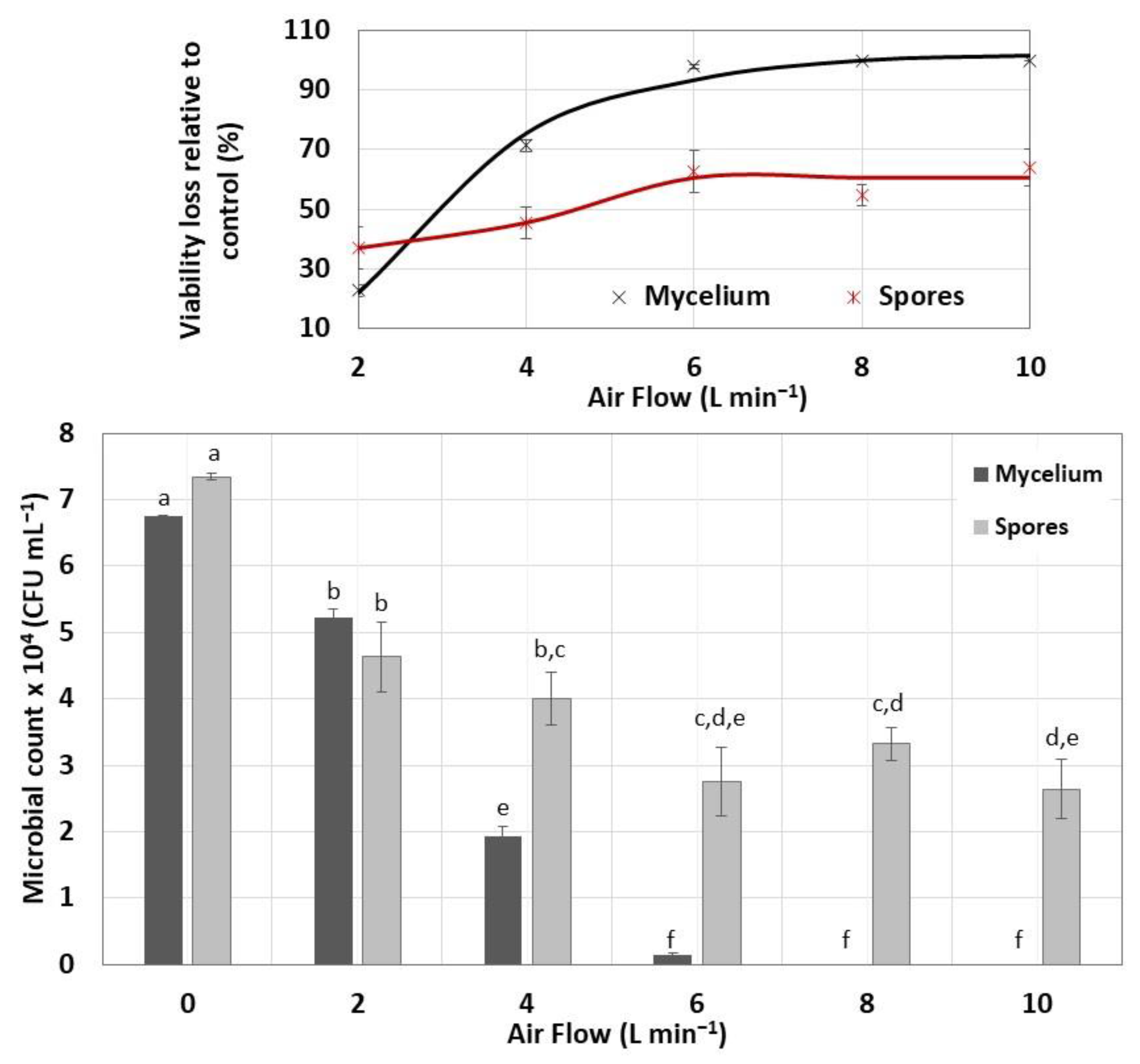
3.4. Viability of Encapsulated and Dehydrated Streptomyces CDBB1232
3.5. Heat Dehydration of Immobilized Biomass
3.6. Effect of Protectors in the Dehydration with Air of Encapsulated Streptomyces CDBB1232 Mycelium
4. Discussion
- Cellular shear damage. The high concentration of the biomass and the high viscosity of the alginate solution demanded vigorous stirring to produce a homogeneous suspension of the heterogeneously sized pellets for the capsule production by extrusion. The high shear force, in addition to causing cell stress, can cause cell damage. The loss of viability due to shearing of cellular material has been already reported by Sabaratnam and Traquiar (2002) [44] and Tamreihao et al. (2016) [60].
- Cellular stress due to mechanical dispersion of pellets. In liquid culture, Streptomyces grows as pellets. The variable diameter of the pellets demanded their mechanical dispersion in order to facilitate their flow through the extrusion device. Mechanical dispersion is a reason for cellular stress and consequently of viability loss [51].
- Stress due to rapid dehydration of immobilized biomass. A high rate of moisture removal was observed during the first 8 h after the dehydration process began and under feeding air flows of 6 L min−1 and higher. Under these air flows, water diffusion coefficients between 8 × 10−5 and 14 × 10−5 m2 s−1 were determined, which were higher than those reported in the literature for fruit dehydration [61]. The viability loss of the immobilized mycelium was registered after the capsule dehydration under air flows equal to or greater than 6 L min−1. It has been suggested that accelerated humidity loss in polymeric materials such as alginate causes the formation of a crust that shrinks rapidly toward the capsule core and subsequently slows the rate of humidity removal [62]. The rapid contraction of the capsule surface can cause cell damage and cell death because of lack of oxygen [63]. In the present work, under an air flow of 10 L min−1, the deceleration of the humidity removal process by contraction of the alginate crust was detected by the change in slope in the humidity loss profile, which decreased from 12 to 3.6 g h−1. The lower loss of the dehydrated mycelium viability under an air flow of 2 L min−1 can be explained by the long strain adaptation process that lasted approximately 10 h before the water loss was recorded.
- Transfer of nutrients and oxygen to the capsule core. Biological material immobilized in alginate can give rise to low percentages of viability considering that the availability of nutrients to the nucleus of the capsule will depend on the cell concentration, the capsule diameter, and the capsule porosity. In the present work, the micellar viability was determined by release of the biological material, as well as by dispersion with grinding of the fresh and dehydrated capsules. Both methods yielded similar viability percentages, although with microbial counts that differed by one power; the highest microbial count was determined in the samples of biological material dispersed by grinding.
- Biomass instability. Manteca et al. (2008) and López-García et al. (2014) [70,71] reported that culture instability can lead to rapid aging of the mycelium and subsequent disintegration of dead hyphae. In the present work, biological material resistant to the encapsulation process was obtained by immobilizing cultured cellular material obtained at the end of its exponential growth phase. Vriezen et al. (2006) and Schoebitz et al. (2012) [24,72] reported that the mycelium harvested in this phase is stable and more resistant to stress caused during handling.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donald, L.; Pipite, A.; Subramani, R.; Owen, J.; Keyzers, R.A.; Taufa, T. Streptomyces: Still the Biggest Producer of New Natural Secondary Metabolites, a Current Perspective. Microbiol. Res. 2022, 13, 418–465. [Google Scholar] [CrossRef]
- Pacios-Michelena, S.; Aguilar González, C.N.; Alvarez-Perez, O.B.; Rodriguez-Herrera, R.; Chávez-González, M.; Arredondo Valdés, R.; Ascacio Valdés, J.A.; Govea Salas, M.; Ilyina, A. Application of Streptomyces Antimicrobial Compounds for the Control of Phytopathogens. Front. Sustain. Food Syst. 2021, 5, 696518. [Google Scholar] [CrossRef]
- Devi, A.P.; Jesumaharaja, G.L.; Balasundaram, K.; Sahana, N.; Battacharya, P.M.; Roy, A.; Bandyopadhyay, S.; Khalko, S. Chapter 19—Streptomyces sp.: A feasible biocontrol agent for sustainable management of crop diseases. In Microbes and Microbial Biotechnology for Green Remediation; Malik, J.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 377–388. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Moradi Pour, M.; Ait Barka, E. A Novel Route for Double-Layered Encapsulation of Streptomyces fulvissimus Uts22 by Alginate-Arabic Gum for Controlling of Pythium aphanidermatum in Cucumber. Agronomy 2022, 12, 655. [Google Scholar] [CrossRef]
- Sousa, J.A.J.; Olivares, F.L. Plant growth promotion by streptomycetes: Ecophysiology, mechanisms and applications. Chem. Biol. Technol. Agric. 2016, 3, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez-Moreno, Z.R.; Vinchira-Villarraga, D.M.; Vergara-Morales, D.I.; Castellanos, L.; Ramos, F.A.; Guarnaccia, C.; Degrassi, G.; Venturi, V.; Moreno-Sarmiento, N. Plant-Growth Promotion and Biocontrol Properties of Three Streptomyces spp. Isolates to Control Bacterial Rice Pathogens. Front. Microbiol. 2019, 10, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubici, G. Streptomyces spp. as biocontrol agents against Fusarium species. CAB Rev. 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant Growth Promoting and Biocontrol Activity of Streptomyces spp. as Endophytes. Int. J. Mol. Sci. 2018, 22, 952. [Google Scholar] [CrossRef] [Green Version]
- Leggett, M.J.; McDonnell, G.; Denyer, S.P.; Setlow, P.; Maillard, J.-Y. Bacterial spore structures and their protective role in biocide resistance. J. Appl. Microbiol. 2012, 113, 485–498. [Google Scholar] [CrossRef]
- McCormick, J.R.; Flärdh, K. Signals and regulators that govern Streptomyces development. FEMS Microbiol. Rev. 2012, 36, 206–231. [Google Scholar] [CrossRef] [Green Version]
- Djejeva, G.; Grigorova, Y.; Stoychev, M. Resistance of spores and vegetative cells of Streptomyces thermovulgaris strain 127 and the glucose isomerase produced by them to temperature and glutaraldehyde. Acta Microbiol. Bulg. 1990, 25, 8–15. [Google Scholar]
- Zacky, F.A.; Ting, A.S.Y. Biocontrol of Fusarium oxysporum f.sp. cubense tropical race 4 by formulated cells and cell-free extracts of Streptomyces griseus in sterile soil environment. Biocontrol Sci. Technol. 2015, 25, 685–696. [Google Scholar] [CrossRef]
- Berninger, T.; González-López, Ó.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb. Biotechnol. 2018, 11, 277–301. [Google Scholar] [CrossRef] [Green Version]
- Myo, E.M.; Ge, B.; Ma, J.; Cui, H.; Liu, B.; Shi, L.; Jiang, M.; Zhang, K. Streptomyces fradiae NKZ-259 and its formulation to enhance plant growth. BMC Microbiol. 2019, 19, 155. [Google Scholar] [CrossRef] [Green Version]
- Aristide, D.; Martial, T.T.P.; Ruth, N.N.E.; Grace, L.B.; Ebenezer, F.T.; Flore, M.P.T.; Thaddee, B. Effects of a Powder Formulation of Streptomyces cameroonensis on Growth and Resistance of Two Cocoa Hybrids from Cameroon against Phytophthora megakarya (Causal Agent of Black Pod Disease). J. Microbiol. Biotechnol. 2022, 32, 160–169. [Google Scholar] [CrossRef]
- Morgan, C.A.; Herman, N.; White, P.A.; Vesey, G. Preservation of micro-organisms by drying; A review. J. Microbiol. Methods 2006, 66, 183–193. [Google Scholar] [CrossRef]
- Ortiz, T.; Ocampo, V.; Prada, L.D.; Franco-Correa, M. Métodos de conservación para actinobacterias con actividad solubilizadora de fósforo. Rev. Colomb. Biotecnol. 2016, 18, 32–39. [Google Scholar] [CrossRef]
- García, A.H. Anhydrobiosis in bacteria: From physiology to applications. J. Biosci. 2011, 36, 939–950. [Google Scholar] [CrossRef]
- Meng, X.; Stanton, C.; Fitzgerald, G.; Daly, C.; Ross, R. Anhydrobiotics: The challenges of drying probiotic cultures. Food Chem. 2008, 106, 1406–1416. [Google Scholar] [CrossRef]
- Yocheva, L.; Najdenova, M.; Doncheva, D.; Antonova-Nikolova, S. Influence of the long-term preservation on some biological features of three Streptomycetes strains, producers of antibiotic substances. J. Cult. Collect. 2002, 3, 25–32. [Google Scholar]
- Bashan, Y.; Hernández, J.; Leyva, L.; Bacilio, M. Alginate microbeads as inoculant carriers for plant growth-promoting bacteria. Biol. Fertil. Soils 2002, 35, 359–368. [Google Scholar] [CrossRef]
- Russo, A.; Basaglia, M.; Tola, E.; Casella, S. Survival, root colonisation and biocontrol capacities of Pseudomonas fluorescens F113 LacZY in dry alginate microbeads. J. Ind. Microbiol. Biotechnol. 2001, 27, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Schoebitz, M.; Simonin, H.; Poncelet, D. Starch filler and osmoprotectants improve the survival of rhizobacteria in dried alginate beads. J. Microencapsul. 2012, 29, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; Raymond, Y.; Arcand, Y. Effects of production methods and protective ingredients on the viability of probiotic Lactobacillus rhamnosus R0011 in air-dried alginate beads. Can. J. Microbiol. 2017, 63, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Strobel, S.A.; Allen, K.; Roberts, C.; Jimenez, D.R.; Scher, H.B.; Jeoh, T. Industrially-Scalable Microencapsulation of Plant Beneficial Bacteria in Dry Cross-Linked Alginate Matrix. Ind. Biotechnol. 2018, 14, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Zamoum, M.; Goudjal, Y.; Sabaou, N.; Mathieu, F.; Zitouni, A. Development of formulations based on Streptomyces rochei strain PTL2 spores for biocontrol of Rhizoctonia solani damping-off of tomato seedlings. Biocontrol Sci. Technol. 2017, 27, 723–738. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhao, Y.; Kaleem, I.; Li, C. Preparation of calcium-alginate microcapsuled microbial fertilizer coating Klebsiella oxytoca Rs-5 and its performance under salinity stress. Eur. J. Soil Biol. 2011, 47, 152–159. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, L.; Qin, S.; Li, C.X. Encapsulation of R. planticola Rs-2 from alginate-starch-bentonite and its controlled release and swelling behavior under simulated soil conditions. J. Ind. Microbiol. Biotechnol. 2012, 39, 317–327. [Google Scholar] [CrossRef]
- Feng, J.; Dou, J.; Wu, Z.; Yin, D.; Wu, W. Controlled Release of Biological Control Agents for Preventing Aflatoxin Contamination from Starch-Alginate Beads. Molecules 2019, 24, 1858. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.T.; Poh, P.E.; Chin, S.K. Microorganism preservation by convective air-drying-A review. Dry. Technol. 2017, 36, 764–779. [Google Scholar] [CrossRef] [Green Version]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998-2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Fathi, F.; Saberi Riseh, R.; Khodaygan, P.; Hosseini, S.; Skorik, Y.A. Microencapsulation of a Pseudomonas Strain (VUPF506) in Alginate-Whey Protein-Carbon Nanotubes and Next-Generation Sequencing Identification of This Strain. Polymers 2021, 13, 4269. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Alenezi, F.N.; Belbahri, L. Recent Advances in Encapsulation Techniques of Plant Growth-Promoting Microorganisms and Their Prospects in the Sustainable Agriculture. Appl. Sci. 2022, 12, 9020. [Google Scholar] [CrossRef]
- Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef]
- Martínez-Cano, B.; Mendoza-Meneses, C.J.; García-Trejo, J.F.; Macías-Bobadilla, G.; Aguirre-Becerra, H.; Soto-Zarazúa, G.M.; Feregrino-Pérez, A.A. Review and Perspectives of the Use of Alginate as a Polymer Matrix for Microorganisms Applied in Agro-Industry. Molecules 2022, 27, 4248. [Google Scholar] [CrossRef]
- Szopa, D.; Mielczarek, M.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Chojnacka, K.; Witek-Krowiak, A. Encapsulation efficiency and survival of plant growth-promoting microorganisms in an alginate-based matrix—A systematic review and protocol for a practical approach. Ind. Crops Prod. 2022, 181, 114846. [Google Scholar] [CrossRef]
- Simó, G.; Fernández-Fernández, E.; Vila-Crespo, J.; Ruipérez, V.; Rodríguez-Nogales, J.M. Research progress in coating techniques of alginate gel polymer for cell encapsulation. Carbohydr. Polym. 2017, 170, 1–14. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Bhatia, S.; Al-Azri, M.S.; Ullah, S.; Najmi, A.; Albratty, M.; Meraya, A.M.; Mohan, S.; Aldawsari, M.F. Effect of Drying Temperature on Physical, Chemical, and Antioxidant Properties of Ginger Oil Loaded Gelatin-Sodium Alginate Edible Films. Membranes 2022, 12, 862. [Google Scholar] [CrossRef]
- Shirling, E.B.; Gottlieb, D. Methods for Characterization of Streptomyces Species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef] [Green Version]
- Kurnianto, M.A.; Kusumaningrum, H.D.; Lioe, H.N. Characterization of Streptomyces Isolates Associated with Estuarine Fish Chanos chanos and Profiling of Their Antibacterial Metabolites-Crude-Extract. Int. J. Microbiol. 2020, 2020, 8851947. [Google Scholar] [CrossRef]
- Mancera-López, M.E.; Izquierdo-Estévez, W.F.; Escalante-Sánchez, A.; Ibarra, J.E.; Barrera-Cortés, J. Encapsulation of Trichoderma harzianum conidia as a method of conidia preservation at room temperature and propagation in submerged culture. Biocontrol Sci. Technol. 2019, 29, 107–130. [Google Scholar] [CrossRef]
- Behfar, A.; Aqajari, N.; Shushizadeh, M.R.; Ramezani, Z.; Ghatrami, E.R. Production of Alginate from Persian Gulf Sargassum angustifolium Seaweeds: Novel Extraction and Characterization Methods. Jundishapur J. Nat. Pharm. Prod. 2022, 17, e106011. [Google Scholar] [CrossRef]
- Sabaratnam, S.; Traquair, J.A. Formulation of a Streptomyces Biocontrol Agent for the Suppression of Rhizoctonia Damping-off in Tomato Transplants. Biol. Control 2002, 23, 245–253. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Castro-Giraldez, M.; Fito, P.J.; Santagapita, P.R. Encapsulation of lactase in Ca(II)-alginate beads: Effect of stabilizers and drying methods. Food Res. Int. 2017, 100, 296–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Celaya, A.L.; Ortiz-García, M.; Vernon-Carter, E.J.; Jauregui-Rincón, J.; Galindo, E.; Serrano-Carreón, L. Spray-Drying Microencapsulation of Trichoderma harzianum Conidias in Carbohydrate Polymers Matrices. Carbohydr. Polym. 2012, 88, 1141–1148. [Google Scholar] [CrossRef]
- Himmelblau, D.H. Basic Principles and Calculations in Chemical Engineering, 3rd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1974; Chapter 3. [Google Scholar]
- Bird, B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; John Wiley & Sons Inc.: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Bérdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Zacchetti, B.; Andrianos, A.; van Dissel, D.; de Ruiter, E.; van Wezel, G.P.; Claessen, D. Microencapsulation extends mycelial viability of Streptomyces lividans 66 and increases enzyme production. BMC Biotechnol. 2018, 18, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, M.C.; Orosco, C.E.; Gordillo, M.A.; Navarro, A.R. In vivo and in vitro antagonism of Streptomyces sp. RO3 against Penicillium digitatum and Geotrichum candidum. Afr. J. Microbiol. Res. 2010, 4, 2451–2456. [Google Scholar] [CrossRef]
- Wardell, J.N.; Stocks, S.M.; Thomas, C.R.; Bushell, M.E. Decreasing the hyphal branching rate of Saccharopolyspora erythraea NRRL 2338 leads to increased resistance to breakage and increased antibiotic production. Biotechnol. Bioeng. 2002, 78, 141–146. [Google Scholar] [CrossRef]
- van Wezel, G.P.; McKenzie, N.L.; Nodwell, J.R. Chapter 5. Applying the genetics of secondary metabolism in model actinomycetes to the discovery of new antibiotics. Methods Enzymol. 2009, 458, 117–141. [Google Scholar] [CrossRef]
- Celler, K.; Picioreanu, C.; van Loosdrecht, M.C.; van Wezel, G.P. Structured morphological modeling as a framework for rational strain design of Streptomyces species. Antonie van Leeuwenhoek 2012, 102, 409–423. [Google Scholar] [CrossRef] [Green Version]
- Chater, K.F.; Biró, S.; Lee, K.J.; Palmer, T.; Schrempf, H. The complex extracellular biology of Streptomyces. FEMS Microbiol. Rev. 2010, 34, 171–198. [Google Scholar] [CrossRef] [Green Version]
- van Wezel, G.P.; McDowall, K.J. The regulation of the secondary metabolism of Streptomyces: New links and experimental advances. Nat. Prod. Rep. 2011, 28, 1311–1333. [Google Scholar] [CrossRef]
- van Dissel, D.; Claessen, D.; van Wezel, G.P. Morphogenesis of Streptomyces in submerged cultures. Adv. Appl. Microbiol. 2014, 89, 1–45. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Mamba, G.; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef]
- Tamreihao, K.; Ningthoujam, D.S.; Nimaichand, S.; Singh, E.S.; Reena, P.; Singh, S.H.; Nongthomba, U. Biocontrol and plant growth promoting activities of a Streptomyces corchorusii strain UCR3-16 and preparation of powder formulation for application as biofertilizer agents for rice plant. Microbiol. Res. 2016, 192, 260–270. [Google Scholar] [CrossRef]
- López-Méndez, E.M.; Ortiz-García-Carrasco, B.; Ruiz-Espinosa, H.; Sampieri-Crod, A.; García-Alvarado, M.A.; Ochoa-Velasco, C.E.; Escobedo-Morales, A.; Ruiz-Lopez, I.I. Effect of shape change and initial geometry on water diffusivity estimation during drying of gel model systems. J. Food Eng. 2018, 216, 52–64. [Google Scholar] [CrossRef]
- Waje, S.S.; Meshram, M.W.; Chaudhary, V.; Pandey, R.; Mahanawar, P.A.; Thorat, B.N. Drying and shrinkage of polymer gels. Braz. J. Chem. Eng. 2005, 22, 209–216. [Google Scholar] [CrossRef] [Green Version]
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Surampalli, R.Y.; Prévost, D. Bio-encapsulation of microbial cells for targeted agricultural delivery. Crit. Rev. Biotechnol. 2011, 31, 211–226. [Google Scholar] [CrossRef]
- Brun-Graeppi, A.K.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.W. Cell microcarriers and microcapsules of stimuli-responsive polymers. J. Control. Release 2011, 149, 209–224. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, A.J. Controlled release of immobilized cells as a strategy to regulate ecological competence of inocula. In Biotechnics/Wastewater. Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 1994; Volume 51. [Google Scholar] [CrossRef]
- Rathore, S.; Desai, P.; Liew, C.V.; Chan, L.W.; Heng, P.W.S. Microencapsulation of microbial cells. J. Food Eng. 2013, 116, 369–381. [Google Scholar] [CrossRef]
- Christenson, L.; Dionne, K.; Lysaught, M. Biomedical application of immobilized cells. In Fundamentals of Animal Cell Encapsulation and Immobilization; Goosen, M.F.A., Ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 7–41. [Google Scholar]
- Cassidy, M.B.; Lee, H.; Trevors, J.T. Environmental applications of immobilized microbial cells: A review. J. Ind. Microbiol. 1996, 16, 79–101. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manteca, A.; Alvarez, R.; Salazar, N.; Yagüe, P.; Sanchez, J. Mycelium Differentiation and Antibiotic Production in Submerged Cultures of Streptomyces coelicolor. Appl. Environ. Microbiol. 2008, 74, 3877–3886. [Google Scholar] [CrossRef] [Green Version]
- López-García, M.T.; Rioseras, B.; Yagüe, P.; Álvarez, J.R.; Manteca, Á. Cell immobilization of Streptomyces coelicolor: Effect on differentiation and actinorhodin production. Int. Microbiol. 2014, 17, 75–80. [Google Scholar] [CrossRef]
- Vriezen, J.A.; de Bruijn, F.J.; Nüsslein, K. Desiccation responses and survival of Sinorhizobium meliloti USDA 1021 in relation to growth phase, temperature, chloride and sulfate availability. Lett. Appl. Microbiol. 2006, 42, 172–178. [Google Scholar] [CrossRef]
- Lobo, C.B.; Juárez Tomás, M.S.; Viruel, E.; Ferrero, M.A.; Lucca, M.E. Development of low-cost formulations of plant growth-promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol. Res. 2019, 219, 12–25. [Google Scholar] [CrossRef]
- Kawakita, R.; Leveau, J.H.J.; Jeoh, T. Comparing Fluidized Bed Spray-Coating and Spray-Drying Encapsulation of Non-Spore-Forming Gram-Negative Bacteria. Ind. Biotechnol. 2021, 17, 283–289. [Google Scholar] [CrossRef]
- Costa, E.; Teixidó, N.; Usall, J.; Fons, E.; Gimeno, V.; Delgado, J.; Viñas, I. Survival of Pantoea agglomerans strain CPA-2 in a spray-drying process. J. Food Prot. 2002, 65, 185–191. [Google Scholar] [CrossRef]
- Sharifi, S.; Rezazad-Bari, M.; Alizadeh, M.; Almasi, H.; Amiri, S. Use of whey protein isolate and gum Arabic for the co-encapsulation of probiotic Lactobacillus plantarum and phytosterols by complex coacervation: Enhanced viability of probiotic in Iranian white cheese. Food Hydrocoll. 2021, 113, 106496. [Google Scholar] [CrossRef]
- Streeter, J.G. Effect of trehalose on survival of Bradyrhizobium japonicum during desiccation. J. Appl. Microbiol. 2003, 95, 484–491. [Google Scholar] [CrossRef]
- Schisler, D.; Slininger, P.; Olsen, N. Appraisal of selected osmoprotectants and carriers for formulating Gram-negative biocontrol agents active against Fusarium dry rot on potatoes in storage. Biol. Control 2016, 98, 1–10. [Google Scholar] [CrossRef]
- Ghandi, A.; Powell, I.B.; Chen, X.D.; Adhikari, B. The Survival of Lactococcus lactis in a Convective-Air-Drying Environment: The Role of Protectant Solids, Oxygen Injury, and Mechanism of Protection. Dry. Technol. 2013, 31, 1661–1674. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Zavaglia, A.; Tymczyszyn, E.; De Antoni, G.; Disalvo, E.A. Action of trehalose on the preservation of Lactobacillus delbrueckii ssp. bulgaricus by heat and osmotic dehydration. J. Appl. Microbiol. 2003, 95, 1315–1320. [Google Scholar] [CrossRef]
- Rueda, B.; Miguélez, E.M.; Hardisson, C.; Manzanal, M.B. Changes in glycogen and trehalose content of Streptomyces brasiliensis hyphae during growth in liquid cultures under sporulating and non-sporulating conditions. FEMS Microbiol. Lett. 2001, 194, 181–185. [Google Scholar] [CrossRef]



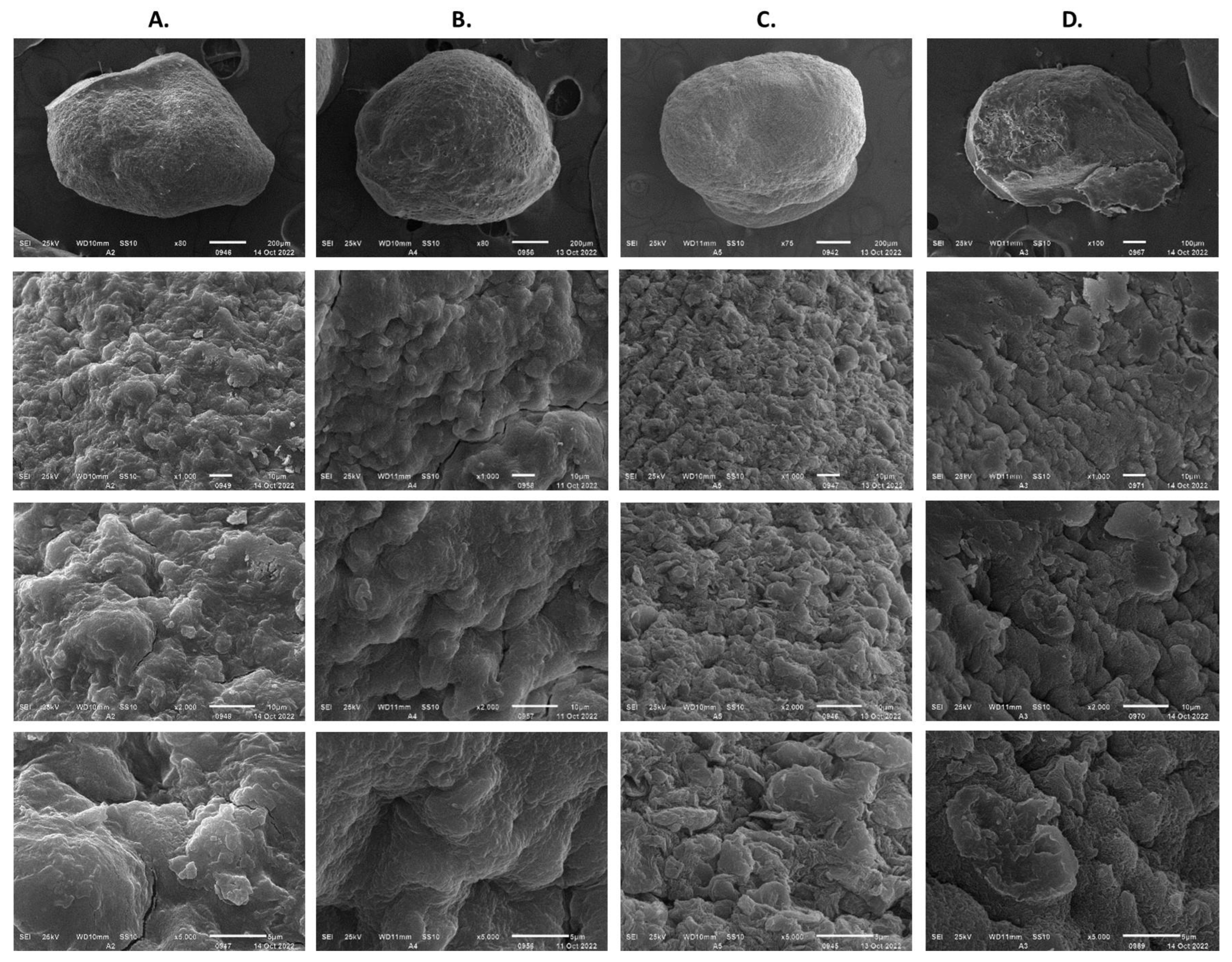
| Protector | Included in: | Encapsulation | Dehydration at 4 L min−1 | Encapsulation and Dehydration |
|---|---|---|---|---|
| Trehalose * YGM | Culture medium Sodium alginate (1.5%) | 100 ± 0 a | 10 ± 1 cde | 13 ± 3 cde |
| YGM + Trehalose | Sodium alginate (1.5%) | 89 ± 7 a | 27 ± 6 c | 24 ± 3 cd |
| YGM + Kaolin | Sodium alginate (1.5%) | 63 ± 6 b | 4 ± 0.3 e | 3 ± 0.1 e |
| YGM Arabic Gum | Sodium alginate (1.5%) Alginate capsule surface | 61 ± 4 b | 100 ± 0 a | 87 ± 5 a |
| YGM | Sodium alginate (1.5%) | 64 ± 5 b | 26 ± 2 c | 16 ± 2 cde |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancera-López, M.E.; Barrera-Cortés, J.; Mendoza-Serna, R.; Ariza-Castolo, A.; Santillan, R. Polymeric Encapsulate of Streptomyces Mycelium Resistant to Dehydration with Air Flow at Room Temperature. Polymers 2023, 15, 207. https://doi.org/10.3390/polym15010207
Mancera-López ME, Barrera-Cortés J, Mendoza-Serna R, Ariza-Castolo A, Santillan R. Polymeric Encapsulate of Streptomyces Mycelium Resistant to Dehydration with Air Flow at Room Temperature. Polymers. 2023; 15(1):207. https://doi.org/10.3390/polym15010207
Chicago/Turabian StyleMancera-López, María Elena, Josefina Barrera-Cortés, Roberto Mendoza-Serna, Armando Ariza-Castolo, and Rosa Santillan. 2023. "Polymeric Encapsulate of Streptomyces Mycelium Resistant to Dehydration with Air Flow at Room Temperature" Polymers 15, no. 1: 207. https://doi.org/10.3390/polym15010207
APA StyleMancera-López, M. E., Barrera-Cortés, J., Mendoza-Serna, R., Ariza-Castolo, A., & Santillan, R. (2023). Polymeric Encapsulate of Streptomyces Mycelium Resistant to Dehydration with Air Flow at Room Temperature. Polymers, 15(1), 207. https://doi.org/10.3390/polym15010207






