Thermal Degradation and Carbonization Mechanism of Fe-Based Metal-Organic Frameworks onto Flame-Retardant Polyethylene Terephthalate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MIL-88B(Fe)
2.3. Preparation of MIL-88B(Fe) and PET Composites
2.4. Characterizations
2.5. Quantum Chemistry Calculation
3. Results and Discussion
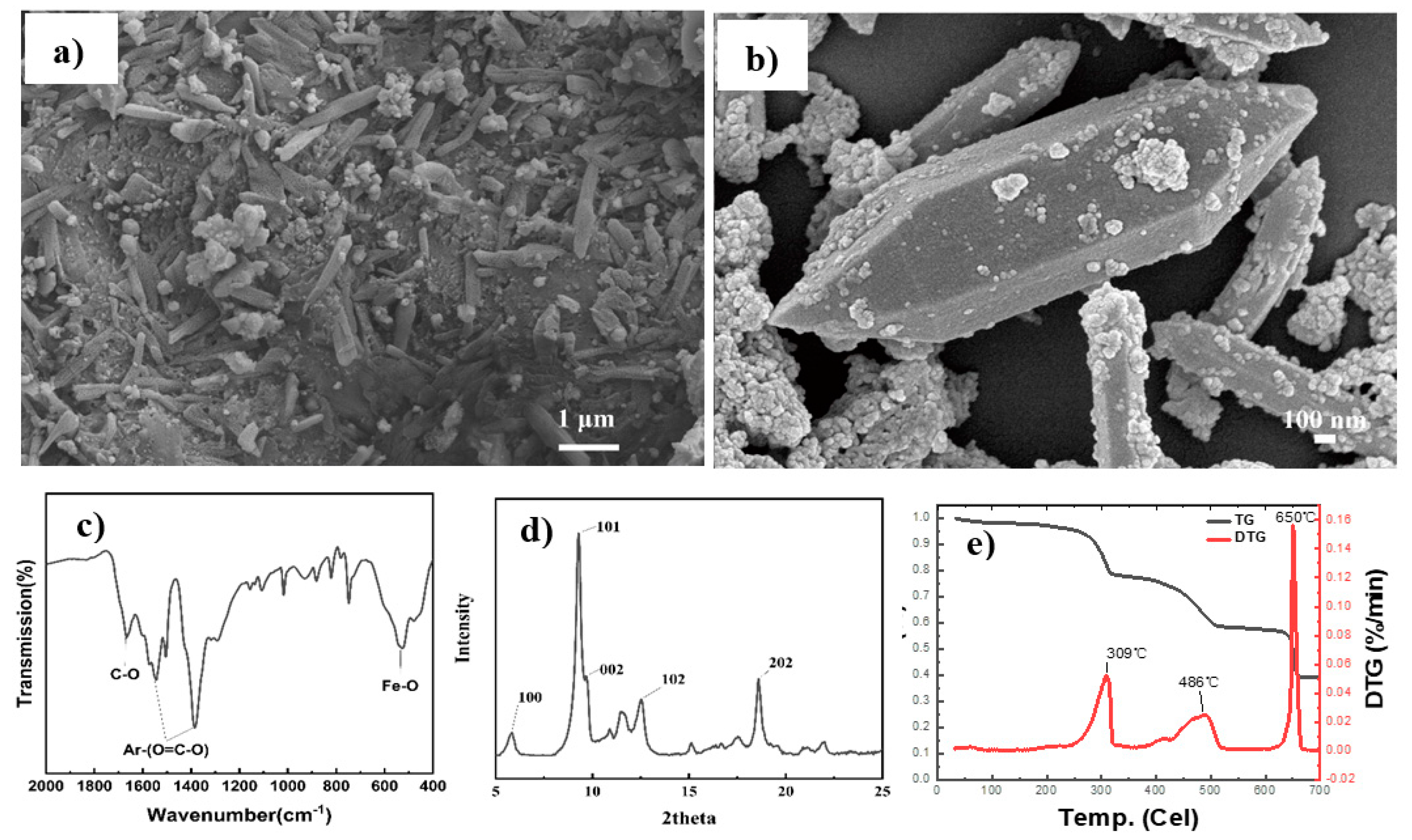
3.1. Characterization of MIL-88B(Fe) and Its PET Composites
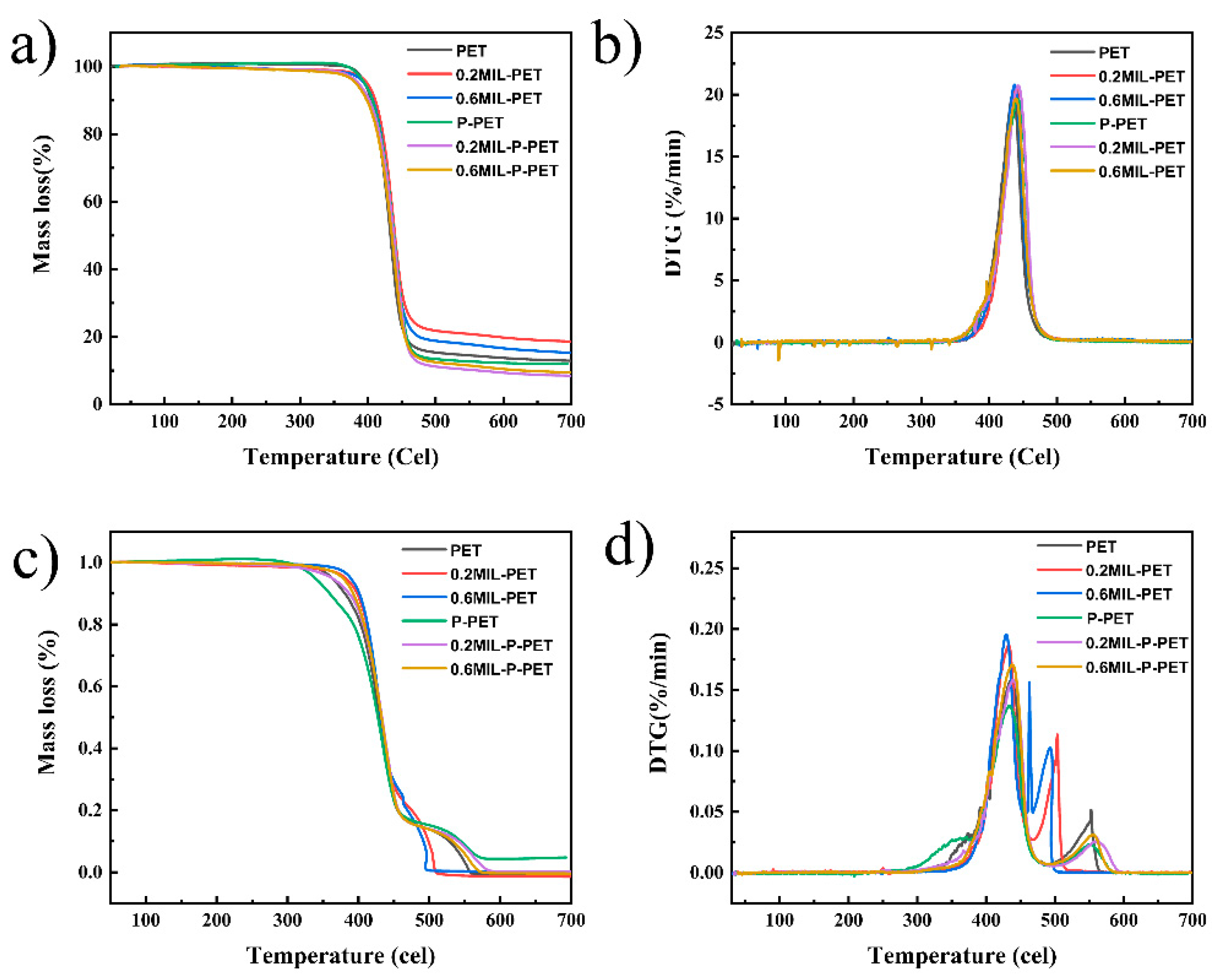
3.2. Thermal Stability
3.3. Flammability of MIL-PET and MIL-P-PET Composites
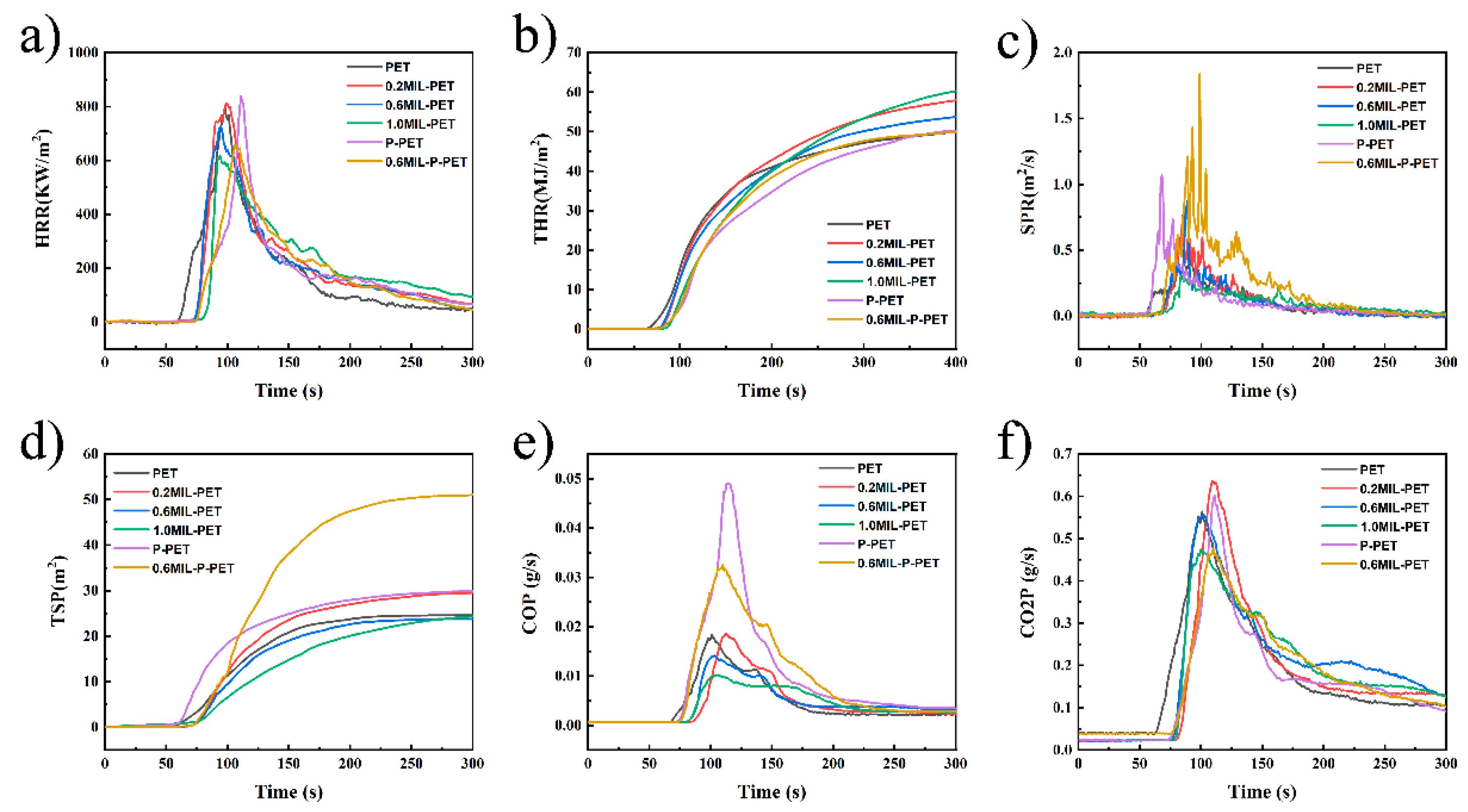
3.4. Forced Combustion of MIL-PET and MIL-P-PET Composites
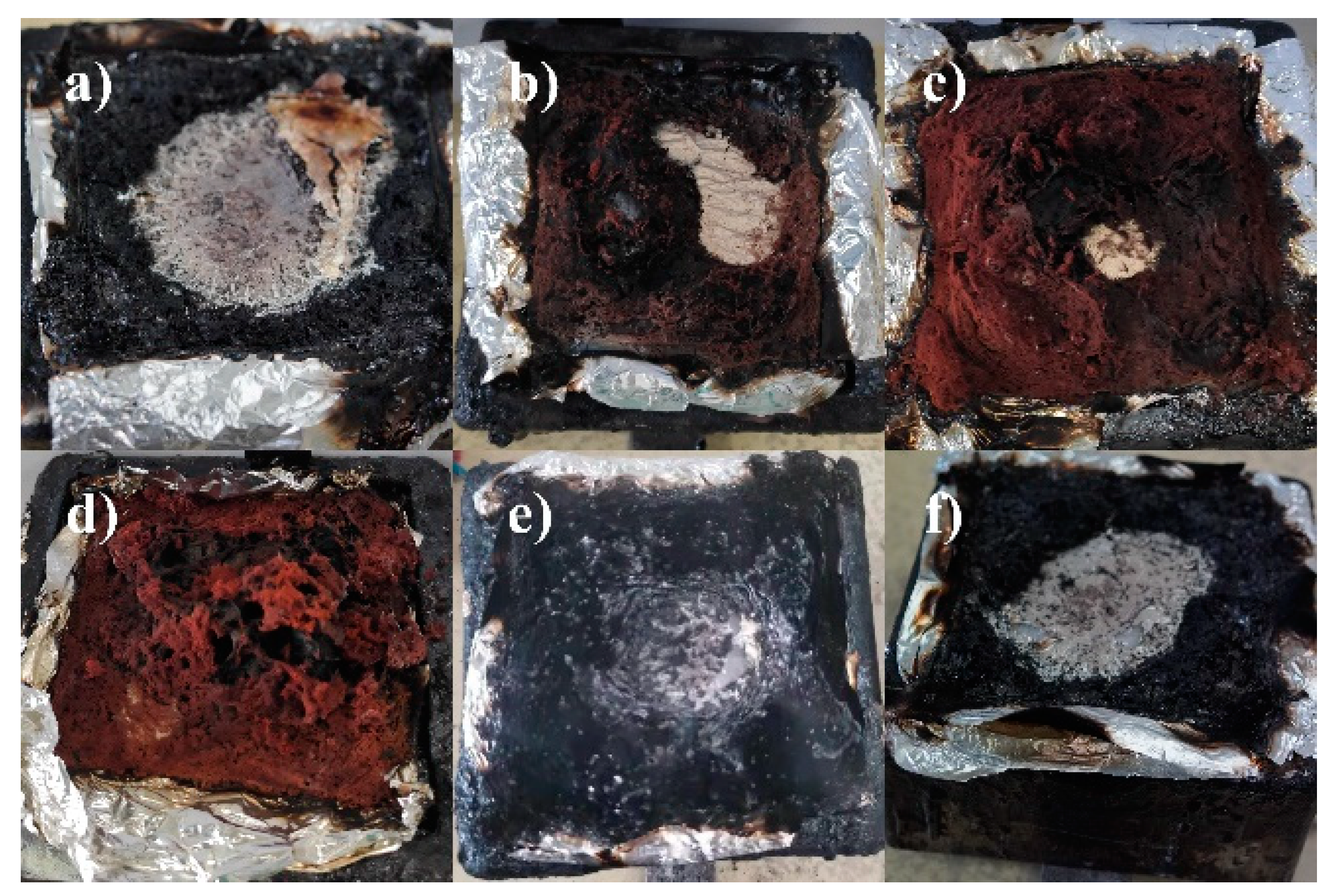
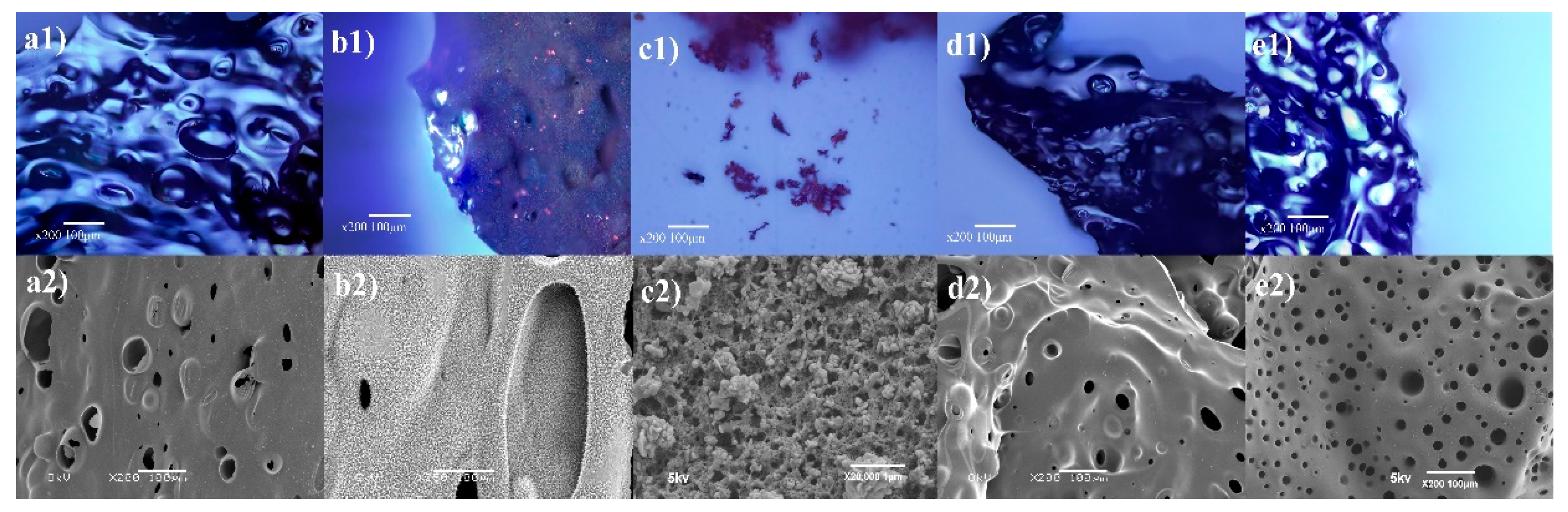
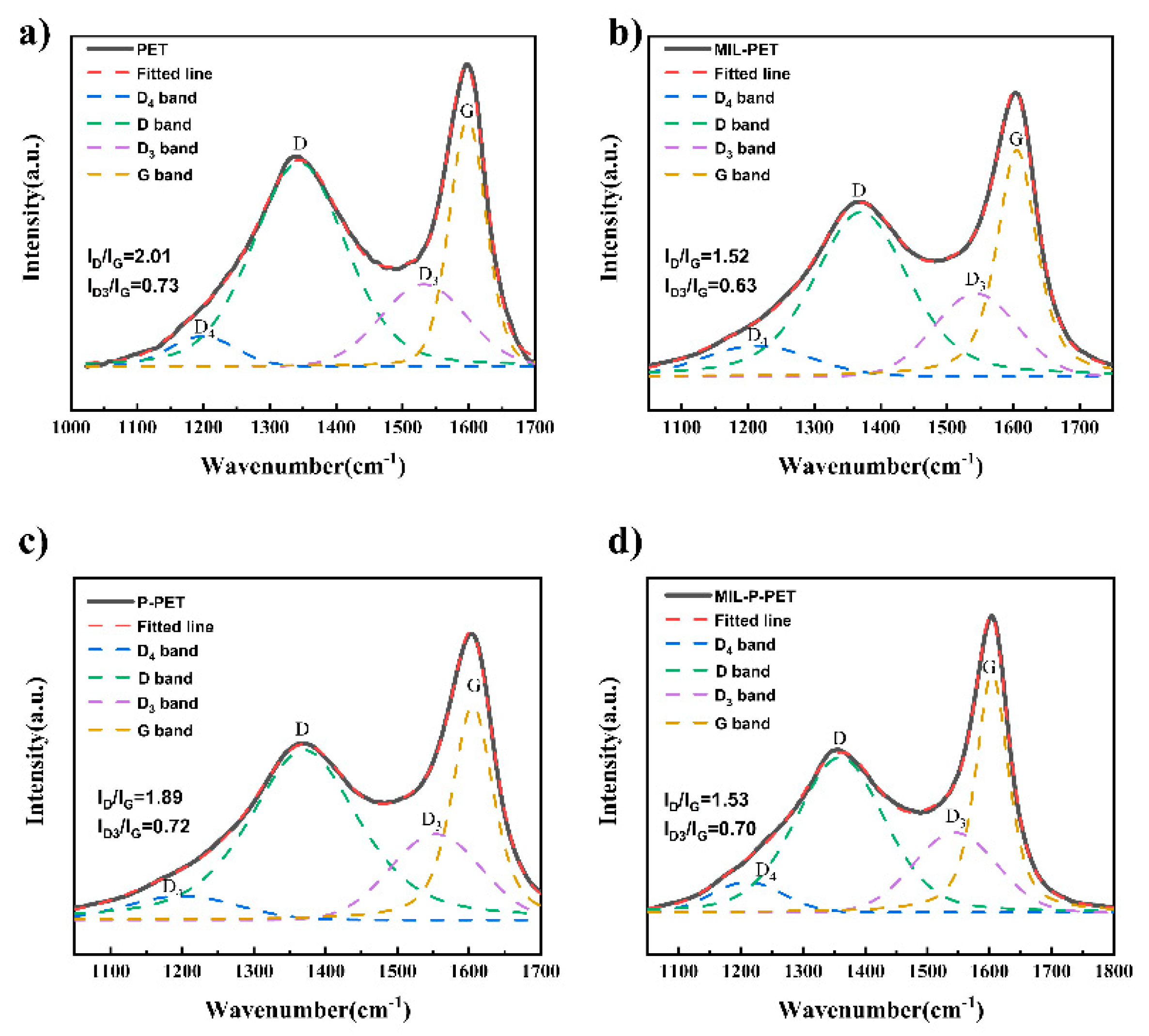
3.5. Char Analysis
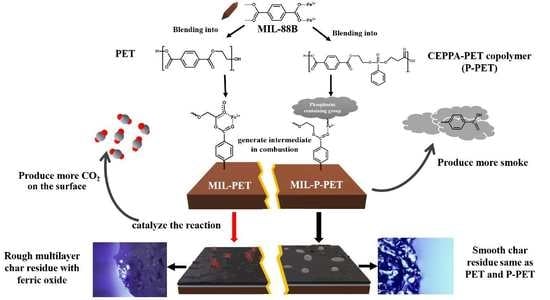
3.6. Flame-Retardant Mechanism
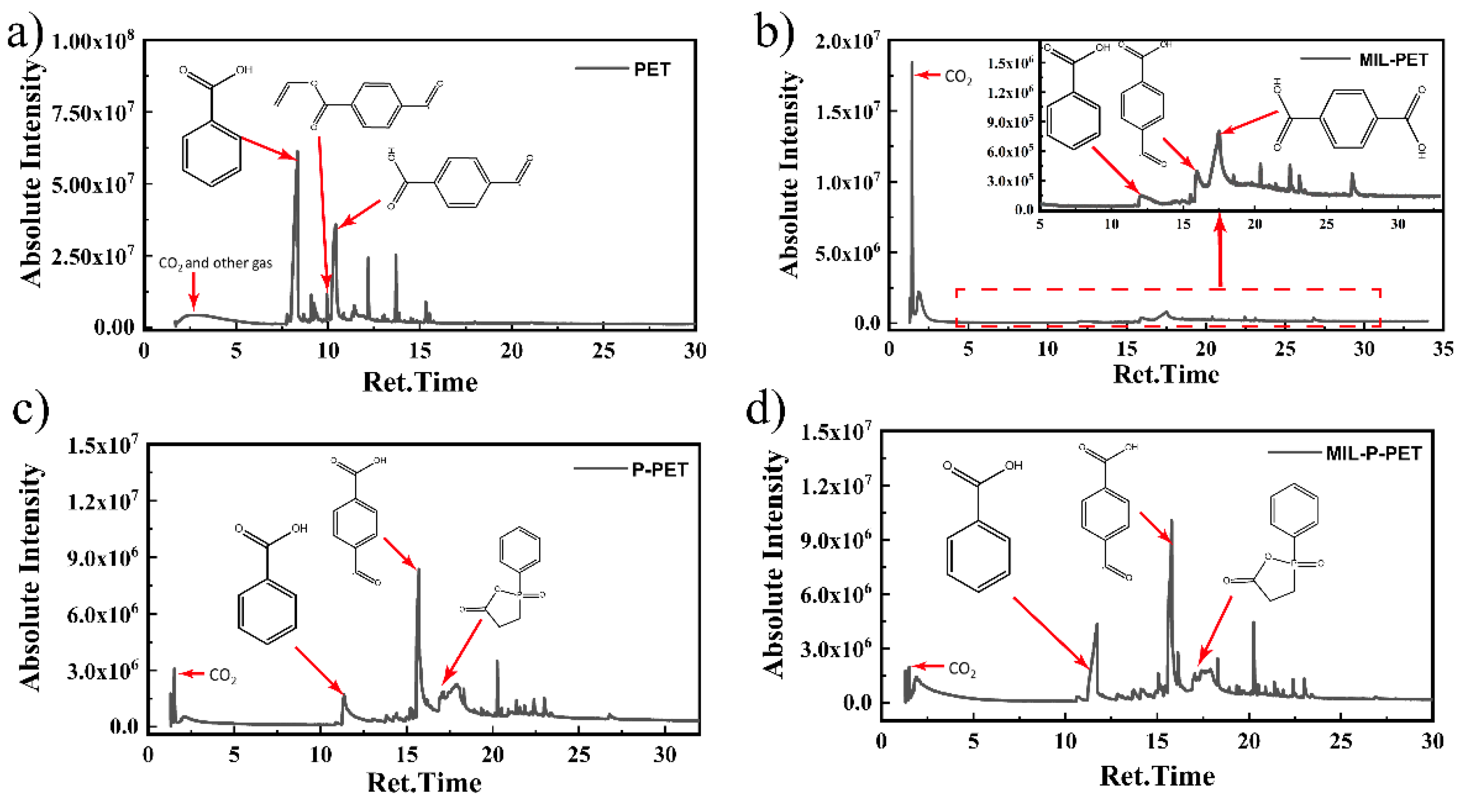
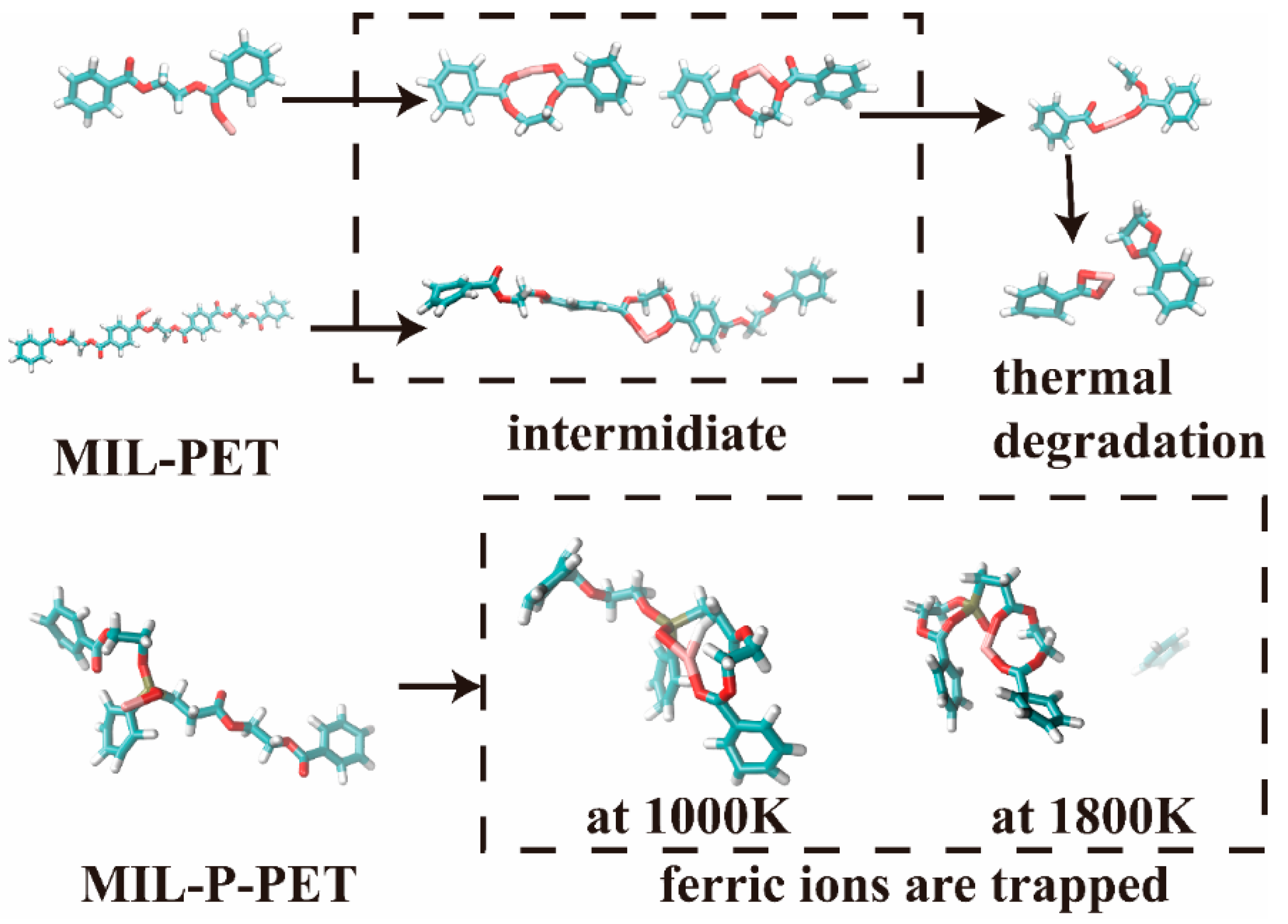
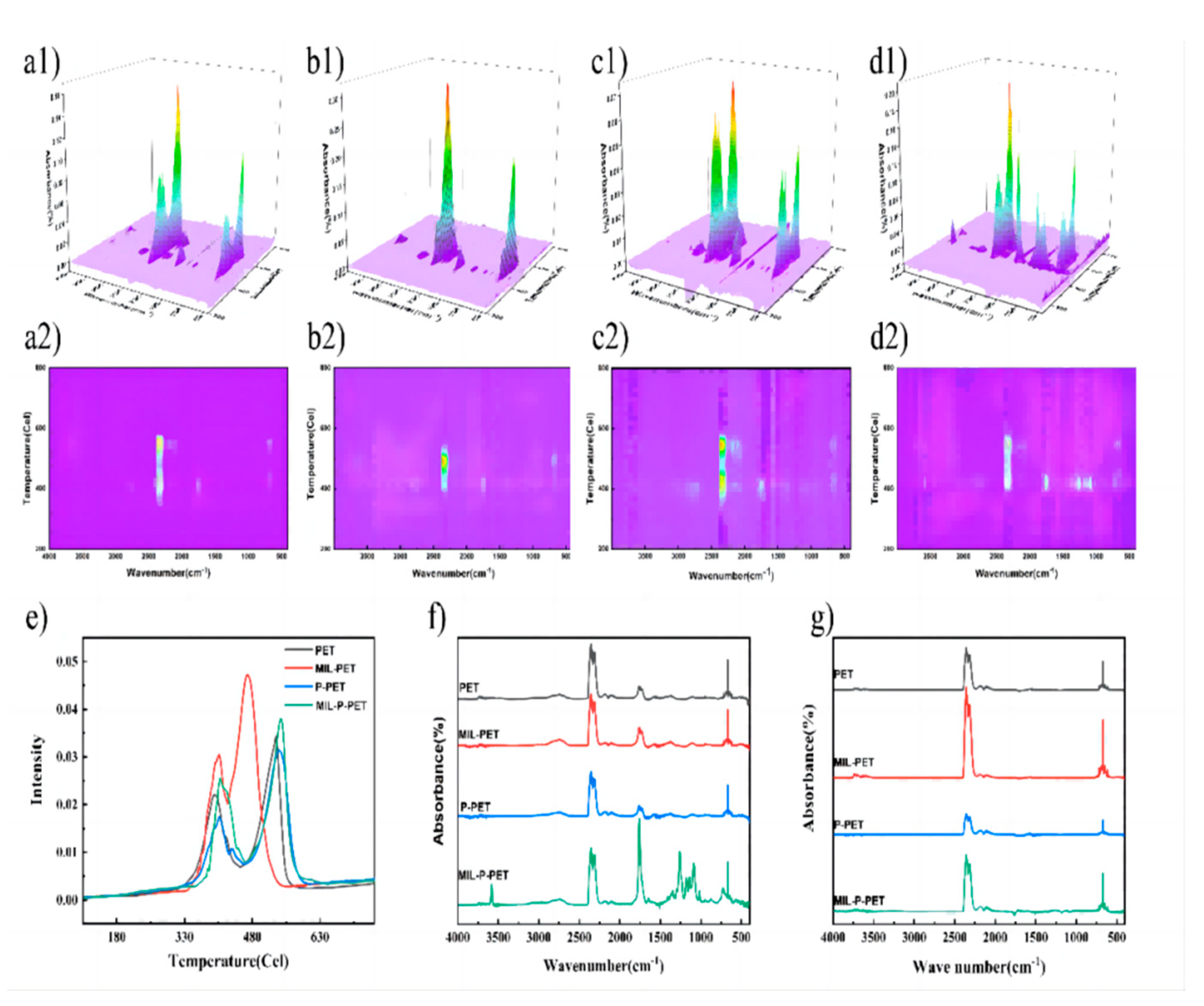
3.7. Thermal Degradation and Carbonization Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, T.-H.; Wu, C.-D.; Kang, S.-H. Thermomechanical properties of polymer nanolithography using atomic force microscopy. Micron 2011, 42, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Niu, M.; Li, J.; Dai, J. Synthesis of a novel microcapsule flame retardant and flame-retardant property of its composites with poly (ethylene terephthalate). J. Polym. Res. 2017, 24, 192. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, M.; Dai, J.; Bai, J.; Xue, B.; Song, Y.; Peng, Y. Flame-retarded polyethylene terephthalate with carbon microspheres/magnesium hydroxide compound flame retardant. Fire Mater. 2018, 42, 794–804. [Google Scholar] [CrossRef]
- Laoutid, F.; Ferry, L.; Lopez-Cuesta, J.M.; Crespy, A. Red phosphorus/aluminium oxide compositions as flame retardants in recycled poly(ethylene terephthalate). Polym. Degrad. Stab. 2003, 82, 357–363. [Google Scholar] [CrossRef]
- Peng, Y.; Niu, M.; Qin, R.; Xue, B.; Shao, M. Study on flame retardancy and smoke suppression of PET by the synergy between Fe2O3 and new phosphorus-containing silicone flame retardant. High Perform. Polym. 2020, 32, 871–882. [Google Scholar] [CrossRef]
- Zhai, G.; Zhou, J.; Xiang, H.; Innocent, M.T.; Yu, S.; Pan, W.; Li, L.; Zhu, M. Combustion forming hollow nanospheres as a ceramic fortress for flame-retardant fiber. Prog. Nat. Sci. Mater. Int. 2021, 31, 239–247. [Google Scholar] [CrossRef]
- Jia, H.; Yao, Y.; Zhao, J.; Gao, Y.; Luo, Z.; Du, P. A novel two-dimensional nickel phthalocyanine-based metal–organic framework for highly efficient water oxidation catalysis. J. Mater. Chem. A 2017, 6, 1188–1195. [Google Scholar] [CrossRef]
- Wang, C.; An, B.; Lin, W. Metal–Organic Frameworks in Solid–Gas Phase Catalysis. ACS Catal. 2018, 9, 130–146. [Google Scholar] [CrossRef]
- Zhou, N.; Su, F.; Guo, C.; He, L.; Jia, Z.; Wang, M.; Jia, Q.; Zhang, Z.; Lu, S. Two-dimensional oriented growth of Zn-MOF-on-Zr-MOF architecture: A highly sensitive and selective platform for detecting cancer markers. Biosens. Bioelectron. 2019, 123, 51–58. [Google Scholar] [CrossRef]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-Organic Framework Nanoparticles in Photodynamic Therapy: Current Status and Perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Abid, H.R.; Tian, H.; Ang, H.-M.; Tade, M.O.; Buckley, C.E.; Wang, S. Nanosize Zr-metal organic framework (UiO-66) for hydrogen and carbon dioxide storage. Chem. Eng. J. 2012, 187, 415–420. [Google Scholar] [CrossRef]
- Park, J.; Yuan, D.; Pham, K.T.; Li, J.-R.; Yakovenko, A.; Zhou, H.-C. Reversible Alteration of CO2 Adsorption upon Photochemical or Thermal Treatment in a Metal–Organic Framework. J. Am. Chem. Soc. 2012, 134, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.Y.; Chen, Y.; Niu, Y.; Williams, K.; Cash, L.; Perez, P.J.; Wojtas, L.; Cai, J.; Chen, Y.S.; Ma, S. Crystal Engineering of an nbo Topology Metal–Organic Framework for Chemical Fixation of CO2 under Ambient Conditions. Angew. Chem. Int. Ed. 2014, 53, 2615–2619. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-L.; Zhou, D.-D.; Zhang, J.; Hu, S.; Haranczyk, M.; Wang, D.-Y. Simultaneous Improvement of Mechanical and Fire-Safety Properties of Polymer Composites with Phosphonate-Loaded MOF Additives. ACS Appl. Mater. Interfaces 2019, 11, 20325–20332. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ma, D.; Li, S.; Qu, W.; Wang, D. Preparation of Zeolitic Imidazolate Frameworks and Their Application as Flame Retardant and Smoke Suppression Agent for Rigid Polyurethane Foams. Polymers 2020, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Hu, W.; Gui, Z.; Hu, Y. Preparation of Metal–Organic Frameworks and Their Application as Flame Retardants for Polystyrene. Ind. Eng. Chem. Res. 2017, 56, 2036–2045. [Google Scholar] [CrossRef]
- Zheng, Y.; Lu, Y.; Zhou, K. A novel exploration of metal–organic frameworks in flame-retardant epoxy composites. J. Therm. Anal. Calorim. 2019, 138, 905–914. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, S.; Pan, Y.-T.; Zhang, S.; Nie, S.; Wei, P.; Zhang, X.; Wang, R.; Wang, D.-Y. Nickel Metal–Organic Framework Derived Hierarchically Mesoporous Nickel Phosphate toward Smoke Suppression and Mechanical Enhancement of Intumescent Flame Retardant Wood Fiber/Poly(lactic acid) Composites. ACS Sustain. Chem. Eng. 2019, 7, 9272–9280. [Google Scholar] [CrossRef]
- Yue, Y.; Yang, S.-Y.; Huang, Y.-L.; Sun, B.; Bian, S.-W. Reduced Graphene Oxide/Polyester Yarns Supported Conductive Metal–Organic Framework Nanorods as Novel Electrodes for All-Solid-State Supercapacitors. Energy Fuels 2020, 34, 16879–16884. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, L.; Li, M.; Liu, M.; Wang, C.; Wang, D.; Fu, S. Interfacial growth of 2D bimetallic metal-organic frameworks on MoS2 nanosheet for reinforcements of polyacrylonitrile fiber: From efficient flame-retardant fiber to recyclable photothermal materials. Chem. Eng. J. 2020, 397, 125410. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Li, C.; Zhou, K. Construction of sandwich-structured CoAl-layered double hydroxide@ zeolitic imidazolate framework-67 (CoAl-LDH@ZIF-67) hybrids: Towards enhancing the fire safety of epoxy resins. RSC Adv. 2018, 8, 36114–36122. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Liu, L.; Qiu, S.; Zhou, X.; Gui, Z.; Hu, Y. DOPO-Modified Two-Dimensional Co-Based Metal–Organic Framework: Preparation and Application for Enhancing Fire Safety of Poly(lactic acid). ACS Appl. Mater. Interfaces 2018, 10, 8274–8286. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Guo, X.; Ma, S.; Xie, J.; Xu, J.; Ma, J. Novel Phosphorus-Nitrogen-Containing Ionic Liquid Modified Metal-Organic Framework as an Effective Flame Retardant for Epoxy Resin. Polymers 2020, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Xin, F.; Zhai, C.; Chen, Y.; Xu, B.; Li, X. The effects of DOPO modified Co-based metalorganic framework on flame retardancy, stiffness and thermal stability of epoxy resin. RSC Adv. 2021, 11, 6781–6790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Z.; Qi, X.; Zhang, W.; Wang, D.-Y. Size tailored bimetallic metal-organic framework (MOF) on graphene oxide with sandwich-like structure as functional nano-hybrids for improving fire safety of epoxy. Compos. Part B Eng. 2020, 188, 107881. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Zhang, L.; Yang, Y.; Wang, D.-Y. Green Synthesis of Biomass Phytic Acid-Functionalized UiO-66-NH2 Hi-erarchical Hybrids toward Fire Safety of Epoxy Resin. ACS Sustain. Chem. Eng. 2019, 8, 994–1003. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Zhang, L.; Molleja, J.G.; Wang, D.-Y. Bimetallic metal-organic frameworks and graphene oxide nano-hybrids for enhanced fire retardant epoxy composites: A novel carbonization mechanism. Carbon 2019, 153, 407–416. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, H.; Guo, J.; Sun, J.; Li, H.; Zhang, S.; Gu, X. Preparation of cobalt-based metal organic framework and its application as synergistic flame retardant in thermoplastic polyurethane (TPU). Compos. Part B Eng. 2019, 182, 107498. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Jian, R.-K.; Wang, D.-Y. Bioinspired iron-loaded polydopamine nanospheres as green flame retardants for epoxy resin via free radical scavenging and catalytic charring. J. Mater. Chem. A 2020, 8, 2529–2538. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yao, D.G.; Ge, M.Q. Reactive melt processing of poly(ethylene terephthalate) waste into calcium terephthalate. Express Polym. Lett. 2021, 15, 153–165. [Google Scholar] [CrossRef]
- Terakado, O.; Hirasawa, M. Effect of metal oxides on the pyrolysis residues of poly(ethylene terephthalate): Formation of carbonaceous submicron, nano-scale filaments and mesoporous compounds. J. Anal. Appl. Pyrolysis 2005, 73, 248–256. [Google Scholar] [CrossRef]
- Shigemoto, I.; Kawakami, T.; Taiko, H.; Okumura, M. A quantum chemical study on the thermal degradation reaction of polyesters. Polym. Degrad. Stab. 2012, 97, 941–947. [Google Scholar] [CrossRef][Green Version]
- Rui, W.; Wenqing, W.; Fanghe, W.; Anying, Z.; Xiuqin, Z.; Deyi, W. Construction of nano-multilayer coatings on copolyester fabrics using UV-grafting mediated layer-by-layer self-assembly for improved anti-droplet and flame retardent performance. Polym. Degrad. Stab. 2021, 183, 109405. [Google Scholar] [CrossRef]
- Ma, T.; Gu, W.; Wang, Y.; Wang, W.; Wang, R. Thermal degradation and flame retardancy prediction of Fe, Al, and Cu-based metal-organic framework and polyethylene terephthalate nanocomposites using DFT calculation. Polymer 2022, 263, 125496. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, H.J.; Cho, Y.J.; Choi, S.; Oh, M. Coordination polymer nanobamboos of {Fe(x)In(1-x)}-MIL-88B: Induced formation of a virtual In-MIL-88B. Chem. A Eur. J. 2014, 20, 5559–5564. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A Robust and Accurate Tight-Binding Quantum Chemical Method for Structures, Vibrational Frequencies, and Noncovalent Interactions of Large Molecular Systems Parametrized for All spd-Block Elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef]
- Dohm, S.; Bursch, M.; Hansen, A.; Grimme, S. Semi-automated transition state localization for organometallic complexes with semi-empirical quantum chemical methods. J. Chem. Theory Comput. 2020, 16, 2002–2012. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Gedler, G.; Antunes, M.; Realinho, V.; Velasco, J.I. Thermal stability of polycarbonate-graphene nanocomposite foams. Polym. Degrad. Stab. 2012, 97, 1297–1304. [Google Scholar] [CrossRef]
- Al-Mulla, A.; Mathew, J.; Al-Omairi, L.; Bhattacharya, S. Thermal decomposition kinetics of tricomponent polyester/polycarbonate systems. Polym. Eng. Sci. 2011, 51, 2335–2344. [Google Scholar] [CrossRef]
- Ma, S.; Liu, W.; Hu, C.; Wang, Z.; Tang, C. Toughening of epoxy resin system using a novel dendritic polysiloxane. Macromol. Res. 2010, 18, 392–398. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Song, L.; Jiao, C. Preparation and thermal properties of a novel UV-cured star polyurethane acrylate coating. Polym. Adv. Technol. 2008, 19, 322–327. [Google Scholar] [CrossRef]
- König, A.; Fehrenbacher, U.; Kroke, E.; Hirth, T. Thermal Decomposition Behavior of the Flame Retardant Melamine in Slabstock Flexible Polyurethane Foams. J. Fire Sci. 2009, 27, 187–211. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Wang, B.; Wen, P.; Song, L.; Hu, Y.; Zhang, P. Comparative Study on the Effect of Beta-Cyclodextrin and Polypseudorotaxane as Carbon Sources on the Thermal Stability and Flame Retardance of Polylactic Acid. Ind. Eng. Chem. Res. 2013, 52, 3287–3294. [Google Scholar] [CrossRef]
- Fateh, T.; Richard, F.; Rogaume, T.; Joseph, P. Experimental and modelling studies on the kinetics and mechanisms of thermal degradation of polymethyl methacrylate in nitrogen and air. J. Anal. Appl. Pyrolysis 2016, 120, 423–433. [Google Scholar] [CrossRef]
- Fernandes, L.; Gaspar, H.; Bernardo, G. Inhibition of thermal degradation of polystyrene by C60 and PCBM: A comparative study. Polym. Test. 2014, 40, 63–69. [Google Scholar] [CrossRef]
- Sheng, C. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity. Fuel 2007, 86, 2316–2324. [Google Scholar] [CrossRef]
- Ma, T.; Wang, R.; Wang, Y.; Gu, W.; Yuan, Y.; Zhang, A.; Wei, J. Studies on the Thermal Degradation Mechanism of Polyethylene Terephthalate and its 2-carboxy Ethyl (phenyl) Phosphinic Acid Copolymers. Polym. Degrad. Stab. 2022, 206, 110185. [Google Scholar] [CrossRef]
- Botelho, G.; Queiros, A.; Liberal, S.; Gijsman, P. Studies on thermal and thermo-oxidative degradation of poly(ethylene ter-ephthalate) and poly(butylene terephthalate). Polym. Degrad. Stab. 2001, 74, 39–48. [Google Scholar] [CrossRef]





| Samples | PTA (phr) | EG (phr) | MIL-88B(Fe) (phr) | CEPPA (phr) |
|---|---|---|---|---|
| PET | 100 | 44.86 | 0 | 0 |
| 0.2-MIL-PET | 100 | 44.86 | 0.2 | 0 |
| 0.6-MIL-PET | 100 | 44.86 | 0.6 | 0 |
| 1.0-MIL-PET | 100 | 44.86 | 1.0 | 0 |
| P-PET | 100 | 44.86 | 0 | 4.5 |
| 0.2-MIL-P-PET | 100 | 44.86 | 0.2 | 4.5 |
| 0.6-MIL-P-PET | 100 | 44.86 | 0.6 | 4.5 |
| Sample | N2 Atmosphere | Air Atmosphere | ||||
|---|---|---|---|---|---|---|
| T5%/°C | Tmax/°C | Char/wt% | T5%/°C | Tmax/°C | Char/wt% | |
| PET | 395.54 | 434.95 | 12.60 | 362.31 | 437.22 | 0.00 |
| 0.2MIL-PET | 399.03 | 439.46 | 18.55 | 381.03 | 431.95 | 0.00 |
| 0.6MIL-PET | 394.35 | 436.92 | 15.17 | 388.61 | 429.22 | 0.00 |
| P-PET | 395.43 | 442.79 | 12.09 | 338.75 | 434.52 | 4.77 |
| 0.2MIL-P-PET | 386.15 | 442.52 | 8.44 | 362.48 | 438.34 | 0.19 |
| 0.6MIL-P-PET | 382.37 | 439.56 | 9.38 | 377.37 | 437.50 | 0.00 |
| Sample | LOI (%) | UL-94 Test | |||
|---|---|---|---|---|---|
| t1(s) | t2(s) | Ignition of Cotton | Rate | ||
| PET | 22 | 32.6 | 25.8 | YES | NR |
| 0.2MIL-PET | 25 | 4.4 | 2.7 | YES | V-2 |
| 0.6MIL-PET | 26 | 3.5 | 2.5 | YES | V-2 |
| 1.0MIL-PET | 27 | 2.3 | 2.3 | YES | V-2 |
| P-PET | 29 | 1.8 | 1.9 | YES | V-2 |
| 0.6MIL-P-PET | 30 | 2.4 | 1.4 | NO | V-0 |
| Sample | PHRR (KW/m2) | THR (MJ/m2) | TSP (m2) | PCOP (g/s) | pCO2P (g/s) |
|---|---|---|---|---|---|
| PET | 800.07 | 49.86 | 24.62 | 0.02 | 0.56 |
| 0.2MIL-PET | 811.49 | 57.79 | 29.51 | 0.02 | 0.64 |
| 0.6MIL-PET | 721.53 | 53.91 | 23.77 | 0.01 | 0.56 |
| 1.0MIL-PET | 615.87 | 60.10 | 24.35 | 0.01 | 0.45 |
| P-PET | 839.30 | 50.20 | 29.97 | 0.05 | 0.60 |
| 0.6MIL-P-PET | 656.79 | 49.83 | 51.02 | 0.03 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Wang, W.; Wang, R. Thermal Degradation and Carbonization Mechanism of Fe-Based Metal-Organic Frameworks onto Flame-Retardant Polyethylene Terephthalate. Polymers 2023, 15, 224. https://doi.org/10.3390/polym15010224
Ma T, Wang W, Wang R. Thermal Degradation and Carbonization Mechanism of Fe-Based Metal-Organic Frameworks onto Flame-Retardant Polyethylene Terephthalate. Polymers. 2023; 15(1):224. https://doi.org/10.3390/polym15010224
Chicago/Turabian StyleMa, Tianyi, Wenqing Wang, and Rui Wang. 2023. "Thermal Degradation and Carbonization Mechanism of Fe-Based Metal-Organic Frameworks onto Flame-Retardant Polyethylene Terephthalate" Polymers 15, no. 1: 224. https://doi.org/10.3390/polym15010224
APA StyleMa, T., Wang, W., & Wang, R. (2023). Thermal Degradation and Carbonization Mechanism of Fe-Based Metal-Organic Frameworks onto Flame-Retardant Polyethylene Terephthalate. Polymers, 15(1), 224. https://doi.org/10.3390/polym15010224