Biopolymer Gels as a Cleaning System for Differently Featured Wooden Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mock-Ups
2.2. Sodium Alginate and Konjac Glucomannan Gels Preparation
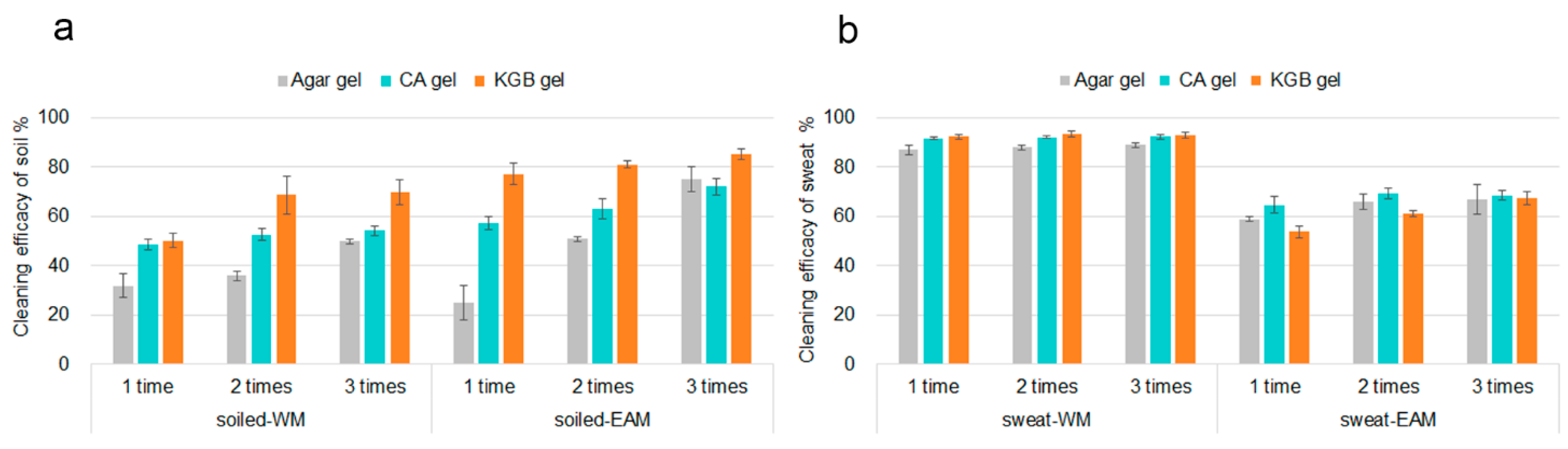
2.3. Cleaning Procedure
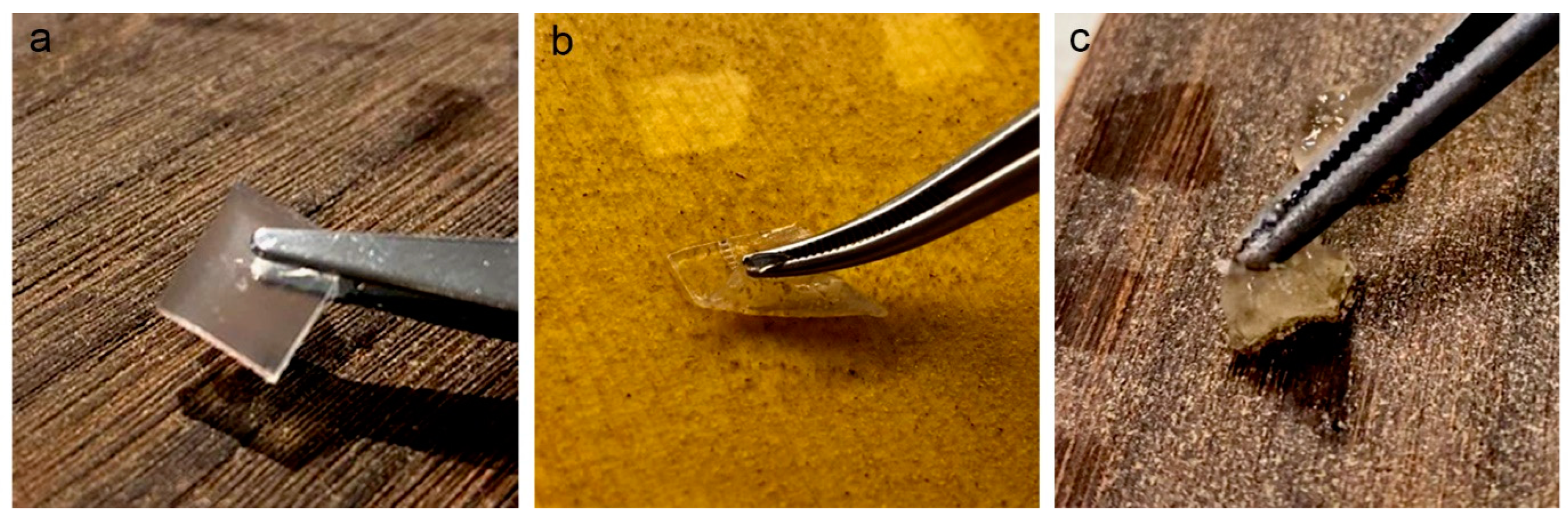
2.4. Mechanical Properties of Fresh and Aged Gels
2.5. X-ray Fluorescence Analysis
2.6. Surface Topography Evaluation
3. Results and Discussion
3.1. Stability of Gels: Mechanical Properties of Fresh and Aged Gels
3.2. Evaluation of the Gels Cleaning Efficacy by XRF Mapping
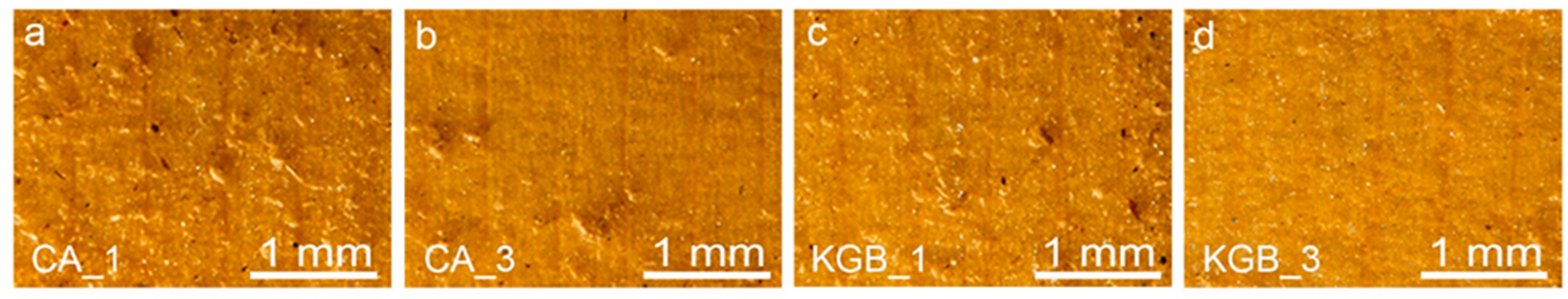
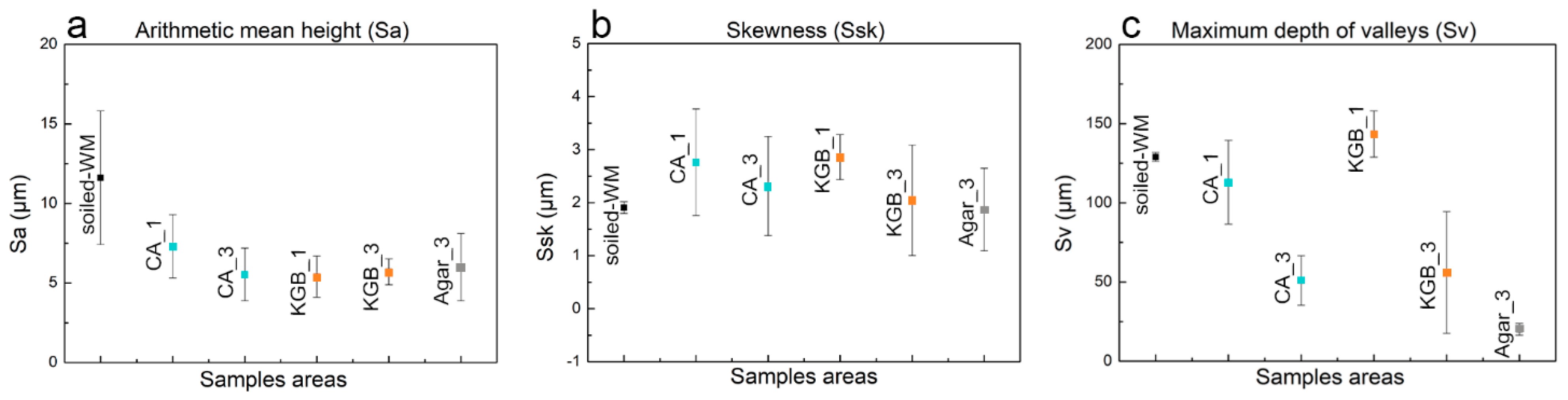
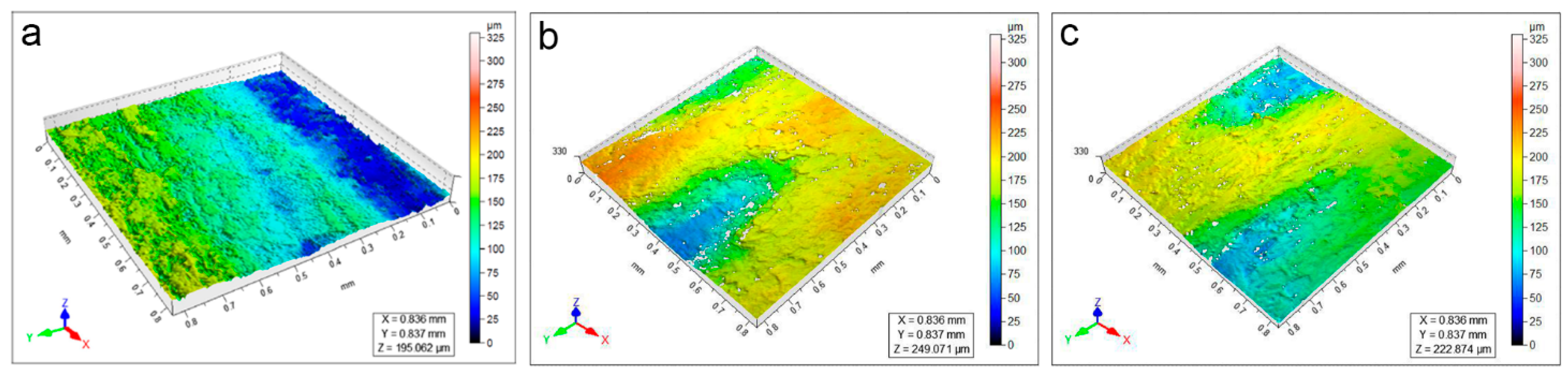
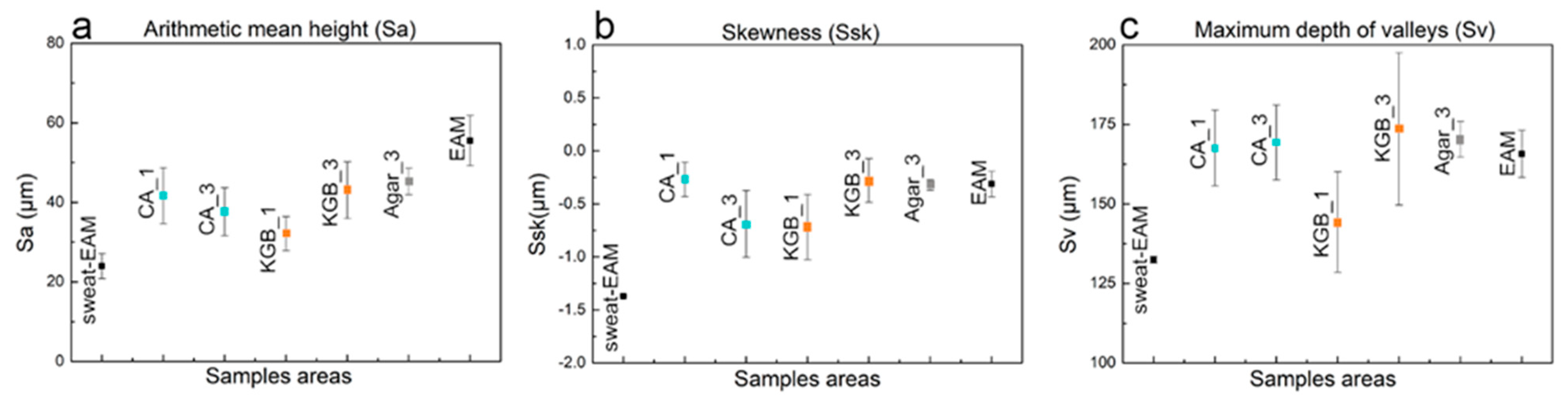
3.3. Surface Topography Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner-Walker, G. The Nature of Cleaning: Physical and Chemical Aspects of Removing Dirt, Stains and Corrosion ArchSci2020 View Project Mineralised Textiles View Project. In Proceedings of the International Symposium on Cultural Heritage Conservation, Tainan, Taiwan, 6–8 November 2012. [Google Scholar]
- Cremonesi, P.; Signorini, E. Un Approccio Alla Pulitura Dei Dipinti Mobili; Prato, I., Ed.; Il Prato: Padova, Italy, 2013; ISBN 9788863361919. [Google Scholar]
- Wolbers, R. Cleaning Painted Surfaces: Aqueous Methods; Archetype Publications Ltd.: London, UK, 2000; ISBN 9781873132364. [Google Scholar]
- Rivers, S.; Umney, N. Conservation of Furniture; Routledge: London, UK, 2007; pp. 1–809. [Google Scholar] [CrossRef]
- Prati, S.; Volpi, F.; Fontana, R.; Galletti, P.; Giorgini, L.; Mazzeo, R.; Mazzocchetti, L.; Samorì, C.; Sciutto, G.; Tagliavini, E. Sustainability in art conservation: A novel bio-based organogel for the cleaning of water sensitive works of art. Pure Appl. Chem. 2018, 90, 239–251. [Google Scholar] [CrossRef]
- Jan van den Berg, K.; Bonaduce, I.; Burnstock, A.; Ormsby, B.; Scharr, M.; Carlyle, L.; Heydenreich, G.; Keune, K. Conservation of Modern Oil Paintings; van den Berg, K.J., Bonaduce, I., Burnstock, A., Ormsby, B., Scharff, M., Carlyle, L., Heydenreich, G., Keune, K., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; ISBN 978-3-030-19253-2. [Google Scholar]
- Casoli, A.; Di Diego, Z.; Isca, C. Cleaning Painted Surfaces: Evaluation of Leaching Phenomenon Induced by Solvents Applied for the Removal of Gel Residues. Environ. Sci. Pollut. Res. 2014, 21, 13252–13263. [Google Scholar] [CrossRef]
- Yamauchi, A. Section 1—Gels: Introduction. In Gels Handbook; Academic Press: Cambridge, MA, USA, 2001; pp. 4–12, eBook; ISBN 9780080532349. [Google Scholar]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Nanotechnologies in the Conservation of Cultural Heritage; Springer Science+Business Media: Dordrecht, the Netherlands, 2015. [Google Scholar]
- Wolbers, R.C. The Use of a Synthetic Soiling Mixture as a Means for Evaluating the Efficacy of Aqueous Cleaning Materials on Painted Surfaces. Conserv. Restaur. Des Biens Cult. Rev. L’araafu 1992, 4, 22–29. [Google Scholar]
- Boccalon, E.; Nocchetti, M.; Pica, M.; Romani, A.; Casciola, M. Composite Sodium Alginate-Ion Exchangers as Cleaning Systems for the Removal of Gypsum Efflorescences. Appl. Clay Sci. 2019, 181, 105216. [Google Scholar] [CrossRef]
- Bartoletti, A.; Barker, R.; Chelazzi, D.; Bonelli, N.; Baglioni, P.; Lee, J.; Angelova, L.V.; Ormsby, B. Reviving WHAAM! A Comparative Evaluation of Cleaning Systems for the Conservation Treatment of Roy Lichtenstein’s Iconic Painting. Herit. Sci. 2020, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Domingues, J.; Bonelli, N.; Giorgi, R.; Baglioni, P. Chemical Semi-IPN Hydrogels for the Removal of Adhesives from Canvas Paintings. Appl. Phys. A Mater. Sci. Process. 2014, 114, 705–710. [Google Scholar] [CrossRef]
- Albano, M.; Grassi, S.; Fiocco, G.; Invernizzi, C.; Rovetta, T.; Licchelli, M.; Marotti, R.; Merlo, C.; Comelli, D.; Malagodi, M. A Preliminary Spectroscopic Approach to Evaluate the Effectiveness of Water- and Silicone-Based Cleaning Methods on Historical Varnished Brass. Appl. Sci. 2020, 10, 3982. [Google Scholar] [CrossRef]
- Guaragnone, T.; Rossi, M.; Chelazzi, D.; Mastrangelo, R.; Severi, M.; Fratini, E.; Baglioni, P. PH-Responsive Semi-Interpenetrated Polymer Networks of PHEMA/PAA for the Capture of Copper Ions and Corrosion Removal. ACS Appl. Mater. Interfaces 2022, 14, 7471–7485. [Google Scholar] [CrossRef]
- Prati, S.; Sciutto, G.; Volpi, F.; Rehorn, C.; Vurro, R.; Blümich, B.; Mazzocchetti, L.; Giorgini, L.; Samorì, C.; Galletti, P.; et al. Cleaning Oil Paintings: NMR Relaxometry and SPME to Evaluate the Effects of Green Solvents and Innovative Green Gels. New J. Chem. 2019, 43, 8229–8238. [Google Scholar] [CrossRef]
- Samorì, C.; Galletti, P.; Giorgini, L.; Mazzeo, R.; Mazzocchetti, L.; Prati, S.; Sciutto, G.; Volpi, F.; Tagliavini, E. The Green Attitude in Art Conservation: Polyhydroxybutyrate–Based Gels for the Cleaning of Oil Paintings. ChemistrySelect 2016, 1, 4502–4508. [Google Scholar] [CrossRef]
- Passaretti, A.; Cuvillier, L.; Sciutto, G.; Guilminot, E.; Joseph, E. Biologically Derived Gels for the Cleaning of Historical and Artistic Metal Heritage. Appl. Sci. 2021, 11, 3405. [Google Scholar] [CrossRef]
- Gueidão, M.; Vieira, E.; Bordalo, R.; Moreira, P. Available Green Conservation Methodologies for the Cleaning of Cultural Heritage: An Overview. Estud. Conserv. e Restauro 2021, 12, 22–44. [Google Scholar] [CrossRef]
- Sansonetti, A.; Bertasa, M.; Canevali, C.; Rabbolini, A.; Anzani, M.; Scalarone, D. A Review in Using Agar Gels for Cleaning Art Surfaces. J. Cult. Herit. 2020, 44, 285–296. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Nada, A.A.; Eid, B.M. Polysaccharide-Based Polymer Gels and Their Potential Applications. Polymer Gels. Gels Horizons: From Science to Smart Materials; Springer: Singapore, 2018; pp. 97–126. [Google Scholar]
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Mater. (Basel) 2021, 14, 1095. [Google Scholar] [CrossRef]
- Cremonesi, P.A.G. Gel Rigidi Polisaccaridici per Il Trattamento Dei Manufatti Artistici; Prato, I., Ed.; Il Prato: Padova, Italy, 2019; ISBN 9788863365016. [Google Scholar]
- Kanth, A.; Singh, M.; Pandey, S.C. Optimizing the Rigidity of Gellan and Agar Gels for of Conservation Science. Int. J. Conserv. Sci. 2018, 9, 451–462. [Google Scholar]
- Scott, C.L. The use of agar as a solvent gel in objects conservation. AIC Objects Spec. Gr. Postprints 2012, 19, 71–83. [Google Scholar]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Yang, D.; Yuan, Y.; Wang, L.; Wang, X.; Mu, R.; Pang, J.; Xiao, J.; Zheng, Y. A Review on Konjac Glucomannan Gels: Microstructure and Application. Int. J. Mol. Sci. 2017, 18, 2250. [Google Scholar] [CrossRef]
- Zhou, N.; Zheng, S.; Xie, W.; Cao, G.; Wang, L.; Pang, J. Konjac Glucomannan: A Review of Structure, Physicochemical Properties, and Wound Dressing Applications. J. Appl. Polym. Sci. 2022, 139, 51780. [Google Scholar] [CrossRef]
- Lee, C.; Volpi, F.; Fiocco, G.; Weththimuni, M.L.; Licchelli, M.; Malagodi, M. Preliminary Cleaning Approach with Alginate and Konjac Glucomannan Polysaccharide Gel for the Surfaces of East Asian and Western String Musical Instruments. Materials 2022, 15, 1100. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, C.; Fiocco, G.; Iwanicka, M.; Kowalska, M.; Targowski, P.; Blümich, B.; Rehorn, C.; Gabrielli, V.; Bersani, D.; Licchelli, M.; et al. Non-Invasive Mobile Technology to Study the Stratigraphy of Ancient Cremonese Violins: OCT, NMR-MOUSE, XRF and Reflection FT-IR Spectroscopy. Microchem. J. 2020, 155, 104754. [Google Scholar] [CrossRef]
- Albano, M.; Comelli, D.; Fiocco, G.; Mattonai, M.; Lucejko, J.J.; Zoia, L.; Colombini, M.P.; Malagodi, M. Chemical Modification of Wood Induced by the Traditional Making Procedures of Bowed String Musical Instruments: The Effect of Alkaline Treatments. Herit. Sci. 2022, 10, 76. [Google Scholar] [CrossRef]
- Fiocco, G.; Invernizzi, C.; Rovetta, T.; Albano, M.; Malagodi, M.; Davit, P.; Gulmini, M. Surfing through the Coating System of Historic Bowed Instruments: A Spectroscopic Perspective. Spectrosc. Eur. 2021, 33, 19. [Google Scholar] [CrossRef]
- Kim, B.G. A Study on the Improvement and Manufacture Method of Traditional Musical Instrument on the Basis of Akhakgwebeom. Soc. Kangwon Prov. Folk 2010, 24, 339–378. [Google Scholar]
- Lee, C.; Jung, H.; Chung, Y. Functional Characteristics of Nakdong Technique Treated on Paulownia Wood Surface. J. Korean Wood Sci. Technol. 2021, 49, 82–92. [Google Scholar] [CrossRef]
- Fiocco, G.; Grassi, S.; Invernizzi, C.; Rovetta, T.; Albano, M.; Davit, P.; Gulmini, M.; Stani, C.; Vaccari, L.; Licchelli, M.; et al. Chemometric Tools to Investigate Complex Synchrotron Radiation FTIR Micro-Spectra: Focus on Historical Bowed Musical Instruments. ACTA IMEKO 2021, 10, 201. [Google Scholar] [CrossRef]
- Albano, M.; Ghirardello, M.; Fiocco, G.; Manzoni, C.; Malagodi, M.; Comelli, D. Complementary Mapping Techniques to Characterize the Wood Finish of Musical Instruments. Eur. Phys. J. Plus 2021, 136, 1054. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Canevari, C.; Legnani, A.; Licchelli, M.; Malagodi, M.; Ricca, M.; Zeffiro, A. Experimental Characterization of Oil-Colophony Varnishes: A Preliminary Study. Int. J. Conserv. Sci. 2016, 7, 813–826. [Google Scholar]
- Vigani, B.; Valentino, C.; Cavalloro, V.; Catenacci, L.; Sorrenti, M.; Sandri, G.; Bonferoni, M.C.; Bozzi, C.; Collina, S.; Rossi, S.; et al. Gellan-Based Composite System as a Potential Tool for the Treatment of Nervous Tissue Injuries: Cross-Linked Electrospun Nanofibers Embedded in a RC-33-Loaded Freeze-Dried Matrix. Pharmaceutics 2021, 13, 164. [Google Scholar] [CrossRef]
- Vigani, B.; Valentino, C.; Sandri, G.; Caramella, C.M.; Ferrari, F.; Rossi, S. Spermidine Crosslinked Gellan Gum-Based “Hydrogel Nanofibers” as Potential Tool for the Treatment of Nervous Tissue Injuries: A Formulation Study. Int. J. Nanomedicine 2022. [CrossRef] [PubMed]
- Said, N.S.; Sarbon, N.M. Physical and Mechanical Characteristics of Gelatin-Based Films as a Potential Food Packaging Material: A Review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurler, J.; Engesland, A.; Poorahmary Kermany, B.; Škalko-Basnet, N. Improved Texture Analysis for Hydrogel Characterization: Gel Cohesiveness, Adhesiveness, and Hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Nussinovitch, A. Production, Properties, and Applications of Hydrocolloid Cellular Solids. Mol. Nutr. Food Res. 2005, 49, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Bezur, A.; Lee, L.; Loubser, M.; Trentelman, K. Handheld XRF in Cultural Heritage: A Practical Workbook for Conservators; Getty Conservation Institute: Los Angeles, CA, USA, 2020; ISBN 978-1-937433-61-1. [Google Scholar]
- Volpi, F.; Fiocco, G.; Rovetta, T.; Invernizzi, C.; Albano, M.; Licchelli, M.; Malagodi, M. New Insights on the Stradivari “Coristo” Mandolin: A Combined Non-Invasive Spectroscopic Approach. Appl. Sci. 2021, 11, 11626. [Google Scholar] [CrossRef]
- Rovetta, T.; Invernizzi, C.; Licchelli, M.; Cacciatori, F.; Malagodi, M. The Elemental Composition of Stradivari’s Musical Instruments: New Results through Non-Invasive EDXRF Analysis. X-Ray Spectrom. 2018, 47, 159–170. [Google Scholar] [CrossRef]
- Zörner, A.; Oertel, S.; Schmitz, B.; Lang, N.; Jank, M.P.M.; Frey, L. Determination of the Selectivity of Printed Wearable Sweat Sensors. In Proceedings of the BIODEVICES 2017—10th International Conference on Biomedical Electronics and Devices, Proceedings; Part of 10th International Joint Conference on Biomedical Engineering Systems and Technologies, BIOSTEC, Porto, Portugal, 21–23 February 2017; SciTePress: Setúbal, Portugal, 2017; Volume 2, pp. 81–87. [Google Scholar]
- Duncan, T.T.; Chan, E.P.; Beers, K.L. Quantifying the ‘Press and Peel’ Removal of Particulates Using Elastomers and Gels. J. Cult. Herit. 2021, 48, 236–243. [Google Scholar] [CrossRef]
- Sadowski, Ł.; Czarnecki, S.; Hoła, J. Evaluation of the Height 3D Roughness Parameters of Concrete Substrate and the Adhesion to Epoxy Resin. Int. J. Adhes. Adhes. 2016, 67, 3–13. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Curtis, R.V.; Bartlett, D.W. Surface Roughness of Impression Materials and Dental Stones Scanned by Non-Contacting Laser Profilometry. Dent. Mater. 2009, 25, 500–505. [Google Scholar] [CrossRef]
- Courard, L.; Nelis, M. Surface Analysis of Mineral Substrates for Repair Works: Roughness Evaluation by Profilometry and Surfometry Analysis. Mag. Concr. Res. 2003, 55, 355–366. [Google Scholar] [CrossRef]
- Garbacz, A.; Courard, L.; Kostana, K. Characterization of Concrete Surface Roughness and Its Relation to Adhesion in Repair Systems. Mater. Charact. 2006, 56, 281–289. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness Parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- De Chiffre, L.; Lonardo, P.; Trumpold, H.; Lucca, D.A.; Goch, G.; Brown, C.A.; Raja, J.; Hansen, H.N. Quantitative Characterisation of Surface Texture. CIRP Ann. Manuf. Technol. 2000, 49, 635–652. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0007850607634581 (accessed on 22 July 2022). [CrossRef]
- Bennett, J.M. Recent Developments in Surface Roughness Characterization. Meas. Sci. Technol. 1992, 3, 1119. [Google Scholar] [CrossRef]
- Feidenhans’l, N.A.; Hansen, P.-E.; Pilný, L.; Madsen, M.H.; Bissacco, G.; Petersen, J.C.; Taboryski, R. Comparison of Optical Methods for Surface Roughness Characterization. Meas. Sci. Technol. 2015, 26, 085208. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Lv, Y.; Qian, K.; Chen, Y.; Qian, X. Preparation of Konjac Glucomannan–Borax Hydrogels with Good Self-Healing Property and PH-Responsive Behavior. J. Polym. Res. 2019, 26, 52. [Google Scholar] [CrossRef]
- Janus, J.; Fauxpoint, G.; Arntz, Y.; Pelletier, H.; Etienne, O. Surface Roughness and Morphology of Three Nanocomposites after Two Different Polishing Treatments by a Multitechnique Approach. Dent. Mater. 2010, 26, 416–425. [Google Scholar] [CrossRef]
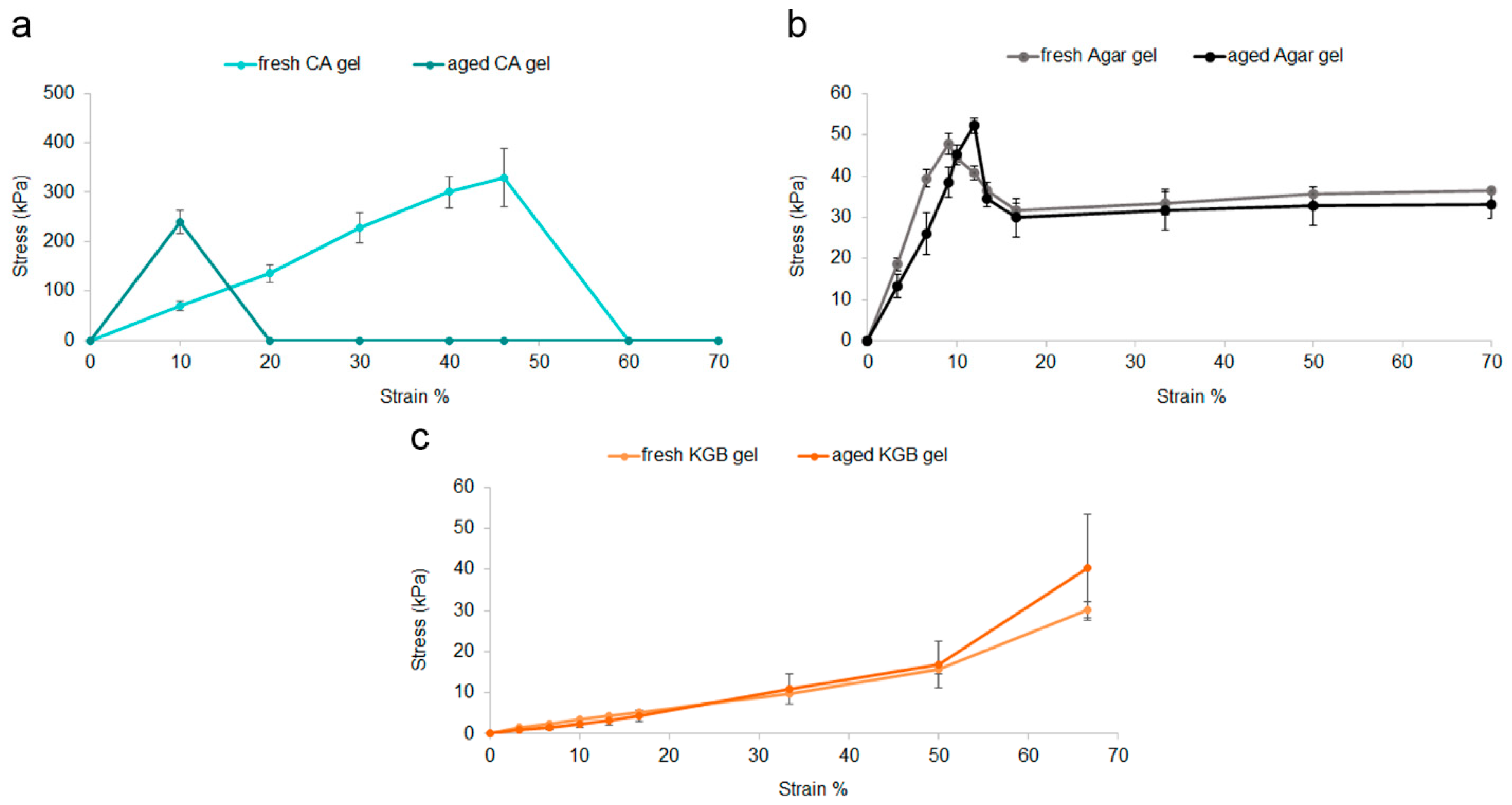




| Classification | Tensile Strength (kPa) | Elongation at Break (%) | AUC | |
|---|---|---|---|---|
| Agar gel | Fresh | 13.4 ± 0.1 * | 11.8 ± 0.1 ° | - |
| Aged | - | - | - | |
| CA gel | Fresh | 328 ± 59 ** | 194 ± 17 # | 10029 ± 1751 § |
| Aged | 239 ± 24 ** | 27 ± 1 # | 2396 ± 250 §§ | |
| Classification | Hardness (kPa) | Young’s Modulus (kPa) | AUC | |
|---|---|---|---|---|
| Agar gel | Fresh | 47 ± 2 * | 556 ± 47 ° | 2361 ± 202 § |
| Aged | 52 ± 2 * | 464 ± 96 ° | 2179 ± 194 § | |
| KGB gel | Fresh | 30 ± 3 ** | 47 ± 0.1 # | 766 ± 15 §§ |
| Aged | 40 ± 13 ** | 30 ± 2 # | 868 ± 264 §§ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Di Turo, F.; Vigani, B.; Weththimuni, M.L.; Rossi, S.; Beltram, F.; Pingue, P.; Licchelli, M.; Malagodi, M.; Fiocco, G.; et al. Biopolymer Gels as a Cleaning System for Differently Featured Wooden Surfaces. Polymers 2023, 15, 36. https://doi.org/10.3390/polym15010036
Lee C, Di Turo F, Vigani B, Weththimuni ML, Rossi S, Beltram F, Pingue P, Licchelli M, Malagodi M, Fiocco G, et al. Biopolymer Gels as a Cleaning System for Differently Featured Wooden Surfaces. Polymers. 2023; 15(1):36. https://doi.org/10.3390/polym15010036
Chicago/Turabian StyleLee, Chaehoon, Francesca Di Turo, Barbara Vigani, Maduka L. Weththimuni, Silvia Rossi, Fabio Beltram, Pasqualantonio Pingue, Maurizio Licchelli, Marco Malagodi, Giacomo Fiocco, and et al. 2023. "Biopolymer Gels as a Cleaning System for Differently Featured Wooden Surfaces" Polymers 15, no. 1: 36. https://doi.org/10.3390/polym15010036









