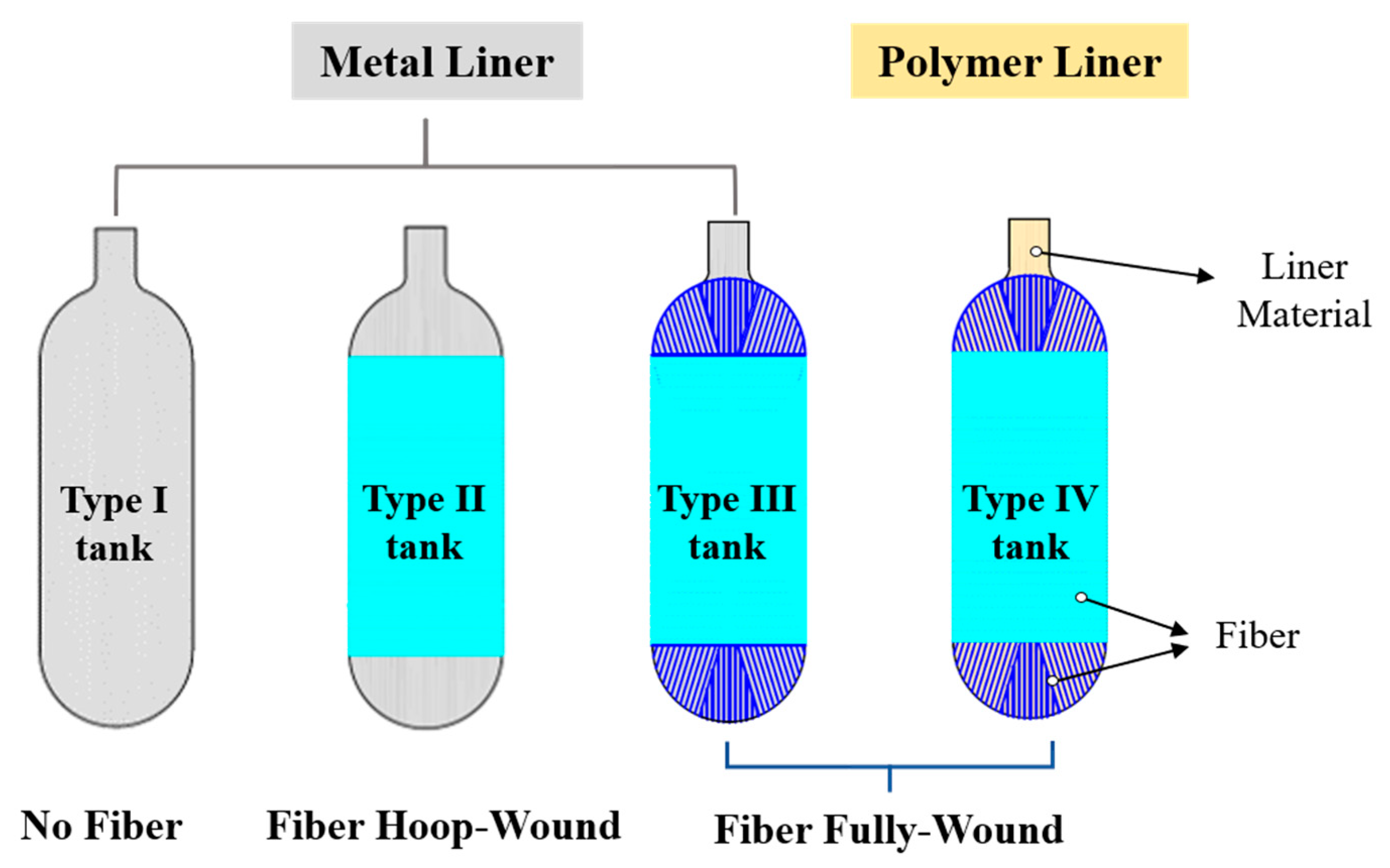
Review of Decompression Damage of the Polymer Liner of the Type IV Hydrogen Storage Tank
Abstract
:1. Introduction
2. Hydrogen Behavior through Polymeric Materials during Decompression
2.1. Gas Permeation through Polymers
- The polymer absorbs the gas on the high-pressure side.
- The gas diffuses inside the polymer matrix.
- The polymer desorbs the gas on the low-pressure side.
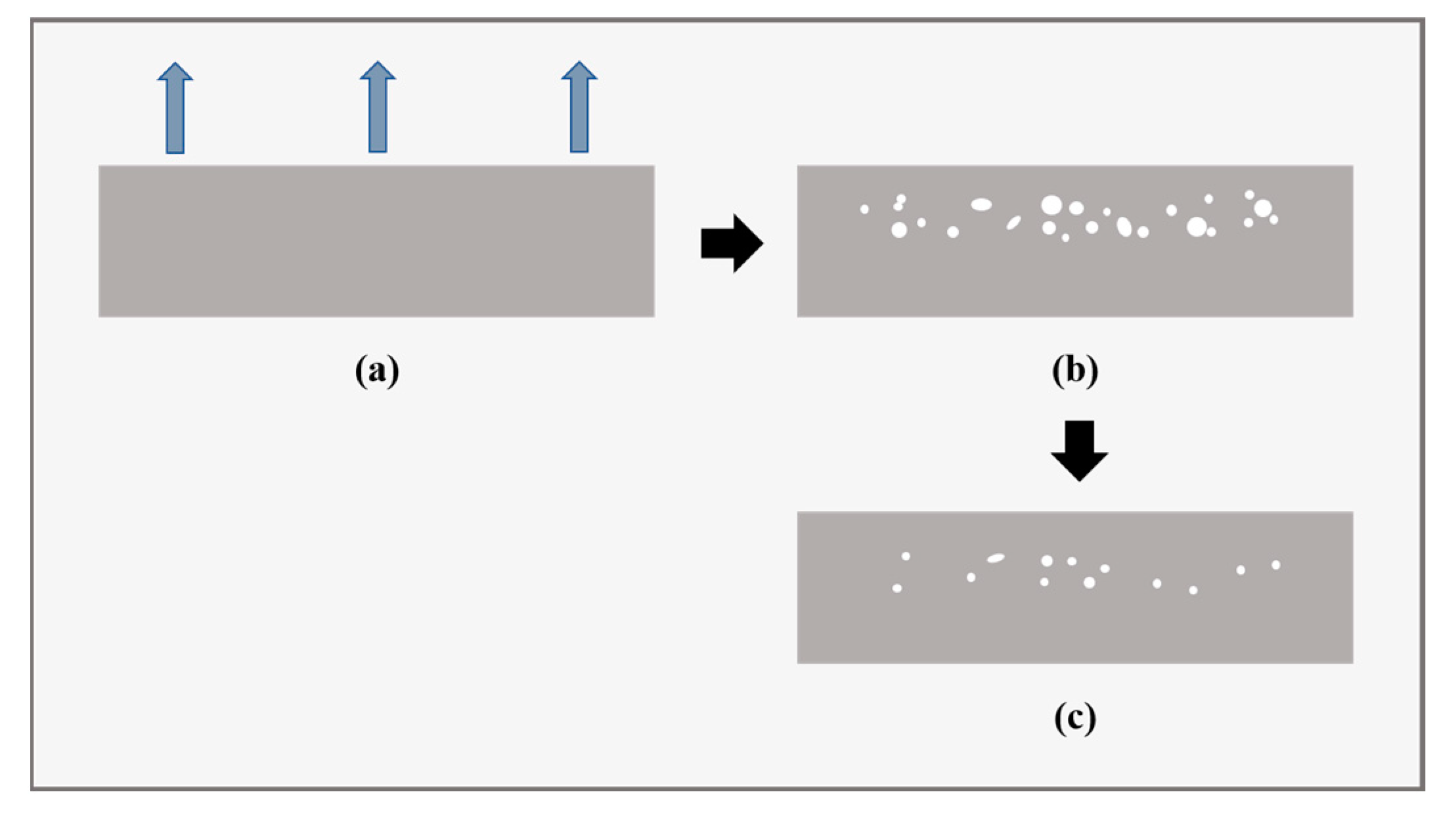
2.2. Formation of Decompression Damage
3. Characterization Methods and Quantitative Evaluations for Internal Damage Morphology in Polymers
3.1. Characterization Methods for Internal Damage of Polymers
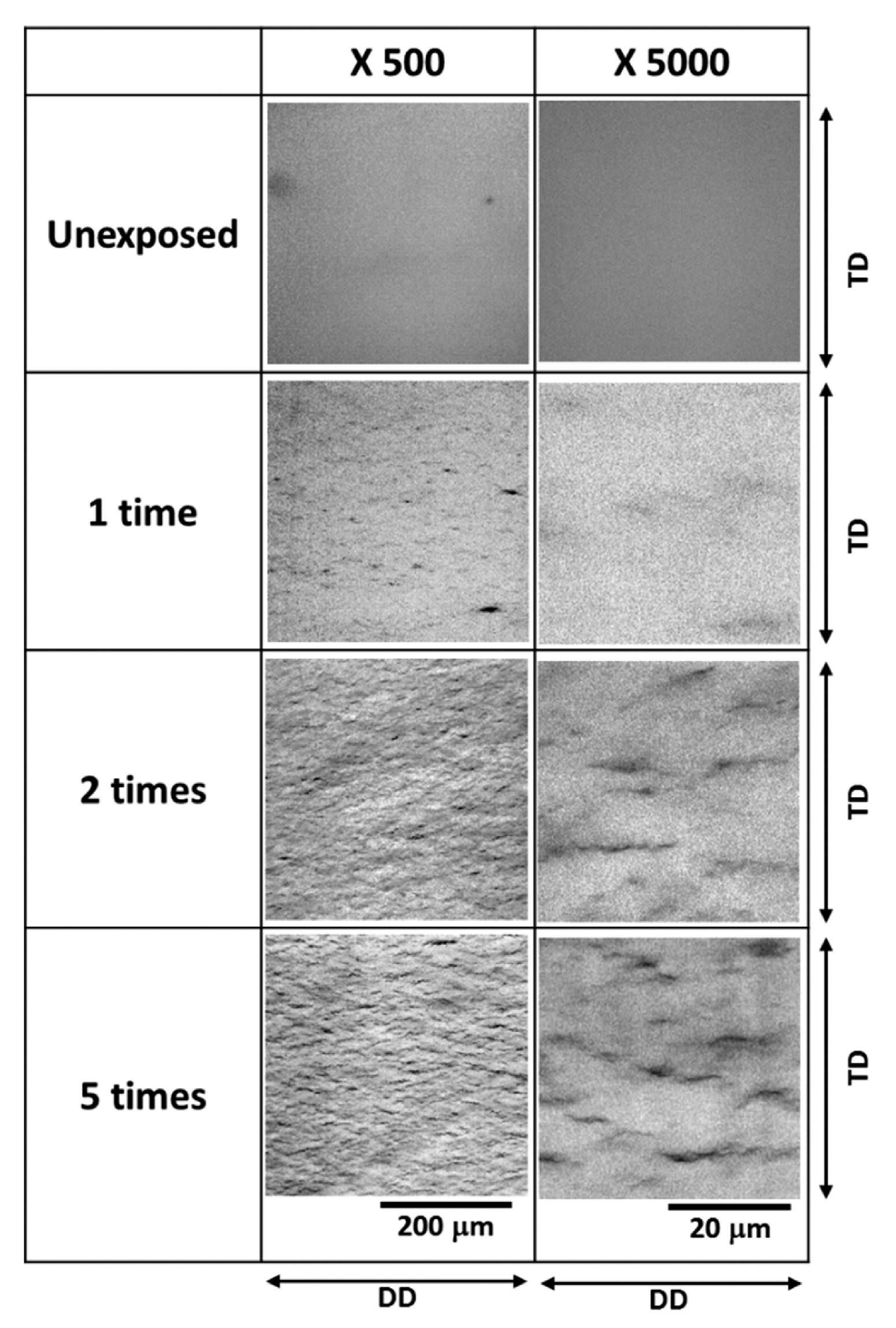
3.1.1. Light Microscope/Camera Observation
3.1.2. Electron Microscopy Observation
3.1.3. Atomic Force Microscope (AFM)
3.1.4. Computed Tomography (CT)—3D Technique
3.1.5. In-Situ Observation
3.2. Quantitative Evaluations for Internal Damage of Polymers
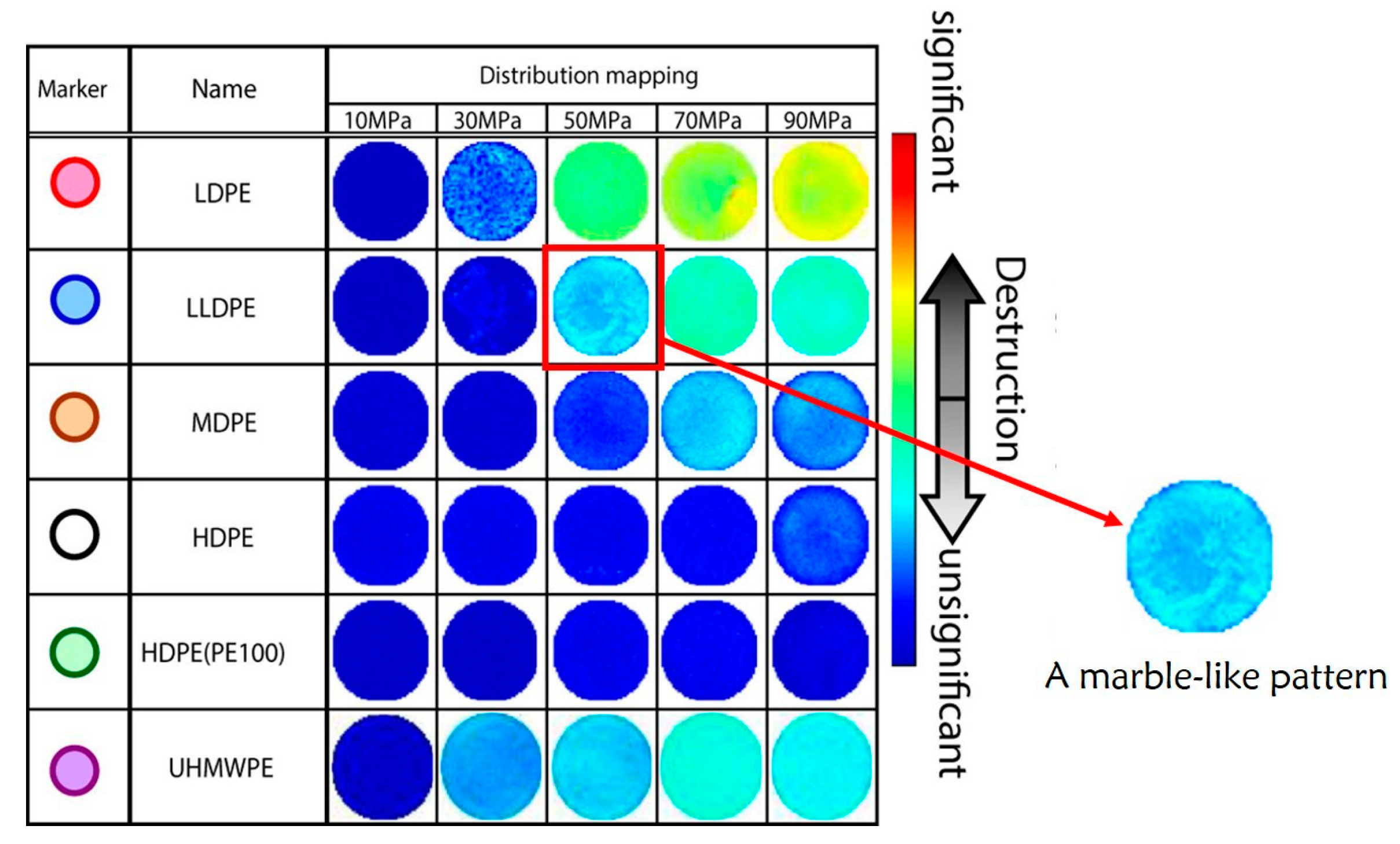
3.2.1. Optical Method
3.2.2. Mechanical Method
3.2.3. Physical Property Method
3.2.4. Acoustic Emission (AE) Method
4. Factors Affecting the Internal Decompression Damage of Polymers
4.1. Factors from the Material Level
4.1.1. Properties of Polymers
4.1.2. Defects Inside Polymers
4.1.3. Additives
4.1.4. Processing Technology
4.1.5. Thickness of Samples
4.2. Operation Conditions Factors
4.2.1. Temperature
4.2.2. Exposure Pressure
4.2.3. Decompression Rate
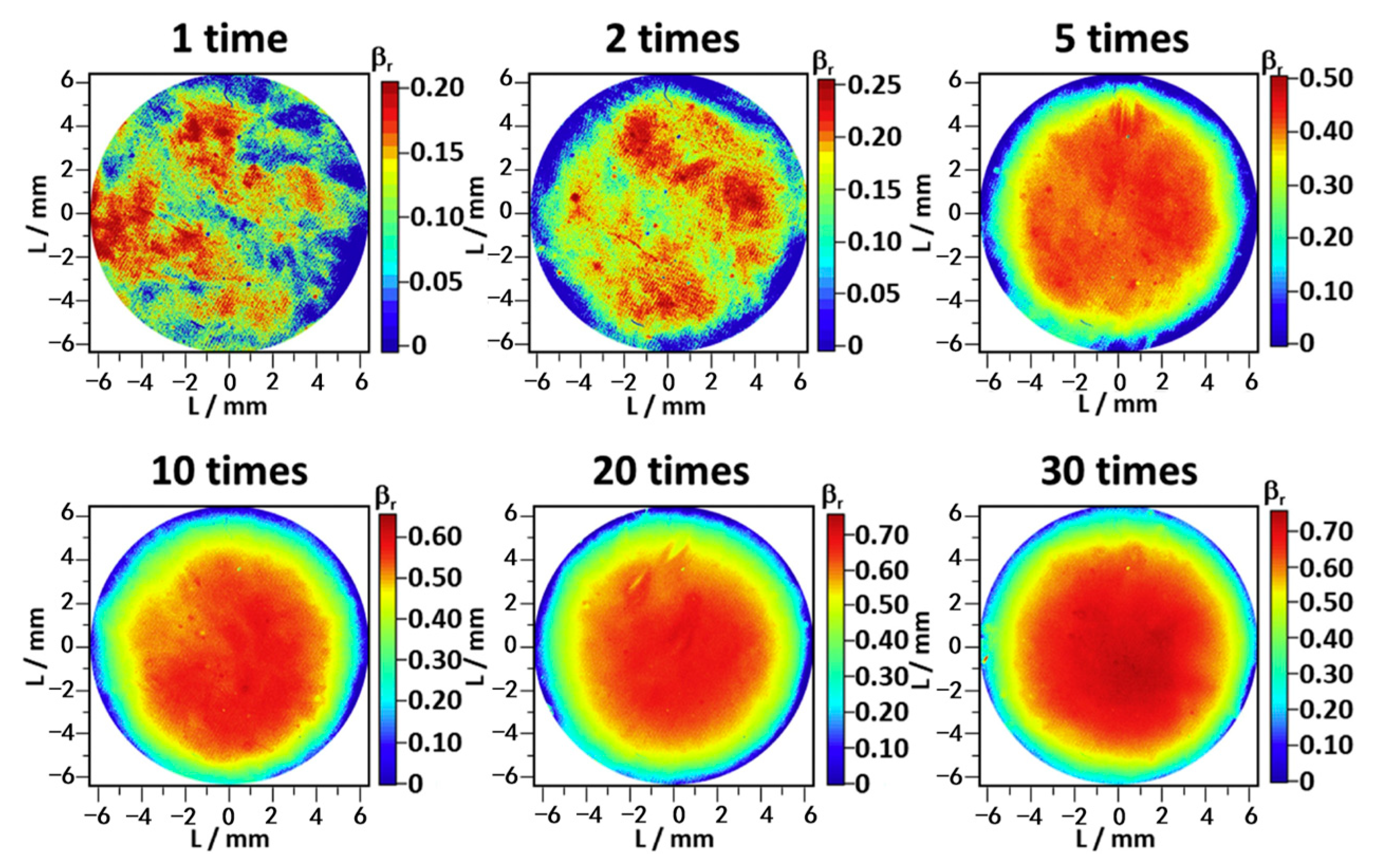
4.2.4. Charge/Discharge Cycle
5. Decompression Damage Predictive Models and Assessments for Polymers
5.1. Decompression Damage Predictive Models for Polymers
5.1.1. Damage Predictive Model for Sudden Fracture
5.1.2. Damage Predictive Model for Blistering
5.2. Decompression Damage Assessments for Polymers
5.2.1. Assessment of Blistering Initiation Limit Pressure
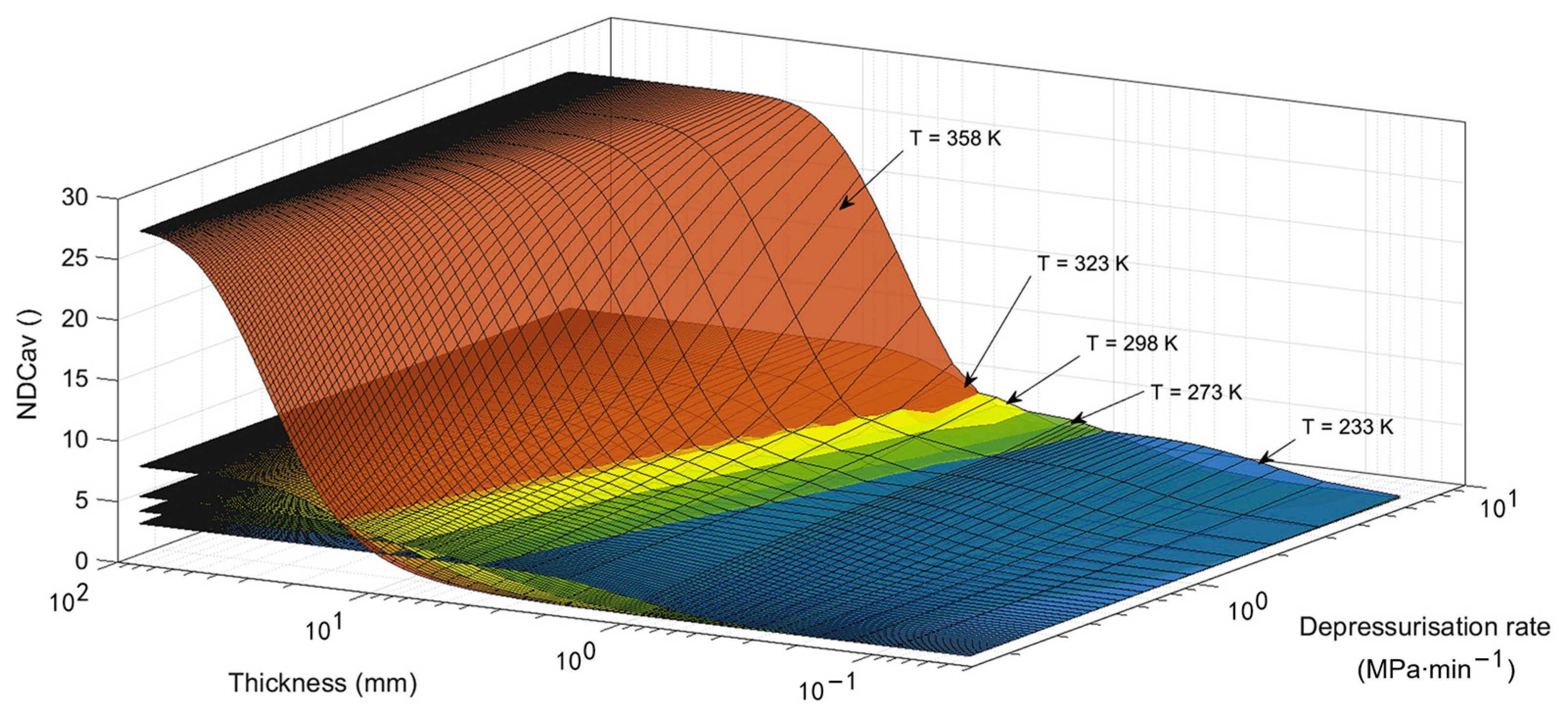
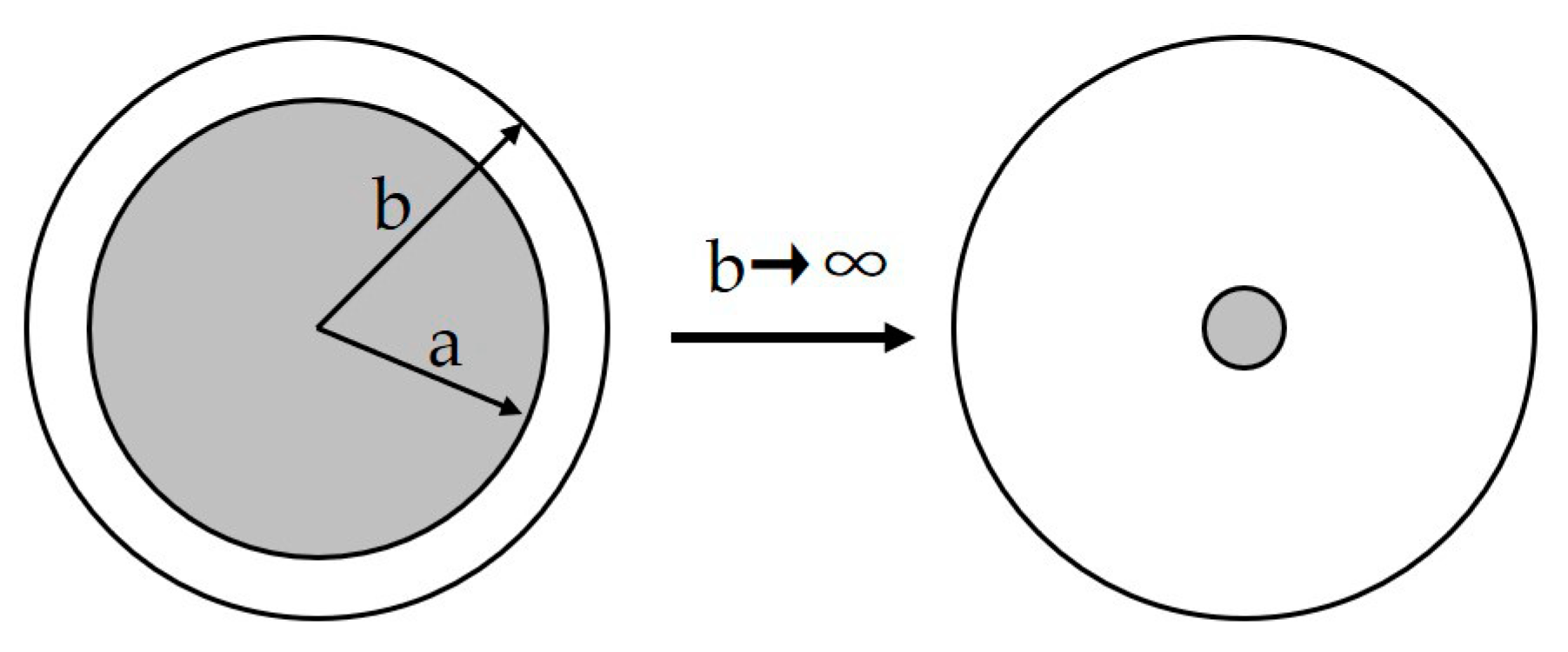
5.2.2. Non-Dimensional Assessment of Decompression Damage
5.2.3. Numerical Assessment of Maximum Decompression Rate
6. Future Research Directions
- The liner design is of interest for damage minimization. Decompression damage is mainly determined by the diffusion and mechanical properties of the liner materials. Firstly, in the liner’s molding process, the molding method and parameters largely affect the material properties at the microscopic level. Molecular orientation is common in injection molding [93] and leads to the anisotropy of materials. The fusion temperature can influence the degree of crystallinity [94]. The effect of the molding process should be further investigated. Secondly, additives play a vital role in changing the properties of polymers and the nature of the damage. The introduction of novel additive strategies is a potential method to obtain desired properties and thereby minimize damage. Furthermore, Klopffer et al. [95] proposed innovative multilayer systems (applying a layer of Ethylene Vinyl Alcohol) and obtained excellent permeation resistance compared to monolayer systems. Therefore, the design of liner materials and systems could be a research interest to reduce the possibility and extent of damage.
- The operational conditions will age the polymers and accelerate material degradation, making the liner more subject to damage. The characteristics of primary crystals of thermoplastics changed after long-term hydrogen exposure at various temperatures [96]. This is considered to be the effect of the annealing temperature. The crystallinity of PE was shown to increase under the high-pressure hydrogen conditions, but was restored to its initial value after decompression [97]. Considering the real practical application of the tanks, the effect of time and hydrogen exposure cycles on polymers’ properties may be prominent. Systematic and detailed research on the effect of operational conditions on material properties related to decompression damage is indispensable.
- Water absorption should be emphasized when PA materials are chosen as the liner of type IV hydrogen storage tanks. It will directly affect the dimensional stability and the physical properties of the products [98]. Polyamide materials contain plenty of amide groups, making them sensitive to water from the environment (typically, the water absorption increases with the density of the amide group) [99]. The formation of hydrogen bonds between water molecules and the polar structure of PA decreases the density of hydrogen bonds between PA polymer chains. As a result, the intermolecular force decreases, and then the mobility of chains increases. It is reported that water absorption significantly impacted some properties of PA and its composites, such as thermal properties [100] and mechanical properties [101,102]. Randhawa et al. [100] utilized the SEM and XRD results of dry PA and water-treated PA to prove the generation of voids and the decrease in crystallinity after water immersion, which were responsible for the degradation of materials’ strength and hardness. In Ksouri et al.’s study, crazing appeared on the surface of long-term water-soaked PA6 and extended to the sample’s interior [103]. Water-induced voids and crazing may promote gas diffusion and weaken materials’ permeation resistance. Thus, the effect of ambient humidity on the decompression damage of polyamide materials cannot be ignored in future research.
- The predictive model is a convenient numerical tool for predicting the decompression damage of materials at specific operational conditions. Some parameters used in current models are the function of temperature and pressure but are considered constants, such as solubility, diffusivity, and yield stress. Therefore, further work could improve the accuracy of parameters and the model complexity to present the damage morphology better. Enriching the database for polymers’ transportation and mechanical properties at various temperatures and pressures is crucial and meaningful work for predictive models.
- The self-healing of polymers is gaining growing interest. Damaged materials can recover their original mechanical properties by self-healing. There are two main approaches to self-healing [104]: (1) intrinsic self-healing, repairing the damage by the chemical bonds inside the polymer itself, and (2) the extrinsic self-healing, which works using the release of healing agents. Blasizik et al. [105] believed that the potential for self-healing exists in thermosets, thermoplastics, and elastomers. Thermoplastics self-heal by joining the surfaces of cracks and then re-entangling polymer chains [106]. The application of self-healing to polymer liners will hinder the propagation of the damage and improve the service life of the type IV tank. Therefore, self-healing could be a future direction in the development of polymer liners for type IV tanks.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Romm, J. The Hype about Hydrogen: Fact and Fiction in the Race to Save the Climate; Bibliovault OAI Repository, the University of Chicago Press: Chicago, IL, USA, 2006. [Google Scholar]
- Lu, J.; Zahedi, A.; Yang, C.S.; Wang, M.Z.; Peng, B. Building the Hydrogen Economy in China: Drivers, Resources and Technologies. Renew. Sustain. Energy Rev. 2013, 23, 543–556. [Google Scholar] [CrossRef]
- Hwang, H.T.; Varma, A. Hydrogen Storage for Fuel Cell Vehicles. Curr. Opin. Chem. Eng. 2014, 5, 42–48. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen Storage and Delivery: Review of the State of the Art Technologies and Risk and Reliability Analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Maus, S.; Hapke, J.; Ranong, C.N.; Wuchner, E.; Friedlmeier, G.; Wenger, D. Filling Procedure for Vehicles with Compressed Hydrogen Tanks. Int. J. Hydrogen Energy 2008, 33, 4612–4621. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Jia, Z.C.; Yuan, Z.M.; Yang, T.; Qi, Y.; Zhao, D.L. Development and Application of Hydrogen Storage. J. Iron Steel Res. Int. 2015, 22, 757–770. [Google Scholar] [CrossRef]
- Rogers, H.C. Hydrogen Embrittlement of Metals: Atomic Hydrogen from a Variety of Sources Reduces the Ductility of Many Metals. Science 1968, 159, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen Embrittlement in Different Materials: A Review. Int. J. Hydrogen Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Landi, D.; Vita, A.; Borriello, S.; Scafa, M.; Germani, M. A Methodological Approach for the Design of Composite Tanks Produced by Filament Winding. Comput.-Aided Appl. 2020, 17, 1229–1240. [Google Scholar] [CrossRef]
- Hua, T.Q.; Ahluwalia, R.K.; Peng, J.K.; Kromer, M.; Lasher, S.; McKenney, K.; Law, K.; Sinha, J. Technical Assessment of Compressed Hydrogen Storage Tank Systems for Automotive Applications. Int. J. Hydrogen Energy 2011, 36, 3037–3049. [Google Scholar] [CrossRef]
- Sirosh, N.; Corbin, R.; Niedzwiecki, A. Hydrogen Composite Tank Project. 2003. Available online: www1.eere.energy.gov/hydrogenandfuelcells/pdfs/iiia1_sirosh.pdf (accessed on 12 March 2023).
- Abele, A.R. Advanced Hydrogen Fuel Systems for Fuel Cell Vehicles. In Proceedings of the 1st International Conference on Fuel Cell Science, Engineering and Technology, Rochester, NY, USA, 21–23 April 2003; pp. 83–87. [Google Scholar]
- Seredynski, P. Hyundai Nexo: Fuel-Cell Refinement, Suv Luxury. 2019. Available online: https://www.sae.org/news/2018/11/2019-hyundai-nexo-fuel-cell-launch (accessed on 12 March 2023).
- Yamashita, A.; Kondo, M.; Goto, S.; Ogami, N. Development of High-Pressure Hydrogen Storage System for the Toyota “Mirai”. In Proceedings of the SAE 2015 World Congress and Exhibition, Detroit, MI, USA, 21–23 April 2015; p. AVL. [Google Scholar]
- Yahashi, H.; Yamashita, A.; Shigemitsu, N.; Goto, S.; Kida, K.; Inou, T. Development of High-Pressure Hydrogen Storage System for New Fcv. In Proceedings of the SAE 2021 WCX Digital Summit, Virtual, Online, 13–15 April 2021; p. AAM. [Google Scholar]
- Su, Y.; Lv, H.; Zhou, W.; Zhang, C. Review of the Hydrogen Permeability of the Liner Material of Type Iv on-Board Hydrogen Storage Tank. World Electr. Veh. J. 2021, 12, 130. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, H.; Kang, H.R.; Zhou, W.; Zhang, C.M. A Literature Review of Failure Prediction and Analysis Methods for Composite High-Pressure Hydrogen Storage Tanks. Int. J. Hydrogen Energy 2019, 44, 25777–25799. [Google Scholar] [CrossRef]
- Wang, X.L.; Tian, M.M.; Chen, X.D.; Xie, P.C.; Yang, J.N.; Chen, J.X.; Yang, W.M. Advances on Materials Design and Manufacture Technology of Plastic Liner of Type Iv Hydrogen Storage Vessel. Int. J. Hydrogen Energy 2022, 47, 8382–8408. [Google Scholar] [CrossRef]
- de Miguel, N.; Cebolla, R.O.; Acosta, B.; Moretto, P.; Harskamp, F.; Bonato, C. Compressed Hydrogen Tanks for on-Board Application: Thermal Behaviour During Cycling. Int. J. Hydrogen Energy 2015, 40, 6449–6458. [Google Scholar] [CrossRef]
- Neto, E.S.B.; Chludzinski, M.; Roese, P.B.; Fonseca, J.S.O.; Amico, S.C.; Ferreira, C.A. Experimental and Numerical Analysis of a Lldpe/Hdpe Liner for a Composite Pressure Vessel. Polym. Test 2011, 30, 693–700. [Google Scholar] [CrossRef]
- Balasooriya, W.; Clute, C.; Schrittesser, B.; Pinter, G. A Review on Applicability, Limitations, and Improvements of Polymeric Materials in High-Pressure Hydrogen Gas Atmospheres. Polym. Rev. 2022, 62, 175–209. [Google Scholar] [CrossRef]
- Stern, S.A.; Fried, J.R. Permeability of Polymers to Gases and Vapors. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007; pp. 1033–1047. [Google Scholar]
- Klopffer, M.H.; Flaconneche, B. Transport Properties of Gases in Polymers: Bibliographic Review. Oil Gas Sci. Technol. 2001, 56, 223–244. [Google Scholar] [CrossRef]
- Flaconneche, B.; Martin, J.; Klopffer, M.H. Transport Properties of Gases in Polymers: Experimental Methods. Oil Gas Sci. Technol.-Rev. D Ifp Energ. Nouv. 2001, 56, 245–259. [Google Scholar] [CrossRef]
- Michaels, A.S.; Bixler, H.J. Flow of Gases through Polyethylene. J. Polym. Sci. 1961, 50, 413–439. [Google Scholar] [CrossRef]
- Kane, M. Permeability, Solubility, and Interaction of Hydrogen in Polymers-An Assessment of Materials for Hydrogen Transport; Savannah River Site (SRS): Aiken, SC, USA, 2008. [Google Scholar]
- Nuruddin, M.; Chowdhury, R.A.; Lopez-Perez, N.; Montes, F.J.; Youngblood, J.P.; Howarter, J.A. Influence of Free Volume Determined by Positron Annihilation Lifetime Spectroscopy (Pals) on Gas Permeability of Cellulose Nanocrystal Films. ACS Appl. Mater. Interfaces 2020, 12, 24380–24389. [Google Scholar] [CrossRef]
- Dewimille, B.; Martin, J.; Jarrin, J. Behavior of Thermoplastic Polymers During Explosive Decompressions in a Petroleum Environment. J. Phys. IV 1993, 3, 1559–1564. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Savvas, T.; Kelly, C.T. Explosive Decompression Failure of Rubbers—a Review of the Origins of Pneumatic Stress-Induced Rupture in Elastomers. Rubber Chem. Technol. 1994, 67, 384–416. [Google Scholar] [CrossRef]
- Yersak, T.A.; Baker, D.R.; Yanagisawa, Y.; Slavik, S.; Immel, R.; Mack-Gardner, A.; Herrmann, M.; Cai, M. Predictive Model for Depressurization-Induced Blistering of Type Iv Tank Liners for Hydrogen Storage. Int. J. Hydrogen Energy 2017, 42, 28910–28917. [Google Scholar] [CrossRef]
- Jaravel, J.; Castagnet, S.; Grandidier, J.C.; Benoit, G. On Key Parameters Influencing Cavitation Damage upon Fast Decompression in a Hydrogen Saturated Elastomer. Polym. Test 2011, 30, 811–818. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ono, H.; Ohyama, K.; Kasai, M.; Kaneko, F.; Nishimura, S. Hydrogen Permeation under High Pressure Conditions and the Destruction of Exposed Polyethylene-Property of Polymeric Materials for High-Pressure Hydrogen Devices (2). Int. J. Hydrogen Energy 2021, 46, 11832–11848. [Google Scholar] [CrossRef]
- Gerland, M.; Boyer, S.A.E.; Castagnet, S. Early Stages of Cavitation in a Stretched and Decompressed Poly(Vinylidene Fluoride) Exposed to Diffusive Hydrogen, Observed by Transmission Electronic Microscopy at the Nanoscale. Int. J. Hydrogen Energy 2016, 41, 1766–1774. [Google Scholar] [CrossRef]
- Yamabe, J.; Nishimura, S. Nanoscale Fracture Analysis by Atomic Force Microscopy of Epdm Rubber Due to High-Pressure Hydrogen Decompression. J. Mater. Sci. 2011, 46, 2300–2307. [Google Scholar] [CrossRef]
- Koga, A.; Yamabe, T.; Sato, H.; Uchida, K.; Nakayama, J.; Yamabe, J.; Nishimura, S. A Visualizing Study of Blister Initiation Behavior by Gas Decompression. Tribol. Online 2013, 8, 68–75. [Google Scholar] [CrossRef]
- Lorge, O.; Briscoe, B.J.; Dang, P. Gas Induced Damage in Poly(Vinylidene Fluoride) Exposed to Decompression. Polymer 1999, 40, 2981–2991. [Google Scholar] [CrossRef]
- El-Sawy, K.M. Inelastic Stability of Tightly Fitted Cylindrical Liners Subjected to External Uniform Pressure. Thin-Walled Struct. 2001, 39, 731–744. [Google Scholar] [CrossRef]
- Naebe, M.; Abolhasani, M.M.; Khayyam, H.; Amini, A.; Fox, B. Crack Damage in Polymers and Composites: A Review. Polym. Rev. 2016, 56, 31–69. [Google Scholar] [CrossRef]
- Yamabe, J.; Koga, A.; Nishimura, S. Failure Behavior of Rubber O-Ring under Cyclic Exposure to High-Pressure Hydrogen Gas. Eng. Fail. Anal. 2013, 35, 193–205. [Google Scholar] [CrossRef]
- Yamabe, J.; Nishimura, S. Influence of Carbon Black on Decompression Failure and Hydrogen Permeation Properties of Filled Ethylene-Propylene-Diene-Methylene Rubbers Exposed to High-Pressure Hydrogen Gas. J. Appl. Polym. Sci. 2011, 122, 3172–3187. [Google Scholar] [CrossRef]
- Ono, H.; Fujiwara, H.; Onoue, K.; Nishimura, S. Influence of Repetitions of the High-Pressure Hydrogen Gas Exposure on the Internal Damage Quantity of High-Density Polyethylene Evaluated by Transmitted Light Digital Image. Int. J. Hydrogen Energy 2019, 44, 23303–23319. [Google Scholar] [CrossRef]
- Baldwin, D. Development of High Pressure Hydrogen Storage Tank for Storage and Gaseous Truck Delivery; Hexagon Lincoln LLC: Lincoln, NE, USA, 2017. [Google Scholar]
- Boyer, S.A.E.; Gerland, M.; Castagnet, S. Gas Environment Effect on Cavitation Damage in Stretched Polyvinylidene Fluoride. Polym. Eng. Sci. 2014, 54, 2139–2146. [Google Scholar] [CrossRef]
- Compatibility of Polymeric Materials Used in the Hydrogen Infrastructure. Available online: https://www.hydrogen.energy.gov/pdfs/review18/scs026_simmons_2018_o.pdf (accessed on 12 March 2023).
- Rosenberg, E.; Brusselle-Dupend, N.; Epsztein, T. A Mesoscale Quantification Method of Cavitation in Semicrystalline Polymers Using X-Ray Microtomography. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2011, 528, 6535–6544. [Google Scholar] [CrossRef]
- Menon, N.C.; Kruizenga, A.M.; San Marchi, C.W.; Campbell, J.; Nissen, A.; Mills, B.E. Polymer Behaviour in High Pressure Hydrogen Helium and Argon Environments as Applicable to the Hydrogen Infrastructure; Sandia National Lab.(SNL-NM): Albuquerque, NM, USA, 2017. [Google Scholar]
- Kulkarni, S.S.; Choi, K.S.; Kuang, W.B.; Menon, N.; Mills, B.; Soulami, A.; Simmons, K. Damage Evolution in Polymer Due to Exposure to High-Pressure Hydrogen Gas. Int. J. Hydrogen Energy 2021, 46, 19001–19022. [Google Scholar] [CrossRef]
- Ono, H.; Nait-Ali, A.; Diallo, O.K.; Benoit, G.; Castagnet, S. Influence of Pressure Cycling on Damage Evolution in an Unfilled Epdm Exposed to High-Pressure Hydrogen. Int. J. Fract. 2018, 210, 137–152. [Google Scholar] [CrossRef]
- Castagnet, S.; Mellier, D.; Nait-Ali, A.; Benoit, G. In-Situ X-Ray Computed Tomography of Decompression Failure in a Rubber Exposed to High-Pressure Gas. Polym. Test 2018, 70, 255–262. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Zakaria, S. Gas-Induced Damage in Elastomeric Composites. J. Mater. Sci. 1990, 25, 3017–3023. [Google Scholar] [CrossRef]
- Castagnet, S.; Ono, H.; Benoit, G.; Fujiwara, H.; Nishimura, S. Swelling Measurement During Sorption and Decompression in a Nbr Exposed to High-Pressure Hydrogen. Int. J. Hydrogen Energy 2017, 42, 19359–19366. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.S.; Song, K. Research on Acoustic Emission Test Method for Metal Fatigue Fracture Based on Wavelet Packets Feature Extraction. In Proceedings of the 10th International Conference on Fracture and Damage Mechanics (FDM 2011), Dubrovnik, Croatia, 19–21 September 2011; pp. 432–435. [Google Scholar]
- Van Tittelboom, K.; De Belie, N.; Lehmann, F.; Grosse, C.U. Acoustic Emission Analysis for the Quantification of Autonomous Crack Healing in Concrete. Constr. Build. Mater. 2012, 28, 333–341. [Google Scholar] [CrossRef]
- Santulli, C. Matrix Cracking Detection by Acoustic Emission in Polymer Composites and Counts/Duration Ratio. E–J Non-Destr. Test 2012, 17, 11. [Google Scholar]
- Yilin, Y.; Gongtian, S.; Junjiao, Z.; Bo, Z.; Yongna, S.; Ke, B. Experiment Research on Tensile Process of Type Iv Gas Cylinder Liner-Hdpe Based on Acoustic Emission. In Proceedings of the Advances in Acoustic Emission Technology, World Conference on Acoustic Emission 2019 (WCAE 2019), Singapore, 5–8 November 2019; pp. 281–295. [Google Scholar]
- Yamabe, J.; Matsumoto, T.; Nishimura, S. Application of Acoustic Emission Method to Detection of Internal Fracture of Sealing Rubber Material by High-Pressure Hydrogen Decompression. Polym. Test 2011, 30, 76–85. [Google Scholar] [CrossRef]
- Ohtsu, M. The History and Development of Acoustic Emission in Concrete Engineering. Mag. Concr. Res. 1996, 48, 321–330. [Google Scholar] [CrossRef]
- Yamabe, J.; Nishimura, S. Influence of Fillers on Hydrogen Penetration Properties and Blister Fracture of Rubber Composites for O-Ring Exposed to High-Pressure Hydrogen Gas. Int. J. Hydrogen Energy 2009, 34, 1977–1989. [Google Scholar] [CrossRef]
- Mason, J.F.; Alkire, J.D. Effect of Rapid Decompression Conditions on Liner Materials. In Proceedings of the Corrosion 2000, Orlando, FL, USA, 26–31 March 2000. [Google Scholar]
- Melnichuk, M.; Thiebaud, F.; Perreux, D. Non-Dimensional Assessments to Estimate Decompression Failure in Polymers for Hydrogen Systems. Int. J. Hydrogen Energy 2020, 45, 6738–6744. [Google Scholar] [CrossRef]
- Hedenqvist, M.; Gedde, U.W. Diffusion of Small-Molecule Penetrants in Semicrystalline Polymers. Prog. Polym. Sci. 1996, 21, 299–333. [Google Scholar] [CrossRef]
- Flaconneche, B.; Martin, J.; Klopffer, M.H. Permeability, Diffusion and Solubility of Gases in Polyethylene, Polyamide 11 and Poly(Vinylidene Fluoride). Oil Gas Sci. Technol.-Rev. D Ifp Energ. Nouv. 2001, 56, 261–278. [Google Scholar] [CrossRef]
- Prachumchon, S. A Study of Hdpe in High Pressure of Hydrogen Gas—Measurement of Permeation Parameters and Fracture Criteria; ProQuest LLC: Ann Arbor, MI, USA, 2012. [Google Scholar]
- Zhang, X.F.; Xie, F.; Pen, Z.L.; Zhang, Y.; Zhang, Y.X.; Zhou, W. Effect of Nucleating Agent on the Structure and Properties of Polypropylene/Poly(Ethylene-Octene) Blends. Eur. Polym. J. 2002, 38, 1–6. [Google Scholar] [CrossRef]
- Mittal, V. Crystallinity, Mechanical Property and Oxygen Permeability of Polypropylene: Effect of Processing Conditions, Nucleating Agent and Compatibilizer. J. Thermoplast. Compos. Mater. 2013, 26, 1407–1423. [Google Scholar] [CrossRef]
- Cui, Y.B.; Kundalwal, S.I.; Kumar, S. Gas Barrier Performance of Graphene/Polymer Nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef]
- Picard, E.; Gerard, J.F.; Espuche, E. Reinforcement of the Gas Barrier Properties of Polyethylene and Polyamide through the Nanocomposite Approach: Key Factors and Limitations. Oil Gas Sci. Technol.-Rev. D Ifp Energ. Nouv. 2015, 70, 237–249. [Google Scholar] [CrossRef]
- Sun, Y.; Lv, H.; Zhou, W.; Zhang, C.M. Research on Hydrogen Permeability of Polyamide 6 as the Liner Material for Type Iv Hydrogen Storage Tank. Int. J. Hydrogen Energy 2020, 45, 24980–24990. [Google Scholar] [CrossRef]
- Ikeda, Y.; Yasuda, Y.; Hijikata, K.; Tosaka, M.; Kohjiya, S. Comparative Study on Strain-Induced Crystallization Behavior of Peroxide Cross-Linked and Sulfur Cross-Linked Natural Rubber. Macromolecules 2008, 41, 5876–5884. [Google Scholar] [CrossRef]
- Monson, L.; Moon, S.I.; Extrand, C.W. Permeation Resistance of Poly(Ether Ether Ketone) to Hydrogen, Nitrogen, and Oxygen Gases. J. Appl. Polym. Sci. 2013, 127, 1637–1642. [Google Scholar] [CrossRef]
- Anovitz, B.S.L.M. Iv.F.1 Lifecycle Verification of Polymeric Storage Liners. Available online: https://www.hydrogen.energy.gov/pdfs/progress13/iv_f_1_smith_2013.pdf (accessed on 12 March 2023).
- Murray, B.R.; Leen, S.B.; Semprimoschnig, C.O.A.; Bradaigh, C.M.O. Helium Permeability of Polymer Materials as Liners for Composite Overwrapped Pressure Vessels. J. Appl. Polym. Sci. 2016, 133, 10. [Google Scholar] [CrossRef]
- Baudet, C.; Grandidier, J.C.; Cangemi, L. A Damage Model for the Blistering of Polyvinylidene Fluoride Subjected to Carbon Dioxide Decompression. J. Mech. Phys. Solids 2011, 59, 1909–1926. [Google Scholar] [CrossRef]
- Melnichuk, M.; Gardavaud, Q.; Thiebaud, F.; Perreux, D. Numerical Assestments of Maximum Depressurisation Rate for Polymer Materials under High-Pressure Hydrogen. Int. J. Hydrogen Energy 2021, 46, 27088–27095. [Google Scholar] [CrossRef]
- Melnichuk, M.; Gardavaud, Q.; Thiebaud, F.; Perreux, D. Temperature Effect in Cavitation Risk Assessments of Polymers for Hydrogen Systems. Int. J. Hydrogen Energy 2020, 45, 23020–23026. [Google Scholar] [CrossRef]
- Xiao, J.S.; Benard, P.; Chahine, R. Estimation of Final Hydrogen Temperature from Refueling Parameters. Int. J. Hydrogen Energy 2017, 42, 7521–7528. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Q.L.; Zhang, J.; Zhang, S.; He, G.P.; Zhang, M.Y.; Su, T.T.; Liang, X.; Huang, C.; Yan, W.H. Review on Studies of the Emptying Process of Compressed Hydrogen Tanks. Int. J. Hydrogen Energy 2021, 46, 22554–22573. [Google Scholar] [CrossRef]
- Melideo, D.; Baraldi, D.; Acosta-Iborra, B.; Cebolla, R.O.; Moretto, P. Cfd Simulations of Filling and Emptying of Hydrogen Tanks. Int. J. Hydrog. Energy 2017, 42, 7304–7313. [Google Scholar] [CrossRef]
- Klopffer, M.H.; Berne, P.; Weber, M.; Castagnet, S.; Hochstetter, G.; Espuche, E. New Materials for Hydrogen Distribution Networks: Materials Development & Technico-Economic Benchmark. In Proceedings of the International Conference on Diffusion in Materials (DIMAT 2011), Dijon, France, 3–8 July 2011; p. 407. [Google Scholar]
- Barth, R.R.; Simmons, K.L.; Marchi, C.S. Polymers for Hydrogen Infrastructure and Vehicle Fuel Systems: Applications, Properties, and Gap Analysis; Sandia National Lab: Livermore, CA, USA, 2013. [Google Scholar]
- Castagnet, S.; Grandidier, J.C.; Comyn, M.; Benoit, G. Hydrogen Influence on the Tensile Properties of Mono and Multi-Layer Polymers for Gas Distribution. Int. J. Hydrogen Energy 2010, 35, 7633–7640. [Google Scholar] [CrossRef]
- Pepin, J.; Laine, E.; Grandidier, J.C.; Castagnet, S.; Blanc-vannet, P.; Papin, P.; Weber, M. Determination of Key Parameters Responsible for Polymeric Liner Collapse in Hyperbaric Type Iv Hydrogen Storage Vessels. Int. J. Hydrogen Energy 2018, 43, 16386–16399. [Google Scholar] [CrossRef]
- Castagnet, S.; Grandidier, J.C.; Comyn, M.; Benoit, G. Mechanical Testing of Polymers in Pressurized Hydrogen: Tension, Creep and Ductile Fracture. Exp. Mech. 2012, 52, 229–239. [Google Scholar] [CrossRef]
- Merah, N.; Saghir, F.; Khan, Z.; Bazoune, A. Effect of Temperature on Tensile Properties of Hdpe Pipe Material. Plast. Rubber Compos. 2006, 35, 226–230. [Google Scholar] [CrossRef]
- Scheichl, R.; Klopffer, M.H.; Benjelloun-Dabaghi, Z.; Flaconneche, B. Permeation of Gases in Polymers: Parameter Identification and Nonlinear Regression Analysis. J. Membr. Sci. 2005, 254, 275–293. [Google Scholar] [CrossRef]
- Alvine, K.J.; Kafentzis, T.A.; Pitman, S.G.; Johnson, K.I.; Skorski, D.; Tucker, J.C.; Roosendaal, T.J.; Dahl, M.E. An in Situ Tensile Test Apparatus for Polymers in High Pressure Hydrogen. Rev. Sci. Instrum. 2014, 85, 8. [Google Scholar] [CrossRef]
- Koga, A.; Uchida, K.; Yamabe, J.; Nishimura, S. Evaluation on High-Pressure Hydrogen Decompression Failure of Rubber O-Ring Using Design of Experiments. Int. J. Automot. Eng. 2011, 2, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Jaravel, J.; Castagnet, S.; Grandidier, J.C.; Gueguen, M. Experimental Real-Time Tracking and Diffusion/Mechanics Numerical Simulation of Cavitation in Gas-Saturated Elastomers. Int. J. Solids Struct. 2013, 50, 1314–1324. [Google Scholar] [CrossRef]
- Kane-Diallo, O.; Castagnet, S.; Nait-Ali, A.; Benoit, G.; Grandidier, J.C. Time-Resolved Statistics of Cavity Fields Nucleated in a Gas-Exposed Rubber under Variable Decompression Conditions—Support to a Relevant Modeling Framework. Polym. Test 2016, 51, 122–130. [Google Scholar] [CrossRef]
- Yamabe, J.; Nishimura, S. Crack Growth Behavior of Sealing Rubber under Static Strain in High-Pressure Hydrogen Gas. J. Solid Mech. Mater. Eng. 2011, 5, 690–701. [Google Scholar] [CrossRef]
- Gent, A.; Tompkins, D. Nucleation and Growth of Gas Bubbles in Elastomers. J. Appl. Phys. 1969, 40, 2520–2525. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Clarendon: Oxford, UK, 1975; pp. 49–56. [Google Scholar]
- Tao, K.S.K.; Yamada, K.; Higashi, S.; Kago, K.; Kuwashiro, S.; Hirano, H.; Takeshita, H.; Tokumitsu, K. Effect of Molding History on Molecular Orientation Relaxation during Physical Aging of Polystyrene Injection Moldings. Int. Polym. Process 2023, 38, 233–243. [Google Scholar] [CrossRef]
- Nasr, A.; Svoboda, P. Influence of Fusion Temperature on Nonisothermal Crystallization Kinetics of Polyamide 6. Polymers 2023, 15, 1952. [Google Scholar] [CrossRef]
- Klopffer, M.H.; Berne, P.; Espuche, E. Development of Innovating Materials for Distributing Mixtures of Hydrogen and Natural Gas. Study of the Barrier Properties and Durability of Polymer Pipes. Oil Gas Sci. Technol.-Rev. D Ifp Energ. Nouv. 2015, 70, 305–315. [Google Scholar] [CrossRef]
- Castagnet, S.; Grandidier, J.C.; Comyn, M.; Benoit, G. Effect of Long-Term Hydrogen Exposure on the Mechanical Properties of Polymers Used for Pipes and Tested in Pressurized Hydrogen. Int. J. Press. Vessel. Pip. 2012, 89, 203–209. [Google Scholar] [CrossRef]
- Fumitoshi, K.; Hirotada, F.; Shin, N. Influence of High-Pressure Hydrogen Gas on Crystalline Polymers. In Proceedings of the 33rd Polymer Degradation Discussion Group, St Julians, Malta, 1–5 September 2019; pp. 1–5. [Google Scholar]
- Sambale, A.K.; Stanko, M.; Emde, J.; Stommel, M. Characterisation and Fe Modelling of the Sorption and Swelling Behaviour of Polyamide 6 in Water. Polymers 2021, 13, 1480. [Google Scholar] [CrossRef]
- Sambale, A.K.; Maisl, M.; Herrmann, H.G.; Stommel, M. Characterisation and Modelling of Moisture Gradients in Polyamide 6. Polymers 2021, 13, 3141. [Google Scholar] [CrossRef]
- Randhawa, K.S.; Patel, A. Influence of Moisture/Water Absorption on Mechanical and Thermal Properties of Polyamide6/Boric Oxide Composites. Pigm. Resin. Technol. 2022, 51, 354–363. [Google Scholar] [CrossRef]
- Eftekhari, M.; Fatemi, A. Tensile Behavior of Thermoplastic Composites Including Temperature, Moisture, and Hygrothermal Effects. Polym. Test 2016, 51, 151–164. [Google Scholar] [CrossRef]
- Guttmann, P.; Pilz, G. Fibre-Reinforced Polyamides and the Influence of Water Absorption on the Mechanical and Thermomechanical Behaviour. In Deformation and Fracture Behaviour of Polymer Materials; Grellmann, W., Langer, B., Eds.; Springer Series in Materials Science; Springer: Berlin, Germany, 2017; Volume 247, pp. 377–388. [Google Scholar]
- Ksouri, I.; De Almeida, O.; Haddar, N. Long Term Ageing of Polyamide 6 and Polyamide 6 Reinforced with 30% of Glass Fibers: Physicochemical, Mechanical and Morphological Characterization. J. Polym. Res. 2017, 24, 12. [Google Scholar] [CrossRef]
- Irzhak, V.I.; Uflyand, I.E.; Dzhardimalieva, G.I. Self-Healing of Polymers and Polymer Composites. Polymers 2022, 14, 5404. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-Healing Polymers and Composites. In Annual Review of Materials Research; Clarke, D.R., Ruhle, M., Zok, F., Eds.; Annual Review of Materials Research; Annual Reviews: Palo Alto, CA, USA, 2010; Volume 40, pp. 179–211. [Google Scholar]
- Caruso, M.M.; Davis, D.A.; Shen, Q.; Odom, S.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Mechanically-Induced Chemical Changes in Polymeric Materials. Chem. Rev. 2009, 109, 5755–5798. [Google Scholar] [CrossRef] [PubMed]




| Mechanical Properties | Evolution | References |
|---|---|---|
| Stiffness | ↓ | [28] |
| Yield stress | ↓ | [28] |
| Rupture stress | ↓ | [28] |
| Modulus | ↓ | [28,36] |
| Elongation at break | ↑ | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Su, Y.; Lv, H.; Liu, M.; Li, W.; Zhang, C. Review of Decompression Damage of the Polymer Liner of the Type IV Hydrogen Storage Tank. Polymers 2023, 15, 2258. https://doi.org/10.3390/polym15102258
Jin Z, Su Y, Lv H, Liu M, Li W, Zhang C. Review of Decompression Damage of the Polymer Liner of the Type IV Hydrogen Storage Tank. Polymers. 2023; 15(10):2258. https://doi.org/10.3390/polym15102258
Chicago/Turabian StyleJin, Zeping, Ying Su, Hong Lv, Min Liu, Wenbo Li, and Cunman Zhang. 2023. "Review of Decompression Damage of the Polymer Liner of the Type IV Hydrogen Storage Tank" Polymers 15, no. 10: 2258. https://doi.org/10.3390/polym15102258





