Impact- and Thermal-Resistant Epoxy Resin Toughened with Acacia Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
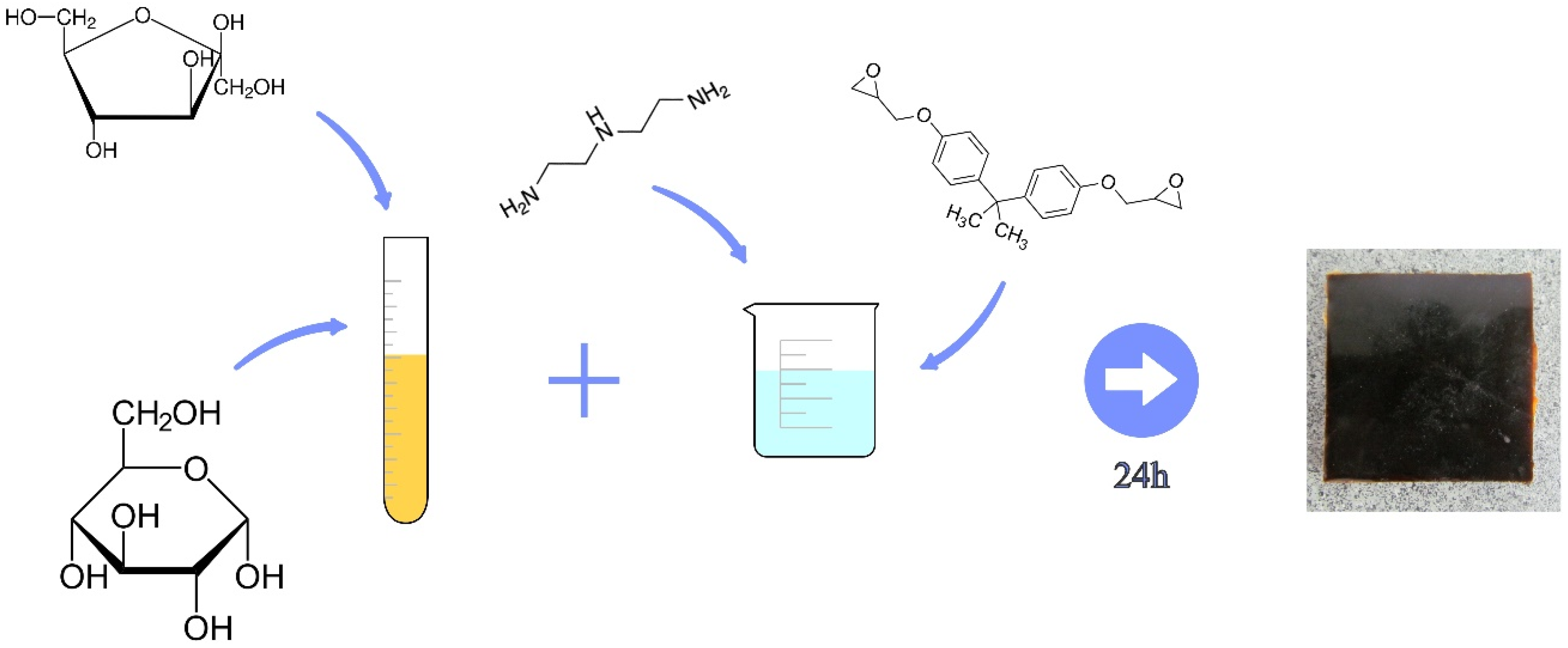
2.2. Preparation of Samples
2.3. Characterization of E and EH
3. Results and Discussion
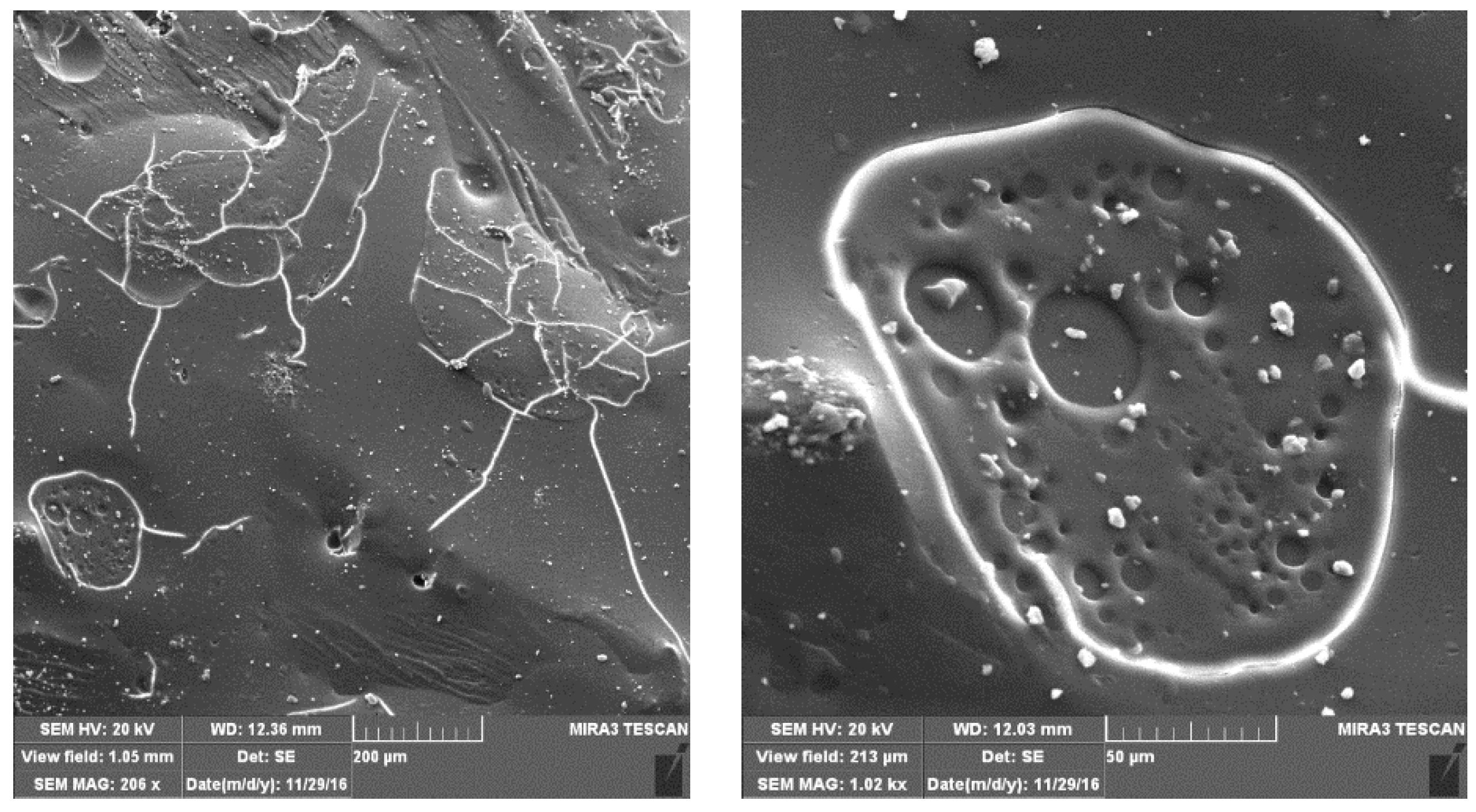
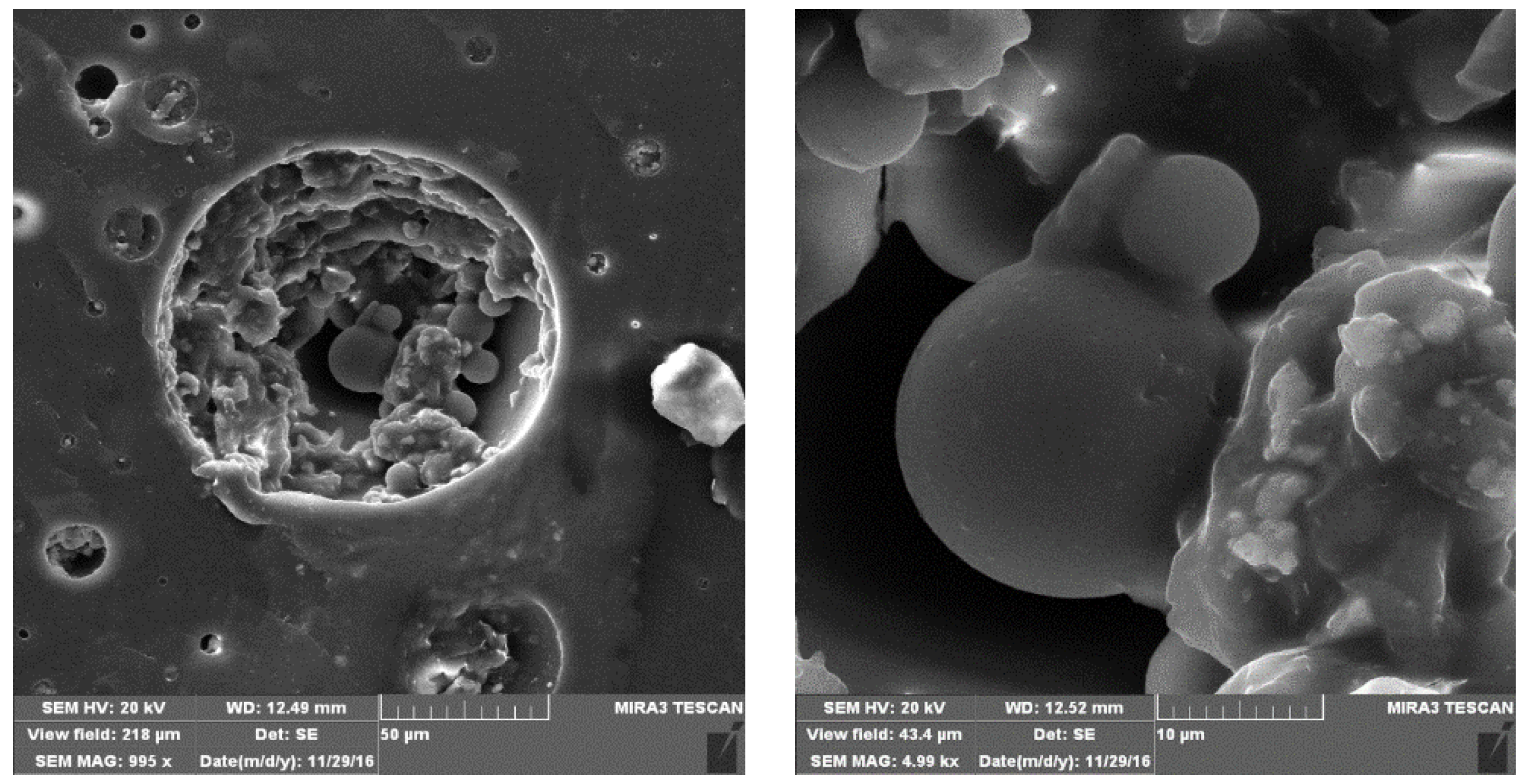
3.1. FESEM Analysis
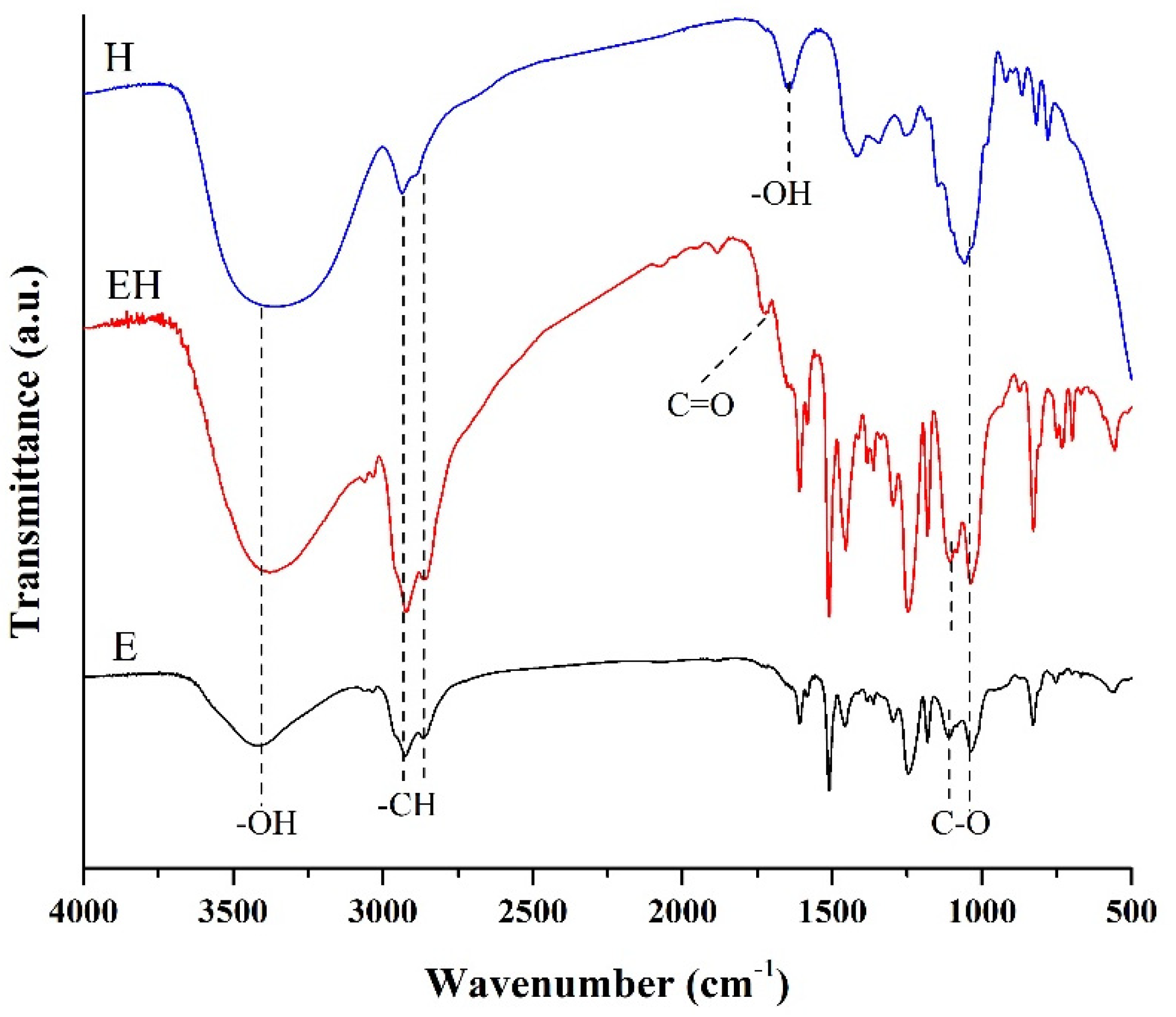
3.2. FTIR Analysis
3.2.1. The Region 4000–1300 cm−1
3.2.2. The Region 1300–400 cm−1
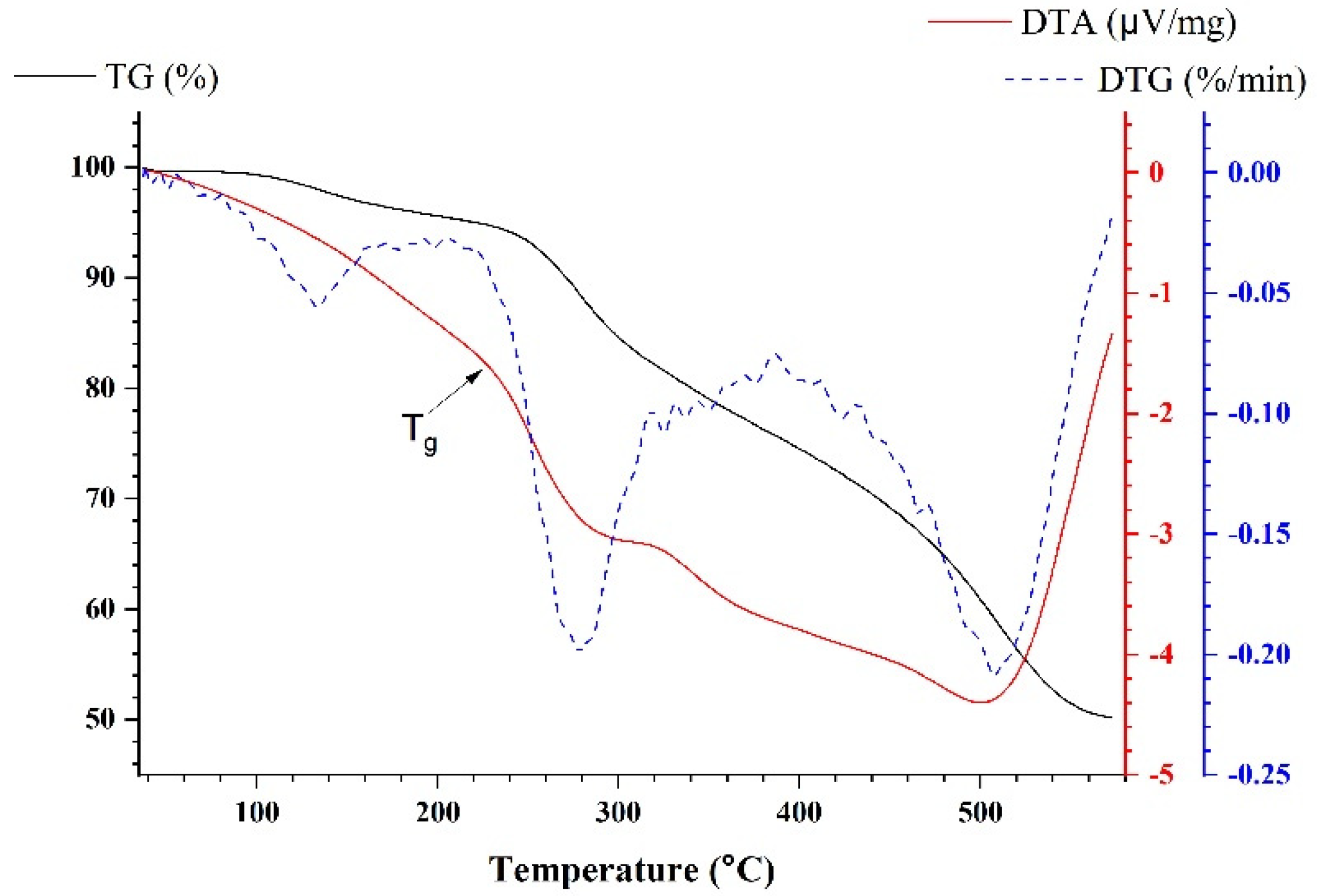
3.3. Thermogravimetric Analysis (TGA)
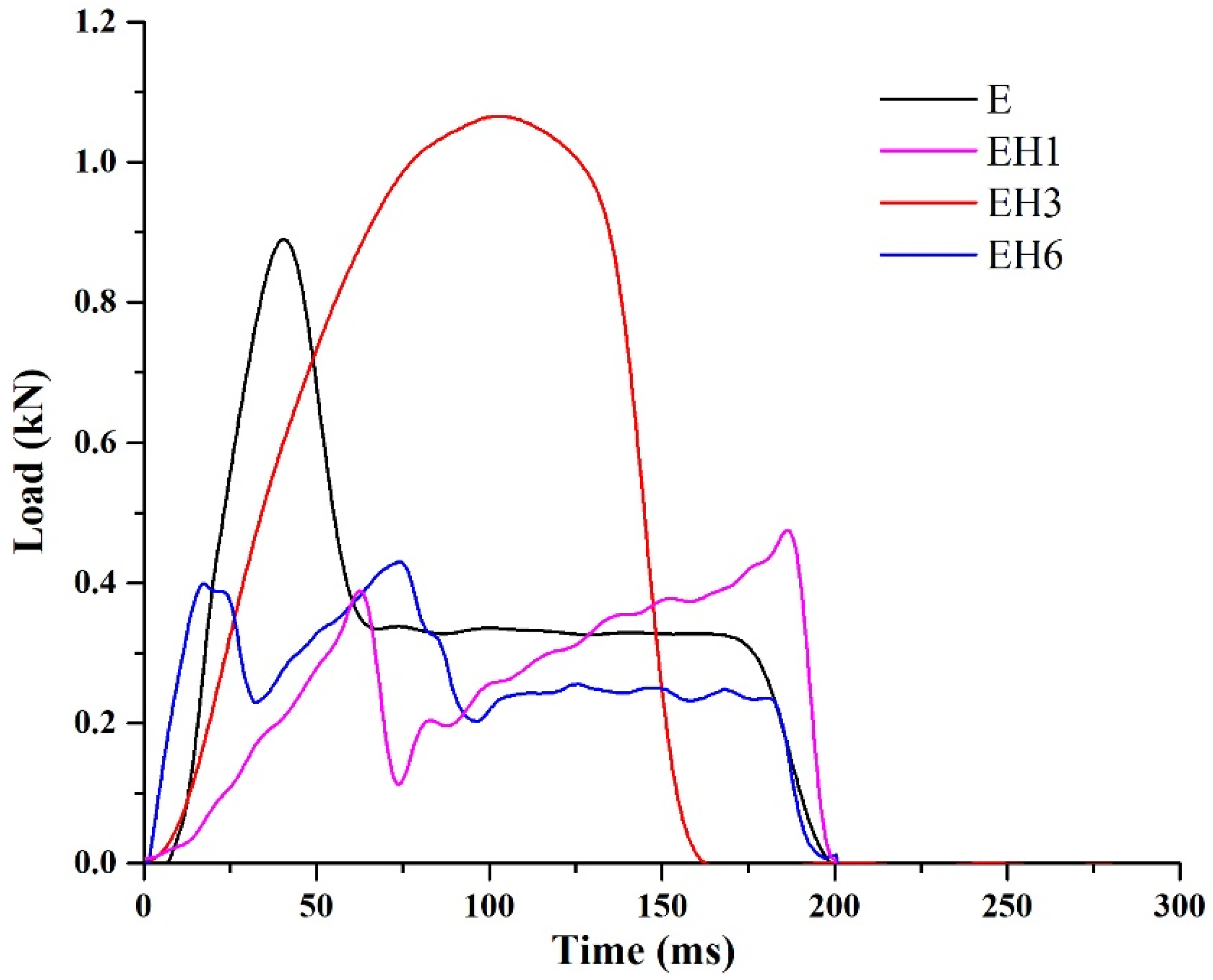
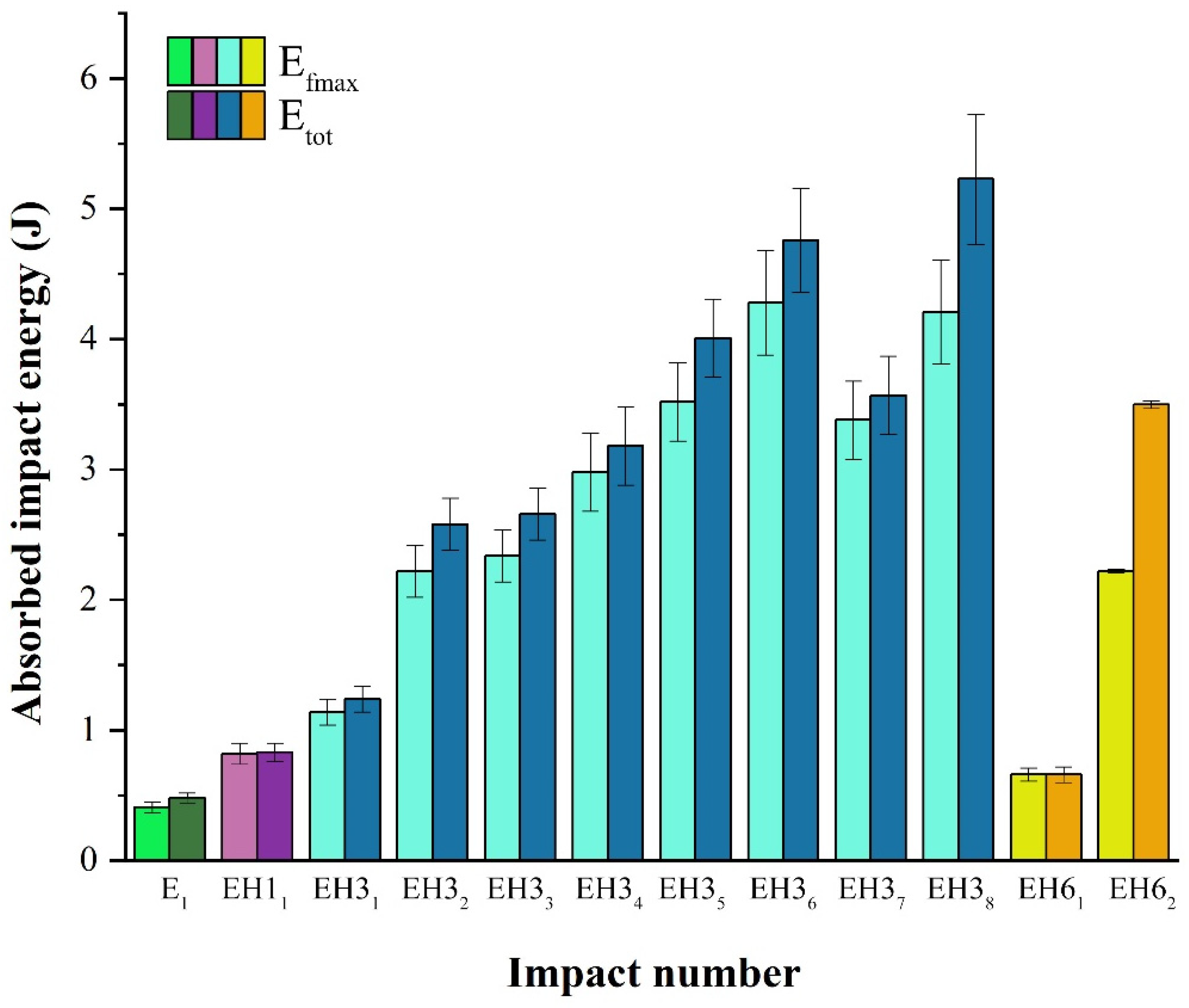
3.4. Impact Test
- The first phase represents the energy absorbed through the initial creation of the crack;
- The second phase represents the energy absorbed during the propagation and development of the crack until the failure of the material.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Westover, A.S.; Baer, B.; Bello, B.H.; Sun, H.; Oakes, L.; Bellan, L.M.; Pint, C.L. Multifunctional high strength and high energy epoxy composite structural supercapacitors with wet-dry operational stability. J. Mater. Chem. A. 2015, 3, 20097–20102. [Google Scholar] [CrossRef]
- Wan, J.; Gan, B.; Li, C.; Molina-Aldareguia, J.; Kalali, E.N.; Wang, X.; Wang, D.Y. A sustainable, eugenol-derived epoxy resin with high biobased content, modulus, hardness and low flammability: Synthesis, curing kinetics and structure–property relationship. Chem. Eng. J. 2016, 284, 1080–1093. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Wang, E.; Zhang, X.; Yuan, R.; Wu, S.; Zhu, Y. Facile preparation of superamphiphobic epoxy resin/modified poly(vinylidene fluoride)/fluorinated ethylene propylene composite coating with corrosion/wear-resistance. Appl. Surf. Sci. 2015, 357, 229–235. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Dai, W.; Song, Y.; Wang, D.; Zeng, L.; Jiang, N. Enhanced thermal and electrical properties of epoxy composites reinforced with graphene nanoplatelets. Polym. Compos. 2015, 36, 556–565. [Google Scholar] [CrossRef]
- Barbosa, A.Q.; da Silva, L.F.M.; Abenojar, J.; Figueiredo, M.; Öchsner, A. Toughness of a brittle epoxy resin reinforced with micro cork particles: Effect of size, amount and surface treatment. Compos. B Eng. 2017, 114, 299–310. [Google Scholar] [CrossRef]
- Wang, R.; Zhuo, D.; Weng, Z.; Wu, L.; Cheng, X.; Zhou, Y.; Wang, J.; Xuan, B. A novel nanosilica/graphene oxide hybrid and its flame retarding epoxy resin with simultaneously improved mechanical, thermal conductivity, and dielectric properties. J. Mater. Chem. A 2015, 3, 9826–9836. [Google Scholar] [CrossRef]
- Wang, T.; Song, B.; Wang, L. A new filler for epoxy resin: Study on the properties of graphite carbon nitride (g-c3n4) reinforced epoxy resin composites. Polymers 2020, 12, 76. [Google Scholar] [CrossRef]
- Ramesh, M. Flax (Linum usitatissimum L.) fibre reinforced polymer composite materials: A review on preparation, properties and prospects. Prog. Mater Sci. 2019, 102, 109–166. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Chen, L.; Rao, W.-H.; Qi, M.; Guo, D.-M.; Liao, W.; Wang, Y.-Z. Latent curing epoxy system with excellent thermal stability, flame retardance and dielectric property. Chem. Eng. J. 2018, 347, 223–232. [Google Scholar] [CrossRef]
- Chee, S.S.; Jawaid, M. The Effect of Bi-Functionalized MMT on Morphology, Thermal Stability, Dynamic Mechanical, and Tensile Properties of Epoxy/Organoclay Nanocomposites. Polymers 2019, 11, 2012. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, Q.; Zhu, H.; Lin, Q.; Wang, C. Impact Resistance Enhancement by Adding Core-Shell Particle to Epoxy Resin Modified with Hyperbranched Polymer. Polymers 2017, 9, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Skorb, E.V.; Andreeva, D.V. Self-healing properties of layer-by-layer assembled multilayers. Polym Int. 2015, 64, 713–723. [Google Scholar] [CrossRef]
- Vinod, A.; Sanjay, M.R.; Suchart, S.; Jyotishkumar, P. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar] [CrossRef]
- Al-Hajaj, Z.; Zdero, R.; Bougherara, H. Mechanical, morphological, and water absorption properties of a new hybrid composite material made from 4 harness satin woven carbon fibres and flax fibres in an epoxy matrix. Compos. A Appl. Sci. Manuf. 2018, 115, 46–56. [Google Scholar] [CrossRef]
- Chee, S.S.; Jawaid, M.; Sultan, M.T.H.; Alothman, O.Y.; Abdullah, L.C. Thermomechanical and dynamic mechanical properties of bamboo/woven kenaf mat reinforced epoxy hybrid composites. Compos. B Eng. 2019, 163, 165–174. [Google Scholar] [CrossRef]
- Alshahrani, H.; Arun Prakash, V.R. Thermal, mechanical and barrier properties of rice husk ash biosilica toughened epoxy biocomposite coating for structural application. Prog. Org. Coat. 2022, 172, 107080. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Kadykova, Y.; Akhmetova, M.; Tastanova, L.; Lopukhova, M. Development and Analysis of the Physicochemical and Mechanical Properties of Diorite-Reinforced Epoxy Composites. Polymers 2021, 13, 2421. [Google Scholar] [CrossRef]
- Oladele, I.O.; Makinde-Isola, B.A.; Adediran, A.A.; Oladejo, M.O.; Owa, A.F.; Olayanju, T.M.A. Mechanical and wear behaviour of pulverised poultry eggshell/sisal fiber hybrid reinforced epoxy composites. Mater. Res. Express 2020, 7, 045304. [Google Scholar] [CrossRef]
- Minugu, O.P.; Gujjala, R.; Shakuntala, O.; Manoj, P.; Chowdary, M.S. Effect of biomass derived biochar materials on mechanical properties of biochar epoxy composites. Proc. IMechE Part C J. Mech. Eng. Sci. 2021, 235, 5626–5638. [Google Scholar] [CrossRef]
- Bezerra, W.B.A.; Monteiro, S.N.; Oliveira, M.S.; da Luz, F.S.; da Costa Garcia Filho, F.; da Cruz Demosthenes, L.C.; Costa, U.O. Processing and characterization of Arapaima gigas scales and their reinforced epoxy composites. J. Mater. Res. Technol. 2020, 9, 3005–3012. [Google Scholar] [CrossRef]
- Asada, C.; Basnet, S.; Otsuka, M.; Sasaki, C.; Nakamura, Y. Epoxy resin synthesis using low molecular weight lignin separated from various lignocellulosic materials. Int. J. Biol. Macromol. 2015, 74, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gu, X. Fabrication of a bi-hydroxyl-bi-DOPO compound with excellent quenching and charring capacities for lignin-based epoxy resin. Int. J. Biol. Macromol. 2022, 205, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, X.; Fu, S. Bio-based epoxy resin from gallic acid and its thermosets toughened with renewable tannic acid derivatives. J. Mater. Sci. 2022, 57, 9493–9507. [Google Scholar] [CrossRef]
- Hollande, L.; Do Marcolino, I.; Balaguer, P.; Domenek, S.; Gross, R.A.; Allais, F. Preparation of Renewable Epoxy-Amine Resins with Tunable Thermo-Mechanical Properties, Wettability and Degradation Abilities from Lignocellulose- and Plant Oils-Derived Components. Front. Chem. 2019, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Fu, Q.; Zhang, T.; Chen, Y.; Tan, J.; Zhou, Y.; Zhu, X. Properties and curing kinetics of epoxy resin toughened by dimer acid diglycidyl ester. Thermochim. Acta. 2021, 699, 178910. [Google Scholar] [CrossRef]
- Hu, F.; Yadav, S.K.; La Scala, J.J.; Throckmorton, J.; Palmese, G.R. Epoxidized soybean oil modified using fatty acids as tougheners for thermosetting epoxy resins: Part 1. J. Appl. Polym. Sci. 2021, 138, e50570. [Google Scholar] [CrossRef]
- Zhang, T.; Tan, J.; Han, X.; Fu, Q.; Chen, M.; Xu, Y.; Zhu, X. Novel epoxy-ended hyperbranched polyether derived from xylitol as sustainable tougheners for epoxy resin. Polym. Test. 2021, 94, 107053. [Google Scholar] [CrossRef]
- Yadav, S.K.; Hu, F.; La Scala, J.J.; Palmese, G.R. Toughening Anhydride-Cured Epoxy Resins Using Fatty Alkyl-Anhydride-Grafted Epoxidized Soybean Oil. ACS Omega 2018, 3, 2641–2651. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Wei, F.; Zhong, L.; Zeng, L.; Zhang, J.; Xu, Z.; Zhang, D.; Miao, M. Epoxidation of agricultural byproduct konjac fly powder and utilization in toughening and strengthening epoxy resin. Ind. Crops Prod. 2020, 146, 112161. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Ball, D.W. The Chemical Composition of Honey. J. Chem. Educ. 2007, 84, 1643–1646. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Abdul Kudus, M.H.; Md Akil, H.; Zamri, M.H. Improvement of Fracture Toughness in Epoxy Nanocomposites through Chemical Hybridization of Carbon Nanotubes and Alumina. Materials 2017, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Demirci, M.; Tarakçıoğlu, N.; Avcı, A.; Akdemir, A.; Demirci, I. Fracture toughness (Mode I) characterization of SiO2 nanoparticle filled basalt/epoxy filament wound composite ring with split-disk test method. Compos. B Eng. 2017, 119, 114–124. [Google Scholar] [CrossRef]
- Rahmani, H.; Eslami-Farsani, R.; Ebrahimnezhad-Khaljiri, H. High velocity impact response of aluminum- carbon fibers-epoxy laminated composites toughened by nano silica and zirconia. Fibers Polym. 2020, 21, 170–178. [Google Scholar] [CrossRef]
- Hao, S.; Yuan, J.; Cui, J.; Yuan, W.; Zhang, H.; Xuan, H. The rapid detection of acacia honey adulteration by alternating current impedance spectroscopy combined with 1H NMR profile. LWT 2022, 161, 113377. [Google Scholar] [CrossRef]
- Wang, H.Y.; Qian, H.; Yao, W.R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Wegener, S.; Kaufmann, M.; Kroh, L.W. Influence of l-pyroglutamic acid on the color formation process of non-enzymatic browning reactions. Food Chem. 2017, 232, 450–454. [Google Scholar] [CrossRef]
- Kozłowicz, K.; Różyło, R.; Gładyszewska, B.; Matwijczuk, A.; Gładyszewski, G.; Chocyk, D.; Samborska, K.; Piekut, J.; Smolewska, M. Identification of sugars and phenolic compounds in honey powders with the use of GC–MS, FTIR spectroscopy, and X-ray diffraction. Sci Rep. 2020, 10, 16269. [Google Scholar] [CrossRef]
- Ciursa, P.; Pauliuc, D.; Dranca, F.; Ropciuc, S.; Oroian, M. Detection of honey adulterated with agave, corn, inverted sugar, maple and rice syrups using FTIR analysis. Food Control. 2021, 130, 108266. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Aboul-Enein, H.Y. Honey Discrimination Using Fourier Transform-Infrared Spectroscopy. Chemistry 2022, 4, 848–854. [Google Scholar] [CrossRef]
- Chowdhury, R.A.; Hosur, M.V.; Nuruddin, M.; Tcherbi-Narteh, A.; Kumar, A.; Boddu, V.; Jeelani, S. Self-healing epoxy composites: Preparation, characterization and healing performance. J. Mater. Res. Technol. 2015, 4, 33–43. [Google Scholar] [CrossRef]
- Radovic, I.; Stajcic, A.; Radisavljevic, A.; Veljkovic, F.; Cebela, M.; Mitic, V.; Radojevic, V. Solvent effects on structural changes in self-healing epoxy composites. Mater. Chem. Phys. 2020, 256, 123761. [Google Scholar] [CrossRef]
- Oracz, J.; Zyzelewicz, D. In vitro antioxidant activity and ftir characterization of high-molecular weight melanoidin fractions from different types of cocoa beans. Antioxidants 2019, 8, 560. [Google Scholar] [CrossRef] [PubMed]
- ElFaham, M.M.; Mostafa, A.M.; Nasr, G.M. Unmanned aerial vehicle (UAV) manufacturing materials: Synthesis, spectroscopic characterization and dynamic mechanical analysis (DMA). J. Mol. Struct. 2020, 1201, 127211. [Google Scholar] [CrossRef]
- Oikonomopoulos, I.; Perraki, T.; Tougiannidis, N. Ftir study of two different lignite lithotypes from neocene achlada lignite deposits in nw greece. Bull. Geol. Soc. Greece. 2017, 43, 2284. [Google Scholar] [CrossRef]
- Anjos, O.; Campos, M.G.; Ruiz, P.C.; Antunes, P. Application of FTIR-ATR spectroscopy to the quantification of sugar in honey. Food Chem. 2014, 169, 218–223. [Google Scholar] [CrossRef]
- Subari, N.; Mohamad Saleh, J.; Md Shakaff, A.Y.; Zakaria, A. A Hybrid Sensing Approach for Pure and Adulterated Honey Classification. Sensors 2012, 12, 14022–14040. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Y.; Meure, S.; Solomon, D. Self-healing polymeric materials: A review of recent developments. Prog. Polym. Sci. 2008, 33, 479–522. [Google Scholar] [CrossRef]
- Radovic, I.M.; Stojanovic, D.B.; Kojovic, A.; Petrovic, M.; Uskokovic, P.S.; Radojevic, V.J.; Aleksic, R.R. Healing efficiency of polystyrene electrospun nanofibers with Grubbs’ catalyst in thermosetting composite. J. Compos. Mater. 2017, 51, 3003–3016. [Google Scholar] [CrossRef]
- Icduygu, M.G.; Asilturk, M.; Yalcinkaya, M.A.; Hamidi, Y.K.; Altan, M.C. Three-Dimensional Nano-Morphology of Carbon Nanotube/Epoxy Filled Poly(methyl methacrylate) Microcapsules. Materials 2019, 12, 1387. [Google Scholar] [CrossRef]
- Bellenger, V.; Fontaine, E.; Fleishmann, A.; Saporito, J.; Verdu, J. Thermogravimetric Study of Amine Cross-Linked Epoxies. Polym. Degrad. Stab. 1984, 9, 195–208. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, H.; Liang, C.; Song, P.; Han, Y.; Han, Y.; Gu, J.; Kong, J.; Pan, D.; Guo, Z. Electromagnetic Interference Shielding MWCNT-Fe3O4@Ag/Epoxy Nanocomposites with Satisfactory Thermal Conductivity and High Thermal Stability. Carbon 2019, 141, 506–514. [Google Scholar] [CrossRef]
- Fei, X.; Wei, W.; Zhao, F.; Zhu, Y.; Luo, J.; Chen, M.; Liu, X. Efficient Toughening of Epoxy-Anhydride Thermosets with a Biobased Tannic Acid Derivative. ACS Sustain. Chem. Eng. 2017, 5, 596–603. [Google Scholar] [CrossRef]
- Nooshkama, M.; Varidi, M.; Vermac, D.K. Functional and biological properties of Maillard conjugates and their potential application in medical and food: A review. Food Res. Int. 2020, 131, 109003. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Gilcher, E.B.; Huber, G.W.; Dumesic, J.A. Synthesis of performance-advantaged polyurethanes and polyesters from biomass-derived monomers by aldol-condensation of 5-hydroxymethyl furfural and hydrogenation. Green Chem. 2021, 23, 4355–4364. [Google Scholar] [CrossRef]
- Engozogho Anris, S.P.; Bi Athomo, A.B.; Safou Tchiama, R.; Santiago-Medina, F.S.; Cabaret, T.; Pizzi, A.; Charrier, B. The condensed tannins of okoume (Aucoumea klaineana pierre): A molecular structure and thermal stability study. Sci. Rep. 2020, 10, 1773. [Google Scholar] [CrossRef] [PubMed]
- Carbas, R.J.C.; Marques, E.A.S.; da Silva, L.F.M.; Lopes, A.M. Effect of Cure Temperature on the Glass Transition Temperature and Mechanical Properties of Epoxy Adhesives. J. Adhes. 2014, 90, 104–119. [Google Scholar] [CrossRef]
- Courtois, A.; Hirsekorn, M.; Benavente, M.; Jaillon, A.; Marcin, L.; Ruiz, E.; Lévesque, M. Viscoelastic behavior of an epoxy resin during cure below the glass transition temperature: Characterization and modeling. J. Compos. Mater. 2018, 53, 155–171. [Google Scholar] [CrossRef]
- Michel, M.; Ferrier, E. Effect of curing temperature conditions on glass transition temperature values of epoxy polymer used for wet lay-up applications. Constr. Build. Mater. 2020, 231, 117206. [Google Scholar] [CrossRef]
- Yu, C.; Xu, Z.; Ma, X.; Zhang, J.; Chen, S.; Miao, M.; Chen, H.; Zhang, D. Hyperbranched polymers containing epoxy and imide structure. Prog. Org. Coat. 2021, 151, 106031. [Google Scholar] [CrossRef]

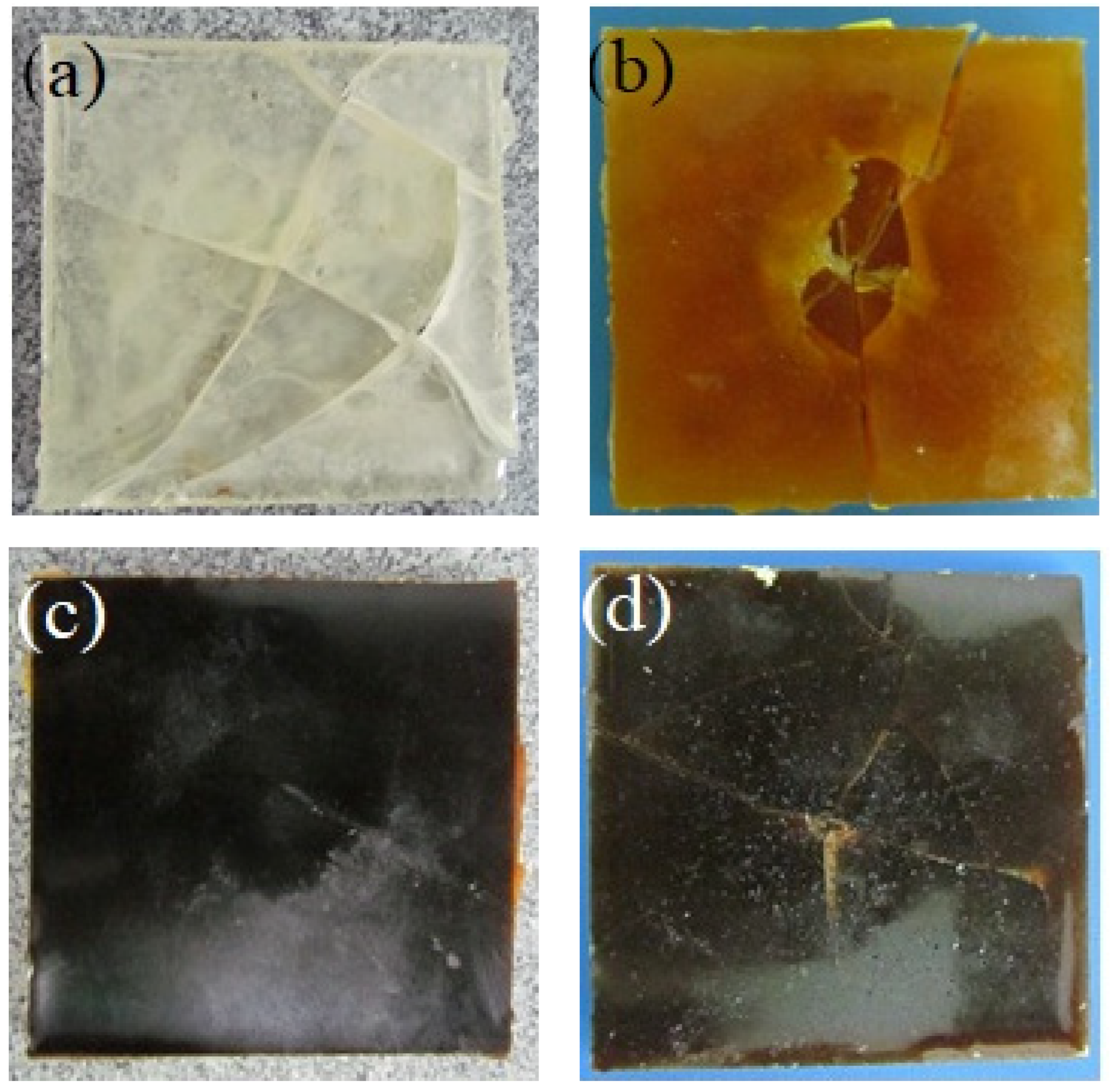


| Sample | EH3 | EH6 | ||
|---|---|---|---|---|
| Impact | Impact Velocity, m/s | Impact Depth, mm | Impact Velocity, m/s | Impact Depth, mm |
| 1 | 0.05 | 1 | 0.05 | 1 |
| 2 | 0.1 | 2 | 1 | 6 |
| 3 | 0.2 | 2 | - | - |
| 4 | 0.2 | 3 | - | - |
| 5 | 0.3 | 3 | - | - |
| 6 | 0.5 | 3 | - | - |
| 7 | 0.5 | 6 | - | - |
| 8 | 1.0 | 6 | - | - |
| T5, °C | T10, °C | T30, °C | Thri, °C | Tg, °C | |
|---|---|---|---|---|---|
| EH3 | 221.5 | 271.1 | 443.5 | 173.8 | 228.0 |
| E | 115.3 | 244.7 | 290.3 | 107.9 | 102.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stajcic, I.; Veljkovic, F.; Petrovic, M.; Veličkovic, S.; Radojevic, V.; Vlahović, B.; Stajcic, A. Impact- and Thermal-Resistant Epoxy Resin Toughened with Acacia Honey. Polymers 2023, 15, 2261. https://doi.org/10.3390/polym15102261
Stajcic I, Veljkovic F, Petrovic M, Veličkovic S, Radojevic V, Vlahović B, Stajcic A. Impact- and Thermal-Resistant Epoxy Resin Toughened with Acacia Honey. Polymers. 2023; 15(10):2261. https://doi.org/10.3390/polym15102261
Chicago/Turabian StyleStajcic, Ivana, Filip Veljkovic, Milos Petrovic, Suzana Veličkovic, Vesna Radojevic, Branislav Vlahović, and Aleksandar Stajcic. 2023. "Impact- and Thermal-Resistant Epoxy Resin Toughened with Acacia Honey" Polymers 15, no. 10: 2261. https://doi.org/10.3390/polym15102261
APA StyleStajcic, I., Veljkovic, F., Petrovic, M., Veličkovic, S., Radojevic, V., Vlahović, B., & Stajcic, A. (2023). Impact- and Thermal-Resistant Epoxy Resin Toughened with Acacia Honey. Polymers, 15(10), 2261. https://doi.org/10.3390/polym15102261









