Unlocking the Potential Use of Reactive POSS as a Coagent for EPDM/PP-Based TPV
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing
2.3. Characterization
3. Results and Discussions
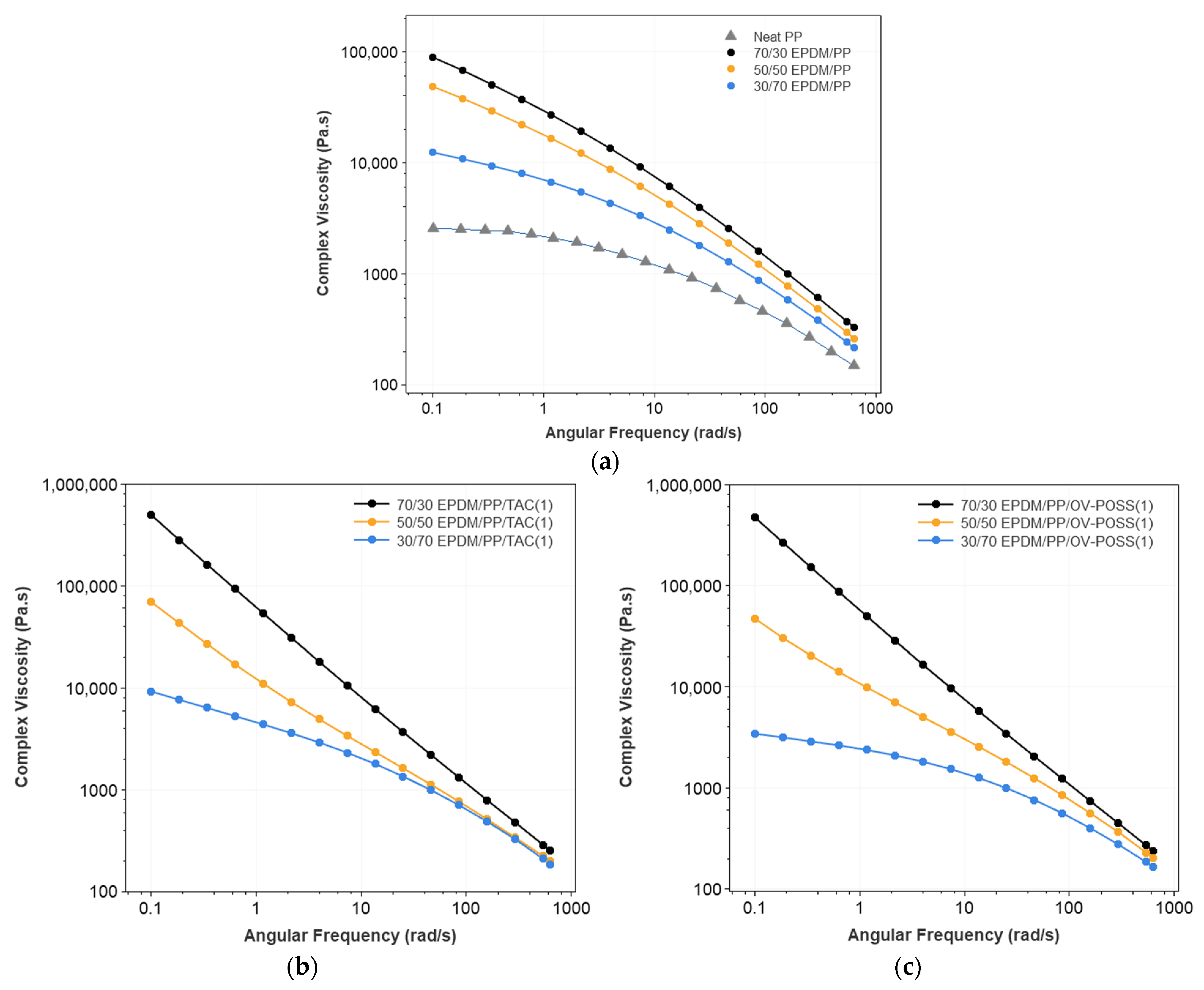
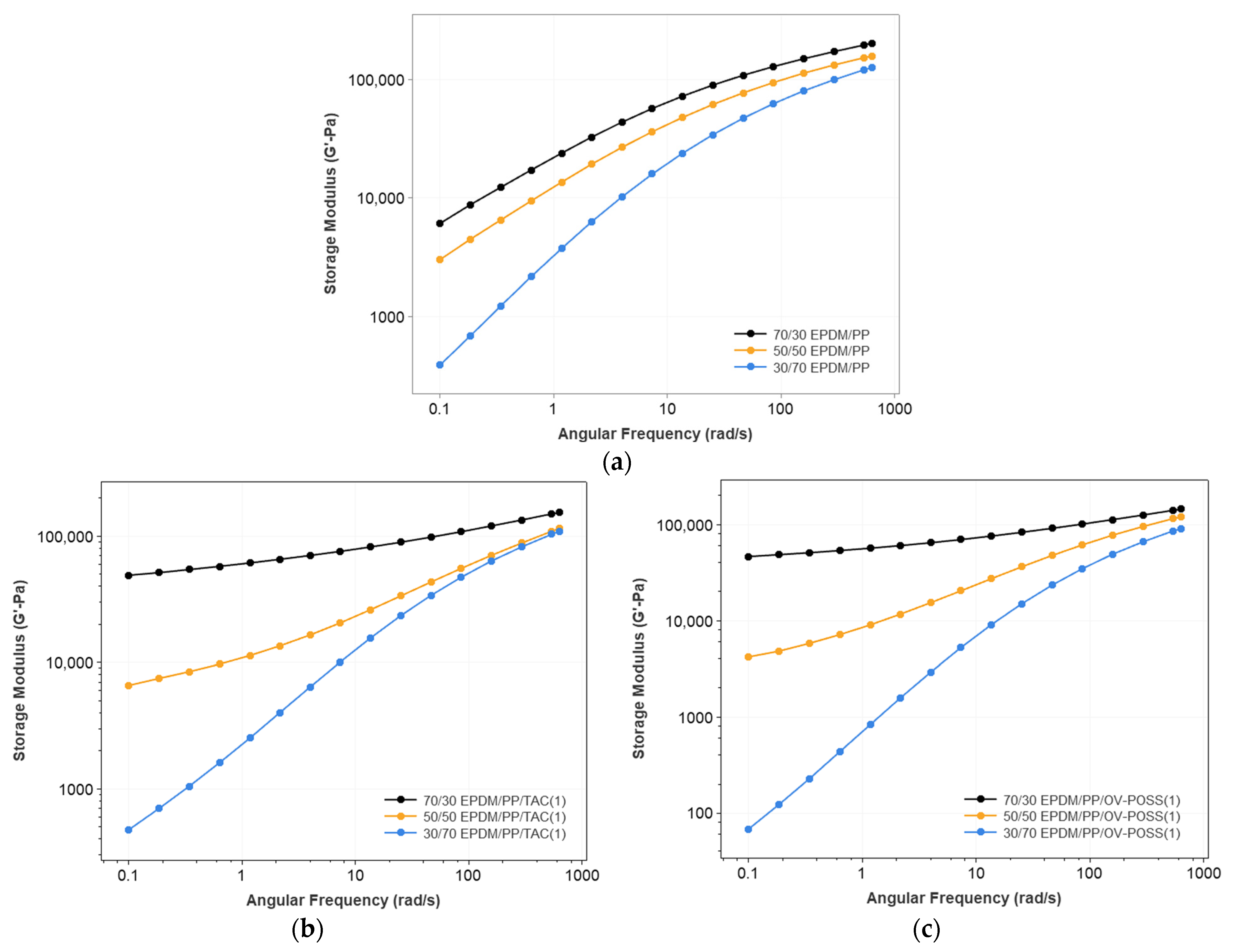
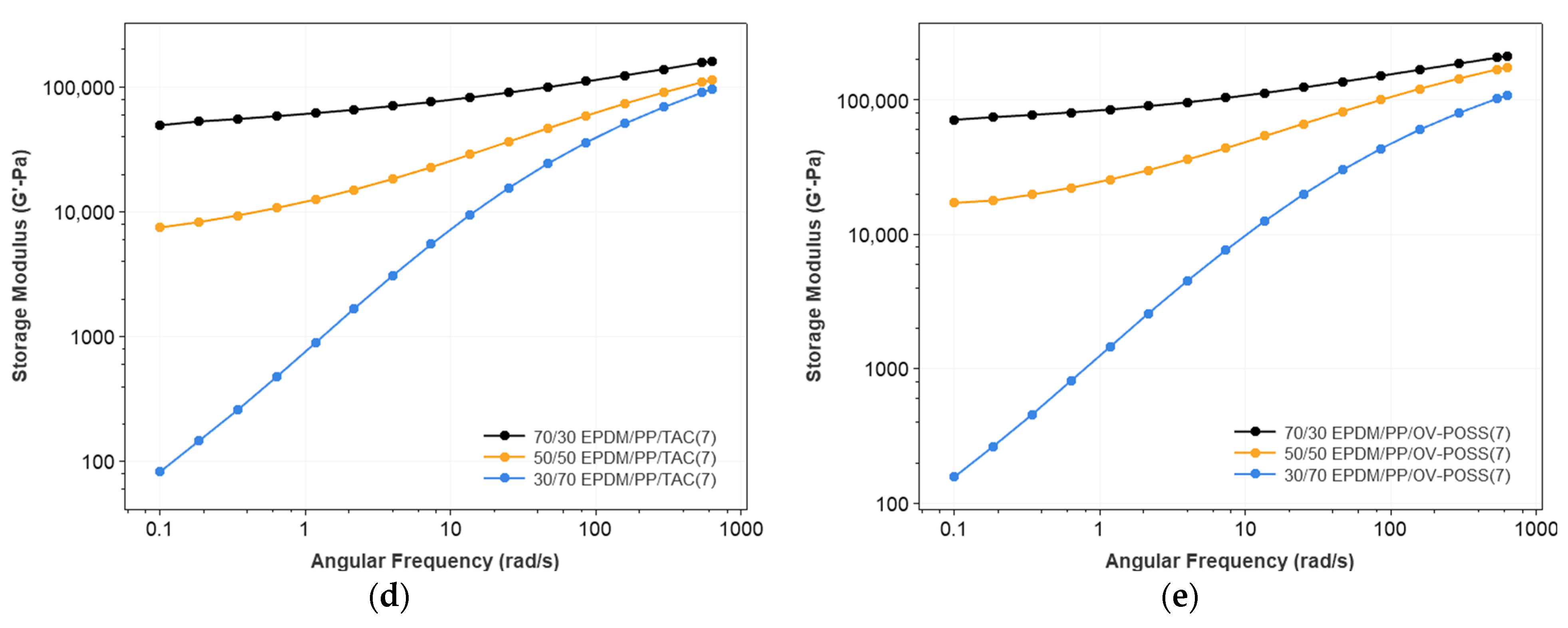
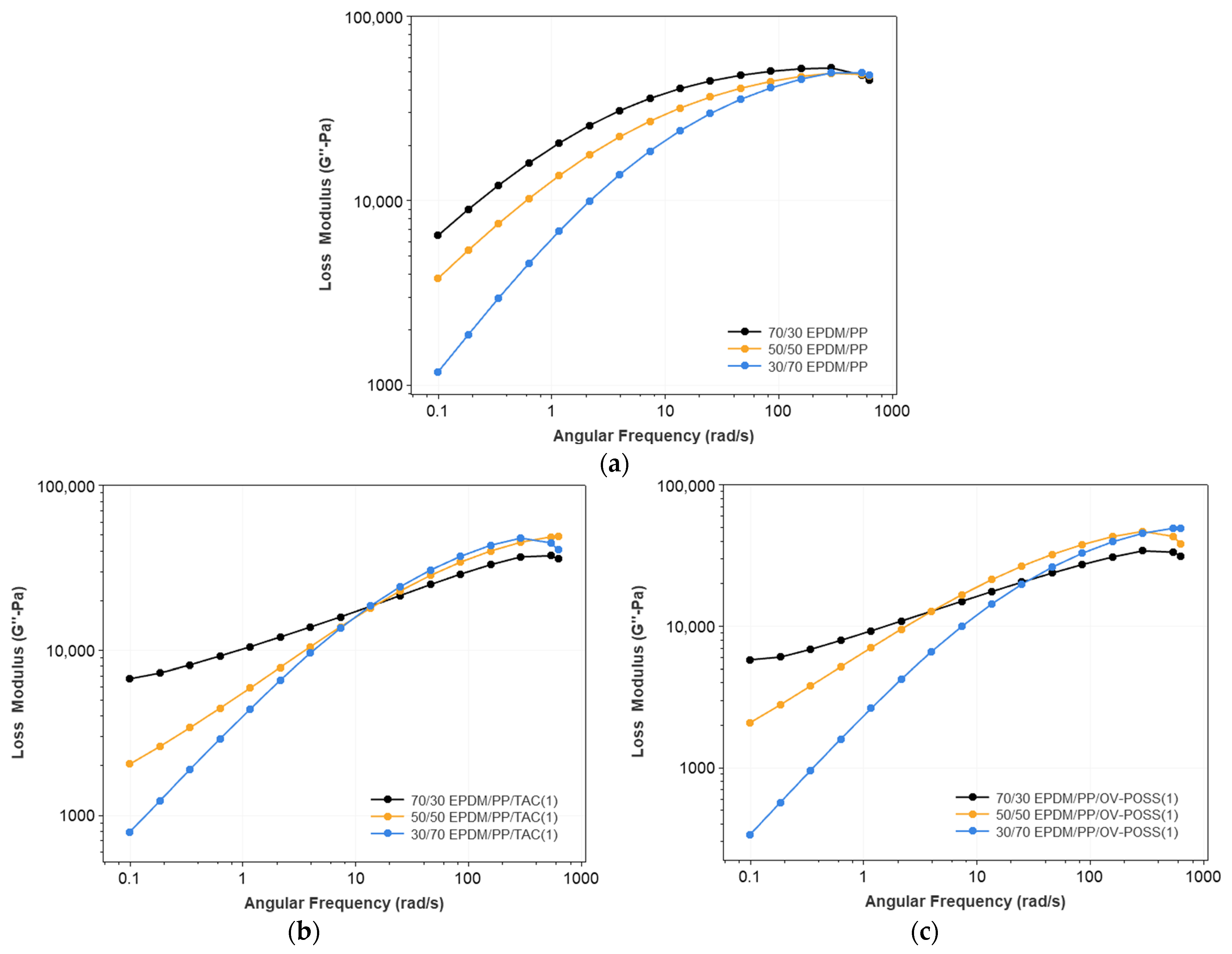
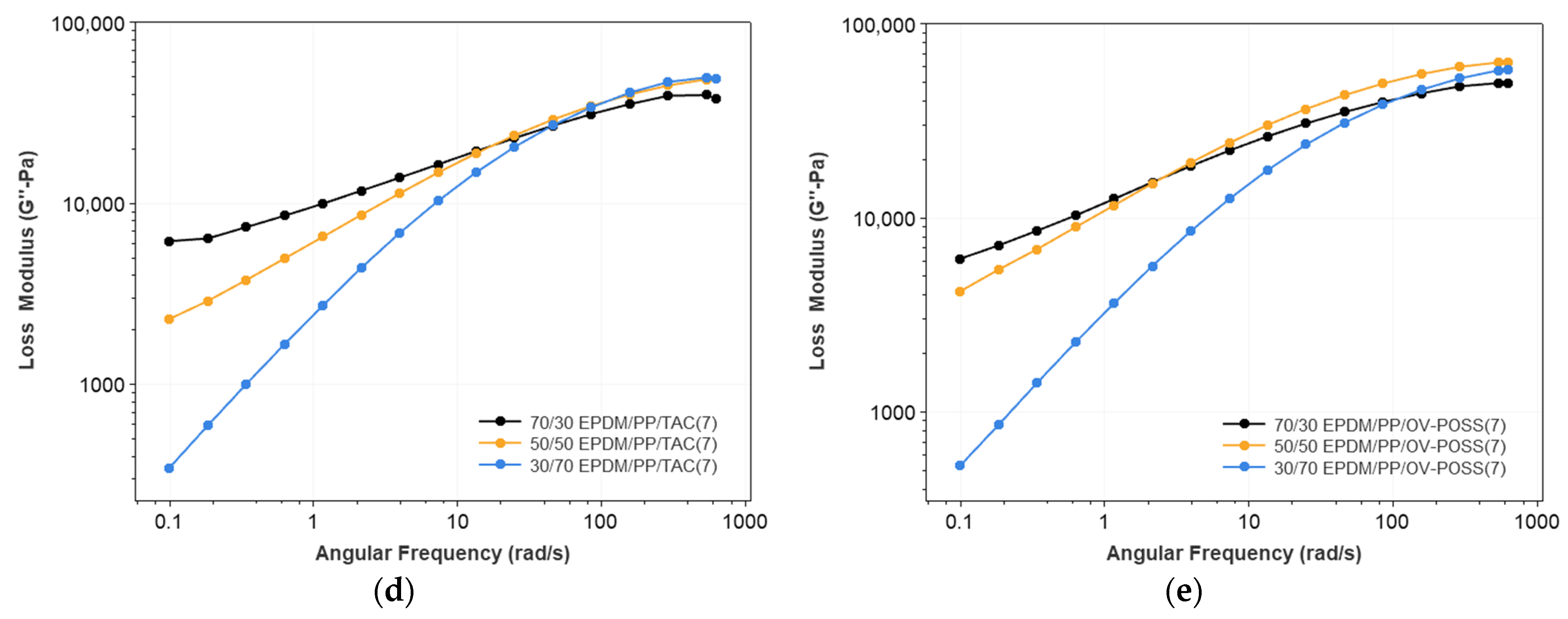
3.1. Rheological Properties of the TPVs
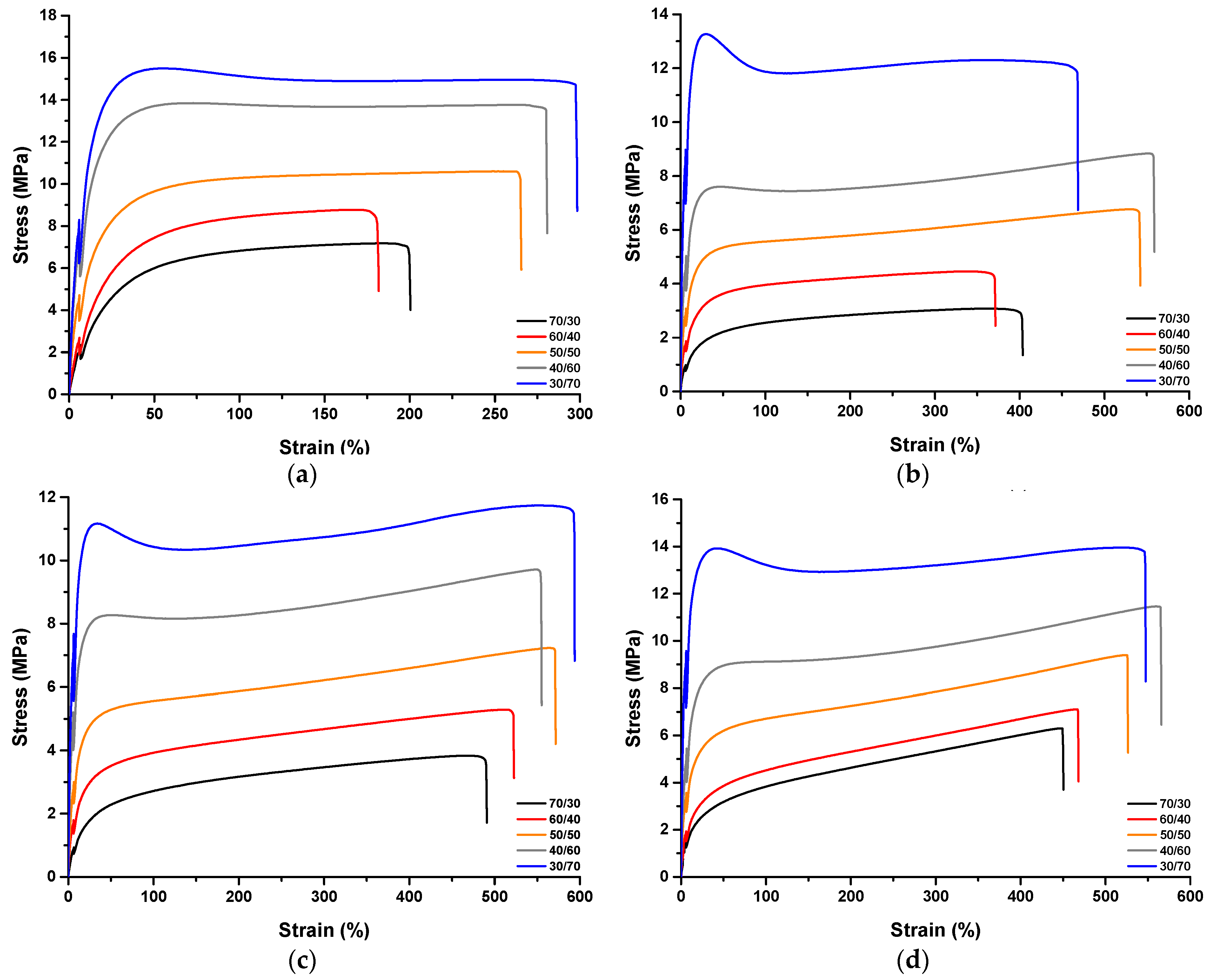
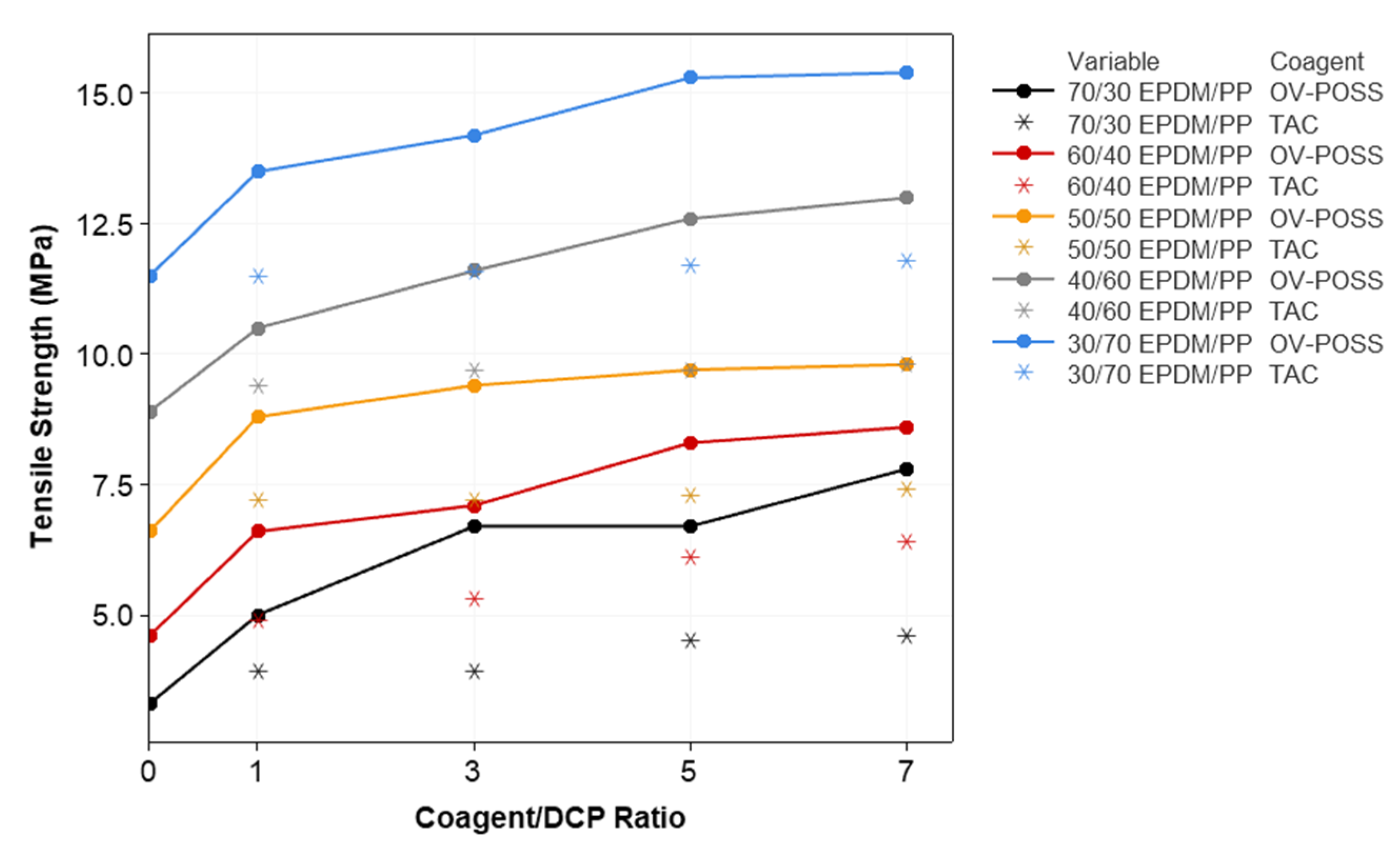
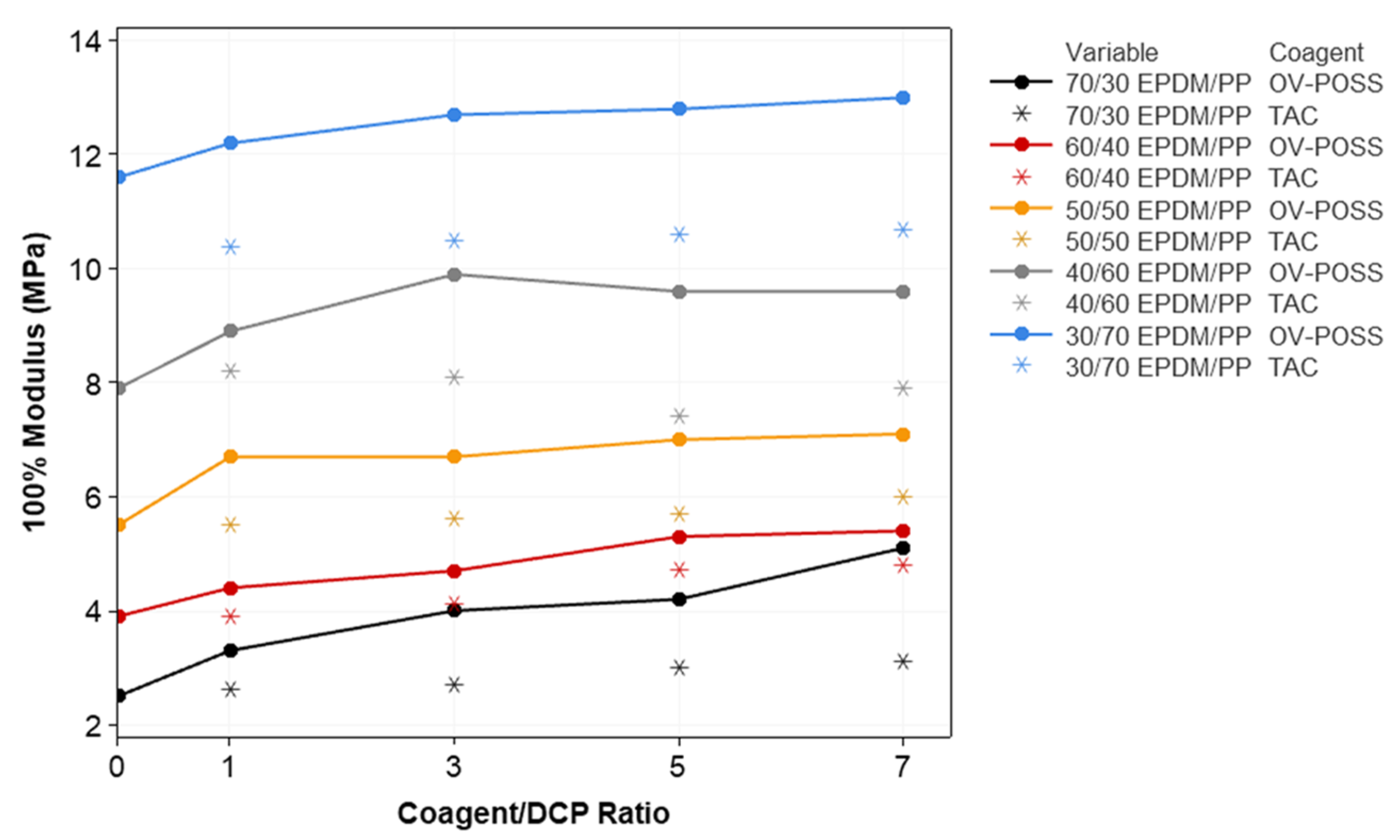
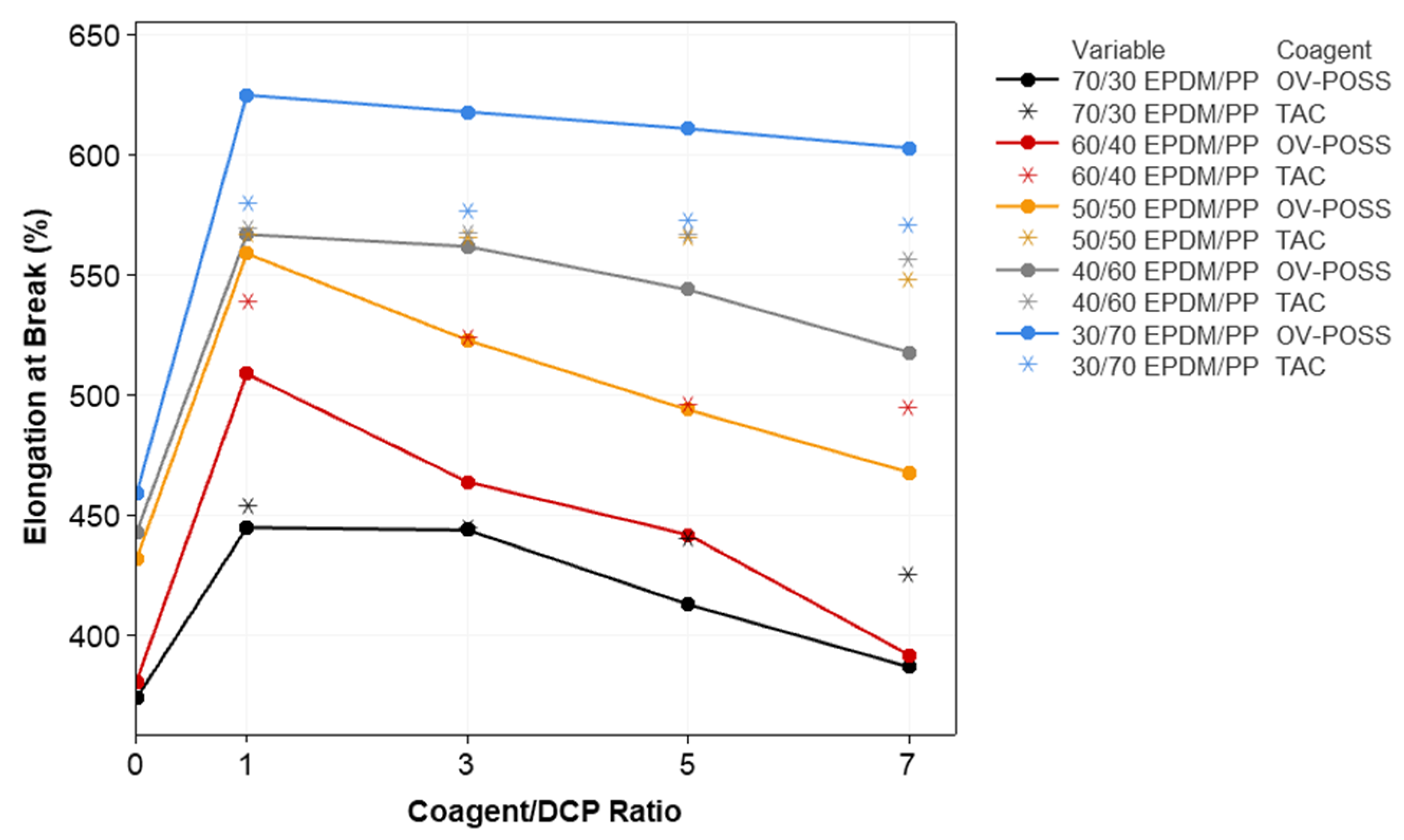
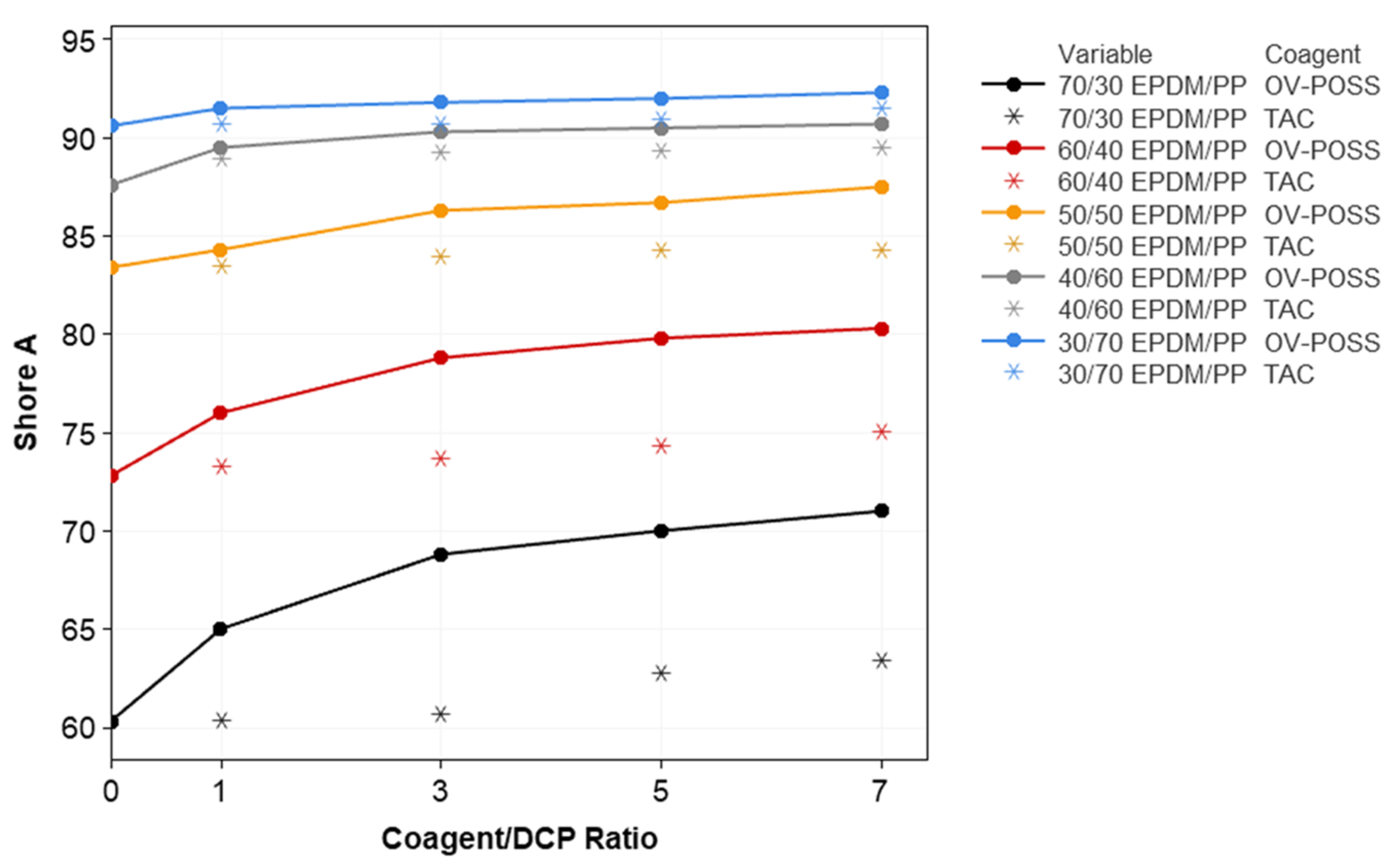
3.2. Mechanical Properties and Crosslink Density of the TPVs
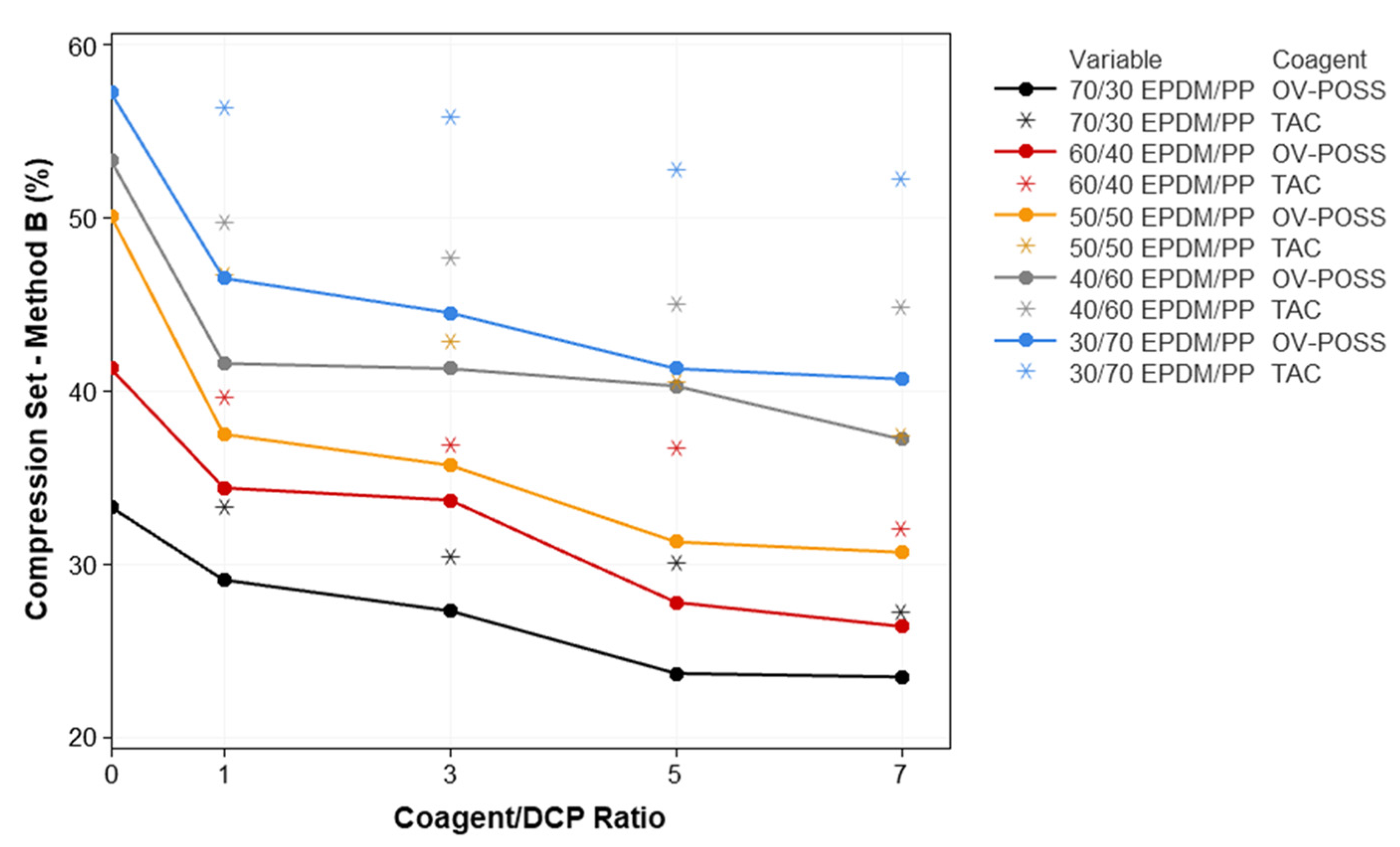
3.3. Thermal Aging Resistance of the TPVs
3.4. Oil Resistance of the TPVs
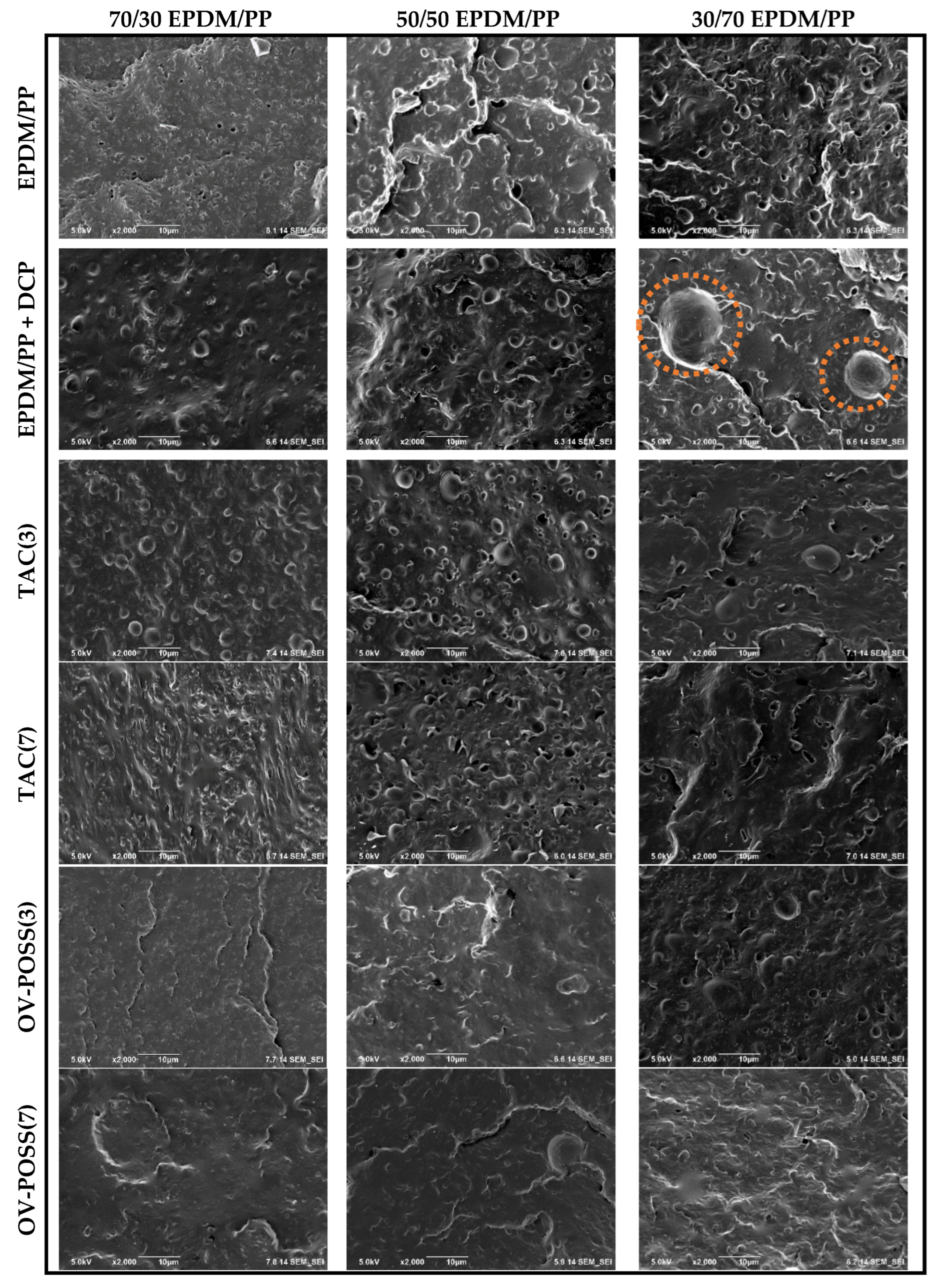
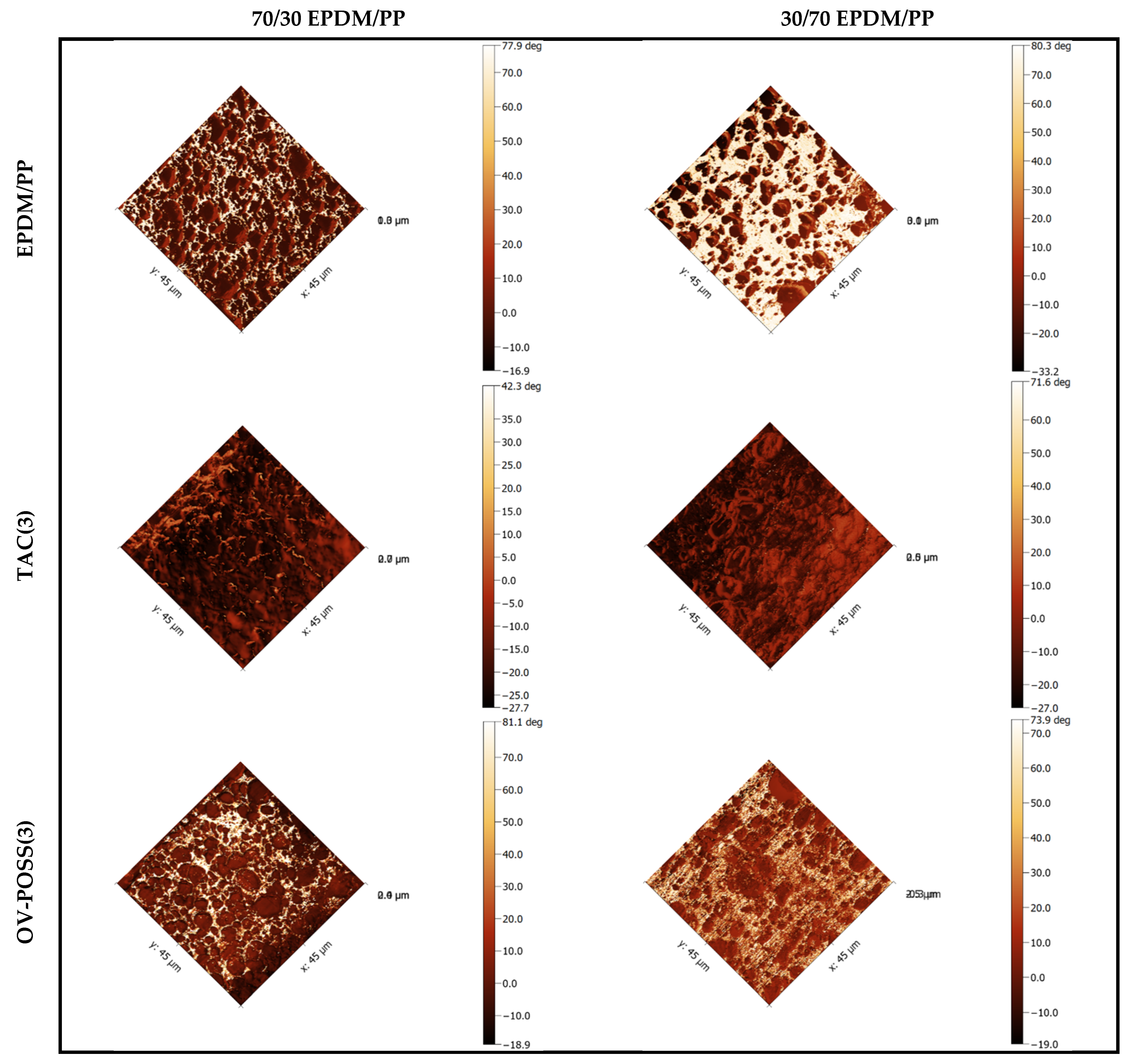
3.5. Morphological Analysis by SEM
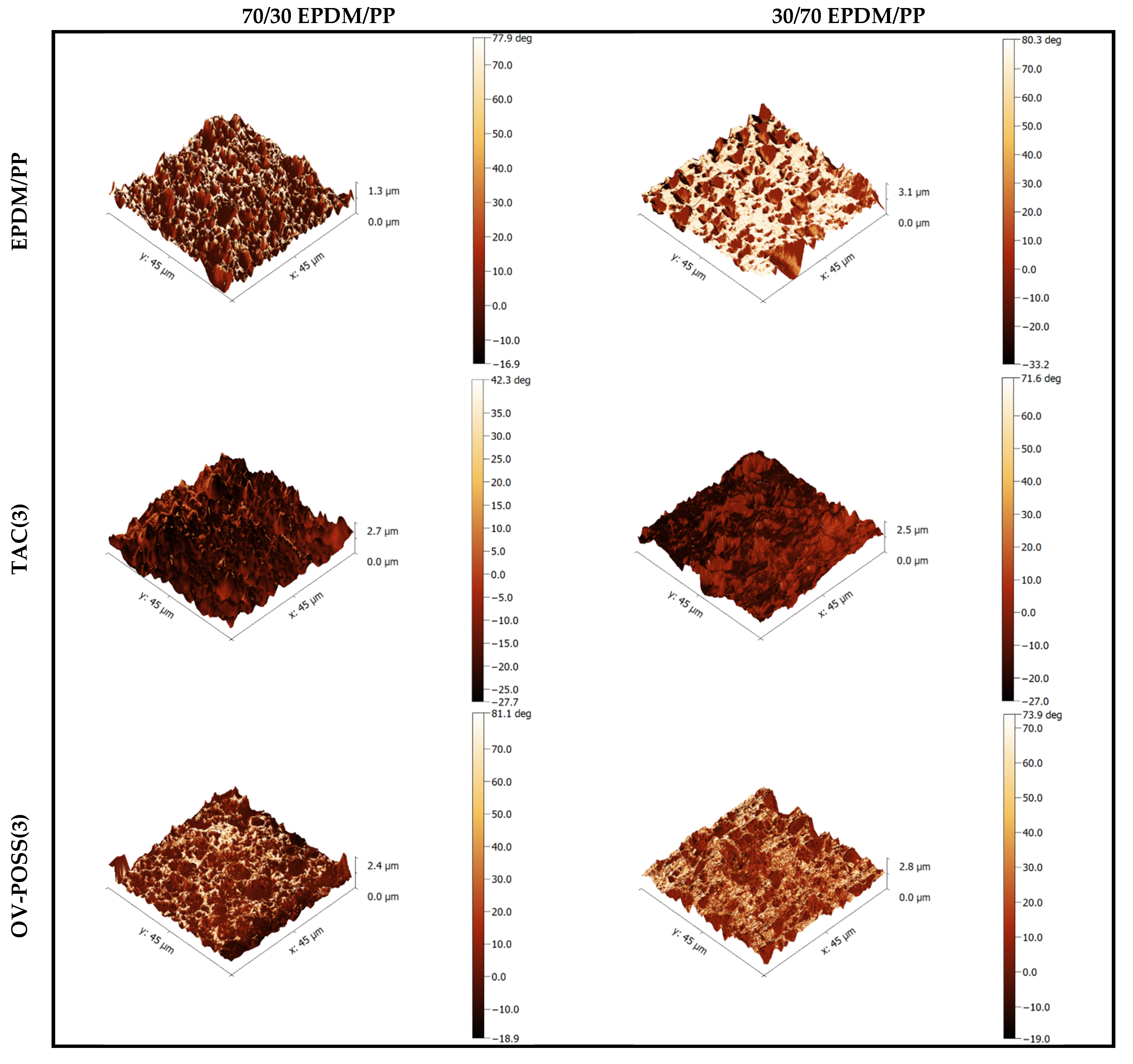
3.6. Morphological Analysis by AFM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Antunes, C.F.; van Duin, M.; Machado, A.V. Morphology and Phase Inversion of EPDM/PP Blends—Effect of Viscosity and Elasticity. Polym. Test. 2011, 30, 907–915. [Google Scholar] [CrossRef]
- Antunes, C.F.; Machado, A.V.; van Duin, M. Morphology Development and Phase Inversion during Dynamic Vulcanisation of EPDM/PP Blends. Eur. Polym. J. 2011, 47, 1447–1459. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Li, J.; Wu, S.; Han, W.; Kang, H.; Fang, Q. Green Thermoplastic Vulcanizates Based on Silicone Rubber and Poly(butylene succinate) via in situ Interfacial Compatibilization. ACS Omega 2021, 6, 4461–4469. [Google Scholar] [CrossRef]
- Nakason, C.; Wannavilai, P.; Kaesaman, A. Effect of Vulcanization System on Properties of Thermoplastic Vulcanizates Based on Epoxidized Natural Rubber/Polypropylene Blends. Polym. Test. 2006, 25, 34–41. [Google Scholar] [CrossRef]
- Nakason, C.; Jarnthong, M.; Kaesaman, A.; Kiatkamjornwong, S. Thermoplastic Elastomers Based on Epoxidized Natural Rubber and High-Density Polyethylene Blends: Effect of Blend Compatibilizers on the Mechanical and Morphological Properties. J. Appl. Polym. Sci. 2008, 109, 2694–2702. [Google Scholar] [CrossRef]
- Mani, S.; Cassagnau, P.; Bousmina, M.; Chaumont, P. Morphology Development in Novel Composition of Thermoplastic Vulcanizates Based on PA12/PDMS Reactive Blends. Macromol. Mater. Eng. 2011, 296, 909–920. [Google Scholar] [CrossRef]
- Tian, M.; Han, J.; Zou, H.; Tian, H.; Wu, H.; She, Q.; Chen, W.; Zhang, L. Dramatic Influence of Compatibility on Crystallization Behavior and Morphology of Polypropylene in NBR/PP Thermoplastic Vulcanizates. J. Polym. Res. 2012, 19, 9745. [Google Scholar] [CrossRef]
- Yao, P.; Wu, H.; Ning, N.; Zhang, L.; Tian, H.; Wu, Y.; Hu, G.-H.; Chan, T.W.; Tian, M. Microstructure and Properties of Bromo-Isobutylene–Isoprene Rubber/Polyamide 12 Thermoplastic Vulcanizate toward Recyclable Inner Liners for Green Tires. RSC Adv. 2016, 6, 30004–30013. [Google Scholar] [CrossRef]
- Babu, R.R.; Naskar, K. Recent Developments on Thermoplastic Elastomers by Dynamic Vulcanization. In Advanced Rubber Composites; Springer: Cambridge, UK, 2010; pp. 219–247. [Google Scholar]
- Nakason, C.; Nuansomsri, K.; Kaesaman, A.; Kiatkamjornwong, S. Dynamic Vulcanization of Natural Rubber/High-Density Polyethylene Blends: Effect of Compatibilization, Blend Ratio and Curing System. Polym. Test. 2006, 25, 782–796. [Google Scholar] [CrossRef]
- Nakason, C.; Jarnthong, M.; Kaesaman, A.; Kiatkamjornwong, S. Influences of Blend Proportions and Curing Systems on Dynamic, Mechanical, and Morphological Properties of Dynamically Cured Epoxidized Natural Rubber/High-Density Polyethylene Blends. Polym. Eng. Sci. 2009, 49, 281–292. [Google Scholar] [CrossRef]
- Nakason, C.; Jamjinno, S.; Kaesaman, A.; Kiatkamjornwong, S. Thermoplastic Elastomer Based on High-Density Polyethylene/Natural Rubber Blends: Rheological, Thermal, and Morphological Properties. Polym. Adv. Technol. 2008, 19, 85–98. [Google Scholar] [CrossRef]
- George, J.; Neelakantan, N.R.; Varughese, K.T.; Thomas, S. Failure Properties of Thermoplastic Elastomers from Polyethylene/Nitrile Rubber Blends: Effect of Blend Ratio, Dynamic Vulcanization, and Filler Incorporation. J. Appl. Polym. Sci. 2006, 100, 2912–2929. [Google Scholar] [CrossRef]
- Soares, B.G.; Almeida, M.S.M.; Deepa Urs, M.V.; Kumaraswamy, G.N.; Ranganathaiah, C.; Siddaramaiah; Mauler, R. Influence of Curing Agent and Compatibilizer on the Physicomechanical Properties of Polypropylene/Nitrile Butadiene Rubber Blends Investigated by Positron Annihilation Lifetime Technique. J. Appl. Polym. Sci. 2006, 102, 4672–4681. [Google Scholar] [CrossRef]
- Wu, H.; Ning, N.; Zhang, L.; Tian, H.; Wu, Y.; Tian, M. Effect of Additives on the Morphology Evolution of EPDM/PP TPVs during Dynamic Vulcanization in a Twin-Screw Extruder. J. Polym. Res. 2013, 20, 266. [Google Scholar] [CrossRef]
- Yao, P.; Wu, H.; Ning, N.; Zhang, L.; Tian, H.; Wu, Y.; Hu, G.; Chan, T.W.; Tian, M. Properties and Unique Morphological Evolution of Dynamically Vulcanized Bromo-Isobutylene-Isoprene Rubber/Polypropylene Thermoplastic Elastomer. RSC Adv. 2016, 6, 11151–11160. [Google Scholar] [CrossRef]
- Van Dyke, J.D.; Gnatowski, M.; Koutsandreas, A.; Burczyk, A. A Study of Dynamic Vulcanization for Polyamide-12 and Chlorobutyl Rubber. J. Appl. Polym. Sci. 2003, 90, 871–880. [Google Scholar] [CrossRef]
- Nicolini, A.; de Campos Rocha, T.L.Á.; Maldaner Jacobi, M.A. Dynamically Vulcanized PP/EPDM Blends: Influence of Curing Agents on the Morphology Evolution. J. Appl. Polym. Sci. 2008, 109, 3093–3100. [Google Scholar] [CrossRef]
- Patermann, S.; Altstädt, V. Influence of Different Crosslinking Systems on the Mechanical and Morphological Properties of Thermoplastic Vulcanizates. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2015; Volume 1664, p. 120002. [Google Scholar]
- Coran, A.Y.; Patel, R. Rubber-Thermoplastic Compositions. Part I. EPDM-Polypropylene Thermoplastic Vulcanizates. Rubber Chem. Technol. 1980, 53, 141–150. [Google Scholar] [CrossRef]
- Ezzati, P.; Ghasemi, I.; Azizi, H.; Karrabi, M. Correclation between the Rheological Behaviour and Morphologies of PP/EPDM Blends in Various Dynamic Vulcanization Systems. Iran. Polym. J. 2008, 17, 265–272. [Google Scholar]
- Wang, R.; Peng, Z.; Fan, P.P. Effect of Peroxide Content on Morphology and Properties of Thermoplastic Vulcanizates Based on PP and NR. Adv. Mater. Res. 2011, 284–286, 1854–1860. [Google Scholar] [CrossRef]
- Varghese, S.; Alex, R.; Kuriakose, B. Natural Rubber-Isotactic Polypropylene Thermoplastic Blends. J. Appl. Polym. Sci. 2004, 92, 2063–2068. [Google Scholar] [CrossRef]
- Thitithammawong, A.; Nakason, C.; Sahakaro, K.; Noordermeer, J. Effect of Different Types of Peroxides on Rheological, Mechanical, and Morphological Properties of Thermoplastic Vulcanizates Based on Natural Rubber/Polypropylene Blends. Polym. Test. 2007, 26, 537–546. [Google Scholar] [CrossRef]
- Thitithammawong, A.; Nakason, C.; Sahakaro, K.; Noordermeer, J.W.M. NR/PP Thermoplastic Vulcanizates: Selection of Optimal Peroxide Type and Concentration in Relation to Mixing Conditions. J. Appl. Polym. Sci. 2007, 106, 2204–2209. [Google Scholar] [CrossRef]
- Endstra, W.C. Aspects of Ethylene-Propylene Based Polymersi. In Proceedings of the International Conference on ìVarious, Leuven, Belgium, 16–17 April 1991. [Google Scholar]
- Henning, S.K.; Boye, W.M. Fundamentals of Curing Elastomers with Peroxides and Coagents II: Understanding the Relationship Between Coagent and Elastomer. Rubber World 2009, 240, 31–39. [Google Scholar]
- Dikland, H.G.; Ruardy, T.; van der Does, L.; Bantjes, A. New Coagents in Peroxide Vulcanization of EPM. Rubber Chem. Technol. 1993, 66, 693–711. [Google Scholar] [CrossRef]
- Cao, L.; Jiang, X.; Ding, J.; Chen, Y. Effect of Zinc Dimethacrylate on Compatibilization and Reinforcement of Peroxide Dynamically Cured PP/EPDM TPVs. J. Bulg. Chem. Commun. 2015, 47, 691–698. [Google Scholar]
- de Risi, F.R.; Noordermeer, J.W.M. Effect of Methacrylate Co-Agents on Peroxide Cured PP/EPDM Thermoplastic Vulcanizates. Rubber Chem. Technol. 2007, 80, 83–99. [Google Scholar] [CrossRef]
- Kodal, M. Dinamik Olarak Vulkanize Edilmiş PP/SR Harmanlarının Mekanik, Isıl ve Morfolojik Özelliklerinin Incelenmesi. DÜMF Mühendislik Derg. 2018, 9, 325–335. [Google Scholar]
- Mali, M.; Kadam, P.; Mhaske, S. Preparation and Characterization of Vinyltrimethoxysilane and Dicumyl Peroxide–Cured (Ethylene Propylene Diene Monomer)/Polypropylene Thermoplastic Vulcanizates. J. Vinyl Addit. Technol. 2017, 23, 312–320. [Google Scholar] [CrossRef]
- Naskar, K.; Noordermeer, J. Dynamically Vulcanized PP/EPDM Blends: Effects of Different Types of Peroxides on the Properties. Rubber Chem. Technol. 2003, 76, 1001–1018. [Google Scholar] [CrossRef]
- Turan, D.; Sirin, H.; Ozkoc, G. Effects of POSS Particles on the Mechanical, Thermal, and Morphological Properties of PLA and Plasticised PLA. J. Appl. Polym. Sci. 2011, 121, 1067–1075. [Google Scholar] [CrossRef]
- Kodal, M.; Sirin, H.; Ozkoc, G. Effects of Reactive and Nonreactive POSS Types on the Mechanical, Thermal, and Morphological Properties of Plasticized Poly(Lactic Acid). Polym. Eng. Sci. 2014, 54, 264–275. [Google Scholar] [CrossRef]
- Kodal, M. Polypropylene/Polyamide 6/POSS Ternary Nanocomposites: Effects of POSS Nanoparticles on the Compatibility. Polymer 2016, 105, 43–50. [Google Scholar]
- Sirin, H.; Turan, D.; Ozkoc, G.; Gurdag, S. POSS Reinforced PET Based Composite Fibers: “Effect of POSS Type and Loading Level”. Compos. Part B Eng. 2013, 53, 395–403. [Google Scholar] [CrossRef]
- Sirin, H.; Kodal, M.; Karaagac, B.; Ozkoc, G. Effects of Octamaleamic Acid-POSS Used as the Adhesion Enhancer on the Properties of Silicone Rubber/Silica Nanocomposites. Compos. Part B Eng. 2016, 98, 370–381. [Google Scholar] [CrossRef]
- Kodal, M.; Şirin, H.; Karaağaç, B.; Özkoç, G. Improved Interfacial Adhesion with the Help of Functional Polyhedral Oligomeric Silsesquioxanes in Silicone Rubber/Rayon Fiber Composites: Physical, Mechanical, Thermal, and Morphological Properties. Polym. Eng. Sci. 2020, 60, 1958–1972. [Google Scholar] [CrossRef]
- Kilic Tuccar, N.; Can, B.N.; Kodal, M.; Ozkoc, G. The Potential Use of Epoxy-POSS as a Reactive Hybrid Compatibilizers for PLA/PBAT Blends: “Effect of PBAT Molecular Weight and POSS Type”. Polym. Eng. Sci. 2020, 60, 398–413. [Google Scholar] [CrossRef]
- Yazıcı, N.; Dursun, S.; Yarıcı, T.; Kılıç, B.; Mert, O.; Karaağaç, B.; Özkoç, G.; Kodal, M. Effect of Octavinyl-Polyhedral Oligomeric Silsesquioxane on the Cross-Linking, Cure Kinetics, and Adhesion Properties of Natural Rubber/Textile Cord Composites. Ind. Eng. Chem. Res. 2020, 59, 1888–1901. [Google Scholar] [CrossRef]
- Yazıcı, N.; Dursun, S.; Yarıcı, T.; Kılıç, B.; Arıcan, M.O.; Mert, O.; Karaağaç, B.; Özkoç, G.; Kodal, M. The Outstanding Interfacial Adhesion between Acrylo-POSS/Natural Rubber Composites and Polyamide-Based Cords: ‘An Environmentally Friendly Alternative to Resorcinol-Formaldehyde Latex Coating’. Polymer 2021, 228, 123880. [Google Scholar] [CrossRef]
- Turgut, G.; Dogan, M.; Tayfun, U.; Ozkoc, G. The Effects of POSS Particles on the Flame Retardancy of Intumescent Polypropylene Composites and the Structure-Property Relationship. Polym. Degrad. Stab. 2018, 149, 96–111. [Google Scholar] [CrossRef]
- Ozimek, J.; Pielichowski, K. Preparation, Microstructure, and Microstructure-Properties Recent Advances in Polyurethane/POSS Hybrids for Biomedical Applications. Molecules 2022, 27, 1–31. [Google Scholar]
- Ayendele, E.; Sarkar, B.; Alexandridis, P. Polyhedral Oligomeric Silsesquioxane (POSS)-Containing Nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar] [CrossRef]
- Wang, M.; Chi, H.; Joshy, K.S.; Wang, F. Progress in the Synthesis of Bifunctionalized Polyhedral Oligomeric Silsesquioxane. Polymers 2019, 11, 2098. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Zaharescu, T. The Effect of Polyhedral Oligomeric Silsesquioxanes (POSSs) Incorporation in Ethylene-propylene-diene-terpolymer (EPDM): A Thermal Study. J. Therm. Anal. Calorim. 2022, 147, 5313–5321. [Google Scholar] [CrossRef]
- Ma, X.; Ji, T.; Zhang, J.; Shen, S.; Wang, S.; Wang, J.; Hou, X.; Yang, S.; Ma, X. A Double-decker Silsesquioxane of Norbornene and Performance of Crosslinking Reactive Modified EPDM Ablation Resistance Composites. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107370. [Google Scholar] [CrossRef]
- Morici, E.; Di Bartolo, A.; Arrigo, R.; Dintcheva, N.T. Double Bond-Functionalized POSS: Dispersion and Crosslinking in Polyethylene-Based Hybrid Obtained by Reactive Processing. Polym. Bull. 2016, 73, 3385–3400. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.L.; Yang, H.; Zheng, Q. Crosslinking of Low Density Polyethylene with Octavinyl Polyhedral Oligomeric Silsesquioxane as the Crosslinker. RSC Adv. 2014, 4, 44030–44038. [Google Scholar] [CrossRef]
- Bicer, E.; Demir, G.K.; Kodal, M.; Ozkoc, G. Investigation of Shape Memory Behavior and Physical Properties of Crosslinked Low Density Polyethylene/OvPOSS/TAIC Composites. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2205, p. 20029. [Google Scholar]
- Demir, G.K.; Bicer, E.; Kodal, M.; Ozkoc, G. Cross-Linked LLDPE Composites in the Presence of POSS Nanoparticles and Poly(Ethylene Glycol) Dimethacrylate Coagent: “Comparison of Physical Properties and Shape Memory Behaviour”. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2205, p. 20028. [Google Scholar]
- Vennemann, N.; Bökamp, K.; Bröker, D. Crosslink Density of Peroxide Cured TPV. Macromol. Symp. 2006, 245–246, 641–650. [Google Scholar] [CrossRef]
- Shafieizadegan Esfahani, A.R.; Abdollahi, M.M.; Katbab, A.A. Effects of Compounding Procedure on Morphology Development, Melt Rheology, and Mechanical Properties of Nanoclay Reinforced Dynamically Vulcanized EPDM/Polypropylene Thermoplastic Vulcanizates. Polym. Eng. Sci. 2016, 56, 914–921. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-linked Polymer Networks I. Rubberlike Elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry, 1st ed.; Cornell University Press: New York, NY, USA, 1953. [Google Scholar]
- Parker, W.O.; Ferrando, A.; Ferri, D.; Canepari, V. Cross-Link Density of a Dispersed Rubber Measured by 129Xe Chemical Shift. Macromolecules 2007, 40, 5787–5790. [Google Scholar] [CrossRef]
- Goharpey, F.; Nazockdast, H.; Katbab, A.A. Relationship between the Rheology and Morphology of Dynamically Vulcanized Thermoplastic Elastomers Based on EPDM/PP. Polym. Eng. Sci. 2005, 45, 84–94. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1980; ISBN 978-0-471-04894-7. [Google Scholar]
- D’Orazio, L.; Mancarella, C.; Martuscelli, E.; Polato, F. Polypropylene/Ethylene-Co-Propylene Blends: Influence of Molecular Structure and Composition of EPR on Melt Rheology, Morphology and Impact Properties of Injection-Moulded Samples. Polymer 1991, 32, 1186–1194. [Google Scholar] [CrossRef]
- Mighri, F.; Huneault, M.A.; Ajji, A.; Ko, G.H.; Watanabe, F. Rheology of EPR/PP Blends. J. Appl. Polym. Sci. 2001, 82, 2113–2127. [Google Scholar] [CrossRef]
- Ning, N.; Li, S.; Wu, H.; Tian, H.; Yao, P.; HU, G.-H.; Tian, M.; Zhang, L. Preparation, Microstructure, and Microstructure-Properties Relationship of Thermoplastic Vulcanizates (TPVs): A Review. Prog. Polym. Sci. 2018, 79, 61–97. [Google Scholar] [CrossRef]
- Ma, P.; Xu, P.; Zhai, Y.; Dong, W.; Zhang, Y.; Chen, M. Biobased Poly(Lactide)/Ethylene- Co -Vinyl Acetate Thermoplastic Vulcanizates: Morphology Evolution, Superior Properties, and Partial Degradability. ACS Sustain. Chem. Eng. 2015, 3, 2211–2219. [Google Scholar] [CrossRef]
- Wu, H.; Tian, M.; Zhang, L.; Tian, H.; Wu, Y.; Ning, N.; Hu, G.-H. Effect of Rubber Nanoparticle Agglomeration on Properties of Thermoplastic Vulcanizates during Dynamic Vulcanization. Polymers 2016, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.R.; Singha, N.K.; Naskar, K. Interrelationships of Morphology, Thermal and Mechanical Properties in Uncrosslinked and Dynamically Crosslinked PP/EOC and PP/EPDM Blends. Express Polym. Lett. 2010, 4, 197–209. [Google Scholar] [CrossRef]
- Ning, N.; Hu, L.; Yao, P.; Wu, H.; Han, J.; Zhang, L.; Tian, H.; Tian, M. Study on the Microstructure and Properties of Bromobutyl Rubber (BIIR)/Polyamide-12 (PA12) Thermoplastic Vulcanizates (TPVs). J. Appl. Polym. Sci. 2016, 133, 43043. [Google Scholar] [CrossRef]
- Mark, J.E. Experimental Determinations of Crosslink Densities. Rubber Chem. Technol. 1982, 55, 762–768. [Google Scholar] [CrossRef]
- Mark, J.E.; Erman, B.; Eirich, F.R. Science and Technology of Rubber, 3rd ed.; Elsevier Academic Press: London, UK, 2005. [Google Scholar]
- Babu, R.R.; Singha, N.K.; Naskar, K. Effects of Mixing Sequence on Peroxide Cured Polypropylene (PP)/Ethylene Octene Copolymer (EOC) Thermoplastic Vulcanizates (TPVs). Part. I. Morphological, Mechanical and Thermal Properties. J. Polym. Res. 2009, 17, 657–671. [Google Scholar] [CrossRef]
- Babu, R.R.; Singha, N.K.; Naskar, K. Dynamically Vulcanized Blends of Polypropylene and Ethylene-Octene Copolymer: Comparison of Different Peroxides on Mechanical, Thermal, and Morphological Characteristics. J. Appl. Polym. Sci. 2009, 113, 1836–1852. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of Particle Size, Particle/Matrix Interface Adhesion and Particle Loading on Mechanical Properties of Particulate–Polymer Composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Zhu, Z.-K.; Yang, Y.; Yin, J.; Qi, Z.-N. Preparation and Properties of Organosoluble Polyimide/Silica Hybrid Materials by Sol-Gel Process. J. Appl. Polym. Sci. 1999, 73, 2977–2984. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Lauke, B. Characterization of Tensile Behaviour of Hybrid Short Glass Fibre/Calcite Particle/ABS Composites. Compos. Part A Appl. Sci. Manuf. 1998, 29, 575–583. [Google Scholar] [CrossRef]
- Fu, S.Y.; Lauke, B. Analysis of Mechanical Properties of Injection Molded Short Glass Fibre (SGF)/Calcite/ABS Composites. J. Mater. Sci. Technol. 1997, 13, 389–396. [Google Scholar]
- Amdouni, N.; Sautereau, H.; Gerard, J.F. Epoxy Composites Based on Glass Beads: Mechanical Properties. J. Appl. Polym. Sci. 1992, 46, 1723–1735. [Google Scholar] [CrossRef]
- Wang, M.; Berry, C.; Braden, M.; Bonfield, W. Young’s and Shear Moduli of Ceramic Particle Filled Polyethylene. J. Mater. Sci. Mater. Med. 1998, 9, 621–624. [Google Scholar] [CrossRef]
- Hsueh, C.-H. Effects of Aspect Ratios of Ellipsoidal Inclusions on Elastic Stress Transfer of Ceramic Composites. J. Am. Ceram. Soc. 1989, 72, 344–347. [Google Scholar] [CrossRef]
- Young, R.J.; Beaumont, P.W.R. Effect of Composition Upon Fracture of Silica Particle-Filled Epoxy–Resin Composites. J. Mater. Sci. 1977, 12, 684–692. [Google Scholar] [CrossRef]
- Pukanszky, B.; Voros, G. Mechanism of Interfacial Interactions in Particulate Filled Composites. Compos. Interfaces 1993, 1, 411–427. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamaguchi, M.; Okubo, M.; Matsumoto, T. Effects of Particle Size on Mechanical and Impact Properties of Epoxy Resin Filled with Spherical Silica. J. Appl. Polym. Sci. 1992, 45, 1281–1289. [Google Scholar] [CrossRef]
- Reynaud, E.; Jouen, T.; Gauthier, C.; Vigier, G.; Varlet, J. Nanofillers in Polymeric Matrix: A Study on Silica Reinforced PA6. Polymer 2001, 42, 8759–8768. [Google Scholar] [CrossRef]
- Ou, Y.; Yang, F.; Yu, Z.-Z. A New Conception on the Toughness of Nylon 6/Silica Nanocomposite Prepared via in Situ Polymerization. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 789–795. [Google Scholar] [CrossRef]
- Liang, J.Z.; Li, R.K.Y.; Tjong, S.C. Tensile Fracture Behaviour and Morphological Analysis of Glass Bead Filled Low Density Polyethylene Composite. Plast. Rubber Compos. Process. Appl. 1997, 26, 278–282. [Google Scholar]
- Varlet, J.; Cavaillé, J.Y.; Perez, J.; Johari, G.P. Dynamic Mechanical Spectrometry of Nylon-12. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 2691–2705. [Google Scholar] [CrossRef]
- Tjong, S.C.; Xu, S.A. Ternary Polymer Composites: PA6,6/Maleated SEBS/Glass Beads. J. Appl. Polym. Sci. 2001, 81, 3231–3237. [Google Scholar] [CrossRef]
- Levita, G.; Marchetti, A.; Lazzeri, A. Fracture of Ultrafine Calcium Carbonate/Polypropylene Composites. Polym. Compos. 1989, 10, 39–43. [Google Scholar] [CrossRef]
- Mali, M.; Marathe, A.; Mhaske, S. Influence of (Methacryloxymethyl)Methyldimethoxysilane on DCP Cured EPDM/PP Thermoplastic Vulcanizates. J. Vinyl Addit. Technol. 2018, 24, 304–313. [Google Scholar] [CrossRef]
- Reffai Syed Ismail, S.M.; Chatterjee, T.; Naskar, K. Development of Novel Polar Thermoplastic Vulcanizates Based on Ethylene Acrylic Elastomer and Polyamide 12 with Special Reference to Heat and Oil Aging. J. Appl. Polym. Sci. 2015, 132, 42655. [Google Scholar] [CrossRef]
- Saleesung, T.; Saeoui, P.; Sirisinha, C. Mechanical and Thermal Properties of Thermoplastic Elastomer Based on Low Density Polyethylene and Ultra-Fine Fully-Vulcanized Acrylonitrile Butadiene Rubber Powder (UFNBRP). Polym. Test. 2010, 29, 977–983. [Google Scholar] [CrossRef]
- Ning, N.; Hua, Y.; Wu, H.; Zhang, L.; Wu, S.; Tian, M.; Tian, H.; Hu, G.-H. Novel Heat and Oil-Resistant Thermoplastic Vulcanizates Based on Ethylene-Vinyl Acetate Rubber/Poly(Vinylidene Fluoride). RSC Adv. 2016, 6, 91594–91602. [Google Scholar] [CrossRef]
- Tian, M.; Han, J.; Wu, H.; Tian, H.; She, Q.; Chen, W.; Zhang, L. Effect of the Compatibility on the Morphology and Properties of Acrylonitrile-Butadiene Rubber/Polypropylene Thermoplastic Vulcanizates. J. Appl. Polym. Sci. 2012, 124, 1999–2006. [Google Scholar] [CrossRef]
- Dutta, J.; Ramachandran, P.; Naskar, K. Scrutinizing the Influence of Peroxide Crosslinking of Dynamically Vulcanized EVA/TPU Blends with Special Reference to Cable Sheathing Applications. J. Appl. Polym. Sci. 2016, 133, 43706. [Google Scholar] [CrossRef]



| Materials | Commercial Name and Manufacturer | Chemical Structure | Physical Properties and Descriptions |
|---|---|---|---|
| Polypropylene (PP) | Moplen HP456J, Lyondell Basel, Belgium | 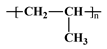 | Homopolymer MFI: 3.4 (230 °C, 2.16 kg/10 min) |
| Ethylene Propylene Diene Monomer (EPDM) | Dutral TER 4548, Versalis, Germany | 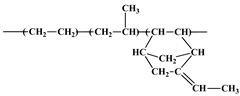 | Mooney Viscosity: 47 MU (1 + 4) 125 °C Ash content: max 0.3% Polypropylene content: 36% ENB content: 4.5% Oil content: 50% paraffin oil |
| Triallyl isocyanate (TAC) | TAC/GR 50, Kettlitz-Chemie GmbH & Co. KG. Germany | 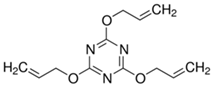 | Density: 1.17 g/cm3 Ash content: 25.5% ± 1.5 Appearance: White, soft grain |
| Dicumyl peroxide (DCP) | Luperox DC40P, Arkema, France | 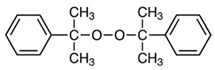 | Molecular weight: 270.4 g/mol Peroxide content: 38–42% Active oxygen content: %2.25–2.49 Density: 0.38 g/cm3 Appearance: White powder |
| POSS | Octavinyl-POSS, Hybrid Plastics Inc., Hattiesburg, MS, USA | 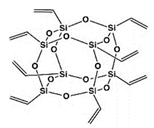 | Molecular weight: 632.31 g/mol Soluble in: THF, Chloroform Appearance: White powder |
| EPDM * (phr) | PP (phr) | DCP (phr) | X (Coagent/DCP Ratio) | Antioxidants (phr) | Abbreviation |
|---|---|---|---|---|---|
| 140 | 30 | 1.75 | 0 | 0.3 | 70/30 EPDM/PP/Coagent(X) |
| 120 | 40 | 1.50 | 1 | 0.4 | 60/40 EPDM/PP/Coagent(X) |
| 100 | 50 | 1.25 | 3 | 0.5 | 50/50 EPDM/PP/Coagent(X) |
| 80 | 60 | 1.00 | 5 | 0.6 | 40/60 EPDM/PP/Coagent(X) |
| 60 | 70 | 0.75 | 7 | 0.7 | 30/70 EPDM/PP/Coagent(X) |
| 70/30 | 60/40 | 50/50 | 40/60 | 30/70 | ||
|---|---|---|---|---|---|---|
| Crosslink density (mol/m3) | EPDM/PP + DCP | 103.0 ± 3.5 | 130.8 ± 9.2 | 208.7 ± 15.0 | 324.8 ± 29.1 | 462.4 ± 24.8 |
| EPDM/PP/TAC(1) | 230.4 ± 18.9 | 250.0 ± 12.8 | 316.4 ± 21.1 | 393.5 ± 21.0 | 479.6 ± 19.0 | |
| EPDM/PP/TAC(3) | 238.7 ± 17.2 | 264.6 ± 14.6 | 334.3 ± 13.9 | 422.8 ± 16.6 | 505.2 ± 20.5 | |
| EPDM/PP/TAC(5) | 239.8 ± 15.6 | 283.3 ± 18.5 | 354.5 ± 15.2 | 437.0 ± 12.2 | 582.1 ± 27.0 | |
| EPDM/PP/TAC(7) | 245.1 ± 10.5 | 323.9 ± 24.2 | 380.9 ± 11.5 | 467.5 ± 18.6 | 601.4 ± 13.2 | |
| EPDM/PP/OV-POSS(1) | 280.1 ± 16.6 | 319.3 ± 24.1 | 393.7 ± 19.5 | 477.8 ± 13.5 | 608.8 ± 36.2 | |
| EPDM/PP/OV-POSS(3) | 328.2 ± 25.6 | 386.5 ± 17.8 | 422.8 ± 20.5 | 559.3 ± 35.4 | 748.9 ± 40.8 | |
| EPDM/PP/OV-POSS(5) | 411.4 ± 14.5 | 484.9 ± 18.2 | 551.7 ± 19.0 | 658.6 ± 18.0 | 778.8 ± 41.3 | |
| EPDM/PP/OV-POSS(7) | 511.6 ± 13.2 | 558.0 ± 34.7 | 661.6 ± 25.0 | 798.4 ± 34.8 | 806.6 ± 50.6 |
| 70/30 | 60/40 | 50/50 | 40/60 | 30/70 | ||
|---|---|---|---|---|---|---|
| Compression Set (%) (70 h. 70 °C) | EPDM/PP | 81.1 ± 2.0 | 82.9 ± 1.1 | 84.0 ± 0.3 | 85.8 ± 7.1 | 87.0 ± 4.2 |
| EPDM/PP + DCP | 52.7 ± 1.8 | 56.5 ± 2.1 | 57.5 ± 1.3 | 62.9 ± 1.1 | 71.7 ± 1.5 | |
| EPDM/PP/TAC(1) | 50.5 ± 0.1 | 57.7 ± 0.5 | 62.8 ± 0.3 | 65.5 ± 4.1 | 73.0 ± 1.1 | |
| EPDM/PP/TAC(3) | 48.1 ± 0.8 | 53.3 ± 2.1 | 62.2 ± 0.9 | 65.3 ± 0.3 | 72.7 ± 1.9 | |
| EPDM/PP/TAC(5) | 47.3 ± 1.2 | 52.4 ± 0.8 | 58.5 ± 0.9 | 65.2 ± 0.8 | 71.6 ± 0.6 | |
| EPDM/PP/TAC(7) | 45.8 ± 1.7 | 47.9 ± 1.5 | 57.9 ± 1.4 | 64.9 ± 0.4 | 69.2 ± 2.6 | |
| EPDM/PP/OV-POSS(1) | 40.6 ± 3.9 | 46.4 ± 0.2 | 53.9 ± 1.1 | 61.0 ± 1.9 | 70.6 ± 3.0 | |
| EPDM/PP/OV-POSS(3) | 39.6 ± 1.3 | 45.5 ± 1.7 | 52.0 ± 0.6 | 60.4 ± 0.3 | 69.5 ± 0.1 | |
| EPDM/PP/OV-POSS(5) | 39.1 ± 0.7 | 44.1 ± 1.6 | 51.8 ± 1.4 | 59.8 ± 0.2 | 69.4 ± 1.4 | |
| EPDM/PP/OV-POSS(7) | 38.6 ± 3.6 | 43.5 ± 1.5 | 51.0 ± 1.5 | 59.5 ± 0.5 | 65.9 ± 2.0 | |
| Changes (%) | EPDM/PP | 68 | 63 | 64 | 63 | 54 |
| EPDM/PP + DCP | 58 | 37 | 15 | 18 | 25 | |
| EPDM/PP/TAC(1) | 52 | 46 | 34 | 32 | 29 | |
| EPDM/PP/TAC(3) | 58 | 44 | 45 | 37 | 30 | |
| EPDM/PP/TAC(5) | 57 | 43 | 44 | 45 | 36 | |
| EPDM/PP/TAC(7) | 68 | 50 | 55 | 45 | 32 | |
| EPDM/PP/OV-POSS(1) | 35 | 26 | 33 | 36 | 34 | |
| EPDM/PP/OV-POSS(3) | 46 | 42 | 39 | 35 | 33 | |
| EPDM/PP/OV-POSS(5) | 34 | 28 | 38 | 44 | 49 | |
| EPDM/PP/OV-POSS(7) | 41 | 29 | 43 | 44 | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çakır, N.Y.; İnan, Ö.; Ergün, M.; Kodal, M.; Özkoç, G. Unlocking the Potential Use of Reactive POSS as a Coagent for EPDM/PP-Based TPV. Polymers 2023, 15, 2267. https://doi.org/10.3390/polym15102267
Çakır NY, İnan Ö, Ergün M, Kodal M, Özkoç G. Unlocking the Potential Use of Reactive POSS as a Coagent for EPDM/PP-Based TPV. Polymers. 2023; 15(10):2267. https://doi.org/10.3390/polym15102267
Chicago/Turabian StyleÇakır, Nazlı Yazıcı, Özgenur İnan, Merve Ergün, Mehmet Kodal, and Güralp Özkoç. 2023. "Unlocking the Potential Use of Reactive POSS as a Coagent for EPDM/PP-Based TPV" Polymers 15, no. 10: 2267. https://doi.org/10.3390/polym15102267
APA StyleÇakır, N. Y., İnan, Ö., Ergün, M., Kodal, M., & Özkoç, G. (2023). Unlocking the Potential Use of Reactive POSS as a Coagent for EPDM/PP-Based TPV. Polymers, 15(10), 2267. https://doi.org/10.3390/polym15102267






