An Overview of the Emerging Technologies and Composite Materials for Supercapacitors in Energy Storage Applications
Abstract
:1. Introduction
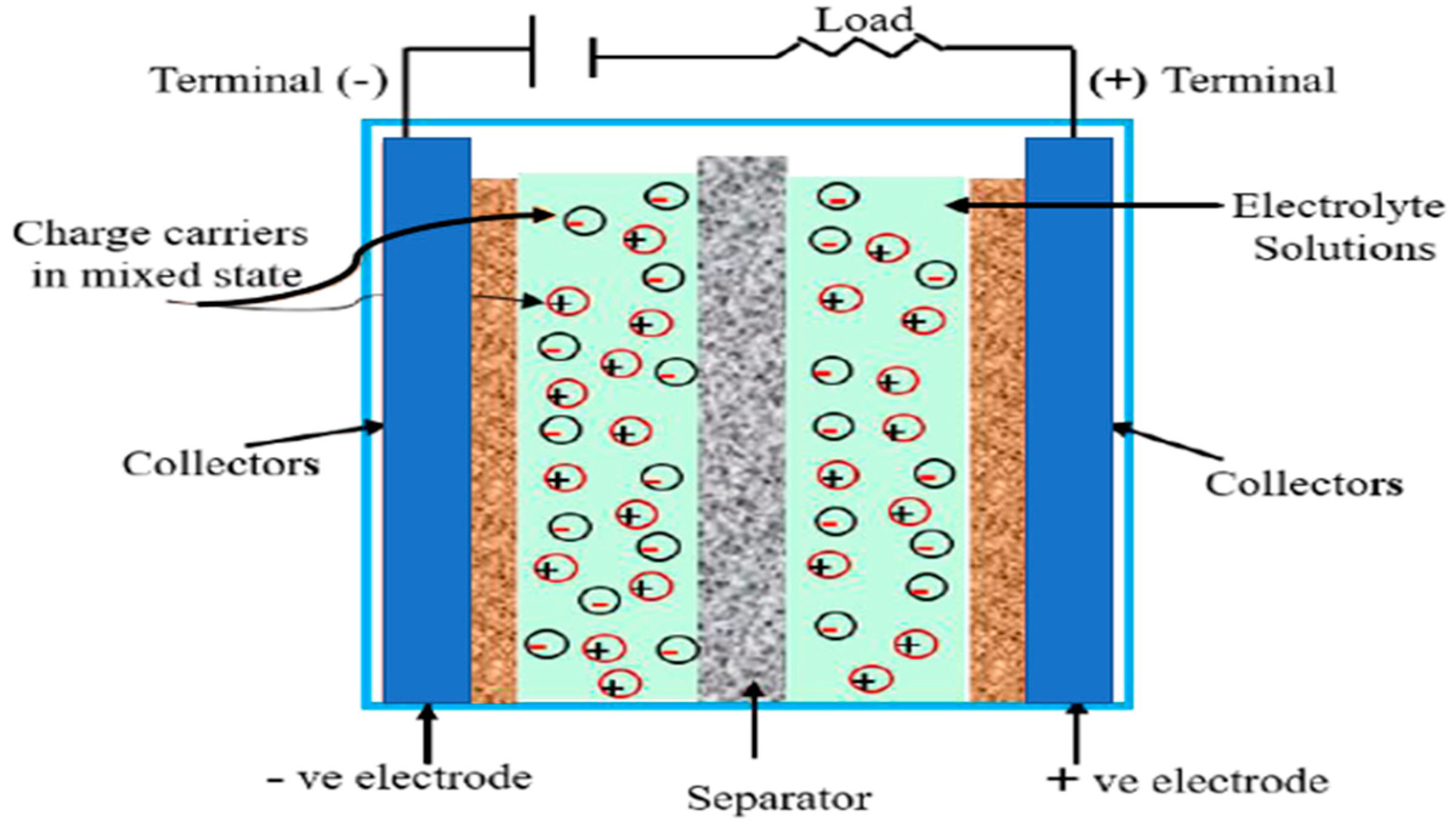
2. Supercapacitors (SCs)
2.1. Electric Double-Layer Capacitors (EDLCs)
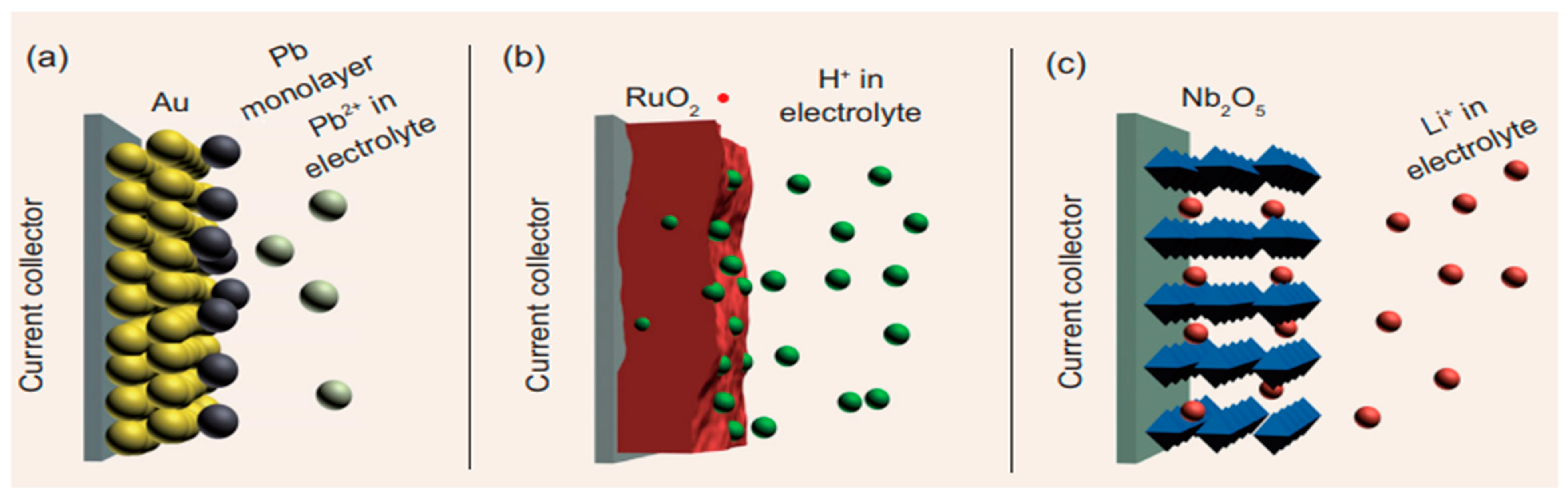
2.2. Pseudocapacitors
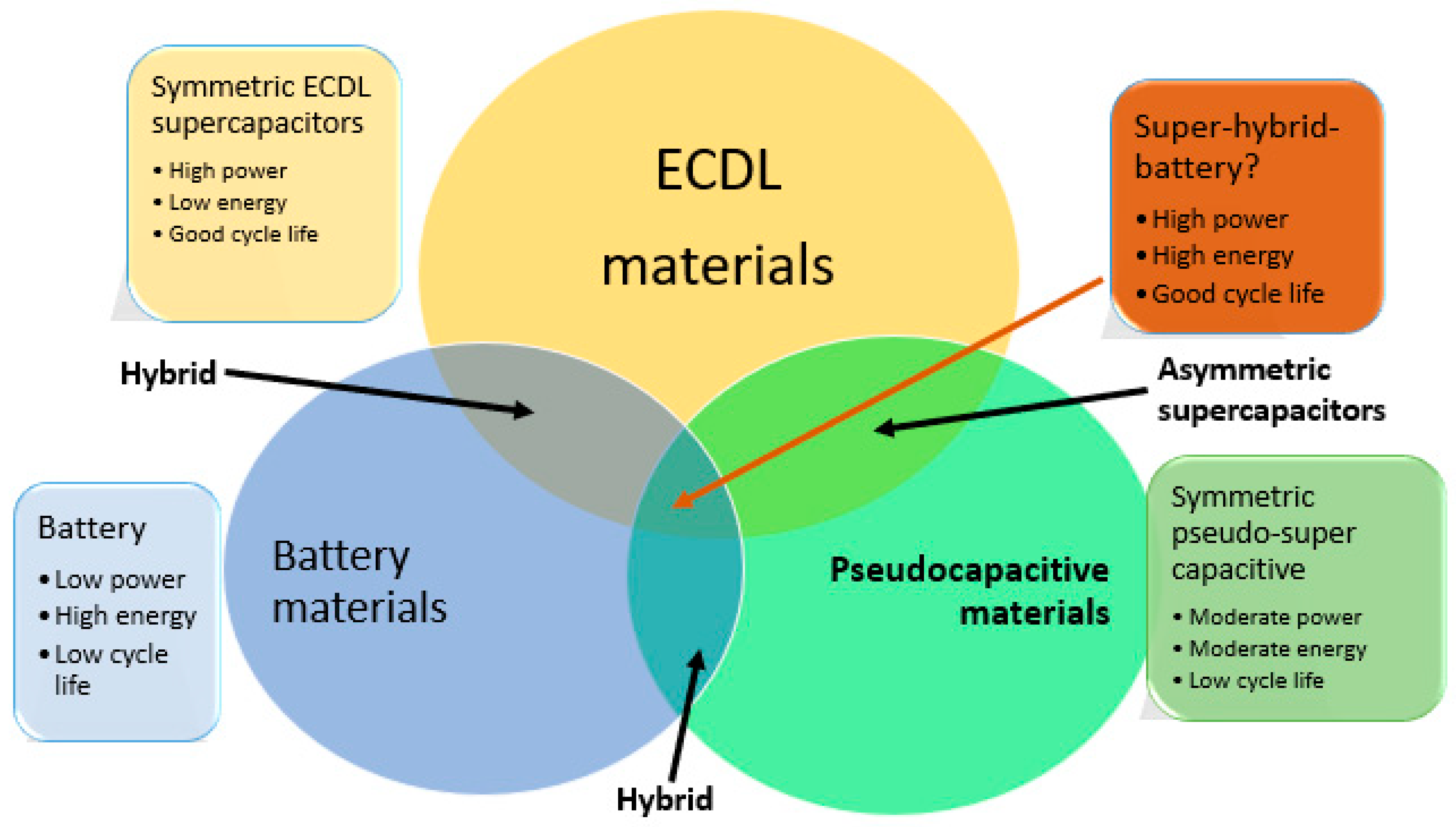
2.3. Hybrid Supercapacitor
3. Materials for Supercapacitors
3.1. Electrode
3.1.1. Carbon Based
3.1.2. Carbon Nanotubes (CNTs)
- Single-walled carbon nanotubes (SWCNTs)
- b.
- Multiple-walled carbon nanotubes (MWCNTs)
3.1.3. Activated Carbons
3.1.4. Carbon Aerogels
3.1.5. Graphene
3.2. Transition Metal-Oxides (TMOs)
3.2.1. Ruthenium Oxide (RuO2)
3.2.2. Manganese Dioxide (MnO2)
3.2.3. Nickel Oxide (NiO)
3.2.4. Cobalt Oxide (Co3O4)
3.3. Conducting Polymers (CPs)
3.4. Perovskite Based
3.5. Electrolyte
4. Applications of Supercapacitors
- Transportation system: Integrating supercapacitors and batteries can provide high-power bursts or boosts for the braking or acceleration systems in the transportation sector, particularly in electric cars. This will also increase the battery pack’s total efficiency and lifespan;
- Backup power systems: Supercapacitors are a viable option for backup power systems in mission-critical settings, such as data acquisition centers, hospitals, and military facilities. In a power failure situation, they can swiftly supply power, enabling the smooth operation of these crucial systems;
- Renewable energy storage: Supercapacitors can store energy from solar and wind sources. This energy can be swiftly released to maintain the power grid’s stability or supply electricity during high demand;
- Consumer devices: Societal growth and advancement in modern technology have recorded significant attention, and the manufacturing of small appliances, such as mobile phone cameras, toys, and remote controls, is at their peaks. Supercapacitors can power these devices. Supercapacitors offer a greater power density than conventional batteries, enabling quicker charging and longer functioning times;
- Aerospace and defense applications: Satellite systems, drones, and missile systems are just a few examples of aerospace and defense applications that use supercapacitors. They are resilient to shock and vibration, and offer reliable and adequate energy storage in challenging conditions;
- Hybrid power system: supercapacitors are a promising alternative for hybrid power systems, which can hybridize fuel cells, batteries, and renewable energy sources needed to provide a dependable and adequate power supply source;
- Energy harvesting: supercapacitors can be employed in energy harvesting devices to store energy generated from vibrations, temperature differences, and light sources.
5. Advantages and Current Challenges of Supercapacitors
- High power density: supercapacitors are suited for tasks requiring short bursts of power due to their high power output features;
- Long life cycle: Supercapacitors are considerably more resilient to charging and discharging cycles than conventional batteries, which usually have a much shorter cycle life. They can withstand hundreds of thousands of processes;
- Wide operating temperature range: supercapacitors can operate over a more extensive temperature range than batteries, making them useful for harsh environments;
- Low maintenance: unlike batteries, which struggle with sulfation and other degradation mechanisms, supercapacitors require low maintenance;
- Light and safe: supercapacitors are safer to use and more easily discarded after use than conventional batteries because they have no heavy metals or dangerous substances;
- High efficiency: supercapacitors can store and release energy with little loss due to their high efficiency.
- Energy density: supercapacitors are less energy dense than batteries. Their applicability for tasks requiring long-term energy storage is thus constrained;
- Cost: supercapacitors are presently more expensive than conventional batteries, restricting their deployment in some applications;
- Leakage current: it is easy and quite possible for supercapacitors to lose current when not in use due to their high current leakage;
- Voltage limitations: supercapacitors can only be used in certain situations because of their lower voltage limits than batteries;
- Limited research: Despite the potential advantages of supercapacitors, research is still in its infancy when compared to the more established battery technologies. Therefore, more research is needed to increase the efficiency and lower the price of supercapacitors.
6. Conclusions and Outlook
- Increased energy density: Supercapacitors are hampered by their lesser energy density compared to batteries, which is one of their main drawbacks. However, considerable research should be devoted to developing novel materials and designs that could significantly boost supercapacitors’ energy densities, to make them more competitive with batteries;
- Development of hybrid systems: Developing hybrid systems that combine the benefits of supercapacitors and battery technologies is crucially essential. These systems provide greater energy density and more extended cycle life than either technology;
- New applications: Supercapacitors are currently employed in a few applications, but they have a broad range of potential uses due to their unique characteristics. For instance, they could be utilized for energy storage in the electrical system to increase the effectiveness of regenerative braking in trains, and to power wearable electronics;
- Development of sustainable materials: Developing eco-friendly and sustainable materials for supercapacitors is significantly growing and becoming a priority, just as it is for all other energy storage technologies. Scientists are considering the use of components such as graphene, carbon nanotubes, and biodegradable plastics for the manufacturing of more sustainable supercapacitors. The sustainability of this aspect is essential, and thus advised;
- Longer life cycle: Supercapacitors have limited life cycles; however, they can endure more charging/discharging cycles than batteries. New materials and designs should be investigated for supercapacitors to reliably operate longer.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amrouche, S.O.; Rekioua, D.; Rekioua, T.; Bacha, S. Overview of energy storage in renewable energy systems. Int. J. Hydrog. Energy 2016, 41, 20914–20927. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.; Wang, R.; Zhu, F.; Lu, Y.; Roskilly, A.P. Experimental investigation on an innovative resorption system for energy storage and upgrade. Energy Convers. Manag. 2017, 138, 651–658. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.S.; Munnings, C.; Bhatt, A.I.; Hollenkamp, A.F. Emerging electrochemical energy conversion and storage technologies. Front. Chem. 2014, 2, 79. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Junior, M.G.; Gonçalves, R.D.F.; Borges, K.C.M.; Rodrigues, M.H.D.M.; Delmonte, M.R.B.; Motta, F.V.; Nascimento, R.M.D. Functional Nanomaterials for Applications in Energy Storage and Conversion. Recent Adv. Complex Funct. Mater. Des. Appl. 2017, 217–237. [Google Scholar]
- Afif, A.; Radenahmad, N.; Cheok, Q.; Shams, S.; Kim, J.H.; Azad, A.K. Ammonia-fed fuel cells: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 60, 822–835. [Google Scholar] [CrossRef]
- Radenahmad, N.; Afif, A.; Petra, P.I.; Rahman, S.M.; Eriksson, S.-G.; Azad, A.K. Proton-conducting electrolytes for direct methanol and direct urea fuel cells—A state-of-the-art review. Renew. Sustain. Energy Rev. 2016, 57, 1347–1358. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, A.K. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar]
- Abdah, M.A.A.M.; Awan, H.T.A.; Mehar, M.; Mustafa, M.N.; Walvekar, R.; Alam, M.W.; Khalid, M.; Umapathi, R.; Chaudhary, V. Advancements in MXene-polymer composites for high-performance supercapacitor applications. J. Energy Storage 2023, 63, 106942. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, H.; Hu, T.; Fan, B.; Wang, X.; Li, Z. Emerging 2D MXenes for supercapacitors: Status, challenges and prospects. Chem. Soc. Rev. 2020, 49, 6666–6693. [Google Scholar] [CrossRef] [PubMed]
- Adedoja, O.S.; Saleh, U.A.; Alesinloye, A.R.; Timiyo, T.-E.J.; Onuigbo, I.F.; Adejuwon, O.O.; Josiah, E. An energy balance and multicriterial approach for the sizing of a hybrid renewable energy system with hydrogen storage. e-Prime 2023, 4, 100146. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar] [CrossRef]
- Wei, L.; Deng, W.; Li, S.; Wu, Z.; Cai, J.; Luo, J. Sandwich-like chitosan porous carbon Spheres/MXene composite with high specific capacitance and rate performance for supercapacitors. J. Bioresour. Bioprod. 2022, 7, 63–72. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Srikesh, G.; Mohan, A.; Arivazhagan, V. In-situ synthesis of Co3O4/graphite nanocomposite for high-performance supercapacitor electrode applications. Appl. Surf. Sci. 2017, 403, 578–583. [Google Scholar]
- Zhang, D.; Tan, C.; Zhang, W.; Pan, W.; Wang, Q.; Li, L. Expanded Graphite-Based Materials for Supercapacitors: A Review. Molecules 2022, 27, 716. [Google Scholar] [CrossRef]
- Low, W.H.; Khiew, P.S.; Lim, S.S.; Siong, C.W.; Ezeigwe, E.R. Recent development of mixed transition metal oxide and graphene/mixed transition metal oxide based hybrid nanostructures for advanced supercapacitors. J. Alloys Compd. 2019, 775, 1324–1356. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Burke, A. R&D considerations for the performance and application of electrochemical capacitors. Electrochim. Acta 2007, 53, 1083–1091. [Google Scholar]
- Kyeremateng, N.A.; Brousse, T.; Pech, D. Microsupercapacitors as miniaturized energy-storage components for on-chip electronics. Nat. Nanotechnol. 2016, 12, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Afif, A.; Rahman, S.M.H.; Azad, A.T.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Nagarajarao, S.H.; Nandagudi, A.; Viswanatha, R.; Basavaraja, B.M.; Santosh, M.S.; Praveen, B.M.; Pandith, A. Recent Developments in Supercapacitor Electrodes: A Mini Review. Chemengineering 2022, 6, 5. [Google Scholar] [CrossRef]
- Adedoja, O.S.; Sadiku, E.R.; Hamam, Y. Prospects of Hybrid Conjugated Polymers Loaded Graphene in Electrochemical Energy Storage Applications. J. Inorg. Organomet. Polym. Mater. 2023, 1–20. [Google Scholar] [CrossRef]
- Navathe, G.; Prasad, S.; Mane, A.; Barge, S.; Dongale, T.; Shaikh, V.; Karanjkar, M.; Teli, S.; Patil, P.; Prasad, N.R. A Critical Review on Design and Development of New Generation Energy Storage Devices. ES Energy Environ. 2022, 17, 11–32. [Google Scholar]
- Mitali, J.; Dhinakaran, S.; Mohamad, A. Energy storage systems: A review. Energy Storage Sav. 2022, 1, 166–216. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Wang, T.; Shao, J.; Wang, D.; Yang, Y.-W. Mesoporous Transition Metal Oxides for Supercapacitors. Nanomaterials 2015, 5, 1667–1689. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, H.-X.; Cao, Y.; Yan, X.-H.; Yan, J.; Gao, H.-L.; Luo, H.-W.; Wang, S.-W.; Jia, X.-D.; Kachalova, L.; et al. Recent advances and challenges of electrode materials for flexible supercapacitors. Co-ord. Chem. Rev. 2021, 438, 213910. [Google Scholar] [CrossRef]
- Kirchner, K.; Ivaništšev, V.; Fedorov, M. Electrical double layer in ionic liquids: Structural transitions from multilayer to monolayer structure at the interface. Electrochim. Acta 2013, 110, 762–771. [Google Scholar] [CrossRef]
- Burke, A. Ultracapacitors: Why, how, and where is the technology. J. Power Sources 2000, 91, 37–50. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van Schalkwijk, W. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.; Braatz, P. Comparison of commercial supercapacitors and high-power lithium-ion batteries for power-assist applications in hybrid electric vehicles: I. Initial characterization. J. Power Sources 2002, 112, 236–246. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.-G.; Tan, J.; Wu, Z.-S.; Gentle, I.; Lu, G.Q.; Cheng, H.-M. Fabrication of Graphene/Polyaniline Composite Paper via In Situ Anodic Electropolymerization for High-Performance Flexible Electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Adedoja, O.S.; Hamam, Y.; Sadiku, R.; Khalaf, B. Applications of Nanomaterials for Water Quality Sustainability: Present Status and Future Trends. Int. J. Sustain. Dev. Plan. 2021, 16, 357–363. [Google Scholar] [CrossRef]
- Anderson, M.A.; Cudero, A.L.; Palma, J. Review on the science and technology of water desalination by capacitive deionization. Electrochim. Acta 2010, 55, 3845–3856. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, M.; Meng, Y.; Fang, P.; Lu, X.; Tong, Y. Iron-Based Supercapacitor Electrodes: Advances and Challenges. Adv. Energy Mater. 2016, 6, 1601053. [Google Scholar] [CrossRef]
- Attia, S.Y.; Mohamed, S.G.; Barakat, Y.F.; Hassan, H.H.; Al Zoubi, W. Supercapacitor electrode materials: Addressing challenges in mechanism and charge storage. Rev. Inorg. Chem. 2021, 42, 53–88. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, X.; Cao, D. Biomass-derived nitrogen-doped porous carbons (NPC) and NPC/ polyaniline composites as high performance supercapacitor materials. Eng. Sci. 2018, 1, 55–63. [Google Scholar] [CrossRef]
- Hou, C.; Hou, J.; Zhang, H.; Ma, Y.; He, X.; Geng, W.; Zhang, Q. Facile synthesis of LiMn0.75Fe0.25PO4/C nanocomposite cathode materials of lithium-ion batteries through microwave sintering. Eng. Sci. 2020, 11, 36–43. [Google Scholar]
- Pan, D.; Luo, S.; Feng, Y.; Zhang, X.; Su, F.; Liu, H.; Liu, C.; Mai, X.; Naik, N.; Guo, Z. Highly thermally conductive 3D BN/MWCNTs/C spatial network composites with improved electrically insulating and flame retardancy prepared by biological template assisted method. Compos. Part B Eng. 2021, 222, 109039. [Google Scholar] [CrossRef]
- Stević, Z.; Rajčić-Vujasinović, M.; Bugarinović, S.; Dekanski, A. Construction and Characterisation of Double Layer Capacitors. Acta Phys. Pol. A 2010, 117, 228–233. [Google Scholar] [CrossRef]
- Harris, F.E.; Alder, B.J. Dielectric Polarization in Polar Substances. J. Chem. Phys. 1953, 21, 1031–1038. [Google Scholar] [CrossRef]
- Tyler, R.W.; Webb, J.H.; York, W.C. Measurements of Electrical Polarization in Thin Dielectric Materials. J. Appl. Phys. 1955, 26, 61–68. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode Materials for Supercapacitors: A Review of Recent Advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Riaz, A.; Sarker, M.R.; Saad, M.H.M.; Mohamed, R. Review on comparison of different energy storage technologies used in micro-energy harvesting, WSNs, low-cost microelectronic devices: Challenges and recommendations. Sensors 2021, 21, 5041. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Liu, L.; Manasa, P.; Kang, L.; Ran, F. Vanadium nitride for aqueous supercapacitors: A topic review. J. Mater. Chem. A 2020, 8, 8218–8233. [Google Scholar] [CrossRef]
- Song, B.; Choi, J.I.; Zhu, Y.; Geng, Z.; Zhang, L.; Lin, Z.; Tuan, C.-C.; Moon, K.-S.; Wong, C.-P. Molecular Level Study of Graphene Networks Functionalized with Phenylenediamine Monomers for Supercapacitor Electrodes. Chem. Mater. 2016, 28, 9110–9121. [Google Scholar] [CrossRef]
- Song, B.; Zhao, J.; Wang, M.; Mullavey, J.; Zhu, Y.; Geng, Z.; Chen, D.; Ding, Y.; Moon, K.-S.; Liu, M.; et al. Systematic study on structural and electronic properties of diamine/triamine functionalized graphene networks for supercapacitor application. Nano Energy 2017, 31, 183–193. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, X.; Sarkar, S.; Zhao, Y. Challenges and opportunities for supercapacitors. APL Mater. 2019, 7, 100901. [Google Scholar] [CrossRef]
- Goubard-Bretesché, N.; Crosnier, O.; Favier, F.; Brousse, T. Improving the Volumetric Energy Density of Supercapacitors. Electrochim. Acta 2016, 206, 458–463. [Google Scholar] [CrossRef]
- Chu, X.; Kinoshita, K. Electrochemical capacitors. In The Electrochemical Society Proceedings Series; Electrochemical Society: Pennington, NJ, USA, 1996; p. 235. [Google Scholar]
- Edwards, I.A.S.; Marsh, H.; Menendez, R. Introduction to Carbon Science; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Yoon, S.; Lee, J.; Hyeon, T.; Oh, S.M. Electric Double-Layer Capacitor Performance of a New Mesoporous Carbon. J. Electrochem. Soc. 2000, 147, 2507–2512. [Google Scholar] [CrossRef]
- Namisnyk, A.; Zhu, J. A Survey of Electrochemical Super-Capacitor Technology. In Proceedings of the Australian Universities Power Engineering Conference, Brisbane, Australia, 26–29 September 2003. [Google Scholar]
- Sparnaay, M.J. The Electric Double Layer, Volume 4; Pergamon Press (Aust.) Pty. Ltd.: Sydney, Australia, 1972. [Google Scholar]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Presser, V. Carbon Nanomaterials; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Futaba, D.; Hata, K.; Yamada, T.; Hiraoka, T.; Hayamizu, Y.; Kakudate, Y.; Tanaike, O.; Hatori, H.; Yumura, M.; Iijima, S. Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes. Nat. Mater. 2006, 5, 987–994. [Google Scholar] [CrossRef]
- Portet, C.; Chmiola, J.; Gogotsi, Y.; Park, S.; Lian, K. Electrochemical characterizations of carbon nanomaterials by the cavity microelectrode technique. Electrochim. Acta 2008, 53, 7675–7680. [Google Scholar] [CrossRef]
- Liu, H.; Jin, L.-H.; He, P.; Wang, C.; Xia, Y. Direct synthesis of mesoporous carbon nanowires in nanotubes using MnO2 nanotubes as a template and their application in supercapacitors. Chem. Commun. 2009, 6813–6815. [Google Scholar] [CrossRef]
- Largeot, C.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Microporous Carbon-Based Electrical Double Layer Capacitor Operating at High Temperature in Ionic Liquid Electrolyte. Electrochem. Solid-State Lett. 2011, 14, A174–A176. [Google Scholar] [CrossRef]
- Niu, C.; Sichel, E.K.; Hoch, R.; Moy, D.; Tennent, H. High power electrochemical capacitors based on carbon nanotube electrodes. Appl. Phys. Lett. 1997, 70, 1480–1482. [Google Scholar] [CrossRef]
- Garthwaite, J. How ultracapacitors work (and why they fall short). Earth2Tech. GigaOM Netw. 2011. [Google Scholar]
- Conway, B.; Birss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from ‘supercapacitor’ to ‘battery’ behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, Y.; Ji, X. NiCo2O4-based materials for electrochemical supercapacitors. J. Mater. Chem. A Mater. 2014, 2, 14759–14772. [Google Scholar] [CrossRef]
- Bryan, A.M.; Santino, L.; Lu, Y.; Acharya, S.; D’arcy, J.M. Conducting Polymers for Pseudocapacitive Energy Storage. Chem. Mater. 2016, 28, 5989–5998. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Mikhailin, A.A.; Bograchev, D.A.; Sosenkin, V.E.; Bagotsky, V.S. Studies of supercapacitor carbon electrodes with high pseudocapacitance. Recent Trend Electrochem. Sci. Technol. 2012, 159. [Google Scholar]
- Wang, J.; Dong, S.; Ding, B.; Wang, Y.; Hao, X.; Dou, H.; Xia, Y.; Zhang, X. Pseudocapacitive materials for electrochemical capacitors: From rational synthesis to capacitance optimization. Natl. Sci. Rev. 2016, 4, 71–90. [Google Scholar] [CrossRef]
- Trasatti, S.; Buzzanca, G. Ruthenium dioxide: A new interesting electrode material. Solid state structure and electrochemical behaviour. J. Electroanal. Chem. Interfacial Electrochem. 1971, 29, A1–A5. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, B.; Wang, J.; Shao, Z. Non-aqueous hybrid supercapacitors fabricated with mesoporous TiO2 microspheres and activated carbon electrodes with superior performance. J. Power Sources 2014, 253, 80–89. [Google Scholar] [CrossRef]
- Lim, E.; Jo, C.; Lee, J. A mini review of designed mesoporous materials for energy-storage applications: From electric double-layer capacitors to hybrid supercapacitors. Nanoscale 2016, 8, 7827–7833. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Li, J.; Luo, Z.; Tang, J.; Zhu, Y. Advanced aqueous energy storage devices based on flower-like nanosheets-assembled Ni0.85Se microspheres and porous Fe2O3 nanospheres. Electrochim. Acta 2019, 302, 449–458. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, K.; Zhitomirsky, I. New colloidal route for electrostatic assembly of oxide nanoparticle—Carbon nanotube composites. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 446, 15–22. [Google Scholar] [CrossRef]
- Yang, X.; Xu, K.; Zou, R.; Hu, J. A Hybrid Electrode of Co3O4@PPy Core/Shell Nanosheet Arrays for High-Performance Supercapacitors. Nano-Micro Lett. 2015, 8, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Halper, M.S.; Ellenbogen, J.C. Supercapacitors: A brief overview. MITRE Corp. McLean Va. USA 2006, 1. [Google Scholar]
- Cericola, D.; Kötz, R. Hybridization of rechargeable batteries and electrochemical capacitors: Principles and limits. Electrochim. Acta 2012, 72, 1–17. [Google Scholar] [CrossRef]
- Laforgue, A.; Simon, P.; Fauvarque, J.F.; Mastragostino, M.; Soavi, F.; Sarrau, J.F.; Lailler, P.; Conte, M.; Rossi, E.; Saguatti, S. Activated Carbon/Conducting Polymer Hybrid Supercapacitors. J. Electrochem. Soc. 2003, 150, A645–A651. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Z.; Jing, M.; Yang, X.; Song, W.; Ji, X. Mesoporous NiCO2S4 nanoparticles as high-performance electrode materials for supercapacitors. J. Power Sources 2015, 273, 584–590. [Google Scholar] [CrossRef]
- Pell, W.G.; Conway, B.E. Peculiarities and requirements of asymmetric capacitor devices based on combination of capacitor and battery-type electrodes. J. Power Sources 2004, 136, 334–345. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Z.; Hu, Z.; Xi, L.; Ji, X.; Liu, Y. 3D interconnected ultrathin cobalt selenide nanosheets as cathode materials for hybrid supercapacitors. Electrochim. Acta 2018, 269, 30–37. [Google Scholar] [CrossRef]
- Xue, W.-D.; Wang, W.-J.; Fu, Y.-F.; He, D.-X.; Zeng, F.-Y.; Zhao, R. Rational synthesis of honeycomb-like NiCO2O4@NiMoO4 core/shell nanofilm arrays on Ni foam for high-performance supercapacitors. Mater. Lett. 2017, 186, 34–37. [Google Scholar] [CrossRef]
- Alguail, A.A. Battery type hybrid supercapacitor based on conducting polymers. Универзитет У Беoграду 2018. [Google Scholar]
- Low, W.H.; Khiew, P.S.; Lim, S.S.; Siong, C.W.; Chia, C.H.; Ezeigwe, E.R. Facile synthesis of graphene-Zn3V2O8 nanocomposite as a high performance electrode material for symmetric supercapacitor. J. Alloys Compd. 2019, 784, 847–858. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Ihns, M.; Li, M.; Hwang, J.Y.; Mousavi, M.F.; Chaney, L.; Lech, A.T.; Kaner, R.B. Engineering three-dimensional hybrid supercapacitors and microsupercapacitors for high-performance integrated energy storage. Proc. Natl. Acad. Sci. USA 2015, 112, 4233–4238. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Li, K.; Li, Y.; Gao, B.; Ding, T.; Zhong, Q.; Su, J.; Gong, L.; Chen, J.; Yuan, L.; et al. High-Performance Hybrid Supercapacitor Based on Graphene-Wrapped Mesoporous T-Nb2O5 Nanospheres Anode and Mesoporous Carbon-Coated Graphene Cathode. ChemElectroChem 2016, 3, 1360–1368. [Google Scholar] [CrossRef]
- Arbizzani, C.; Mastragostino, M.; Soavi, F. New trends in electrochemical supercapacitors. J. Power Sources 2001, 100, 164–170. [Google Scholar] [CrossRef]
- Balducci, A.; Bardi, U.; Caporali, S.; Mastragostino, M.; Soavi, F. Ionic liquids for hybrid supercapacitors. Electrochem. Commun 2004, 6, 566–570. [Google Scholar] [CrossRef]
- Du, P.; Hu, X.; Yi, C.; Liu, H.C.; Liu, P.; Zhang, H.-L.; Gong, X. Self-Powered Electronics by Integration of Flexible Solid-State Graphene-Based Supercapacitors with High Performance Perovskite Hybrid Solar Cells. Adv. Funct. Mater. 2015, 25, 2420–2427. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding Three-Dimensional Graphene/MnO2 Composite Networks As Ultralight and Flexible Supercapacitor Electrodes. ACS Nano 2012, 7, 174–182. [Google Scholar] [CrossRef]
- Gou, J.; Xie, S.; Liu, C. Hollow sphere NiS2 as high-performance hybrid supercapacitor electrode materials. In Proceedings of the AIP Conference Proceedings, Xi’an, China, 10–11 December 2016; p. 20040. [Google Scholar]
- Sot, M.; Kiamahalleh, M.V.; Najafpour, G.; Zein, S.H.S. Optimization of specific capacitance for hybrid supercapacitor material based on nickel-manganese oxides/multiwalled carbon nanotubes/poly (3, 4-ethylenedioxythiophene) using response surface methodology. World Appl. Sci. J. Spec. Issue Carbon Nanotub. 2010, 9, 14–20. [Google Scholar]
- Chen, W.; Yu, H.; Lee, S.-Y.; Wei, T.; Li, J.; Fan, Z. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for energy storage: Review of electrode materials and methods of increasing capacitance for supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Electrophoretic nanotechnology of graphene–carbon nanotube and graphene–polypyrrole nanofiber composites for electrochemical supercapacitors. J. Colloid Interface Sci. 2013, 407, 474–481. [Google Scholar] [CrossRef]
- Shi, K.; Yang, X.; Cranston, E.D.; Zhitomirsky, I. Efficient Lightweight Supercapacitor with Compression Stability. Adv. Funct. Mater. 2016, 26, 6437–6445. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Fabrication of Polypyrrole-Coated Carbon Nanotubes Using Oxidant–Surfactant Nanocrystals for Supercapacitor Electrodes with High Mass Loading and Enhanced Performance. ACS Appl. Mater. Interfaces 2013, 5, 13161–13170. [Google Scholar] [CrossRef]
- Shi, K.; Giapis, K.P. Scalable Fabrication of Supercapacitors by Nozzle-Free Electrospinning. ACS Appl. Energy Mater. 2018, 1, 296–300. [Google Scholar] [CrossRef]
- Doennig, D.; Pentcheva, R. Control of orbital reconstruction in (LaAlO3) M/(SrTiO3) N (001) quantum wells by strain and confinement. Sci. Rep. 2015, 5, 7909. [Google Scholar] [CrossRef]
- Zhang, L.; Hui, K.N.; Hui, K.S.; Lee, H. High-performance hybrid supercapacitor with 3D hierarchical porous flower-like layered double hydroxide grown on nickel foam as binder-free electrode. J. Power Sources 2016, 318, 76–85. [Google Scholar] [CrossRef]
- Lokhande, C.D.; Dubal, D.P.; Joo, O.-S. Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 2011, 11, 255–270. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, S.-I.; Yoon, J.-C.; Jang, J.-H. Chemical Vapor Deposition of Mesoporous Graphene Nanoballs for Supercapacitor. ACS Nano 2013, 7, 6047–6055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Gallant, B.M.; Byon, H.R.; Hammond, P.T.; Shao-Horn, Y. Nanostructured carbon-based electrodes: Bridging the gap between thin-film lithium-ion batteries and electrochemical capacitors. Energy Environ. Sci. 2011, 4, 1972–1985. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.; Dai, S. Carbon Materials for Chemical Capacitive Energy Storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2018, 141, 467–480. [Google Scholar] [CrossRef]
- Aqel, A.; Abou El-Nour, K.M.M.; Ammar, R.A.A.; Al-Warthan, A. Carbon nanotubes, science and technology part (I) structure, synthesis and characterisation. Arab. J. Chem. 2012, 5, 1–23. [Google Scholar] [CrossRef]
- Prasad, R.D.; Charmode, N.; Shrivastav, O.P.; Prasad, S.R.; Moghe, A.; Samant, A.; Sarvalkar, P.D.; Prasad, N.R.; Iari, P.F.S.S. A Review on Concept of Nanotechnology in Veterinary Medicine. ES Food Agrofor. 2021, 4, 28–60. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Novoselova, I.A.; Kuleshov, S.; Volkov, S.V.; Bykov, V. Electrochemical synthesis, morphological and structural characteristics of carbon nanomaterials produced in molten salts. Electrochim. Acta 2016, 211, 343–355. [Google Scholar] [CrossRef]
- Manikandan, N.; Suresh Kumar, V.P.; Siva Murugan, S.; Rathis, G.; Vishnu Saran, K.; Shabariganesh, T.K. Carbon nanotubes and their properties-The review. Mater. Today Proc. 2021, 47, 4682–4685. [Google Scholar]
- Maksoud, M.I.A.A.; Fahim, R.A.; Shalan, A.E.; Elkodous, M.A.; Olojede, S.O.; Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Awed, A.S.; Ashour, A.H.; et al. Advanced materials and technologies for supercapacitors used in energy conversion and storage: A review. Environ. Chem. Lett. 2020, 19, 375–439. [Google Scholar] [CrossRef]
- Oraon, R.; De Adhikari, A.; Tiwari, S.K.; Nayak, G.C. Enhanced Specific Capacitance of Self-Assembled Three-Dimensional Carbon Nanotube/Layered Silicate/Polyaniline Hybrid Sandwiched Nanocomposite for Supercapacitor Applications. ACS Sustain. Chem. Eng. 2016, 4, 1392–1403. [Google Scholar] [CrossRef]
- Cheng, F.; Yang, X.; Zhang, S.; Lu, W. Boosting the supercapacitor performances of activated carbon with carbon nanomaterials. J. Power Sources 2020, 450, 227678. [Google Scholar] [CrossRef]
- Mastragostino, M.; Arbizzani, C.; Soavi, F. Conducting polymers as electrode materials in supercapacitors. Solid State Ion. 2002, 148, 493–498. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Di Bartolomeo, A. Fatih Sen Enhanced electrochemical performance of MnNi2O4/rGO nanocomposite as pseudocapacitor electrode material and methanol electro-oxidation catalyst. Nanotechnology 2021, 32, 325707. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, H.; Fu, Q. Recent progress on PEDOT:PSS based polymer blends and composites for flexible electronics and thermoelectric devices. Mater. Chem. Front. 2020, 4, 3130–3152. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, A.; Pan, Y.; Yu, C.; Zou, Y.; Zhou, Y.; Chen, Q.; Wu, S. Facile synthesis of a Co3O4@carbon nanotubes/polyindole composite and its application in all-solid-state flexible supercapacitors. J. Mater. Chem. A 2015, 3, 13011–13015. [Google Scholar] [CrossRef]
- Abdah, M.A.A.M.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Des. 2019, 186, 108199. [Google Scholar] [CrossRef]
- Pan, D.; Dong, J.; Yang, G.; Su, F.; Chang, B.; Liu, C.; Zhu, Y.-C.; Guo, Z. Ice template method assists in obtaining carbonized cellulose/boron nitride aerogel with 3D spatial network structure to enhance the thermal conductivity and flame retardancy of epoxy-based composites. Adv. Compos. Hybrid Mater. 2021, 5, 58–70. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Nie, P.; Xu, G.; Shi, M.; Wang, J.; Wu, Y.; Fu, R.; Dou, H.; Zhang, X. Progress of nanostructured electrode materials for supercapacitors. Adv. Sustain. Syst. 2018, 2, 1700110. [Google Scholar] [CrossRef]
- Rangom, Y.; Tang, X.; Nazar, L.F. Carbon Nanotube-Based Supercapacitors with Excellent ac Line Filtering and Rate Capability via Improved Interfacial Impedance. ACS Nano 2015, 9, 7248–7255. [Google Scholar] [CrossRef]
- Zhou, S. Solvent granularity in the differential electrical capacitance of supercapacitor and mechanism analysis. Phys. A Stat. Mech. Its Appl. 2019, 533, 121905. [Google Scholar] [CrossRef]
- Hao, L.; Li, X.; Zhi, L. Carbonaceous Electrode Materials for Supercapacitors. Adv. Mater. 2013, 25, 3899–3904. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Cheng, Y.; Hobson, T.; Liu, J. Design and Synthesis of Hierarchical MnO2 Nanospheres/Carbon Nanotubes/Conducting Polymer Ternary Composite for High Performance Electrochemical Electrodes. Nano Lett. 2010, 10, 2727–2733. [Google Scholar] [CrossRef]
- Su, H.; Wang, T.; Zhang, S.; Song, J.; Mao, C.; Niu, H.; Jin, B.; Wu, J.; Tian, Y. Facile synthesis of polyaniline/TiO2/graphene oxide composite for high performance supercapacitors. Solid State Sci. 2012, 14, 677–681. [Google Scholar] [CrossRef]
- Renault, S.; Gottis, S.; Barrès, A.-L.; Courty, M.; Chauvet, O.; Dolhem, F.; Poizot, P. A green Li–organic battery working as a fuel cell in case of emergency. Energy Environ. Sci. 2013, 6, 2124–2133. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Kalam, A.A.; Park, S.; Seo, Y.; Bae, J. High-Efficiency Supercapacitor Electrodes of CVD-grown Graphenes Hybridized with Multiwalled Carbon Nanotubes. Bull. Korean Chem. Soc. 2015, 36, 2111–2115. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Mohanty, A.; Balasingam, S.K.; Kim, S.J.; Ramadoss, A. Comprehensive Insight into the Mechanism, Material Selection and Performance Evaluation of Supercapatteries. Nano-Micro Lett. 2020, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M. Possibility of utilizing agriculture biomass as a renewable and sustainable future energy source. Heliyon 2022, 8, e08905. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Horie, Y.; Ozaki, S.; Matsuzawa, Y.; Suezaki, H.; Kim, C.; Miyashita, N.; Endo, M. Correlation between the pore and solvated ion size on capacitance uptake of PVDC-based carbons. Carbon 2004, 42, 1491–1500. [Google Scholar] [CrossRef]
- Gamby, J.; Taberna, P.; Simon, P.; Fauvarque, J.; Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J. Power Sources 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Liu, N.; Shen, J.; Liu, D. Activated high specific surface area carbon aerogels for EDLCs. Microporous Mesoporous Mater. 2013, 167, 176–181. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Dash, R.; Gogotsi, Y. Effect of pore size and surface area of carbide derived carbons on specific capacitance. J. Power Sources 2006, 158, 765–772. [Google Scholar] [CrossRef]
- Zhi, M.; Yang, F.; Meng, F.; Li, M.; Manivannan, A.; Wu, N. Effects of Pore Structure on Performance of An Activated-Carbon Supercapacitor Electrode Recycled from Scrap Waste Tires. ACS Sustain. Chem. Eng. 2014, 2, 1592–1598. [Google Scholar] [CrossRef]
- Pang, M.; Long, G.; Jiang, S.; Ji, Y.; Han, W.; Wang, B.; Liu, X.; Xi, Y. One pot low-temperature growth of hierarchical δ-MnO2 nanosheets on nickel foam for supercapacitor applications. Electrochim. Acta 2015, 161, 297–304. [Google Scholar] [CrossRef]
- Wen, J.; Chen, X.; Huang, M.; Yang, W.; Deng, J. Core–shell-structured MnO2@carbon spheres and nitrogen-doped activated carbon for asymmetric supercapacitors with enhanced energy density. J. Chem. Sci. 2019, 132, 6. [Google Scholar] [CrossRef]
- Mousavi, M.P.S.; Wilson, B.E.; Kashefolgheta, S.; Anderson, E.L.; He, S.; Bühlmann, P.; Stein, A. Ionic Liquids as Electrolytes for Electrochemical Double-Layer Capacitors: Structures that Optimize Specific Energy. ACS Appl. Mater. Interfaces 2016, 8, 3396–3406. [Google Scholar] [CrossRef]
- Araby, S.; Qiu, A.; Wang, R.; Zhao, Z.; Wang, C.-H.; Ma, J. Aerogels based on carbon nanomaterials. J. Mater. Sci. 2016, 51, 9157–9189. [Google Scholar] [CrossRef]
- Raghavendra, K.V.G.; Vinoth, R.; Zeb, K.; Gopi, C.V.M.; Sambasivam, S.; Kummara, M.R.; Obaidat, I.M.; Kim, H.J. An intuitive review of supercapacitors with recent progress and novel device applications. J. Energy Storage 2020, 31, 101652. [Google Scholar] [CrossRef]
- Chien, H.-C.; Cheng, W.-Y.; Wang, Y.-H.; Lu, S.-Y. Ultrahigh Specific Capacitances for Supercapacitors Achieved by Nickel Cobaltite/Carbon Aerogel Composites. Adv. Funct. Mater. 2012, 22, 5038–5043. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Wu, Z.; Li, L.; Lin, Z.; Song, B.; Li, Z.; Moon, K.-S.; Wong, C.-P.; Bai, S.-L. Alternating current line-filter based on electrochemical capacitor utilizing template-patterned graphene. Sci. Rep. 2015, 5, 10983. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.; Li, Y.; Wang, H.; Mousavi, M.F.; Kaner, R.B. Graphene-based materials for flexible supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.I.; Narita, A.; Chen, Q.; Mics, Z.; Turchinovich, D.; Kläui, M.; Bonn, M.; Müllen, K. Photoswitchable Micro-Supercapacitor Based on a Diarylethene-Graphene Composite Film. J. Am. Chem. Soc. 2017, 139, 9443–9446. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, E.; Li, X.; Yu, Y.; Qu, J.; Yu, Z.-Z. Direct Reduction of Graphene Oxide by Ni Foam as a High-Capacitance Supercapacitor Electrode. ACS Appl. Mater. Interfaces 2016, 8, 2297–2305. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Lu, L.; Leung, S.-F.; Chen, D.; Chen, X.; Fan, Z.; Shen, G.; Li, D. Integrated Photo-supercapacitor Based on Bi-polar TiO2Nanotube Arrays with Selective One-Side Plasma-Assisted Hydrogenation. Adv. Funct. Mater. 2013, 24, 1840–1846. [Google Scholar] [CrossRef]
- Baro, M.; Jaidev; Ramaprabhu, S. Conductive and nitrogen-enriched porous carbon nanostructure derived from poly (para-phenylenediamine) for energy conversion and storage applications. Appl. Surf. Sci. 2020, 503, 144069. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Zheng, J.P.; Cygan, P.J.; Jow, T.R. Hydrous Ruthenium Oxide as an Electrode Material for Electrochemical Capacitors. J. Electrochem. Soc. 1995, 142, 2699–2703. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Chen, W.; Hedhili, M.N.; Cha, D.; Alshareef, H.N. Enhanced rate performance of mesoporous Co3O4 nanosheet supercapacitor electrodes by hydrous RuO2 nanoparticle decoration. ACS Appl. Mater. Interfaces 2014, 6, 4196–4206. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, D.; Dabo, P.; Guay, D.; Sherwood, P. XPS investigations of thermally prepared RuO2 electrodes in reductive conditions. Electrochim. Acta 2003, 48, 4245–4252. [Google Scholar] [CrossRef]
- Galizzioli, D.; Tantardini, F.; Trasatti, S. Ruthenium dioxide: A new electrode material. I. Behaviour in acid solutions of inert electrolytes. J. Appl. Electrochem. 1974, 4, 57–67. [Google Scholar] [CrossRef]
- Liu, T.; Pell, W.; Conway, B. Self-discharge and potential recovery phenomena at thermally and electrochemically prepared RuO2 supercapacitor electrodes. Electrochim. Acta 1997, 42, 3541–3552. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, X.G. NiO-based composite electrode with RuO2 for electrochemical capacitors. Electrochim. Acta 2004, 49, 229–232. [Google Scholar] [CrossRef]
- Li, X.; Gan, W.; Zheng, F.; Li, L.; Zhu, N.; Huang, X. Preparation and electrochemical properties of RuO2/polyaniline electrodes for supercapacitors. Synth. Met. 2012, 162, 953–957. [Google Scholar] [CrossRef]
- Pusawale, S.; Deshmukh, P.; Gunjakar, J.; Lokhande, C. SnO2–RuO2 composite films by chemical deposition for supercapacitor application. Mater. Chem. Phys. 2013, 139, 416–422. [Google Scholar] [CrossRef]
- Yong-gang, W.; Xiao-gang, Z. Preparation and electrochemical capacitance of RuO2/TiO2 nanotubes composites. Electrochim. Acta 2004, 49, 1957–1962. [Google Scholar] [CrossRef]
- Liu, X.; Pickup, P.G. Ru oxide supercapacitors with high loadings and high power and energy densities. J. Power Sources 2008, 176, 410–416. [Google Scholar] [CrossRef]
- Sun, S.; Wang, P.; Wu, Q.; Wang, S.; Fang, S. Template-free synthesis of mesoporous MnO2 under ultrasound irradiation for supercapacitor electrode. Mater. Lett. 2014, 137, 206–209. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Li, Z.-S.; Huang, Y.-G.; Li, Q.-Y.; Wang, X.-Y. A novel hybrid supercapacitor based on spherical activated carbon and spherical MnO2 in a non-aqueous electrolyte. J. Mater. Chem. 2010, 20, 3883–3889. [Google Scholar] [CrossRef]
- Song, Z.; Liu, W.; Zhao, M.; Zhang, Y.; Liu, G.; Yu, C.; Qiu, J. A facile template-free synthesis of α-MnO2 nanorods for supercapacitor. J. Alloys Compd. 2013, 560, 151–155. [Google Scholar] [CrossRef]
- Nayak, P.K.; Munichandraiah, N. Mesoporous MnO2 synthesized by using a tri-block copolymer for electrochemical supercapacitor studies. Microporous Mesoporous Mater. 2011, 143, 206–214. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, X.; Li, Y.-G.; Tian, B.-Z.; Zhao, D.-Y.; Jiang, Z.-Y. Synthesis and Electrochemical Properties of Semicrystalline Gyroidal Mesoporous MnO2. Chin. J. Chem. 2006, 24, 835–839. [Google Scholar] [CrossRef]
- Devaraj, S.; Gabriel, G.S.; Gajjela, S.R.; Balaya, P. Mesoporous MnO2 and Its Capacitive Behavior. Electrochem. Solid-State Lett. 2012, 15, A57–A59. [Google Scholar] [CrossRef]
- Nayak, P.K.; Munichandraiah, N. Rapid sonochemical synthesis of mesoporous MnO2 for supercapacitor applications. Mater. Sci. Eng. B 2012, 177, 849–854. [Google Scholar] [CrossRef]
- Ming, B.; Li, J.; Kang, F.; Pang, G.; Zhang, Y.; Chen, L.; Xu, J.; Wang, X. Microwave–hydrothermal synthesis of birnessite-type MnO2 nanospheres as supercapacitor electrode materials. J. Power Sources 2012, 198, 428–431. [Google Scholar] [CrossRef]
- Kim, B.K.; Chabot, V.; Yu, A. Carbon nanomaterials supported Ni(OH)2/NiO hybrid flower structure for supercapacitor. Electrochim. Acta 2013, 109, 370–380. [Google Scholar] [CrossRef]
- Wu, M.; Gao, J.; Zhang, S.; Chen, A. Synthesis and characterization of aerogel-like mesoporous nickel oxide for electrochemical supercapacitors. J. Porous Mater. 2006, 13, 407–412. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, X.; Su, L.; Gao, B.; Shen, L. Facile synthesis and self-assembly of hierarchical porous NiO nano/micro spherical superstructures for high performance supercapacitors. J. Mater. Chem. 2009, 19, 5772–5777. [Google Scholar] [CrossRef]
- Li, X.; Xiong, S.; Li, J.; Bai, J.; Qian, Y. Mesoporous NiO ultrathin nanowire networks topotactically transformed from α-Ni(OH)2 hierarchical microspheres and their superior electrochemical capacitance properties and excellent capability for water treatment. J. Mater. Chem. 2012, 22, 14276–14283. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.X.; Li, H.H.; Su, L.; Wei, J.P.; Zhou, Z. Mesoporous slit-structured NiO for high-performance pseudocapacitors. Phys. Chem. Chem. Phys. 2012, 14, 11048–11052. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, B.; Guan, D.; Pei, K.; Chen, Z.; Yang, M.; Dong, X. Preparation of nanospherical porous NiO by a hard template route and its supercapacitor application. Mater. Lett. 2014, 135, 172–175. [Google Scholar] [CrossRef]
- Meher, S.K.; Justin, P.; Rao, G.R. Microwave-Mediated Synthesis for Improved Morphology and Pseudocapacitance Performance of Nickel Oxide. ACS Appl. Mater. Interfaces 2011, 3, 2063–2073. [Google Scholar] [CrossRef]
- Han, D.; Jing, X.; Wang, J.; Yang, P.; Song, D.; Liu, J. Porous lanthanum doped NiO microspheres for supercapacitor application. J. Electroanal. Chem. 2012, 682, 37–44. [Google Scholar] [CrossRef]
- Lu, Q.; Lattanzi, M.W.; Chen, Y.; Kou, X.; Li, W.; Fan, X.; Unruh, K.M.; Chen, J.G.; Xiao, J.Q. Titelbild: Supercapacitor Electrodes with High-Energy and Power Densities Prepared from Monolithic NiO/Ni Nanocomposites (Angew. Chem. 30/2011). Angew. Chem. 2011, 123, 6803. [Google Scholar] [CrossRef]
- Yuan, C.; Yang, L.; Hou, L.; Shen, L.; Zhang, F.; Li, D.; Zhang, X. Large-scale Co3O4 nanoparticles growing on nickel sheets via a one-step strategy and their ultra-highly reversible redox reaction toward supercapacitors. J. Mater. Chem. 2011, 21, 18183–18185. [Google Scholar] [CrossRef]
- Lee, K.K.; Chin, W.S.; Sow, C.H. Cobalt-based compounds and composites as electrode materials for high-performance electrochemical capacitors. J. Mater. Chem. A 2014, 2, 17212–17248. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, A.; Liu, S.; Zhao, J.; Fang, S.; Jia, D.; Li, F. Free-standing and porous hierarchical nanoarchitectures constructed with cobalt cobaltite nanowalls for supercapacitors with high specific capacitances. J. Power Sources 2012, 219, 140–146. [Google Scholar] [CrossRef]
- Zhong, J.-H.; Wang, A.-L.; Li, G.-R.; Wang, J.-W.; Ou, Y.-N.; Tong, Y.-X. Co3O4/Ni(OH)2 composite mesoporous nanosheet networks as a promising electrode for supercapacitor applications. J. Mater. Chem. 2012, 22, 5656–5665. [Google Scholar] [CrossRef]
- Cao, L.; Lü, M.; Li, H.-L. Preparation of Mesoporous Nanocrystalline Co3O4 and Its Applicability of Porosity to the Formation of Electrochemical Capacitance. J. Electrochem. Soc. 2005, 152, A871–A875. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Wang, X.; Yang, X.; Lu, L. Preparation and electrochemical properties of mesoporous Co3O4 crater-like microspheres as supercapacitor electrode materials. Curr. Appl. Phys. 2010, 10, 1422–1426. [Google Scholar] [CrossRef]
- Wang, X.; Sumboja, A.; Khoo, E.; Yan, C.; Lee, P.S. Cryogel synthesis of hierarchical interconnected macro-/mesoporous Co3O4 with superb electrochemical energy storage. J. Phys. Chem. C 2012, 116, 4930–4935. [Google Scholar] [CrossRef]
- Liu, X.; Long, Q.; Jiang, C.; Zhan, B.; Li, C.; Liu, S.; Zhao, Q.; Huang, W.; Dong, X. Facile and green synthesis of mesoporous Co3O4 nanocubes and their applications for supercapacitors. Nanoscale 2013, 5, 6525–6529. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Yang, L.; Hou, L.; Shen, L.; Zhang, X.; Lou, X.W. Growth of ultrathin mesoporous Co3O4 nanosheet arrays on Ni foam for high-performance electrochemical capacitors. Energy Environ. Sci. 2012, 5, 7883–7887. [Google Scholar] [CrossRef]
- Zhi, J.; Deng, S.; Zhang, Y.; Wang, Y.; Hu, A. Embedding Co3O4 nanoparticles in SBA-15 supported carbon nanomembrane for advanced supercapacitor materials. J. Mater. Chem. A 2013, 1, 3171–3176. [Google Scholar] [CrossRef]
- Padmanathan, N.; Selladurai, S.; Razeeb, K.M. Ultra-fast rate capability of a symmetric supercapacitor with a hierarchical Co3O4 nanowire/nanoflower hybrid structure in non-aqueous electrolyte. RSC Adv. 2015, 5, 12700–12709. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Liu, B.; Yu, G.; Hou, X.; Chen, D.; Shen, G. NiCO2O4 nanowire arrays supported on Ni foam for high-performance flexible all-solid-state supercapacitors. J. Mater. Chem. A 2012, 1, 2468–2473. [Google Scholar] [CrossRef]
- Seetharamappa, J.; Yellappa, S.; D’souza, F. The Chalkboard—Carbon Nanotubes: Next Generation of Electronic Materials. Electrochem. Soc. Interface 2006, 15, 23–61. [Google Scholar] [CrossRef]
- Bredas, J.L.; Street, G.B. Polarons, bipolarons, and solitons in conducting polymers. Accounts Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- De Oliveira, M.A.; Dos Santos, H.F.; De Almeida, W.B. Structure and torsional potential of p-phenylthiophene: A theoretical comparative study. Phys. Chem. Chem. Phys. 2000, 2, 3373–3380. [Google Scholar] [CrossRef]
- Hu, Z.-R.; Li, D.-D.; Kim, T.-H.; Kim, M.-S.; Xu, T.; Ma, M.-G.; Choi, S.-E.; Si, C. Lignin-Based/Polypyrrole Carbon Nanofiber Electrode With Enhanced Electrochemical Properties by Electrospun Method. Front. Chem. 2022, 10, 49. [Google Scholar] [CrossRef]
- Senthilkumar, B.; Thenamirtham, P.; Selvan, R.K. Structural and electrochemical properties of polythiophene. Appl. Surf. Sci. 2011, 257, 9063–9067. [Google Scholar] [CrossRef]
- Alesary, H.F.; Ismail, H.; Khudhair, A.F.; Mohammed, M. Effects of Dopant Ions on the Properties of Polyaniline Conducting Polymer. Orient. J. Chem. 2018, 34, 2525–2533. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, X.; Kong, J. Polyaniline nanofibers prepared with hydrogen peroxide as oxidant. Synth. Met. 2007, 157, 269–275. [Google Scholar] [CrossRef]
- Keskinen, J.; Tuurala, S.; Sjödin, M.; Kiri, K.; Nyholm, L.; Flyktman, T.; Strømme, M.; Smolander, M. Asymmetric and symmetric supercapacitors based on polypyrrole and activated carbon electrodes. Synth. Met. 2015, 203, 192–199. [Google Scholar] [CrossRef]
- Lermusiaux, L.; Mazel, A.; Carretero-Genevrier, A.; Sanchez, C.; Drisko, G.L. Metal-Induced Crystallization in Metal Oxides. Accounts Chem. Res. 2022, 55, 171–185. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, B.; Sun, Y.; Yang, H.; Zhang, X. Structure, morphology and electrochemical properties of LaxSr1−xCo0.1Mn0.9O3-δ perovskite nanofibers prepared by electrospinning method. J Alloys Compd. 2015, 624, 31–39. [Google Scholar] [CrossRef]
- Lang, X.; Mo, H.; Hu, X.; Tian, H. Supercapacitor performance of perovskite La1−xSrxMnO3. Dalton Trans. 2017, 46, 13720–13730. [Google Scholar] [CrossRef] [PubMed]
- Wilde, P.; Guther, T.; Oesten, R.; Garche, J. Strontium ruthenate perovskite as the active material for supercapacitors. J. Electroanal. Chem. 1999, 461, 154–160. [Google Scholar] [CrossRef]
- Wohlfahrt-Mehrens, M.; Schenk, J.; Wilde, P.; Abdelmula, E.; Axmann, P.; Garche, J. New materials for supercapacitors. J. Power Sources 2002, 105, 182–188. [Google Scholar] [CrossRef]
- Balamurugan, C.; Arunkumar, S.; Lee, D.-W. Hierarchical 3D nanostructure of GdInO3 and reduced-graphene-decorated GdInO3 nanocomposite for CO sensing applications. Sens. Actuators B Chem. 2016, 234, 155–166. [Google Scholar] [CrossRef]
- Rai, A.; Sharma, A.; Thakur, A.K. Evaluation of aluminium doped lanthanum ferrite based electrodes for supercapacitor design. Solid State Ionics 2014, 262, 230–233. [Google Scholar] [CrossRef]
- Ji, J.-H.; Lee, J.-W.; Chung, H.; Koh, J.-H. (Na, K) NbO3–CaCu3Ti4O12 perovskite composites for supercapacitor based piezoelectric devices. Ceram. Int. 2016, 42, 4978–4983. [Google Scholar] [CrossRef]
- Hwang, D.K.; Kim, S.; Lee, J.-H.; Hwang, I.-S.; Kim, I.-D. Phase evolution of perovskite LaNiO3 nanofibers for supercapacitor application and p-type gas sensing properties of LaOCl--NiO composite nanofibers. J. Mater. Chem. 2011, 21, 1959–1965. [Google Scholar] [CrossRef]
- Arjun, N.; Pan, G.-T.; Yang, T.C. The exploration of Lanthanum based perovskites and their complementary electrolytes for the supercapacitor applications. Results Phys. 2017, 7, 920–926. [Google Scholar] [CrossRef]
- Lokhande, C.; Gujar, T.; Shinde, V.; Mane, R.S.; Han, S.-H. Electrochemical supercapacitor application of pervoskite thin films. Electrochem. Commun. 2007, 9, 1805–1809. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, B.; Sun, Y.; Yang, H.; Zhang, X. Sr-doped Lanthanum Nickelate Nanofibers for High Energy Density Supercapacitors. Electrochimica Acta 2015, 174, 41–50. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Su, C.; Zhou, W.; Liu, M.; Shao, Z. Perovskite SrCo0.9Nb0.1O3-δ as an anion-intercalated electrode material for supercapacitors with ultrahigh volumetric energy density. Angew. Chem. 2016, 128, 9728–9731. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, B.; Sun, Y.; Yang, H.; Zhang, X. Symmetric/Asymmetric Supercapacitor Based on the Perovskite-type Lanthanum Cobaltate Nanofibers with Sr-substitution. Electrochim. Acta 2015, 178, 398–406. [Google Scholar] [CrossRef]
- Gajewski, P.; Béguin, F. Hydrogel–Polymer Electrolyte for Electrochemical Capacitors with High Volumetric Energy and Life Span. Chemsuschem 2020, 13, 1876–1881. [Google Scholar] [CrossRef]
- Gao, H.; Lian, K. Proton-conducting polymer electrolytes and their applications in solid supercapacitors: A review. RSC Adv. 2014, 4, 33091–33113. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C. A review of polymer electrolytes: Fundamental, approaches and applications. Ionics 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Masarapu, C.; Wang, L.-P.; Li, X.; Wei, B. Tailoring Electrode/Electrolyte Interfacial Properties in Flexible Supercapacitors by Applying Pressure. Adv. Energy Mater. 2012, 2, 546–552. [Google Scholar] [CrossRef]





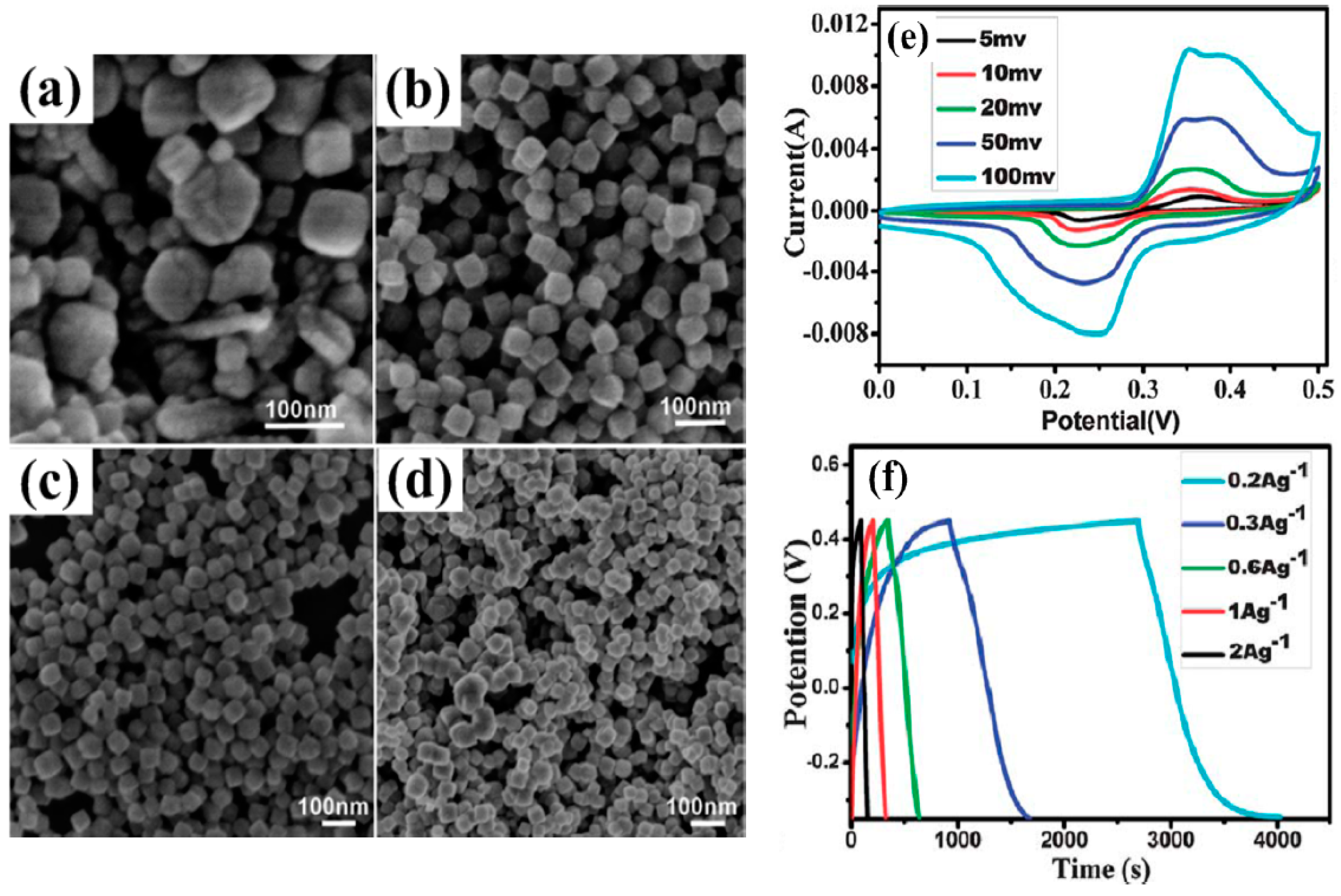
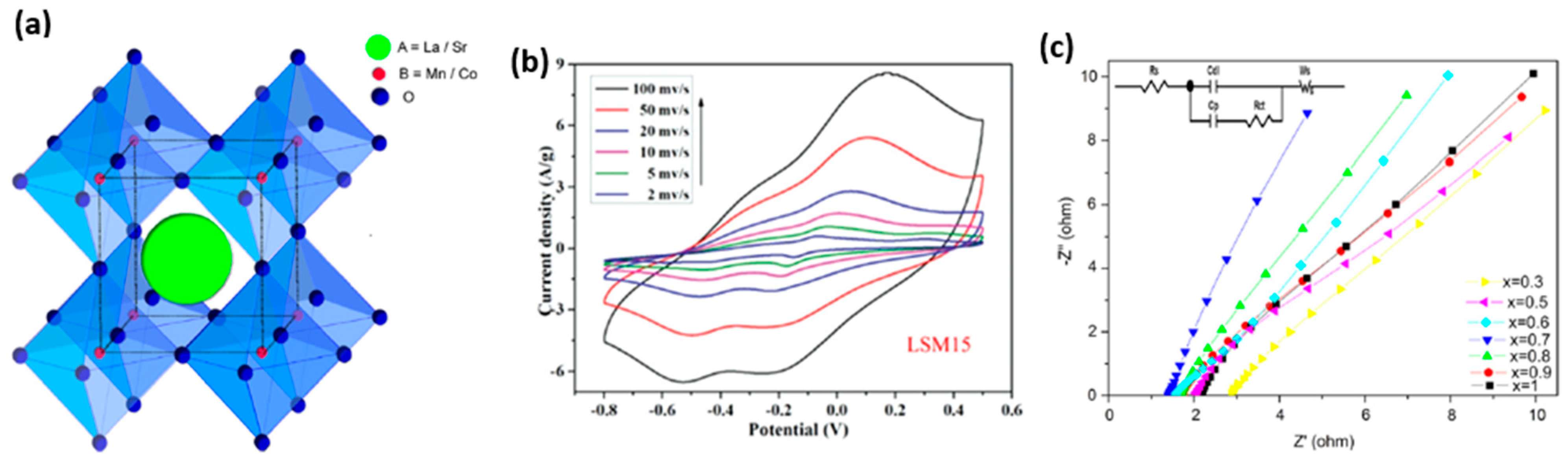

| S/N | Electrode Materials | Specific Capacitance (Fg−1) | Power Density (Wkg−1) | Energy Density (Whkg−1) | References |
|---|---|---|---|---|---|
| 1 | PEDOX-PSS + CNTs | 85–150 | 100–3000 | >0.92 | [125] |
| 2 | PEDOX-PSS + SWCNTs | 104 | 825 | 7 | [129] |
| 3 | RuO2 + SWCNTs | 1715 | - | - | [130] |
| 4 | MnO2 + PANI + Carbon | 695 | - | - | [133] |
| 5 | MnO2 + CNT + PEDOS-PSS | 200 | - | - | [134] |
| 6 | PANI + TiO2 + graphene oxide | 1020 | - | - | [135] |
| 7 | Polymers + MWCNTs | 296 | - | - | [136] |
| 8 | PEDOT + MWCNTs | 199 | - | - | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adedoja, O.S.; Sadiku, E.R.; Hamam, Y. An Overview of the Emerging Technologies and Composite Materials for Supercapacitors in Energy Storage Applications. Polymers 2023, 15, 2272. https://doi.org/10.3390/polym15102272
Adedoja OS, Sadiku ER, Hamam Y. An Overview of the Emerging Technologies and Composite Materials for Supercapacitors in Energy Storage Applications. Polymers. 2023; 15(10):2272. https://doi.org/10.3390/polym15102272
Chicago/Turabian StyleAdedoja, Oluwaseye Samson, Emmanuel Rotimi Sadiku, and Yskandar Hamam. 2023. "An Overview of the Emerging Technologies and Composite Materials for Supercapacitors in Energy Storage Applications" Polymers 15, no. 10: 2272. https://doi.org/10.3390/polym15102272
APA StyleAdedoja, O. S., Sadiku, E. R., & Hamam, Y. (2023). An Overview of the Emerging Technologies and Composite Materials for Supercapacitors in Energy Storage Applications. Polymers, 15(10), 2272. https://doi.org/10.3390/polym15102272







