Experimental Study of Thermal and Fire Reaction Properties of Glass Fiber/Bismaleimide Composites for Aeronautic Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization and Measurement
3. Results
3.1. Thermal Decomposition Characteristics
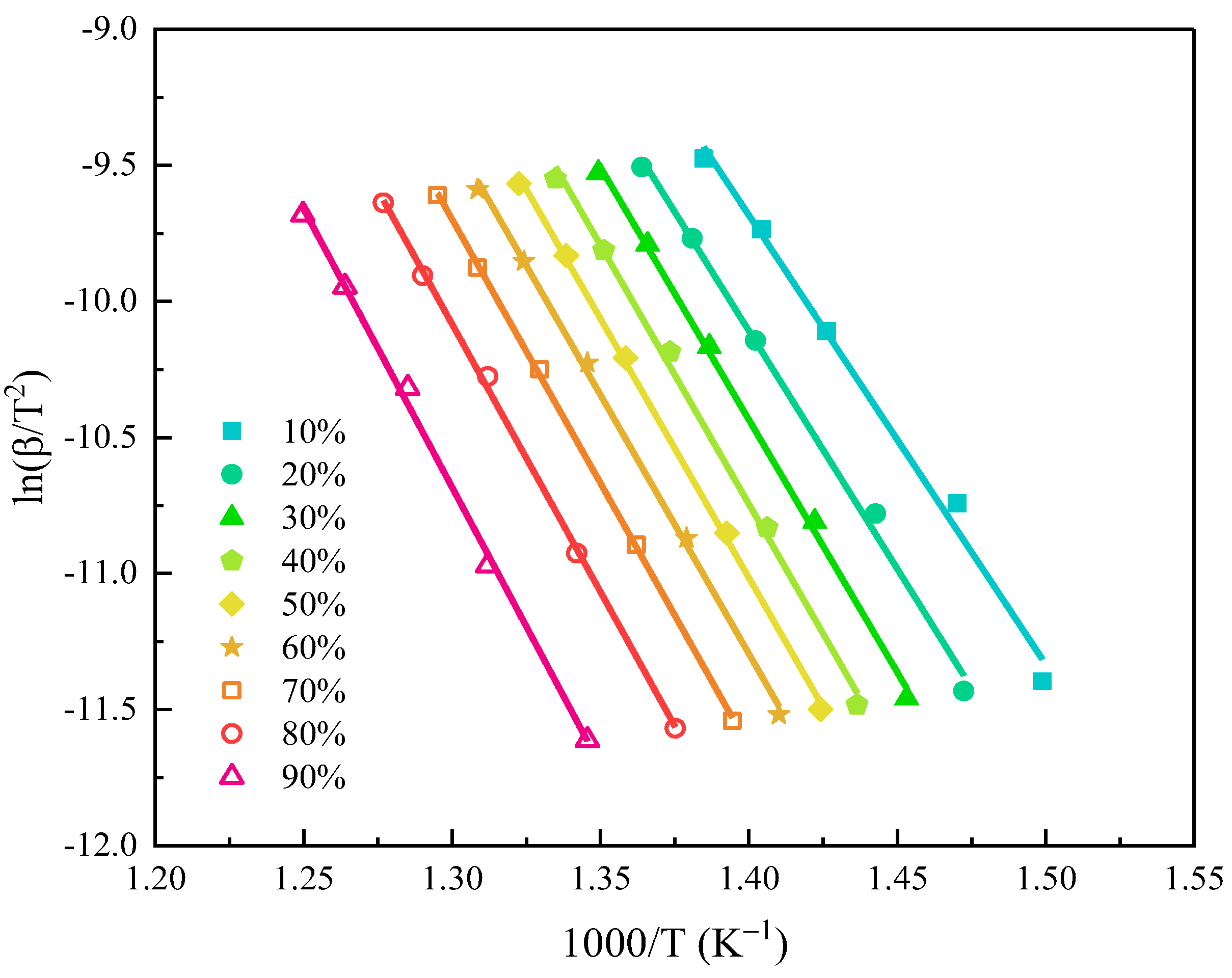
3.2. Pyrolysis Kinetic Analysis
3.3. TG–FTIR Analysis
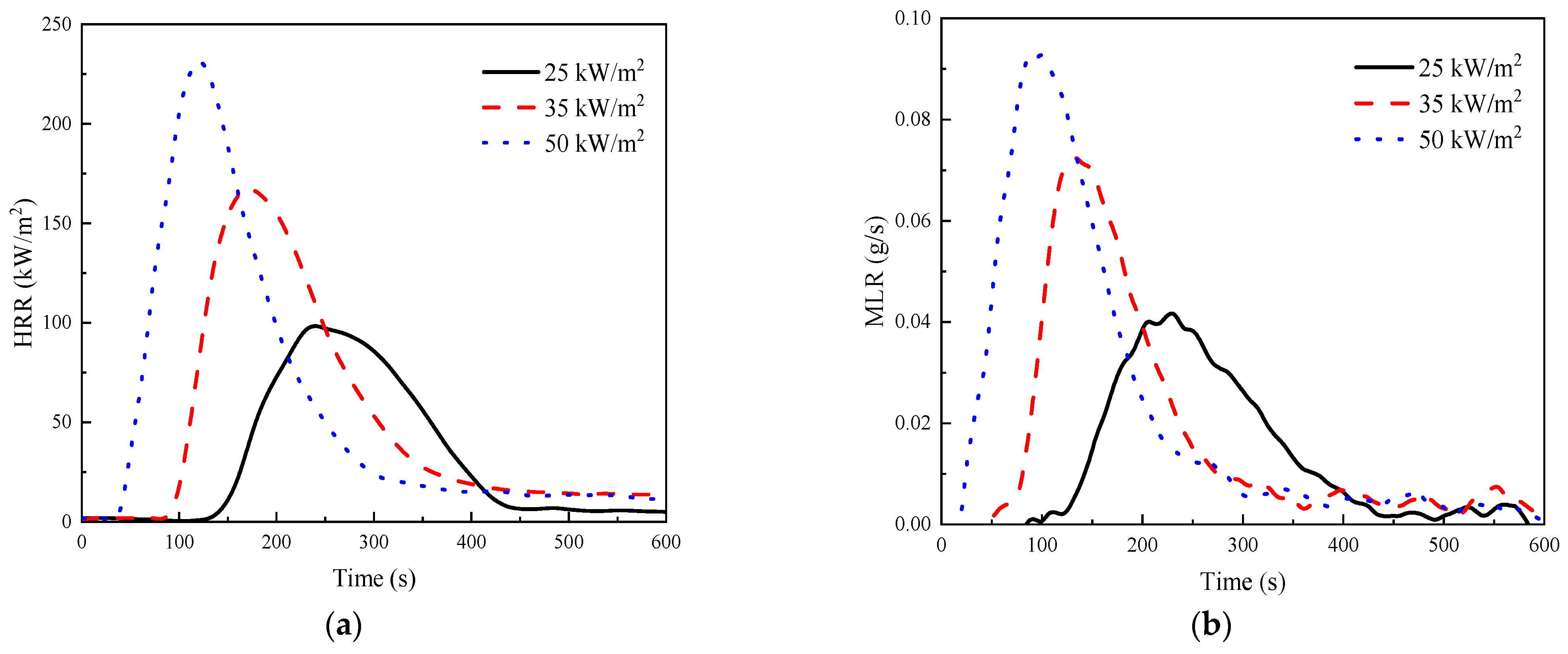
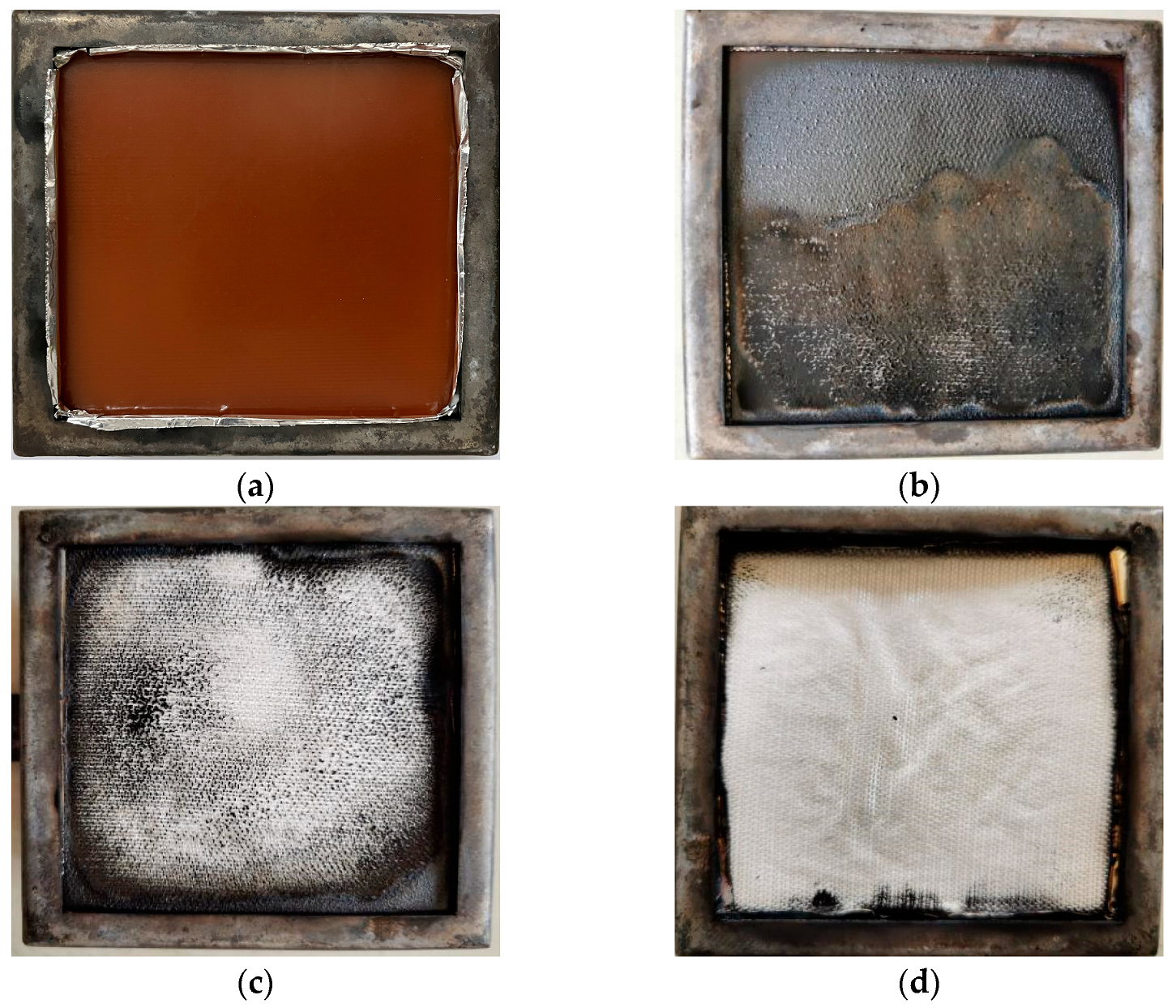
3.4. Cone Calorimeter Tests
3.5. LOI Tests
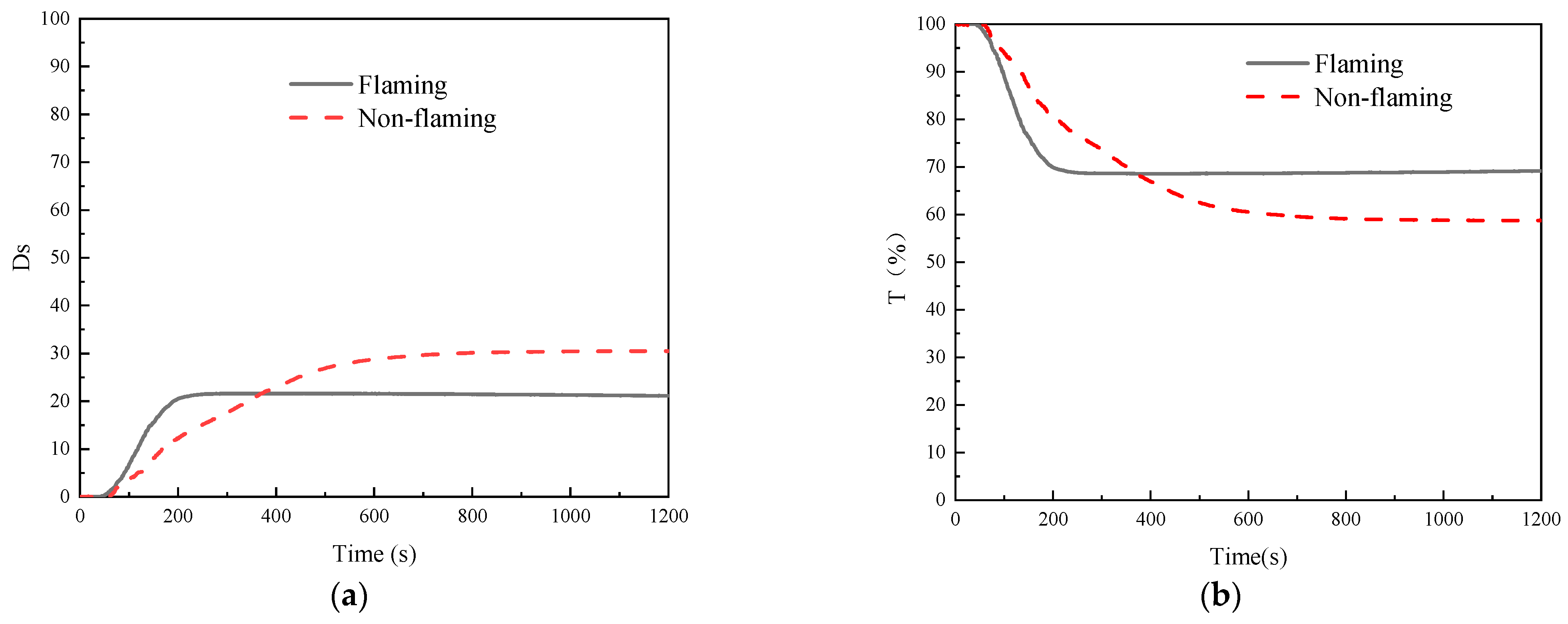
3.6. Smoke Density Tests
4. Conclusions
- There was only one pyrolysis step of GF/BMI composites in the nitrogen atmosphere, and only the BMI matrix underwent pyrolytic reaction with decomposition gas products consisting mainly of CO2, H2O, CH4, NOx, SO2, etc. With the increase of heating rate, the initial decomposition temperature, the final decomposition temperature, and the maximum mass loss rate temperature all moved toward high temperature in different degrees. In addition, the average activation energy calculated by the KAS method was up to 156 kJ/mol. The initial decomposition temperature and activation energy were both higher than those of GF/epoxy composites, indicating that GF/BMI composites had good thermal stability.
- The results of the cone calorimeter test showed that the greater the heat flux, the fuller the combustion, the shorter the time threshold values, and the higher the heat release rate, the total heat release under 25 kW/m2 was only 54% of the value under 50 kW/m2. Although the LOI gradually decreased with the experimental temperature from 20 to 220 °C, the value always remained no less than 39.0%, reaching the B1 fire resistance level. The smoke density test revealed that GF/BMI composites were superior to GF/epoxy composites in terms of smoke production characteristics. The maximum specific optical density in 4 min was greater, whereas the time to maximum specific optical density and time to critical specific optical density were both shorter in flaming mode than those in non-flaming mode, demonstrating that the flaming mode posed a more serious smoke threat in the early stage of the fire than the non-flaming mode. In general, high fire intensity weakened some retardant properties.
- The fire performance index, total heat release index, total smoke production index, and toxic gas production index used for evaluating fire hazard showed an overall more serious state with the increase in heat flux. Similarly, the average accumulation rate and the smoke obscuration index were also higher in the flaming mode. The effects of heat and smoke in a fire scene were more intense with the coupling of open flames and thermal radiation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.B.; Zhang, B.Y.; Xing, L.Y. Application and Development of Advanced Polymer Matrix Composites. Mater. China 2009, 28, 2–12. [Google Scholar]
- Soutis, C. Fibre reinforced composites in aircraft construction. Prog. Aeosp. Sci. 2005, 41, 143–151. [Google Scholar] [CrossRef]
- Dutton, S.; Kelly, D.; Baker, A. Composite Materials for Aircraft Structures, 2nd ed.; America Institute of Aeronautics and Astronautics: Washington, DC, USA, 2004. [Google Scholar]
- Choi, I.; Kim, J.G.; Lee, D.G.; Seo, I.S. Aramid/epoxy composites sandwich structures for low-observable radomes. Compos. Sci. Technol. 2011, 71, 1632–1638. [Google Scholar] [CrossRef]
- Deluca, E.; Prifti, J.; Betheney, W.; Chou, S.C. Ballistic impact damage of S 2-glass-reinforced plastic structural armor. Compos. Sci. Technol. 1998, 58, 1453–1461. [Google Scholar] [CrossRef]
- Goubalt, P.; Mayes, S. Comparative Analysis of Metal and Composite Materials for the Primary Structures of a Patrol Craft. Nav. Eng. J. 1996, 5, 387–397. [Google Scholar] [CrossRef]
- Vallbo, S. Material Selection Considerations for Polymer Composite Structures in Naval Ship Applications. J. Sandw. Struct. Mater. 2005, 7, 413–429. [Google Scholar] [CrossRef]
- Chen, X.X.; Yuan, L.; Zhang, Z.Y.; Wang, H.; Liang, G.Z.; Gu, A.J. New glass fiber/bismaleimide composites with significantly improved flame retardancy, higher mechanical strength and lower dielectric loss. Compos. B. Eng. 2015, 71, 96–102. [Google Scholar] [CrossRef]
- Kurihara, Y. Polymer matrix composite materials in automobile industries. Adv. Compos. Mater. 1995, 4, 209–219. [Google Scholar] [CrossRef]
- Poorzeinolabedin, M.; Golzar, M. Improving the Woven Glass/Epoxy Composite for Automobile Exterior Body Cover. Mater. Manuf. Process. 2011, 26, 562–566. [Google Scholar] [CrossRef]
- Advanced Composites Center of Aviation Industry Corporation of China. Aeronautical Composite Technology; Aviation Industry Press: Beijing, China, 2013. [Google Scholar]
- Shen, Z. Design Handbook of Composite Structure; Aviation Industry Press: Beijing, China, 2004. [Google Scholar]
- Xu, Y.Y.; Sun, X.D.; Shen, R.Q.; Wang, Z.; Wang, Q.S. Thermal behavior and smoke characteristics of glass/epoxy laminate and its foam core sandwich composite. J. Therm. Anal. Calorim. 2020, 141, 1173–1182. [Google Scholar] [CrossRef]
- Häublein, M.; Demleitner, M.; Altstädt, V. Fire behavior and flame-retardant properties of application-oriented fiber-reinforced polymers (FRPs). In Composite Materials Manufacturing, Properties and Applications; Low, I., Dong, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 15, pp. 383–417. [Google Scholar]
- Liang, G.Z.; Gu, A.J.; Lan, L.W.; Zhang, M.X.; Lv, Y. Modified Bismaleimide Resins for High Performance Radomes. Aerosp. Mater. Technol. 1996, 6, 14–17. [Google Scholar]
- Zhao, Q. Handbook of Advanced Composite Materials; China Machine Press: Beijing, China, 2003. [Google Scholar]
- Zhao, Y.; Liu, W.; Seah, L.K.; Chai, G.B. Delamination growth behavior of a woven E-glass/bismaleimide composite in seawater environment. Compos. B. Eng. 2016, 106, 332–343. [Google Scholar] [CrossRef]
- Zhao, Y.; Seah, L.K.; Chai, G.B. Effects of seawater exposure on mode II fatigue delamination growth of a woven E-glass/bismaleimide composite. J. Reinf. Plast. Compos. 2016, 35, 138–150. [Google Scholar]
- Varma, I.K.; Sharma, S. Glass Fabric Reinforced Bismaleimide Composites: Effect of Elastomer/ Reactive Diluent on Properties. J. Compos Mater. 1986, 20, 308–320. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.Y.; Zhao, H.W.; Zhou, B.Q.; Ren, R. Effect of organo-montmorillonite on mechanical properties of bismaleimide composites reinforced by glass fibre and carbon fibre. Plast. Rubber Compos. 2015, 44, 142–147. [Google Scholar] [CrossRef]
- Zeng, X.L.; Yu, S.H.; Lai, M.B.; Sun, R.; Wong, C.P. Tuning the mechanical properties of glass fiber-reinforced bismaleimide–triazine resin composites by constructing a flexible bridge at the interface. Sci. Technol. Adv. Mater. 2013, 14, 65001. [Google Scholar] [CrossRef]
- Ambika Devi, K.; Reghunadhan Nair, C.P.; Viswanathan Ashari, G.; Ninan, K.N. Properties of Glass Laminates of Novolac Epoxy—Diallyl Bisphenol A—Bismaleimide Resins. Polym. Polym. Compos. 2008, 16, 199–208. [Google Scholar]
- Zhang, Z.Y.; Li, Y.; Liang, G.Z.; Gu, A.J. Fabrication and origin of flame retarding glass fiber/bismaleimide resin composites with high thermal stability, good mechanical properties, and a low dielectric constant and loss for high frequency copper clad laminates. RSC Adv. 2016, 6, 19638–19646. [Google Scholar] [CrossRef]
- Liang, G.Z.; Zhao, L.; Meng, J.R. Integrated Properties Improvement of Glass/Epoxy FR-4 Copper Clad Laminates via the Modification by Bismaleimide. Polym.-Plast. Technol. Eng. 2005, 43, 1553–1567. [Google Scholar] [CrossRef]
- Chen, S.M.; Jiang, H.M.; Liu, Z.J. Determination of thermal decomposition kinetic parameters of glass-fiber/epoxy composite. High Power Laser Part. Beams 2010, 22, 1969–1972. [Google Scholar] [CrossRef]
- Drukker, E.; Green, A.K.; Marom, G. Mechanical and chemical consequences of through thickness thermal gradients in polyimide matrix composite materials. Compos. Pt. A-Appl. Sci. Manuf. 2003, 34, 125–133. [Google Scholar] [CrossRef]
- Razgon, A.; Sukenik, C.N. Ceramic Coatings for Fiber Matrix Composites: Titania Thin Films on Bismaleimide-glass Fiber Composites. J. Mater. Res. 2005, 20, 2544–2552. [Google Scholar] [CrossRef]
- Kourtides, D.A.; Gilwee, W.J., Jr.; Parker, J.A. Thermochemical characterization of some thermally stable thermoplastic and thermoset polymers. Polym. Eng. Sci. 1979, 19, 24–29. [Google Scholar] [CrossRef]
- Kourtides, D.A.; Gilwee, W.J., Jr.; Parker, J.A. Thermal response of composite panels. Eng. Sci. 1979, 19, 226–231. [Google Scholar] [CrossRef]
- ASTM E662-17a; Standard Test Method for Specific Optical Density of Smoke Generated by Solid Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- Quintiere, J. Fundamentals of Fire Phenomena; John Wiley & Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Yu, Q.; Chen, P.; Gao, Y.; Mu, J.J.; Chen, Y.W.; Lu, C.; Liu, D. Effects of vacuum thermal cycling on mechanical and physical properties of high performance carbon/bismaleimide composite. Mater. Chem. Phys. 2011, 130, 1046–1053. [Google Scholar] [CrossRef]
- Bai, Y.P.; Wei, Y.Z.; Zhang, Z.Q. Study on the Relationship between the Structure of Bismaleimide and its Thermal Stability. Mater. Rep. 1995, 1, 58–61. [Google Scholar]
- Torrecillas, R.; Regnier, N.; Mortaigne, B. Thermal degradation of bismaleimide and bisnadimide networks—Products of thermal degradation and type of crosslinking points. Polym. Degrad. Stabil. 1996, 51, 307–318. [Google Scholar] [CrossRef]
- Torrecillas, R.; Baudry, A.; Dufay, J.; Mortaigne, B. Thermal degradation of high performance polymers—Influence of structure on polyimide thermostability. Polym. Degrad. Stabil. 1996, 54, 267–274. [Google Scholar] [CrossRef]
- Howell, B.A. Utilization of thermogravimetry in the study of reaction mechanism. J. Therm. Anal. Calorim. 2008, 93, 27–34. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J. Influence of thermal aging on decomposition behavior and kinetic mechanism of commercial proton exchange membranes. Thermochim. Acta 2020, 690, 178664. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Shen, R.Q.; Parker, T.; Zhang, Y.; Wang, Z.; Wang, Q.S. Thermal behavior and kinetics study of carbon/epoxy resin composites. Polym. Compos. 2019, 40, 4530–4546. [Google Scholar] [CrossRef]
- Carmona, V.B.; Campos, A.D.; Marconcini, J.M.; Mattoso, L.H.C. Kinetics of thermal degradation applied to biocomposites with TPS, PCL and sisal fibers by non-isothermal procedures. J. Therm. Anal. Calorim. 2014, 115, 153–160. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal degradation kinetics of plastics and model selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
- Chrissafis, K. Kinetics of thermal degradation of polymers: Complementary use of isoconversional and model-fitting methods. J. Therm. Anal. Calorim. 2009, 95, 273–283. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Zhang, Q.; Pan, W.P.; Lee, W.M. The thermal analysis of bismaleimide/graphite fiber composite by TG/FTIR. Thermochim. Acta 1993, 226, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.H.; Chen, P.; Yu, Q.; Zhu, N.B.; Wang, B.C.; Zhang, J.X.; Li, J.F. Synthesis and properties of chain-extended bismaleimide resins containing phthalide cardo structure. Polym. Int. 2010, 59, 1665–1672. [Google Scholar] [CrossRef]
- Fedelich, N. Evolved Gas Analysis; Carl Hanser Verlag: Munich, Germany, 2020. [Google Scholar]
- Perng, L.H. Thermal degradation mechanism of poly(arylene sulfone)s by stepwise Py-GC/MS. J. Polym. Sci. Pol. Chem. 2000, 38, 583–593. [Google Scholar] [CrossRef]
- Mouritz, A.; Gibson, A. Fire Properties of Polymer Composite Materials; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Harada, T. Time to ignition, heat release rate and fire endurance time of wood in cone calorimeter test. Fire Mater. 2001, 25, 161–167. [Google Scholar] [CrossRef]
- Babrauskas, V. Ignition Handbook Principles and Applications to Fire Safety Engineering, Fire Investigation, Risk Management and Forensic Science; Fire Science Publishers: Washington, DC, USA, 2003. [Google Scholar]
- Ma, Q.J.; Chen, J.C.; Zhang, H. Heat release rate determination of pool fire at different pressure conditions. Fire Mater. 2018, 42, 620–626. [Google Scholar] [CrossRef]
- Babrauskas, V.; Peacock, R.D. Heat release rate: The single most important variable in fire hazard. Fire Saf. J. 1992, 18, 255–272. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, P.; Mu, J.J.; Gao, Y.; Liu, D. Surface molecular degradation of high performance carbon/bismaleimide composites induced by proton irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms 2011, 269, 318–323. [Google Scholar] [CrossRef]
- Shu, Z.; Xu, X.; Li, X. Evaluation on Fire Performance of Polymer Materials: Cone Calorimeter Method; Chemical Industry Press: Beijing, China, 2007. [Google Scholar]
- GB 8624-2012; Classification for Burning Behavior of Building Materials and Products. China National Standardization Administration: Beijing, China, 2012.
- Gaskill, J.R.; Veith, C.R. Smoke opacity from certain woods and plastics. Fire Technol. 1968, 4, 185–195. [Google Scholar] [CrossRef]




| β (°C/min) | To (°C) | Tf (°C) | Tp (°C) | MLRp (%/min) |
|---|---|---|---|---|
| 5 | 364 | 500 | 424 | 0.02 |
| 10 | 379 | 520 | 441 | 0.05 |
| 20 | 396 | 536 | 457 | 0.10 |
| 30 | 404 | 551 | 470 | 0.15 |
| 40 | 414 | 560 | 481 | 0.20 |
| α (%) | E (kJ/mol) | R2 |
|---|---|---|
| 10 | 137 | 0.9896 |
| 20 | 145 | 0.99471 |
| 30 | 154 | 0.99754 |
| 40 | 158 | 0.99596 |
| 50 | 158 | 0.99883 |
| 60 | 158 | 0.99794 |
| 70 | 161 | 0.99957 |
| 80 | 164 | 0.9986 |
| 90 | 170 | 0.99685 |
| Heat Flux (kW/m2) | TTI (s) | pHRR (kW/m2) | Time-to-pHRR (s) | THR (MJ/m2) | TSR (m2/m2) | RW (%) |
|---|---|---|---|---|---|---|
| 25 | 126 | 104.9 | 225 | 18.6 | 775.2 | 79.4 |
| 35 | 85 | 193.1 | 157 | 24.6 | 844.9 | 73.8 |
| 50 | 38 | 242.0 | 116 | 34.3 | 1259.1 | 65.6 |
| Heat Flux (kW/m2) | FPI (m2 s/kW) | THRI6min (MJ/m2) | TSPI6min (m2/s) | ToxPI6min (g/s) |
|---|---|---|---|---|
| 25 | 0.56 | 1.25 | 2.82 | 0.18 |
| 35 | 0.54 | 1.35 | 2.87 | 0.57 |
| 50 | 0.33 | 1.50 | 3.04 | 0.69 |
| Temperature (°C) | LOI (%) |
|---|---|
| 20 | 47.8 |
| 50 | 46.5 |
| 100 | 43.3 |
| 150 | 40.8 |
| 220 | 39.0 |
| Fire Pattern | Dm | tDm (min) | 4Dm | t16 (min) | R (1/min) | SOI (1/min) |
|---|---|---|---|---|---|---|
| Flaming | 21.64 | 7.62 | 21.36 | 2.56 | 9.54 | 48.39 |
| Non-flaming | 30.56 | 19.23 | 14.63 | 4.48 | 3.73 | 15.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Qu, F.; Wang, Z.; Xiong, X.; Xu, Y. Experimental Study of Thermal and Fire Reaction Properties of Glass Fiber/Bismaleimide Composites for Aeronautic Application. Polymers 2023, 15, 2275. https://doi.org/10.3390/polym15102275
Li G, Qu F, Wang Z, Xiong X, Xu Y. Experimental Study of Thermal and Fire Reaction Properties of Glass Fiber/Bismaleimide Composites for Aeronautic Application. Polymers. 2023; 15(10):2275. https://doi.org/10.3390/polym15102275
Chicago/Turabian StyleLi, Gang, Fang Qu, Zhi Wang, Xuhai Xiong, and Yanying Xu. 2023. "Experimental Study of Thermal and Fire Reaction Properties of Glass Fiber/Bismaleimide Composites for Aeronautic Application" Polymers 15, no. 10: 2275. https://doi.org/10.3390/polym15102275






