Multifunctional Properties of Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Epoxy Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
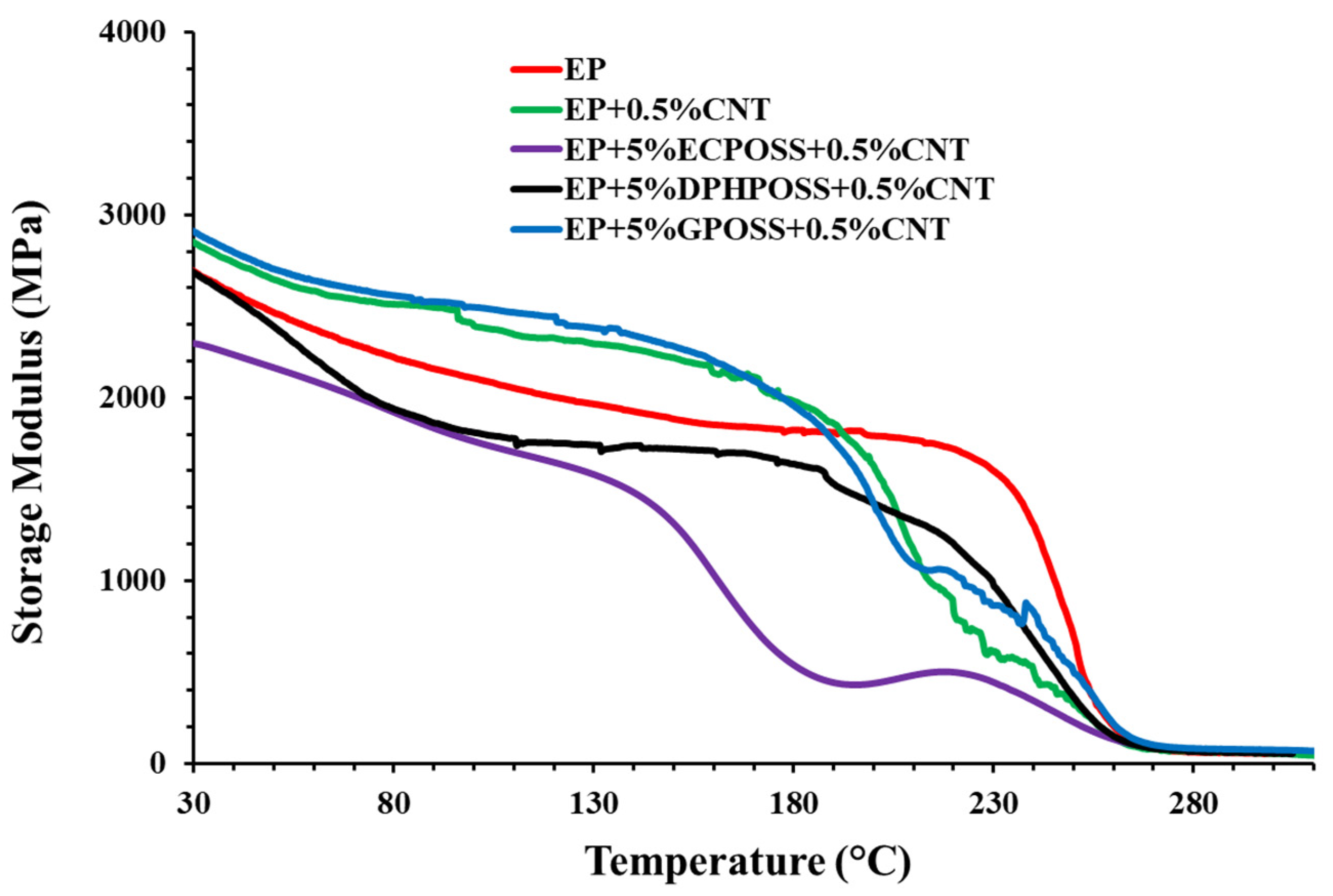
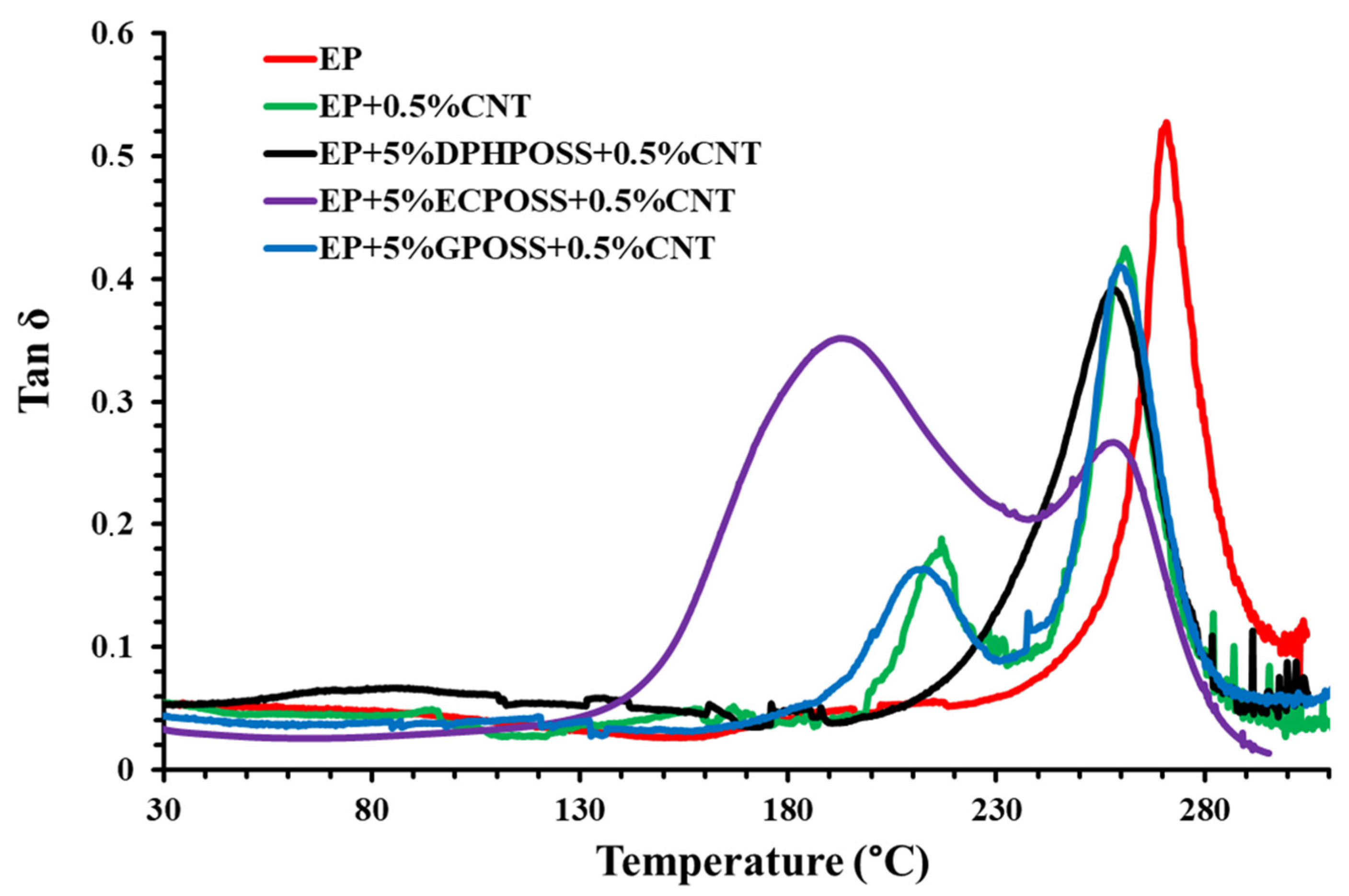
3.1. Dynamic Mechanical Behavior of the Epoxy Samples
DMA Storage Modulus and Loss Factor (tanδ) Curves of the Epoxy Samples
3.2. FTIR Investigation of the Epoxy Samples
Curing Behavior Evaluation of the Epoxy Samples before and after Curing Treatment
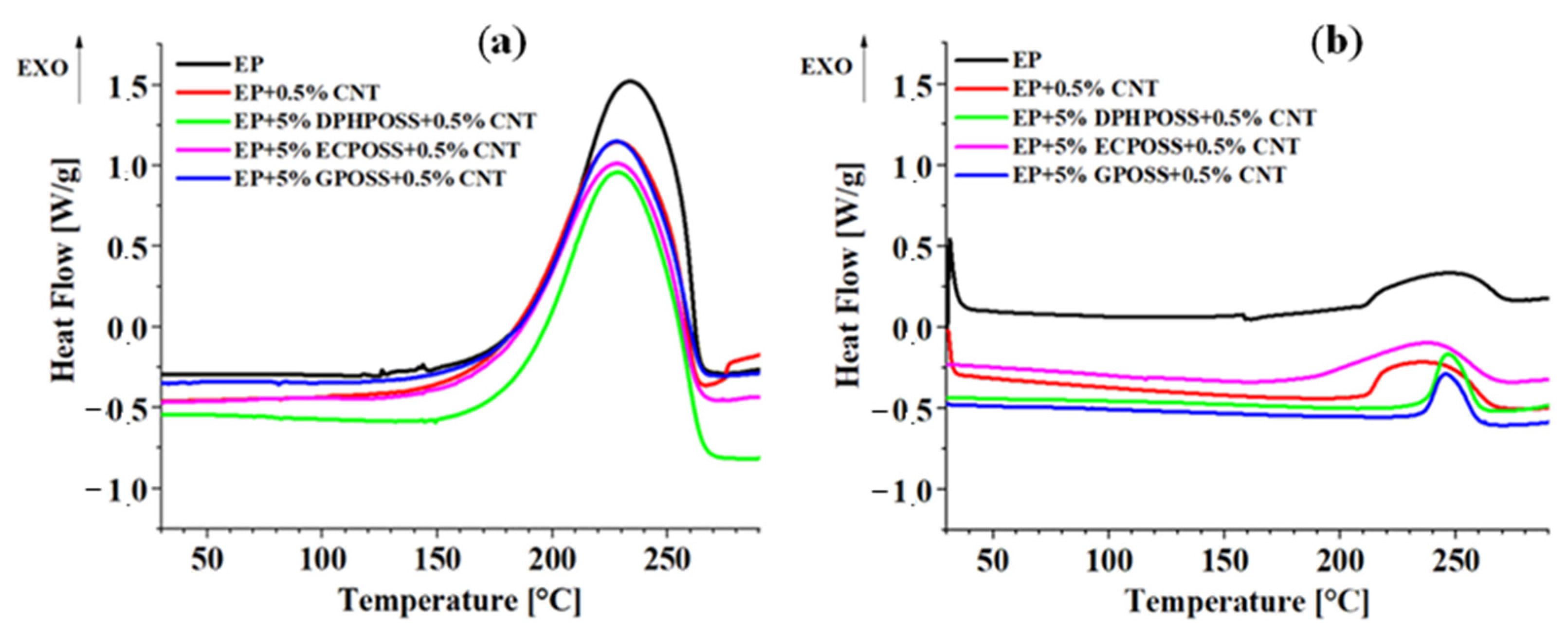
3.3. Thermal Behavior of the Epoxy Samples
3.3.1. Differential Scanning Calorimetry (DSC) Analysis
3.3.2. Thermogravimetric Analysis (TGA)
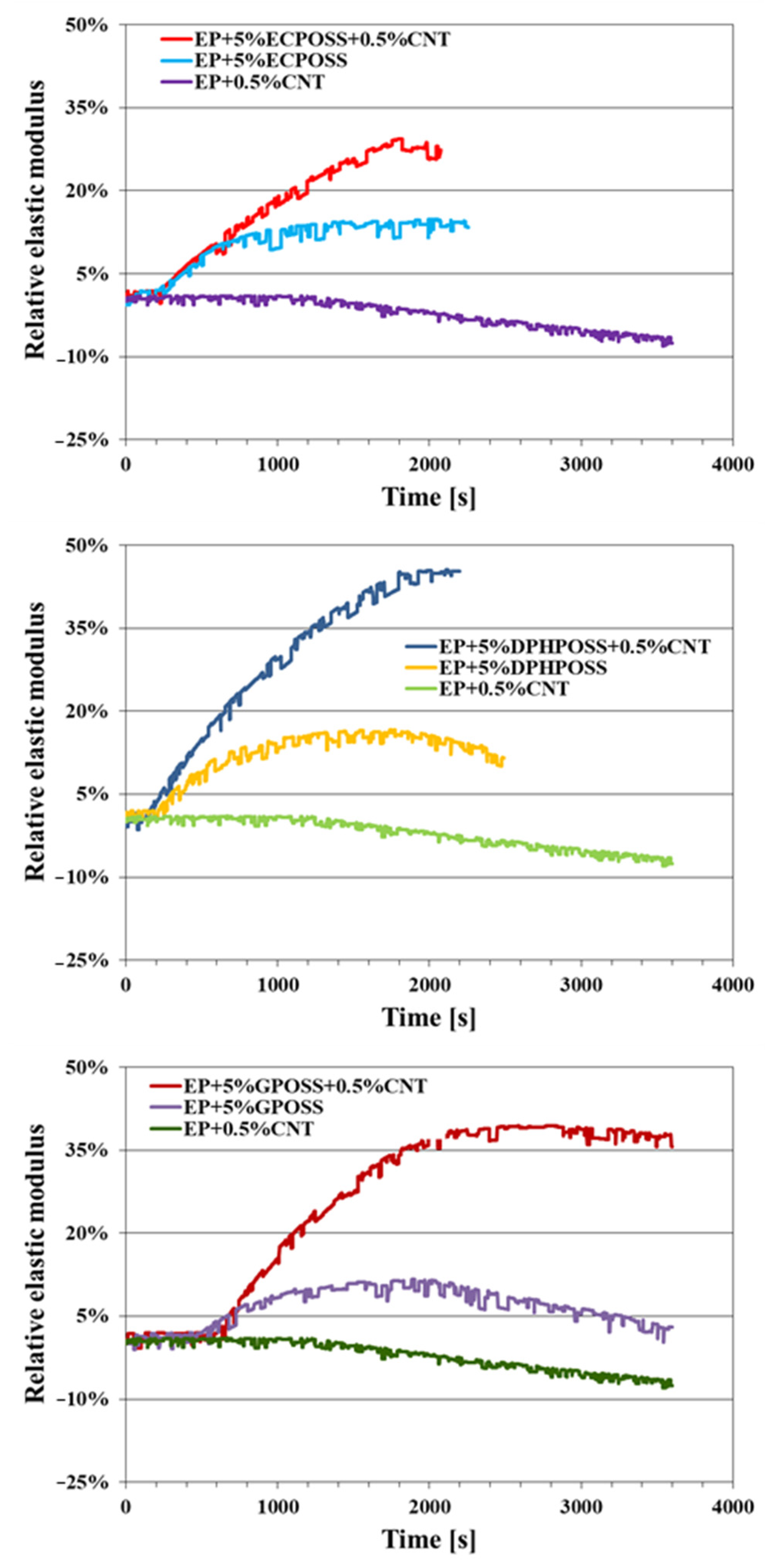
3.4. Self-Healing Efficiency of the Epoxy Samples
Evaluation of the Relative Elastic Modulus versus Time of the Epoxy Samples
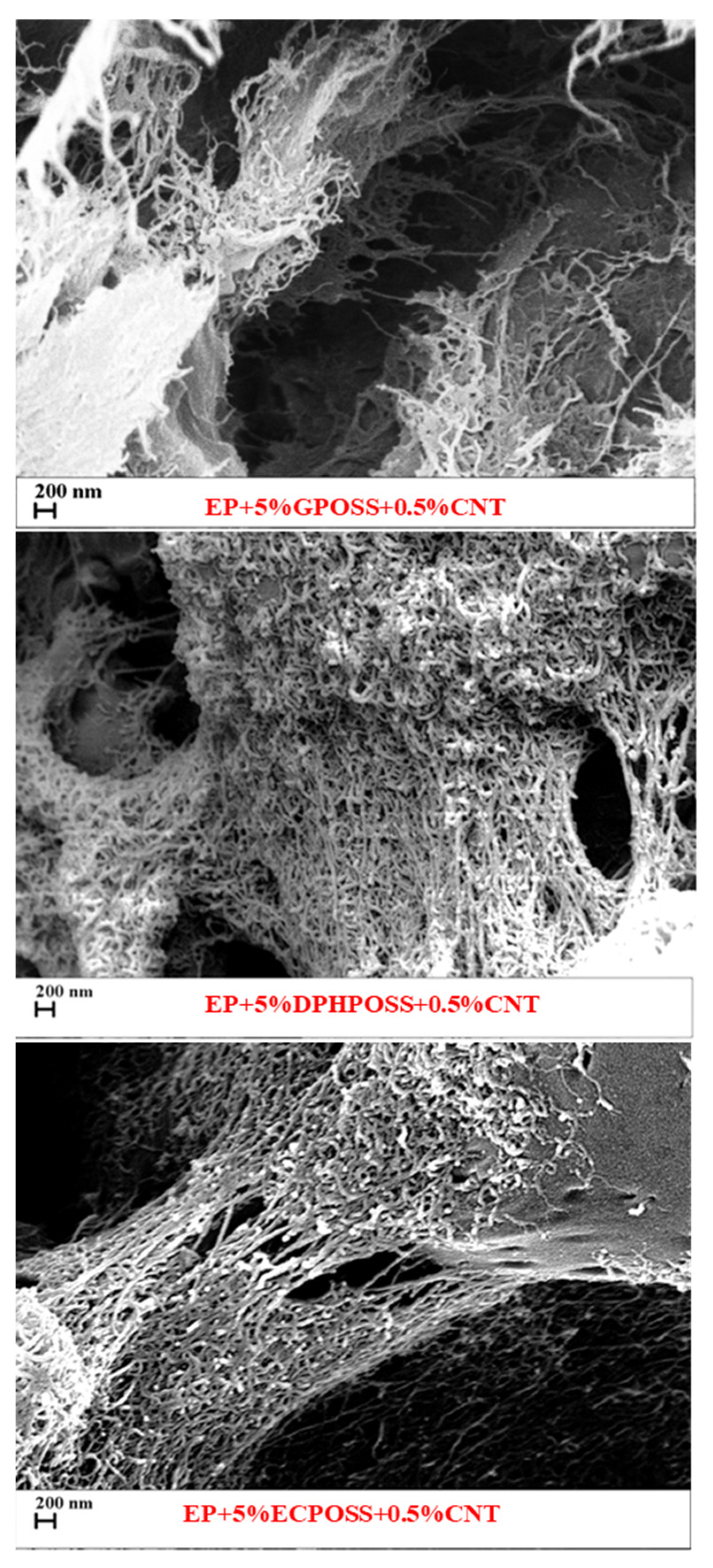
3.5. FESEM Morphological Investigation of the Epoxy Samples
Evaluation of the Nanofiller Dispersion and the Nanofiller–Epoxy Matrix Interfacial Interactions
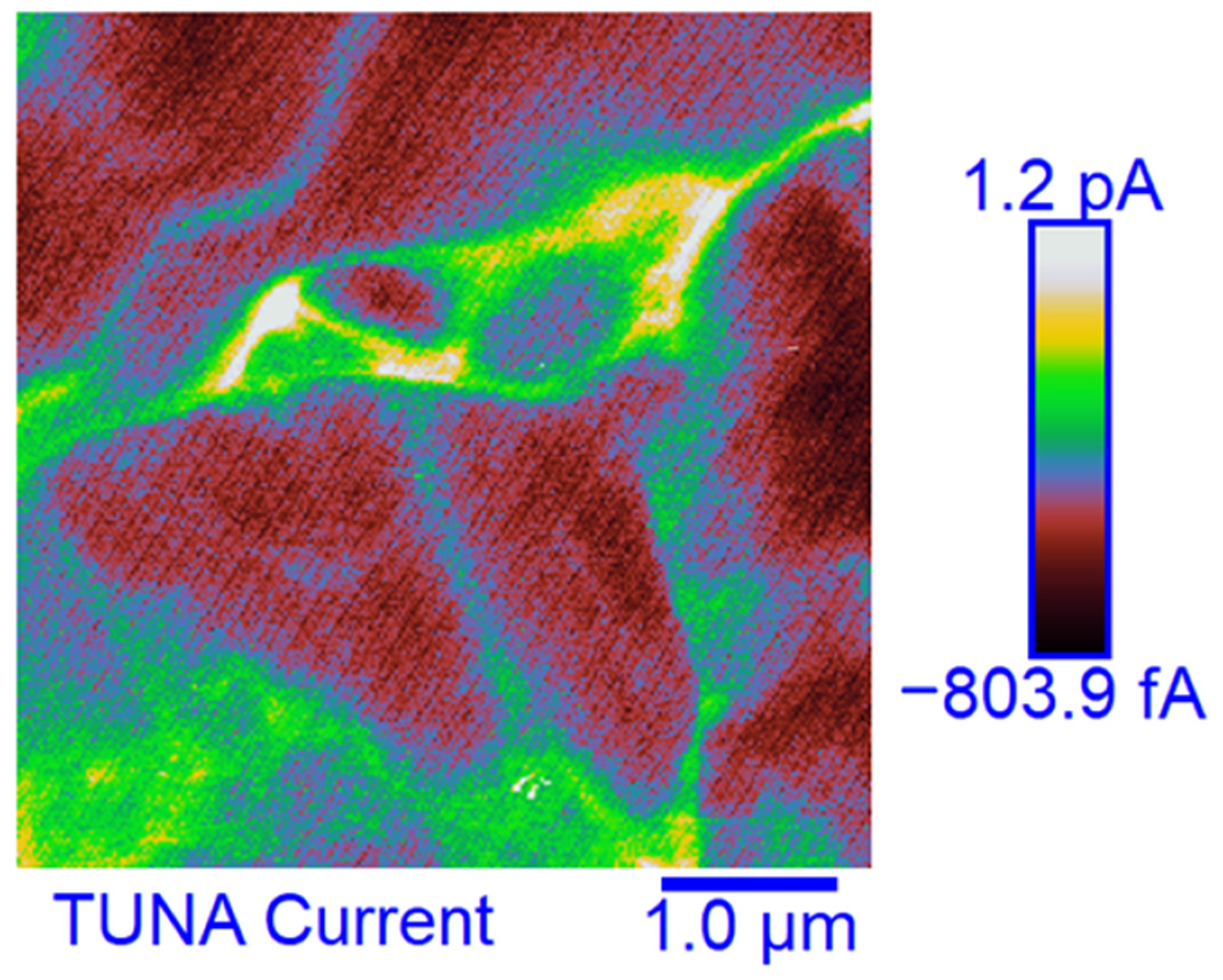
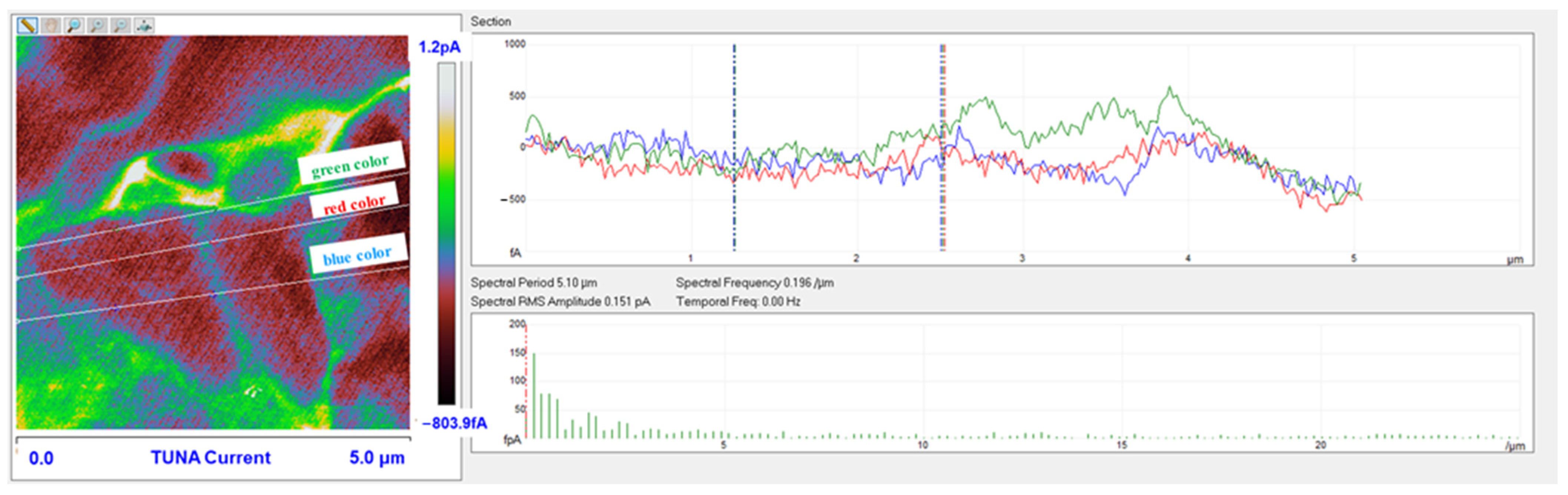
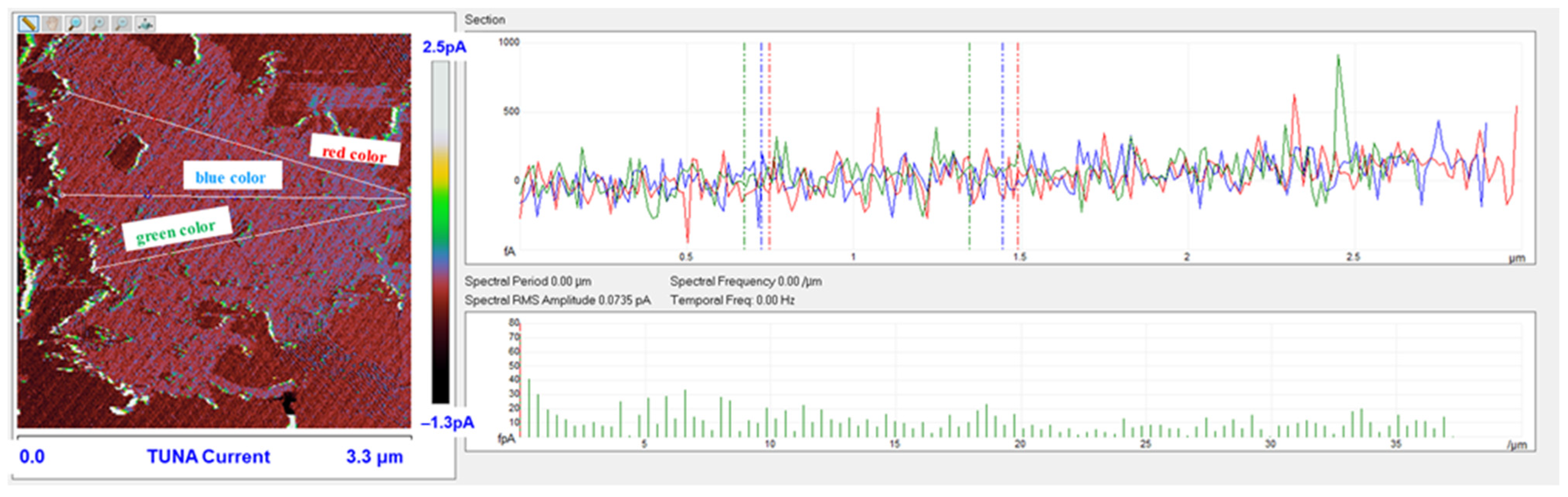
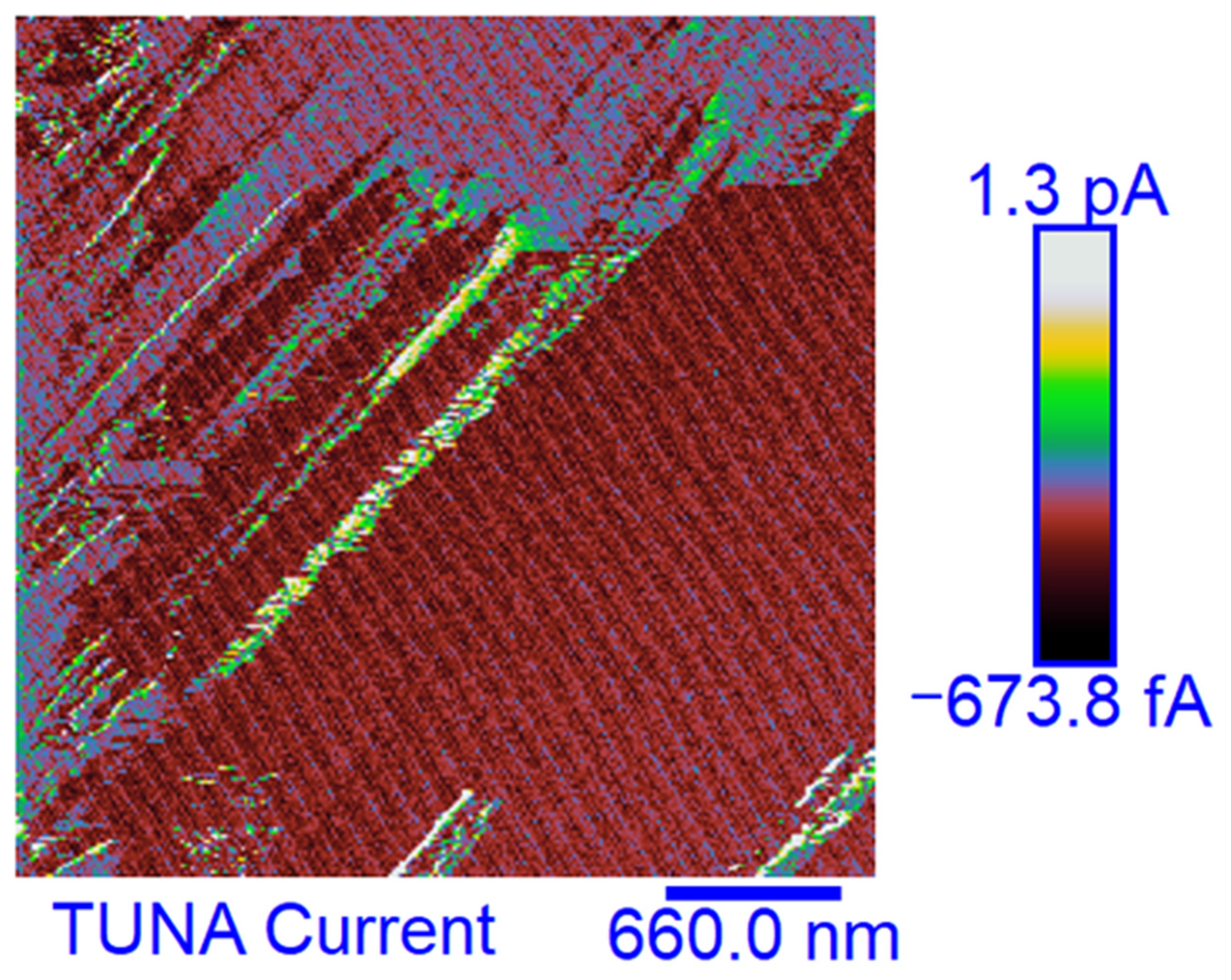
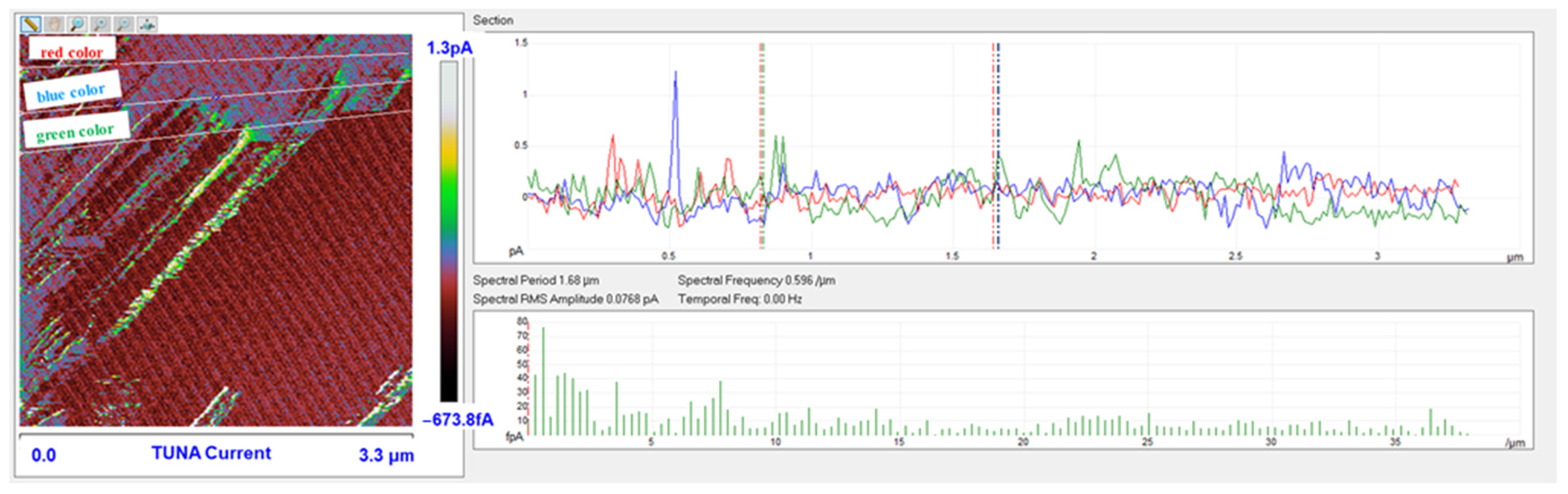
3.6. TUNA Morphological Investigation of the Epoxy Samples
Mapping of the Conductive Nanofiller Distribution and Local Electrical Properties of the Nanometric Domains
4. Conclusions
- Dynamic mechanical analysis (DMA) shows that, among the multifunctional nanocomposites, the highest storage modulus values over the whole investigated temperature range were recorded for the sample EP+5%GPOSS+0.5%CNT, loaded with CNTs and GPOSS, which is molecularly solubilized in the hosting liquid epoxy precursors due to the presence of oxirane rings in GPOSS structure, thus creating a continuous crosslinking network in the epoxy matrix; this nanocomposite manifests a trend of the storage modulus similar to that of the sample EP+0.5%CNT which contains only the CNTs;
- All multifunctional formulations have a Tg centered at values close to 260 °C, which fully satisfy structural requirements;
- The calorimetric data well explain the mechanical behavior of the analyzed samples;
- For all three multifunctional nanohybrids, the bulk electrical conductivity values of 10−3 S/m are consistent with the electric current values measured by the TUNA analysis;
- For the samples EP+5%GPOSS+0.5%CNT and EP+5%DPHPOSS+0.5%CNT, a thermostability similar to that shown by the unfilled resin EP was observed, whereas an increase in thermostability with respect to the EP resin was observed for the EP+0.5%CNT and EP+5%ECPOSS+0.5%CNT samples, especially in the second stage of the degradation process;
- The combined action of POSS with CNTs has allowed obtaining the highest values of self-healing efficiency (30%, 45%, 40% for EP+5%ECPOSS+0.5%CNT, EP+5%DPHPOSS+0.5%CNT, EP+5%GPOSS+0.5%CNT, respectively) if compared to those measured for samples containing only ECPOSS, GPOSS, DPHPOSS in the absence of CNTs (15% for EP+5%ECPOSS and EP+5%DPHPOSS, 10% for EP+5%GPOSS);
- The sample EP+0.5%CNT containing only carbon nanotubes shows no self-healing efficiency.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Al Kiey, S.A.; Hasanin, M.S. Green and facile synthesis of nickel oxide-porous carbon composite as improved electrochemical electrodes for supercapacitor application from banana peel waste. Environ. Sci. Pollut. Res. 2021, 28, 66888–66900. [Google Scholar] [CrossRef]
- Hasanin, M.; Mwafy, E.A.; Youssef, A.M. Electrical Properties of Conducting Tertiary Composite Based on Biopolymers and Polyaniline. J. Bio. Tribo Corros. 2021, 7, 133. [Google Scholar] [CrossRef]
- Ibrahim, S.; Hasanin, M.; Ahmed, H.Y.; Abdellatif, F.H.H. Poly(amidoamine)/cellulose based bio-composites as potential anticancer bio-compatible polymers. Polym. Bull. 2022, 79, 8807–8822. [Google Scholar] [CrossRef]
- Raimondo, M.; Donati, G.; Milano, G.; Guadagno, L. Hybrid composites based on carbon nanotubes and graphene nanosheets outperforming their single-nanofiller counterparts. FlatChem 2022, 36, 100431. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Tacconi, N.R.D.; Chenthamarakshall, C.R. Semiconductor-based composite materials preparation, propertiesand performance. Chem. Mater. 2001, 13, 2765–2782. [Google Scholar] [CrossRef]
- Gomez-Romero, P. Hybrid Organic-Inorganic Materials-In Search of Synergic Activity. Adv. Mater. 2001, 13, 163–174. [Google Scholar] [CrossRef]
- Ellsworth, M.W.; Gin, D.L. Recent Advances in the Design and Synthesis of Polymer–Inorganic Nanocomposites. Polym. News 1999, 24, 331–341. [Google Scholar]
- Gnanasekaran, D.; Madhavan, K.; Reddy, B.S.R. Developments of polyhedral oligomeric silsesquioxanes (POSS), POSS nanocomposites and their applications: A review. J. Sci. Ind. Res. 2009, 68, 437–464. [Google Scholar]
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Mokarian-Tabari, P.; Furukawa, H.; Khosla, A. Review—Organic-Inorganic Hybrid Functional Materials: An Integrated Platform for Applied Technologies. J. Electrochem. Soc. 2018, 165, B3137–B3156. [Google Scholar] [CrossRef]
- Szeluga, U.; Kumanek, B.; Trzebicka, B. Synergy in hybrid polymer/nanocarbon composites. A review. Compos. A Appl. Sci. Manuf. 2015, 73, 204–231. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Weissleder, R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug. Deliv. Rev. 2008, 60, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Yazman, Ş.; Uyaner, M.; Karabörk, F.; Akdemir, A. Effects of nano reinforcing/matrix interaction on chemical, thermal and mechanical properties of epoxy nanocomposites. J. Compos. Mater. 2021, 55, 4257–4272. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Nassar, M.; Hassan, Y.R.; Piszczyk, Ł.; Saeb, M.R.; Kot-Wasik, A. Sustainable multifunctional zinc oxide quantum dots-aided double-layers security paper sheets. Heliyon 2023, 9, e14695. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, P.; Chockalingam, S.; Rattan, S.; Gupta, B.K. Tunable Mechanical, Electrical, and Thermal Properties of Polymer Nanocomposites through GMA Bridging at Interface. ACS Omega 2018, 3, 3675–3687. [Google Scholar] [CrossRef] [PubMed]
- Gimenes Benega, M.A.; Silva, W.M.; Schnitzler, M.C.; Espanhol Andrade, R.J.; Ribeiro, H. Improvements in thermal and mechanical properties of composites based on epoxy-carbon nanomaterials—A brief landscape. Polymer Test. 2021, 98, 107180. [Google Scholar] [CrossRef]
- Jancar, J.; Douglas, J.F.; Starr, F.W.; Kumar, S.K.; Cassagnau, P.; Lesser, A.J.; Sternstein, S.S.; Buehler, M.J. Current issues in research on structure–property relationships in polymer nanocomposites. Polymer 2010, 51, 3321–3343. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Schadler, L.S.; Braun, P.V. Nanocomposite Science and Technology; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Idumah, C.I.; Obele, C.M. Understanding interfacial influence on properties of polymer nanocomposites. Surf. Interfaces 2021, 22, 100879. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Godara, S.S.; Mahato, P.K. Potential Applications of Hybrid Nanocomposites. Mater. Today Proc. 2019, 18, 5327–5331. [Google Scholar] [CrossRef]
- García-Martínez, J.-M.; Collar, E.P. Organic–Inorganic Hybrid Materials. Polymers 2021, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Ansari, M.M.; Tewari, D.; Gaur, M.; Yadav, A.B.; García-Betancourt, M.-L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and Nanostructure Synthesis and Controlled Growth Methods. Nanomaterials 2022, 12, 3226. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, H.; Mei, L.; Dou, K.; Jiang, Y.; Sun, Z.; Wang, S.; Hasanin, M.S.; Deng, J.; Zhou, Q. Fucoidan-derived carbon dots against Enterococcus faecalis biofilm and infected dentinal tubules for the treatment of persistent endodontic infections. J. Nanobiotechnol. 2022, 20, 321. [Google Scholar] [CrossRef]
- Ulus, H.; Üstün, T.; Şahin, Ö.S.; Karabulut, S.E.; Eskizeybek, V.; Avcı, A. Low-velocity impact behavior of carbon fiber/epoxy multiscale hybrid nanocomposites reinforced with multiwalled carbon nanotubes and boron nitride nanoplates. J. Compos. Mater. 2016, 50, 761–770. [Google Scholar] [CrossRef]
- Neto, J.S.S.; Banea, M.D.; Cavalcanti, D.K.K.; Queiroz, H.F.M.; Aguiar, R.A.A. Analysis of mechanical and thermal properties of epoxy multiwalled carbon nanocomposites. J. Compos. Mater. 2020, 54, 4831–4840. [Google Scholar] [CrossRef]
- Raimondo, M.; Naddeo, C.; Vertuccio, L.; Bonnaud, L.; Dubois, P.; Binder, W.H.; Sorrentino, A.; Guadagno, L. Multifunctionality of structural nanohybrids: The crucial role of carbon nanotube covalent and non-covalent functionalization in enabling high thermal, mechanical and self-healing performance. Nanotechnology 2020, 31, 225708. [Google Scholar] [CrossRef]
- Guadagno, L.; Vertuccio, L.; Naddeo, C.; Raimondo, M.; Barra, G.; De Nicola, F.; Volponi, R.; Lamberti, P.; Spinelli, G.; Tucci, V. Electrical Current Map and Bulk Conductivity of Carbon Fiber-Reinforced Nanocomposites. Polymers 2019, 11, 1865. [Google Scholar] [CrossRef]
- Raimondo, M.; Naddeo, C.; Vertuccio, L.; Lafdi, K.; Sorrentino, A.; Guadagno, L. Carbon-Based Aeronautical Epoxy Nanocomposites: Effectiveness of Atomic Force Microscopy (AFM) in Investigating the Dispersion of Different Carbonaceous Nanoparticles. Polymers 2019, 11, 832. [Google Scholar] [CrossRef]
- Polydoropoulou, P.V.; Katsiropoulos, C.V.; Pantelakis, S.G.; Raimondo, M.; Guadagno, L. A critical assessment of multifunctional polymers with regard to their potential use in structural applications. Compos. Part. B Eng. 2019, 157, 150–162. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Barra, G.; Vertuccio, L.; Sorrentino, A.; Binder, W.H.; Kadlec, M. Development of self-healing multifunctional materials. Compos. Part. B Eng. 2017, 128, 30–38. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Goyat, M.S.; Kumar, A. A review on the effect of oxide nanoparticles, carbon nanotubes, and their hybrid structure on the toughening of epoxy nanocomposites. J. Mater. Sci. 2022, 57, 13202–13232. [Google Scholar] [CrossRef]
- Benra, J.; Forero, S. Epoxy resins reinforced with carbon nanotubes. Lightweight des worldw 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Mostovoy, A.; Yakovlev, A.; Tseluikin, V.; Lopukhova, M. Epoxy Nanocomposites Reinforced with Functionalized Carbon Nanotubes. Polymers 2020, 12, 1816. [Google Scholar] [CrossRef]
- Garg, A.; Chalak, H.D.; Belarbi, M.O.; Zenkour, A.M.; Sahoo, R. Estimation of carbon nanotubes and their applications as reinforcing composite materials–An engineering review. Compos. Struct. 2021, 272, 114234. [Google Scholar] [CrossRef]
- Romero, M.; Mombrú, D.; Pignanelli, F.; Faccio, R.; Mombrú, A.W. Hybrid Organic-Inorganic Materials and Interfaces With Mixed Ionic-Electronic Transport Properties: Advances in Experimental and Theoretical Approaches. Front. Chem. 2022, 10, 892013. [Google Scholar] [CrossRef] [PubMed]
- Garcés, J.M.; Moll, D.J.; Bicerano, J.; Fibiger, R.; McLeod, D.G. Polymeric nano composite for automotive applications. Adv. Mater. 2000, 12, 1835–1839. [Google Scholar] [CrossRef]
- Kurahatti, R.V.; Surendranathan, A.O.; Kori, S.A.; Singh, N.; Ramesh Kumar, A.V.; Srivastava, S. Defence Applications of Polymer nanocomposites. Def. Sci. J. 2010, 60, 551–563. [Google Scholar] [CrossRef]
- Yahyaei, H.; Mohseni, M.; Ghanbari, H. POSS Hybrid Materials for Medical Applications. In Polymer/POSS Nanocomposites and Hybrid Materials; Kalia, S., Pielichowski, K., Eds.; Springer: Cham, Switzerland, 2018; pp. 373–394. [Google Scholar] [CrossRef]
- Awad, Z.K.; Aravinthan, T.; Zhuge, Y.; Gonzalez, F. A review of optimization techniques used in the design of fiber composite structures for civil engineering applications. Mater. Des. 2012, 33, 534–544. [Google Scholar] [CrossRef]
- Ayatullah Hosne Asif, A.K.M.; Hasan, M.Z. Application of Nanotechnology in Modern Textiles: A Review. Int. J. Eng. Technol. 2018, 8, 227–231. [Google Scholar] [CrossRef]
- Honarvar, Z.; Hadian, Z.; Mashayekh, M. Nanocomposites in food packaging applications and their risk assessment for health. Electron. Physician 2016, 8, 2531–2538. [Google Scholar] [CrossRef] [PubMed]
- Gururaja, M.N.; Hari Rao, A.N. A Review on Recent Applications and Future Prospectus of Hybrid Composites. Int. J. Soft Comput. Eng. 2012, 1, 352–355. [Google Scholar]
- Navrotskaya, A.G.; Aleksandrova, D.D.; Krivoshapkina, E.F.; Sillanpää, M.; Krivoshapkin, P.V. Hybrid Materials Based on Carbon Nanotubes and Nanofibers for Environmental Applications. Front. Chem. 2020, 8, 546. [Google Scholar] [CrossRef] [PubMed]
- John, Ł.; Ejfler, J. A Brief Review on Selected Applications of Hybrid Materials Based on Functionalized Cage-like Silsesquioxanes. Polymers 2023, 15, 1452. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; He, C.; Russell, T.P.; Wang, D. Epoxy-polyhedral oligomeric silsesquioxanes (POSS) nanocomposite vitrimers with high strength, toughness, and efficient relaxation. Giant 2020, 4, 100035. [Google Scholar] [CrossRef]
- Divakaran, N.; Kale, M.B.; Dhamodharan, D.; Mubarak, S.; Wu, L.; Wang, J. Effect of POSS-Modified Montmorillonite on Thermal, Mechanical, and Electrical Properties of Unsaturated Polyester Nanocomposites. Polymers 2020, 12, 2031. [Google Scholar] [CrossRef]
- Raimondo, M.; Russo, S.; Guadagno, L.; Longo, P.; Chirico, S.; Mariconda, A.; Bonnaud, L.; Murariu, O.; Dubois, P. Effect of incorporation of POSS compounds and phosphorous hardeners on thermal and fire resistance of nanofilled aeronautic resins. RSC Adv. 2015, 5, 10974–10986. [Google Scholar] [CrossRef]
- Strakowska, A.; Czlonka, S.; Strzelec, K. POSS compounds as a modifiers for rigid polyurethane foams (composites). Polymers 2019, 11, 1092. [Google Scholar] [CrossRef]
- Guadagno, L.; Pantelakis, S.; Strohmayer, A.; Raimondo, M. High-Performance Properties of an Aerospace Epoxy Resin Loaded with Carbon Nanofibers and Glycidyl Polyhedral Oligomeric Silsesquioxane. Aerospace 2022, 9, 222. [Google Scholar] [CrossRef]
- Min, D.; Cui, H.; Hai, Y.; Li, P.; Xing, Z.; Zhang, C.; Li, S. Interfacial regions and network dynamics in epoxy/POSS nanocomposites unravelling through their effects on the motion of molecular chains. Compos. Sci. Technol. 2020, 199, 108329. [Google Scholar] [CrossRef]
- Florea, N.M.; Lungu, A.; Badica, P.; Craciun, L.; Enculescu, M.; Ghita, D.G.; Ionescu, C.; Zgirian, R.G.; Iovu, H. Novel nanocomposites based on epoxy resin/epoxy-functionalized polydimethylsiloxane reinforced with POSS. Compos. Part. B Eng. 2015, 75, 226–234. [Google Scholar] [CrossRef]
- Gamal Mohamed, M.; Tsai, M.-Y.; Wang, C.-F.; Huang, C.-F.; Danko, M.; Dai, L.; Chen, T.; Kuo, S.-W. Multifunctional Polyhedral Oligomeric Silsesquioxane (POSS) Based Hybrid Porous Materials for CO2 Uptake and Iodine Adsorption. Polymers 2021, 13, 221. [Google Scholar] [CrossRef] [PubMed]
- Duraibabu, D.; Kumar, S.A.; Alagar, M. Tetra functional epoxy/polyhedral oligomeric silsesquioxane (POSS) nanocomposites with enhanced mechanical, thermal, anticorrosion and dielectric properties. J. Plast. Film. Sheet. 2023, 39, 52–79. [Google Scholar] [CrossRef]
- Loman-Cortes, P.; Binte Huq, T.; Vivero-Escoto, J.L. Use of Polyhedral Oligomeric Silsesquioxane (POSS) in Drug Delivery, Photodynamic Therapy and Bioimaging. Molecules 2021, 26, 6453. [Google Scholar] [CrossRef]
- Dhanapal, D.; Srinivasan, A.; Rajarathinam, M.; Muthukaruppan, A. Evaluation of augmented thermal, thermo-mechanical, mechanical properties of nano alumina reinforced TGDDM epoxy nanocomposites. High. Perform. Polym. 2023, 35, 313–323. [Google Scholar] [CrossRef]
- Chi, H.; Zhang, G.; Wang, N.; Wang, Y.; Li, T.; Wang, F.; Ye, C. Enhancing the mechanical strength and toughness of epoxy resins with linear POSS nano-modifiers. Nanoscale Adv. 2022, 4, 1151. [Google Scholar] [CrossRef]
- Florea, N.M.; Lungu, A.; Balanuca, B.; Badica, P.; Craciun, L.; Damian, C.M.; Enculescu, M.; Ionescu, C.; Tihan, G.; Iovu, H. Effect of polyhedral oligomeric silsesquioxane nanoreinforcement on the properties of epoxy resin/monoglycidylether-terminated poly(dimethylsiloxane) nanocomposites. High. Perform. Polym. 2016, 28, 724–734. [Google Scholar] [CrossRef]
- Chabane, H.; Livi, S.; Benes, H.; Ladavière, C.; Ecorchard, P.; Duchet-Rumeau, J.; Gérard, J.F. Polyhedral oligomeric silsesquioxane-supported ionic liquid for designing nanostructured hybrid organic-inorganic networks. Eur. Polym. J. 2019, 114, 332–337. [Google Scholar] [CrossRef]
- Raimondo, M.; Guadagno, L.; Vertuccio, L.; Naddeo, C.; Barra, G.; Spinelli, G.; Lamberti, P.; Tucci, V.; Lafdi, K. Electrical conductivity of carbon nanofiber reinforced resins: Potentiality of Tunneling Atomic Force Microscopy (TUNA) technique. Compos. Part. B Eng. 2018, 143, 148–160. [Google Scholar] [CrossRef]
- Guadagno, L.; Aliberti, F.; Longo, R.; Raimondo, M.; Pantani, R.; Sorrentino, A.; Catauro, M.; Vertuccio, L. Electrical anisotropy controlled heating of acrylonitrile butadiene styrene 3D printed parts. Mater. Des. 2023, 225, 111507. [Google Scholar] [CrossRef]
- Raimondo, M.; Naddeo, C.; Catauro, M.; Guadagno, L. Thermo-mechanical properties and electrical mapping of nanoscale domains of carbon-based structural resins. J. Therm. Anal. Calorim. 2022, 147, 5473–5481. [Google Scholar] [CrossRef]
- Raimondo, M.; Calabrese, E.; Binder, W.H.; Michael, P.; Rana, S.; Guadagno, L. Tunneling Atomic Force Microscopy Analysis of Supramolecular Self-Responsive Nanocomposites. Polymers 2021, 13, 1401. [Google Scholar] [CrossRef] [PubMed]
- Nobile, M.R.; Raimondo, M.; Naddeo, C.; Guadagno, L. Rheological and Morphological Properties of Non-Covalently Functionalized Graphene-Based Structural Epoxy Resins with Intrinsic Electrical Conductivity and Thermal Stability. Nanomaterials 2020, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, M.; Guadagno, L.; Speranza, V.; Bonnaud, L.; Dubois, P.; Lafdi, K. Multifunctional graphene/POSS epoxy resin tailored for aircraft lightning strike protection. Compos. Part. B Eng. 2018, 140, 44–56. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Vertuccio, L.; Barra, G.; Arena, M.; Viscardi, M. Vibro-acoustic characteristics of multifunctional carbon fiber reinforced panel. Def. Technol. 2022; article in press. [Google Scholar] [CrossRef]
- Guadagno, L.; Vertuccio, L.; Sorrentino, A.; Raimondo, M.; Naddeo, C.; Vittoria, V.; Iannuzzo, G.; Calvi, E.; Russo, S. Mechanical and barrier properties of epoxy resin filled with multi-walled carbon nanotubes. Carbon 2009, 47, 2419–2430. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Barra, G.; Vertuccio, L.; Russo, S.; Lafdi, K.; Tucci, V.; Spinelli, G.; Lamberti, P. Influence of carbon nanoparticles/epoxy matrix interaction on mechanical, electrical and transport properties of structural advanced materials. Nanotechnology 2017, 28, 094001. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Vertuccio, L.; Naddeo, C.; Barra, G.; Longo, P.; Lamberti, P.; Spinelli, G.; Tucci, V. Morphological, rheological and electrical properties of composites filled with carbon nanotubes functionalized with 1-pyrenebutyric acid. Compos. B. Eng. 2018, 147, 12–21. [Google Scholar] [CrossRef]





| ΔHT | ΔHRes | DC | |
|---|---|---|---|
| [J/g] | [J/g] | [%] | |
| EP | 659.29 | 46.15 | 93 |
| EP+0.5%CNT | 571.91 | 62.91 | 89 |
| EP+5%DPHPOSS+0.5%CNT | 479.11 | 28.21 | 94 |
| EP+5%ECPOSS+0.5%CNT | 510.09 | 71.56 | 86 |
| EP+5%GPOSS+0.5%CNT | 568.59 | 51.17 | 91 |
| Air Flow | Nitrogen Flow | |||||
|---|---|---|---|---|---|---|
| Td5% | Td10% | Residue at 900 °C | Td5% | Td10% | Residue at 900 °C | |
| EP | 358.94 | 373.07 | 1.44 | 349.37 | 369.69 | 0.7009 |
| EP+0.5%CNT | 362.94 | 373.94 | 3.97 | 360.21 | 372.34 | 28.11 |
| EP+5%DPHPOSS +0.5%CNT | 363.12 | 376.67 | 4.31 | 365.62 | 378.22 | 28.57 |
| EP+5%ECPOSS +0.5%CNT | 366.23 | 377.55 | 4.15 | 366.70 | 376.698 | 28.02 |
| EP+5%GPOSS +0.5%CNT | 359.60 | 373.36 | 3.65 | 359.83 | 374.032 | 20.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guadagno, L.; Sorrentino, A.; Longo, R.; Raimondo, M. Multifunctional Properties of Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Epoxy Nanocomposites. Polymers 2023, 15, 2297. https://doi.org/10.3390/polym15102297
Guadagno L, Sorrentino A, Longo R, Raimondo M. Multifunctional Properties of Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Epoxy Nanocomposites. Polymers. 2023; 15(10):2297. https://doi.org/10.3390/polym15102297
Chicago/Turabian StyleGuadagno, Liberata, Andrea Sorrentino, Raffaele Longo, and Marialuigia Raimondo. 2023. "Multifunctional Properties of Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Epoxy Nanocomposites" Polymers 15, no. 10: 2297. https://doi.org/10.3390/polym15102297
APA StyleGuadagno, L., Sorrentino, A., Longo, R., & Raimondo, M. (2023). Multifunctional Properties of Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Epoxy Nanocomposites. Polymers, 15(10), 2297. https://doi.org/10.3390/polym15102297









