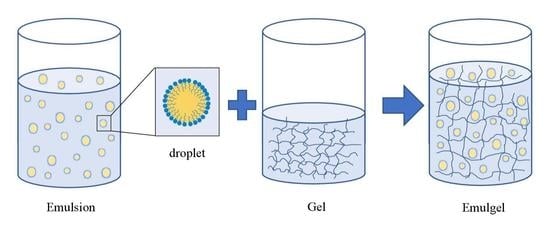
Emulgels: Promising Carrier Systems for Food Ingredients and Drugs
Abstract
:1. Introduction
2. Formulation of Emulgel
2.1. Gelling Agents
2.2. Oil Phase
2.3. Aqueous Phase
2.4. Emulsifiers
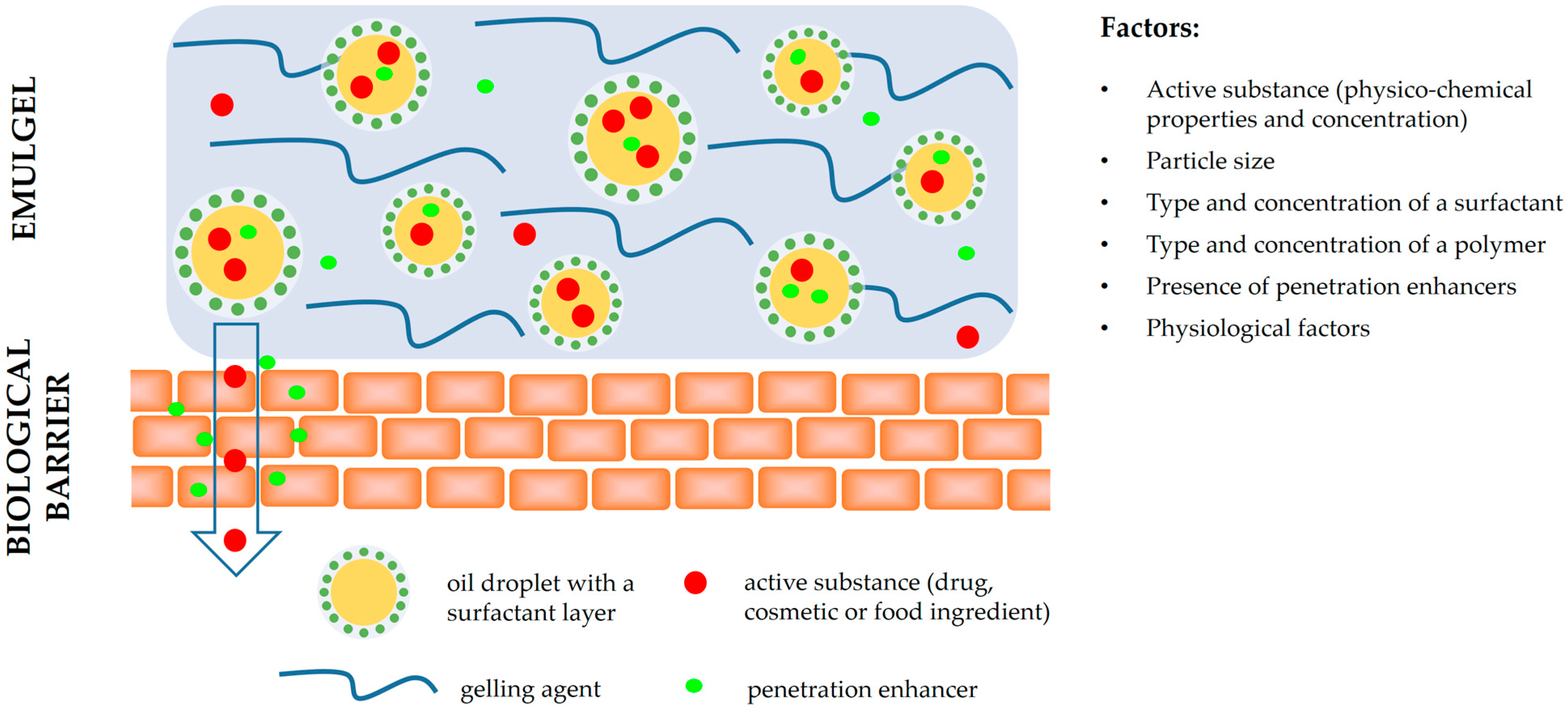
2.5. Penetration Enhancers
- These agents should be non-toxic, non-irritating, and non-allergenic;
- They should be pharmacologically inactive. Consequently, they should not bind to receptors;
- They should have predictable and reproducible activity and duration of effect;
- They should allow drugs into the body while preventing the loss of endogenous material from the body;
- They should be compatible with both excipients and active ingredients;
- They should possess cosmetic acceptability and be appropriate for the skin.
2.6. pH Adjustment
2.7. Preservatives
2.8. Humectants
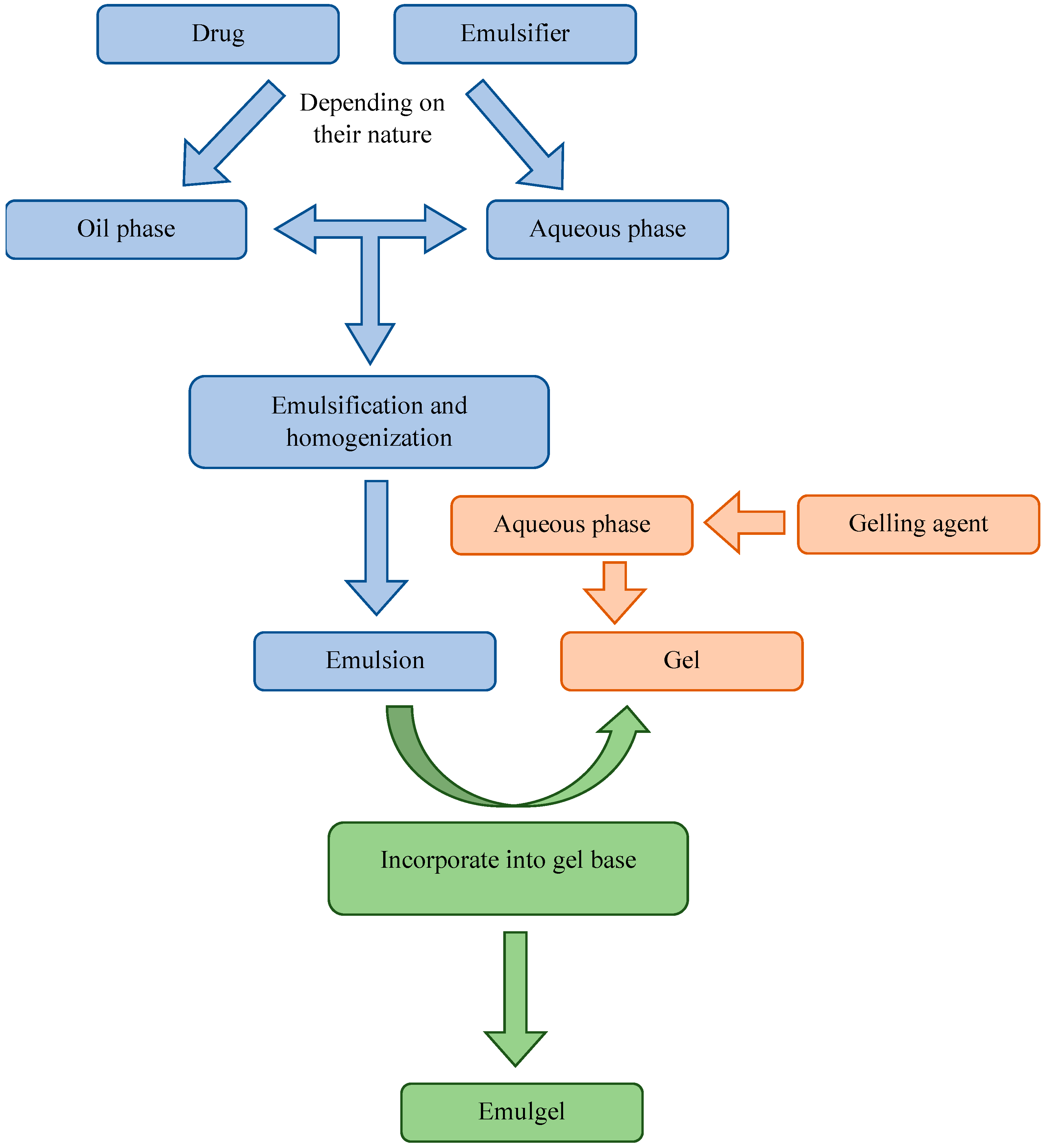
3. Preparation Processes of Emulgels
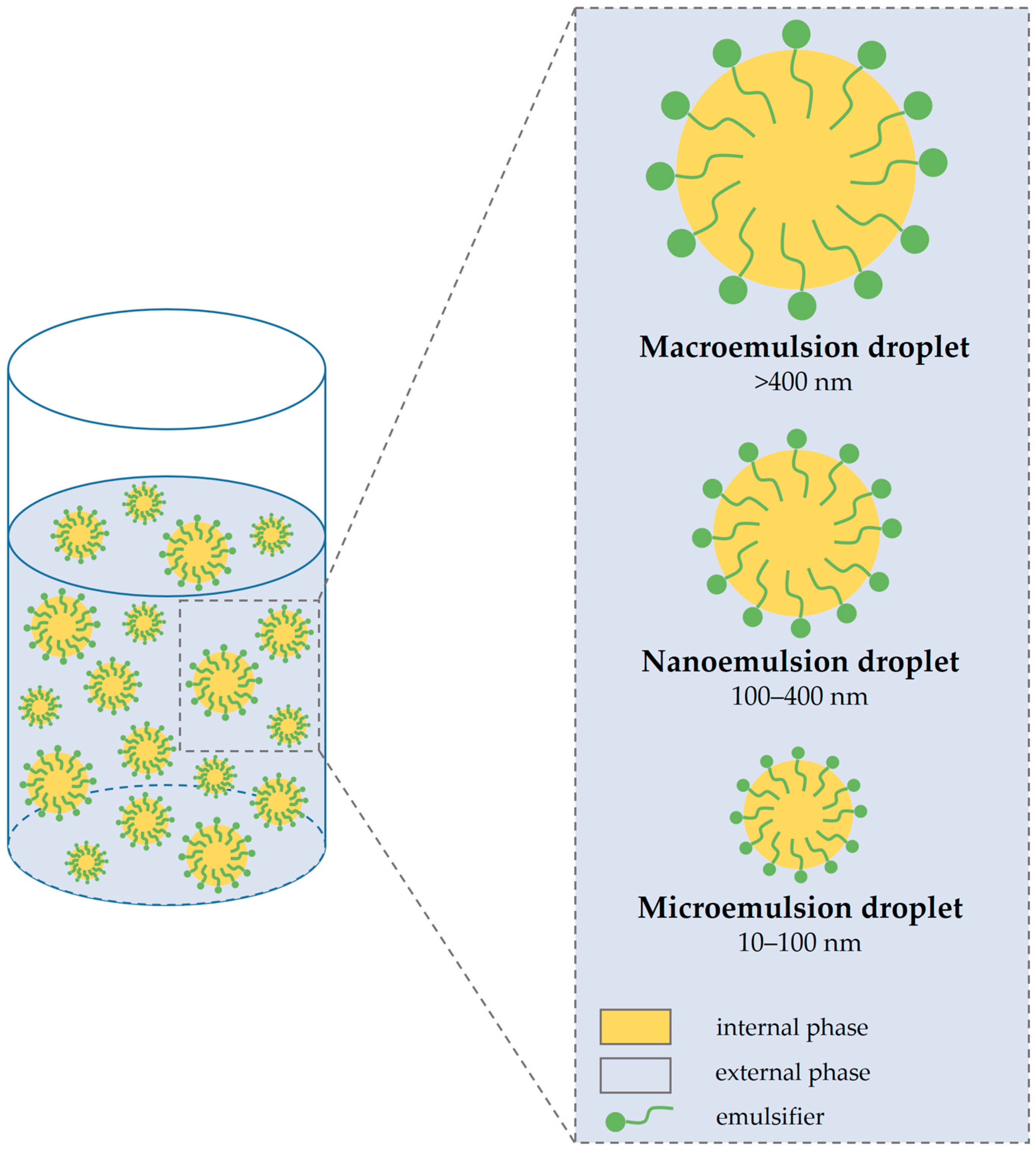
4. Types of Emulgel
4.1. Macroemulsion Gel
4.2. Nanoemulgel
4.3. Microemulsion Gel
5. Advantages of Emulgel Formulations
6. Disadvantages of Emulgel Formulations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trucillo, P. Drug Carriers: Classification, Administration, Release Profiles, and Industrial Approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Nayak, A.K.; Ahmad, S.A.; Beg, S.; Ara, T.J.; Hasnain, M.S. Drug delivery: Present, past, and future of medicine. In Applications of Nanocomposite Materials in Drug Delivery; Inamuddin, A., Mohammad, A., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 255–282. ISBN 978-0-12-813741-3. [Google Scholar]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to Supercritical Fluids. Processes 2020, 8, 1022. [Google Scholar] [CrossRef]
- Voronin, D.V.; Abalymov, A.A.; Svenskaya, Y.I.; Lomova, M.V. Key Points in Remote-Controlled Drug Delivery: From the Carrier Design to Clinical Trials. Int. J. Mol. Sci. 2021, 22, 9149. [Google Scholar] [CrossRef] [PubMed]
- Malavi, S.; Kumbhar, P.; Manjappa, A.; Chopade, S.; Patil, O.; Udichi, K.; Dwivedi, J.; Disouza, J. Topical Emulgel: Basic Considerations in Development and Advanced Research. Indian J. Pharm. Sci. 2022, 84, 1105–1115. [Google Scholar] [CrossRef]
- Husain, I.H.; Wankhade, V.P. Emulgel: Modern tool for topical drug delivery. Eur. J. Biomed. Pharm. Sci. 2020, 7, 492–500. [Google Scholar]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Talat, M.; Zaman, M.; Khan, R.; Jamshaid, M.; Akhtar, M.; Mirza, A.Z. Emulgel: An effective drug delivery system. Drug Dev. Ind. Pharm. 2021, 47, 1193–1199. [Google Scholar] [CrossRef]
- Sastri, T.K.; Gupta, V.N.; Chakraborty, S.; Madhusudhan, S.; Kumar, H.; Chand, P.; Jain, V.; Veeranna, B.; Gowda, D.V. Novel Gels: An Emerging Approach for Delivering of Therapeutic Molecules and Recent Trends. Gels 2022, 8, 316. [Google Scholar] [CrossRef]
- Kankane, M.; Nigam, V.; Modi, S.; Jain, S.; Adhikari, P. Emulgel: A dual release system for hydrophobic drug delivery. World J. Biol. Pharm. Health Sci. 2022, 12, 335–347. [Google Scholar] [CrossRef]
- Lu, G.W.; Gao, P. Chapter 3—Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; Kulkarni, V.S., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 59–94. ISBN 978-0-8155-2025-2. [Google Scholar]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Liu, L.; Javed, H.U.; Xiao, J. Engineering Emulsion Gels as Functional Colloids Emphasizing Food Applications: A Review. Front. Nutr. 2022, 9, 890188. [Google Scholar] [CrossRef]
- Lu, Y.; Mao, L.; Hou, Z.; Miao, S.; Gao, Y. Development of emulsion gels for the delivery of functional food ingredients: From structure to functionality. Food Eng. Rev. 2019, 11, 245–258. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Yuan, F.; Gao, Y.; Mao, L. Emulsion gels with different proteins at the interface: Structures and delivery functionality. Food Hydrocoll. 2021, 116, 106637. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Naqash, F.; Rashid, R. Oleogels: Promising alternatives to solid fats for food applications. Food Hydrocoll. Health 2022, 2, 100058. [Google Scholar] [CrossRef]
- Corstens, M.N.; Berton-Carabin, C.C.; Elichiry-Ortiz, P.T.; Hol, K.; Troost, F.J.; Masclee, A.A.; Schroën, K. Emulsion-alginate beads designed to control in vitro intestinal lipolysis: Towards appetite control. J. Funct. Foods 2017, 34, 319–328. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Bellissimo, N.; Singh, H.; Rousseau, D. Modulating fat digestion through food structure design. Prog. Lipid Res. 2017, 68, 109–118. [Google Scholar] [CrossRef]
- Sharma, V.; Nayak, S.K.; Paul, S.R.; Choudhary, B.; Ray, S.S.; Pal, K. Emulgels. In Polymeric Gels; Pal, K., Banerjee, I., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 251–264. ISBN 978-0-08-102179-8. [Google Scholar]
- Patil, P.B.; Datir, S.K.; Saudagar, R.B. A review on topical gels as drug delivery system. J. Drug Deliv. Ther. 2019, 9, 989–994. [Google Scholar]
- Dubey, A.; Prabhu, P.; Kamath, J.V. Nano Structured lipid carriers: A Novel Topical drug delivery system. Int. J. PharmTech Res. 2012, 4, 705–714. [Google Scholar]
- Apolinário, A.C.; Salata, G.C.; de Souza, M.M.; Chorilli, M.; Lopes, L.B. Rethinking breast cancer chemoprevention: Technological advantages and enhanced performance of a nanoethosomal-based hydrogel for topical administration of fenretinide. AAPS PharmSciTech. 2022, 23, 104. [Google Scholar] [CrossRef]
- Javed, N.; Hussain Shah, S.N.; Saeed, J.; Nisar, M.; Javed, H.; Riaz, R.; Karim, K. Formulation development and evaluation of transdermal emulgel using Aloevera extract and natural penetration enhancers. Pak. J. Pharm. Sci. 2022, 35, 953–964. [Google Scholar] [PubMed]
- Erni, P.; Windhab, E.J.; Fischer, P. Emulsion drops with complex interfaces: Globular versus flexible proteins. Macromol. Mater. Eng. 2011, 296, 249–262. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent Advances in Lipid-Based Vesicles and Particulate Carriers for Topical and Transdermal Application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, M.; Nie, G. Biomembrane-based nanostructures for cancer targeting and therapy: From synthetic liposomes to natural biomembranes and membrane-vesicles. Adv. Drug Deliv. Rev. 2021, 178, 113974. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Xu, Y.; Zhou, Z.; Li, Y.; Ling, W.; Song, W. Bio-Inspired Drug Delivery Systems: From Synthetic Polypeptide Vesicles to Outer Membrane Vesicles. Pharmaceutics 2023, 15, 368. [Google Scholar] [CrossRef]
- Son, G.H.; Lee, B.J.; Cho, C.W. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J. Pharm. Investig. 2017, 47, 287–296. [Google Scholar] [CrossRef]
- Song, W.; Zhang, Y.; Yu, D.G.; Tran, C.H.; Wang, M.; Varyambath, A.; Kim, J.; Kim, I. Efficient Synthesis of Folate-Conjugated Hollow Polymeric Capsules for Accurate Drug Delivery to Cancer Cells. Biomacromolecules 2021, 22, 732–742. [Google Scholar] [CrossRef]
- Kar, M.; Chourasiya, Y.; Maheshwari, R.; Tekade, R.K. Chapter 2—Current Developments in Excipient Science: Implication of Quantitative Selection of Each Excipient in Product Development. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 29–83. ISBN 978-0-12-817909-3. [Google Scholar]
- Shah, H.; Jain, A.; Laghate, G.; Prabhudesai, D. Chapter 32—Pharmaceutical excipients. In Remington, 23rd ed.; Adejare, A., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 633–643. ISBN 978-0-12-820007-0. [Google Scholar]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics 2023, 15, 164. [Google Scholar] [CrossRef]
- Vlaia, L.; Coneac, G.; Olariu, I.; Vlaia, V.; Lupuleasa, D. Cellulose-Derivatives-Based Hydrogels as Vehicles for Dermal and Transdermal Drug Delivery. In Emerging Concepts in Analysis and Applications of Hydrogels; Majee, S.B., Ed.; IntechOpen: Rijeka, Croatia, 2016; pp. 159–200. [Google Scholar]
- Mirgorodskaya, A.; Kushnazarova, R.; Pavlov, R.; Valeeva, F.; Lenina, O.; Bushmeleva, K.; Kuryashov, D.; Vyshtakalyuk, A.; Gaynanova, G.; Petrov, K.; et al. Supramolecular Tools to Improve Wound Healing and Antioxidant Properties of Abietic Acid: Biocompatible Microemulsions and Emulgels. Molecules 2022, 27, 6447. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, J.; Liu, Y.; Peng, F.; Wang, X.; Wang, C.; Li, M.; Xu, H. Gel properties and formation mechanism of soy protein isolate gels improved by wheat bran cellulose. Food Chem. 2020, 324, 126876. [Google Scholar] [CrossRef]
- Li, A.; Gong, T.; Hou, Y.; Yang, X.; Guo, Y. Alginate-stabilized thixotropic emulsion gels and their applications in fabrication of low-fat mayonnaise alternatives. Int. J. Biol. Macromol. 2020, 146, 821–831. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Maidannyk, V.; Miao, S. Effect of concentrations of alginate, soy protein isolate and sunflower oil on water loss, shrinkage, elastic and structural properties of alginate-based emulsion gel beads during gelation. Food Hydrocoll. 2020, 108, 105998. [Google Scholar] [CrossRef]
- Leon, A.M.; Medina, W.T.; Park, D.J.; Aguilera, J.M. Properties of microparticles from a whey protein isolate/alginate emulsion gel. Food Sci. Technol. Int. 2018, 24, 414–423. [Google Scholar] [CrossRef]
- He, E.; Li, H.; Li, X.; Wu, X.; Lei, K.; Diao, Y. Transdermal Delivery of Indirubin-Loaded Microemulsion Gel: Preparation, Characterization and Anti-Psoriatic Activity. Int. J. Mol. Sci. 2022, 23, 3798. [Google Scholar] [CrossRef]
- Khan, B.A.; Ali, A.; Hosny, K.M.; Halwani, A.A.; Almehmady, A.M.; Iqbal, M.; Alharbi, W.S.; Abualsunun, W.A.; Bakhaidar, R.B.; Murshid, S.S.A.; et al. Carbopol emulgel loaded with ebastine for urticaria: Development, characterization, in vitro and in vivo evaluation. Drug Deliv. 2022, 29, 52–61. [Google Scholar] [CrossRef]
- Ahmad, J.; Gautam, A.; Komath, S.; Bano, M.; Garg, A.; Jain, K. Topical Nano-emulgel for Skin Disorders: Formulation Approach and Characterization. Recent Pat. Antiinfect. Drug Discov. 2019, 14, 36–48. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Kotta, S.; Ahmad, J.; Akhter, S.; Alam, M.S.; Khan, M.A.; Awan, Z.; Sivakumar, P.M. Improved Analgesic and Anti-Inflammatory Effect of Diclofenac Sodium by Topical Nanoemulgel: Formulation Development—In Vitro and In Vivo Studies. J. Chem. 2020, 2020, 4071818. [Google Scholar] [CrossRef]
- Oliveira, R.S.; da Silva, D.F.; Mota, S.; Garrido, J.; Garrido, E.M.; Lobo, J.M.S.; Almeida, I.F. Design of an Emulgel for Psoriasis Focused on Patient Preferences. Appl. Sci. 2022, 12, 3260. [Google Scholar] [CrossRef]
- Mazurkevičiūtė, A.; Matulytė, I.; Ivaškienė, M.; Žilius, M. Assessment of Physical, Mechanical, Biopharmaceutical Properties of Emulgels and Bigel Containing Ciclopirox Olamine. Polymers 2022, 14, 2783. [Google Scholar] [CrossRef]
- Khan, B.A.; Ahmad, S.; Khan, M.K.; Hosny, K.M.; Bukhary, D.M.; Iqbal, H.; Murshid, S.S.; Halwani, A.A.; Alissa, M.; Menaa, F. Fabrication and Characterizations of Pharmaceutical Emulgel Co-Loaded with Naproxen-Eugenol for Improved Analgesic and Anti-Inflammatory Effects. Gels 2022, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Elghamry, H.A.; Khalifa, N.E.; Khojali, W.M.A.; Khafagy, E.-S.; Lila, A.S.A.; El-Horany, H.E.-S.; El-Housiny, S. Ginger Extract-Loaded Sesame Oil-Based Niosomal Emulgel: Quality by Design to Ameliorate Anti-Inflammatory Activity. Gels 2022, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Goci, E.; Haloci, E.; Xhulaj, S.; Malaj, L. Formulation and in vitro evaluation of diclofenac sodium gel. Int. J. Pharm. Pharm. Sci. 2014, 6, 259–261. [Google Scholar]
- Abdallah, M.H.; Abu Lila, A.S.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Preparation, characterization and evaluation of anti-inflammatory and anti-nociceptive effects of brucine-loaded nanoemulgel. Colloids Surf. B Biointerfaces 2021, 205, 111868. [Google Scholar] [CrossRef]
- Begur, M.; Pai, V.; Gowda, D.V.; Srivastava, A.; Raghundan, H.V.; Shinde, C.G.; Manusri, N. Enhanced permeability of Cyclosporine from a transdermally applied nanoemulgel. Der Pharm. Sin. 2015, 6, 69–79. [Google Scholar]
- Khule, P.K.; Gilhotra, R.M.; Nitalikar, M.M.; More, V.V. Formulation and Evaluation of Itraconazole Emulgel for Various Fungal Infections. Asian J. Pharm. 2019, 13, 19–22. [Google Scholar] [CrossRef]
- Mantovani, R.A.; Cavallieri, Â.L.F.; Cunha, R.L. Gelation of oil-in-water emulsions stabilized by whey protein. J. Food Eng. 2016, 175, 108–116. [Google Scholar] [CrossRef]
- Jin, I.H.; Kim, J.E.; Seo, J.H.; Lee, S.P. Physicochemical properties of soy protein isolate gels emulsified with various oils using a microbial transglutaminase. Food Sci. Biotechnol. 2013, 22, 129–136. [Google Scholar] [CrossRef]
- Varma, K.; Kohli, S. Formulation and characterization of faropenem sodium emulgel for topical application. Eur. J. Biomed. Pharm. Sci. 2018, 5, 254–262. [Google Scholar]
- Nikumbh, K.V.; Sevankar, S.G.; Patil, M.P. Formulation development, in vitro and in vivo evaluation of microemulsion-based gel loaded with ketoprofen. Drug Deliv. 2015, 22, 509–515. [Google Scholar] [CrossRef]
- De Lafuente, Y.; Ochoa-Andrade, A.; Parente, M.E.; Palena, M.C.; Jimenez-Kairuz, A.F. Preparation and evaluation of caffeine bioadhesive emulgels for cosmetic applications based on formulation design using QbD tools. Int. J. Cosmet. Sci. 2020, 42, 548–556. [Google Scholar] [CrossRef]
- Salih, Z.T.; Gawhari, F.; Rajab, N.A. Preparation, release, rheology and stability of piroxicam emulgel. Int. J. Appl. Pharm. 2018, 10, 26–29. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, J.; Antil, M.; Kumar, V. Emulgel-novel topical drug delivery system—A comprehensive review. Int. J. Pharm. Sci. Res. 2016, 7, 4733–4742. [Google Scholar]
- Alexander, A.; Khichariya, A.; Gupta, S.; Patel, R.J.; Giri, T.K.; Tripathi, D.K. Recent expansions in an emergent novel drug delivery technology: Emulgel. J. Control. Release 2013, 171, 122–132. [Google Scholar] [CrossRef]
- Hardenia, A.; Jayronia, S.; Jain, S. Emulgel: An emergent tool in topical drug delivery. Int. J. Pharm. Sci. Res. 2014, 5, 1653–1660. [Google Scholar]
- Dantas, M.G.; Reis, S.A.; Damasceno, C.M.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; Almeida, J.R. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar] [CrossRef]
- Khattak, R.Z.; Nawaz, A.; Alnuwaiser, M.A.; Latif, M.S.; Rashid, S.A.; Khan, A.A.; Alamoudi, S.A. Formulation, In Vitro Characterization and Antibacterial Activity of Chitosan-Decorated Cream Containing Bacitracin for Topical Delivery. Antibiotics 2022, 11, 1151. [Google Scholar] [CrossRef]
- Daood, N.M.; Jassim, E.Z.; Ghareeb, M.M.; Zeki, H. Studying the effect of different gelling agent on the preparation and characterization of metronidazole as topical emulgel. Asian J. Pharm. Clin. Res. 2019, 12, 571–577. [Google Scholar] [CrossRef]
- Shahin, M.; Hady, S.A.; Hammad, M.; Mortada, N. Novel jojoba oil-based emulsion gel formulations for clotrimazole delivery. AAPS PharmSciTech 2011, 12, 239–247. [Google Scholar] [CrossRef]
- Abdel-Bary, A.; Shalaby, S.; Abdel-Aal, S. Formulation and stability of chloramphenicol gel and emulgel. Bull. Fac. Pharm. Cairo Univ. 2001, 39, 89–99. [Google Scholar]
- Singh, S.; Singh, I. Evolving Implementation of Emulgel as a Topical Drug Delivery System: A Systematic Review. Cur. Res. Pharm. Sci. 2022, 12, 121–131. [Google Scholar] [CrossRef]
- Chaudhari, A.S.; Vispute, S.K. A Comprehensive Review on Emulgel—Novel Drug Delivery System. Int. J. Pharm. Pharm. Res. 2021, 22, 249–272. [Google Scholar]
- Ghorbanzadeh, M.; Farhadian, N.; Golmohammadzadeh, S.; Karimi, M.; Ebrahimi, M. Formulation, clinical and histopathological assessment of microemulsion based hydrogel for UV protection of skin. Colloids Surf. B Biointerfaces 2019, 179, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Santagati, L.M. Use of Vegetable Oils to Improve the Sun Protection Factor of Sunscreen Formulations. Cosmetics 2019, 6, 25. [Google Scholar] [CrossRef]
- Ibrar, M.; Ayub, Y.; Nazir, R.; Irshad, M.; Hussain, N.; Saleem, Y.; Ahmad, M. Garlic and ginger essential oil-based neomycin nano-emulsions as effective and accelerated treatment for skin wounds’ healing and inflammation: In-vivo and in-vitro studies. Saudi Pharm. J. 2022, 30, 1700–1709. [Google Scholar] [CrossRef]
- Khullar, R.; Kumar, D.; Seth, N.; Saini, S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm. J. 2012, 20, 63–67. [Google Scholar] [CrossRef]
- Montenegro, L.; Rapisarda, L.; Ministeri, C.; Puglisi, G. Effects of Lipids and Emulsifiers on the Physicochemical and Sensory Properties of Cosmetic Emulsions Containing Vitamin, E. Cosmetics 2015, 2, 35–47. [Google Scholar] [CrossRef]
- Dhawas, V.; Dhabarde, D.; Patil, S. Emulgel: A Comprehensive Review for Novel Topical Drug Delivery System. Int. J. Recent Sci. Res. 2020, 11, 38134–38138. [Google Scholar]
- Begum, S.; Chetty, C.; Pavithra, B.; Akhila, B.; Gayathri, C.; Ruksar, S.; Sravani, T.; Voleti, V. A Review on emulgels—A novel approach for topical drug delivery. Asian J. Pharm. Res. Dev. 2019, 7, 70–77. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, Y.; Tu, Z.; Hu, Y.; He, C. Emulsion Properties during Microencapsulation of Cannabis Oil Based on Protein and Sucrose Esters as Emulsifiers: Stability and Rheological Behavior. Foods 2022, 11, 3923. [Google Scholar] [CrossRef]
- Kim, W.; Wang, Y.; Selomulya, C. Dairy and plant proteins as natural food emulsifiers. Trends Food Sci. Technol. 2020, 105, 261–272. [Google Scholar] [CrossRef]
- Lin, D.; Sun, L.C.; Chen, Y.L.; Liu, G.M.; Miao, S.; Cao, M.J. Peptide/protein hydrolysate and their derivatives: Their role as emulsifying agents for enhancement physical and oxidative stability of emulsions. Trends Food Sci. Technol. 2022, 129, 11–24. [Google Scholar] [CrossRef]
- Tang, C.H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef]
- Biviano, M.D.; Böni, L.J.; Berry, J.D.; Fischer, P.; Dagastine, R.R. Viscoelastic characterization of the crosslinking of β-lactoglobulin on emulsion drops via microcapsule compression and interfacial dilational and shear rheology. J. Colloid Interface Sci. 2021, 583, 404–413. [Google Scholar] [CrossRef]
- Kontogiorgos, V. Polysaccharides at fluid interfaces of food systems. Adv. Colloid Interface Sci. 2019, 270, 28–37. [Google Scholar] [CrossRef]
- Lu, S.; Yang, D.; Wang, M.; Yan, M.; Qian, Y.; Zheng, D.; Qiu, X. Pickering emulsions synergistic-stabilized by amphoteric lignin and SiO2 nanoparticles: Stability and pH-responsive mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124158. [Google Scholar] [CrossRef]
- Raju, K.; Sneha, G.; Khatoon, R.; Ashwini, M.; Shirisha, G.; Ajay, B.; Bongoni, R.N. Formulation and Evaluation of Ornidazole Topical Emulgel. World J. Pharm. Pharm. Sci. 2019, 8, 1179–1197. [Google Scholar]
- Khan, B.A.; Ullah, S.; Khan, M.K.; Alshahrani, S.M.; Braga, V.A. Formulation and evaluation of Ocimum basilicum-based emulgel for wound healing using animal model. Saudi Pharm. J. 2020, 28, 1842–1850. [Google Scholar] [CrossRef]
- Balata, G.F.; Shamardl, H.E.; Abd Elmoneim, H.M.; Hakami, A.A.; Almodhwahi, M.A. Propolis emulgel: A natural remedy for burn and wound. Drug Dev. Ind. Pharm. 2018, 44, 1797–1808. [Google Scholar] [CrossRef]
- Manian, M.; Jain, P.; Vora, D.; Banga, A.K. Formulation and Evaluation of the In Vitro Performance of Topical Dermatological Products Containing Diclofenac Sodium. Pharmaceutics 2022, 14, 1892. [Google Scholar] [CrossRef]
- Kola-Mustapha, A.T.; Khalid-Salako, F.A. Herbal emulgels incorporated with Cola millenii K. Schum stem bark ethanol extract potentially for the management of rheumatoid arthritis in-vitro. Phytomedicine Plus 2021, 1, 100033. [Google Scholar] [CrossRef]
- Pranali, S.; Charushila, S.; Sayali, C.; Namrata, M. Design and characterisation of emulgel of an antifungal drug. J. Pharm. Sci. Res. 2019, 11, 2357–2361. [Google Scholar]
- Zhang, X.; Chen, X.; Gong, Y.; Li, Z.; Guo, Y.; Yu, D.; Pan, M. Emulsion gels stabilized by soybean protein isolate and pectin: Effects of high intensity ultrasound on the gel properties, stability and β-carotene digestive characteristics. Ultrason. Sonochem. 2021, 79, 105756. [Google Scholar] [CrossRef] [PubMed]
- Paglarini, C.S.; Martini, S.; Pollonio, M.A. Physical properties of emulsion gels formulated with sonicated soy protein isolate. Int. J. Food Sci. 2019, 54, 451–459. [Google Scholar] [CrossRef]
- Suman, D.; Beena, K. Emugel for topical drug delivery: A novel approach. GSC Biol. Pharm. Sci. 2020, 11, 104–114. [Google Scholar] [CrossRef]
- Yadav, S.; Wairkar, S.; Invally, M.; Ranade, S. Topical emulgel of tolnaftate with penetration enhancer: Development, characterisation and antifungal activity. Indian J. Med. Res. Pharm. Sci. 2017, 4, 28–35. [Google Scholar] [CrossRef]
- Mwangi, A.N.; Njogu, P.M.; Maru, S.M.; Njuguna, N.M.; Njaria, P.M.; Kiriiri, G.K.; Mathenge, A.W. Meloxicam emulgels for topical management of rheumatism: Formulation development, in vitro and in vivo characterization. Saudi Pharm. J. 2021, 29, 351–360. [Google Scholar] [CrossRef]
- Charyulu, N.R.; Joshi, P.; Dubey, A.; Shetty, A. Emulgel: A boon for enhanced topical drug delivery. J. Young Pharm. 2021, 13, 76–79. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Sasseville, D. Hypersensitivity to preservatives. Dermatol. Ther. 2004, 17, 251–263. [Google Scholar] [CrossRef]
- Dréno, B.; Zuberbier, T.; Gelmetti, C.; Gontijo, G.; Marinovich, M. Safety review of phenoxyethanol when used as a preservative in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 15–24. [Google Scholar] [CrossRef]
- De Oliveira Schmitt, P.; Fischer, A.F.; da Silva, R.M.L.; Cruz, A.B. Compatibility and Efficiency of Preservatives in Emulsive Cosmetics Containing High Surfactant Content. Braz. J. Pharm. Sci. 2022, 58, e191088. [Google Scholar] [CrossRef]
- Puschmann, J.; Herbig, M.E.; Müller-Goymann, C.C. Correlation of antimicrobial effects of phenoxyethanol with its free concentration in the water phase of o/w-emulsion gels. Eur. J. Pharm. Biopharm. 2018, 131, 152–161. [Google Scholar] [CrossRef]
- Srivastava, N.; Patel, D.K.; Rai, V.K.; Pal, A.; Yadav, N.P. Development of emulgel formulation for vaginal candidiasis: Pharmaceutical characterization, in vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2018, 48, 490–498. [Google Scholar] [CrossRef]
- Shankar, D.; Gajanan, S.; Suresh, J.; Dushyant, G. Formulation and evaluation of luliconazole emulgel for topical drug delivery. Int. Res. J. Sci. Eng. 2018, 3, 85–89. [Google Scholar]
- Kitisin, T.; Muangkaew, W.; Ampawong, S.; Sansurin, N.; Thitipramote, N.; Sukphopetch, P. Development and efficacy of tryptophol-containing emulgel for reducing subcutaneous fungal nodules from Scedosporium apiospermum eumycetoma. Res. Pharm. Sci. 2022, 17, 707–722. [Google Scholar] [CrossRef]
- Sabry, H.S.; Al-Shohani, A.D.H.; Mahmood, S.Z. Formulation and Evaluation of Levofloxacin and Betamethasone Ophthalmic Emulgel. J. Pharm. Bioallied Sci. 2021, 13, 205–211. [Google Scholar] [CrossRef]
- Sreevidya, V.S. An overview on emulgel. Int. J. Pharm. Phytopharm. Res. 2019, 9, 92–97. [Google Scholar]
- Anand, K.; Ray, S.; Rahman, M.; Shaharyar, A.; Bhowmik, R.; Bera, R.; Karmakar, S. Nano-emulgel: Emerging as a Smarter Topical Lipidic Emulsion-based Nanocarrier for Skin Healthcare Applications. Recent Pat. Antiinfect. Drug Discov. 2019, 14, 16–35. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, B.; Fan, Y.; Wang, M.; Kebebe, D.; Li, J.; Liu, Z. Traditional Chinese medicine combined with hepatic targeted drug delivery systems: A new strategy for the treatment of liver diseases. Biomed. Pharmacother. 2019, 117, 109128. [Google Scholar] [CrossRef]
- Ali, A.; Ansari, V.A.; Ahmad, U.; Akhtar, J.; Jahan, A. Nanoemulsion: An Advanced Vehicle for Efficient Drug Delivery. Drug Res. 2017, 67, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Shaikh, I.A.; Abdel-Wahab, B.A.; Nourein, I.H.; Ahmad, J. Thymoquinone Loaded Topical Nanoemulgel for Wound Healing: Formulation Design and In-Vivo Evaluation. Molecules 2021, 26, 3863. [Google Scholar] [CrossRef]
- Kumar, N.S.G.; Nallaguntla, L.; Kulkarn, G.S. A review on: Microemulgel as a topical drug delivery system. Int. J. Pharm. Res. Appl. 2021, 6, 1542–1549. [Google Scholar]
- Sharma, A.K.; Garg, T.; Goyal, A.K.; Rath, G. Role of microemuslsions in advanced drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Gradzielski, M.; Duvail, M.; de Molina, P.M.; Simon, M.; Talmon, Y.; Zemb, T. Using microemulsions: Formulation based on knowledge of their mesostructure. Chem. Rev. 2021, 121, 5671–5740. [Google Scholar] [CrossRef]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef]
- Scomoroscenco, C.; Teodorescu, M.; Raducan, A.; Stan, M.; Voicu, S.N.; Trica, B.; Ninciuleanu, C.M.; Nistor, C.L.; Mihaescu, C.I.; Petcu, C.; et al. Novel Gel Microemulsion as Topical Drug Delivery System for Curcumin in Dermatocosmetics. Pharmaceutics 2021, 13, 505. [Google Scholar] [CrossRef]
- Tanaji, D.N. Emulgel: A comprehensive review for topical delivery of hydrophobic drugs. Asian J. Pharm. 2018, 12, 382–393. [Google Scholar] [CrossRef]
- Sah, S.K.; Badola, A.; Nayak, B.K. Emulgel: Magnifying the application of topical drug delivery. Indian J. Pharm. Biol. Res. 2017, 5, 25–33. [Google Scholar] [CrossRef]
- Lakshmi, S.S.; Divya, R.; Rao, S.Y.; Kumari, K.P.; Deepthi, K. Emulgel-Novel Trend in Topical Drug Delivery System—Review Article. Res. J. Pharm. Technol. 2021, 14, 2903–2906. [Google Scholar] [CrossRef]
- Patel, B.M.; Kuchekar, A.B.; Pawar, S.R. Emulgel Approach to Formulation Development: A Review. Biosci. Biotechnol. Res. Asia 2021, 18, 459–465. [Google Scholar] [CrossRef]
- Sushma, G.; Pravalika, T.; Sri, B.R.; Priyanaka, P.; Priya, P.V.; Sharma, J.V.C. Emulgels—A Novel Approach for Topical Drug Delivery. Int. J. Pharm. Sci. Rev. Res. 2021, 67, 142–147. [Google Scholar] [CrossRef]
- Baibhav, J.; Gurpreet, S.; Rana, A.C.; Seema, S.; Vikas, S. Emulgel: A comprehensive review on the recent advances in topical drug delivery. Int. Res. J. Pharm. 2011, 2, 66–70. [Google Scholar]
- Jain, S.K.; Bajapi, P.; Modi, S.K.; Gupta, P. A review on emulgel, as a novel trend in topical drug delivery system. Recent Trends Pharm. Sci. Res. 2019, 1, 30–39. [Google Scholar] [CrossRef]

| Gelling Agents | Concentration Used (%w/w) | Type | Reference |
|---|---|---|---|
| Carbopol 934 | 0.5–2.0 | synthetic | [41] |
| Carbopol 940 | 0.75–2.0 | synthetic | [42] |
| 1.0 | [43] | ||
| Carbopol 980 | 0.5 | synthetic | [44] |
| 0.7 | [45] | ||
| Poloxamer 407 | 12.5 | synthetic | [46] |
| Sepineo-P600 | 6.0 | synthetic | [47] |
| HPMC | 4.0–6.0 | semi-synthetic | [48] |
| HEC * | 2.5 | semi-synthetic | [49] |
| NaCMC | 1.0 | semi-synthetic | [50] |
| Guar gum | 0.5 | natural | [51] |
| Xanthan gum | 0.75 and 1.0 | natural | [52] |
| Whey protein isolate | 5.0 | natural | [53] |
| Soybean protein isolate | 10.0 | natural | [54] |
| Alginate | 2.0 | natural | [38] |
| Combination of Carbopol 934 and 940 | 0.5–2.0 and 1.0–2.0 | synthetic | [55] |
| Combination of Carbopol 934 or 940 and HPMC | 1.0 and 0.5–1 | synthetic and semi-synthetic | [56] |
| Combination of Carbomer interpolymer type A and Xanthan | 0.45 and 0.20 | synthetic and natural | [57] |
| Combination of Xanthan gum and Chitosan | 1.5 and 2.0 | natural | [58] |
| Emulsifiers | Concentration (%w/w) | Type | Reference |
|---|---|---|---|
| Tween 80 | 1.0 | nonionic | [83] |
| Combination of Tween 80 and Span 80 | 0.30 or 0.50 and 0.45 or 0.75 | nonionic | [84] |
| 1.0 and 1.5 | [85] | ||
| 2.0 and 3.0 | |||
| Combination of Tween 60 and Span 60 | 0.5 and 4.50 | nonionic | [86] |
| Combination of Tween 20 and Span 20 | 0.5 and 1.0 | nonionic | [87] |
| 0.4 and 0.3 | [88] | ||
| 1.0 and 3.0 | [64] | ||
| Soybean protein isolate | 7.0 | amphiphilic | [89] |
| 4.0 | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milutinov, J.; Krstonošić, V.; Ćirin, D.; Pavlović, N. Emulgels: Promising Carrier Systems for Food Ingredients and Drugs. Polymers 2023, 15, 2302. https://doi.org/10.3390/polym15102302
Milutinov J, Krstonošić V, Ćirin D, Pavlović N. Emulgels: Promising Carrier Systems for Food Ingredients and Drugs. Polymers. 2023; 15(10):2302. https://doi.org/10.3390/polym15102302
Chicago/Turabian StyleMilutinov, Jovana, Veljko Krstonošić, Dejan Ćirin, and Nebojša Pavlović. 2023. "Emulgels: Promising Carrier Systems for Food Ingredients and Drugs" Polymers 15, no. 10: 2302. https://doi.org/10.3390/polym15102302
APA StyleMilutinov, J., Krstonošić, V., Ćirin, D., & Pavlović, N. (2023). Emulgels: Promising Carrier Systems for Food Ingredients and Drugs. Polymers, 15(10), 2302. https://doi.org/10.3390/polym15102302