A Flexible Sensor with Excellent Environmental Stability Using Well-Designed Encapsulation Structure
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Conductive Hydrogel
2.2.1. Preparation of Polyaniline (PANI) Solution
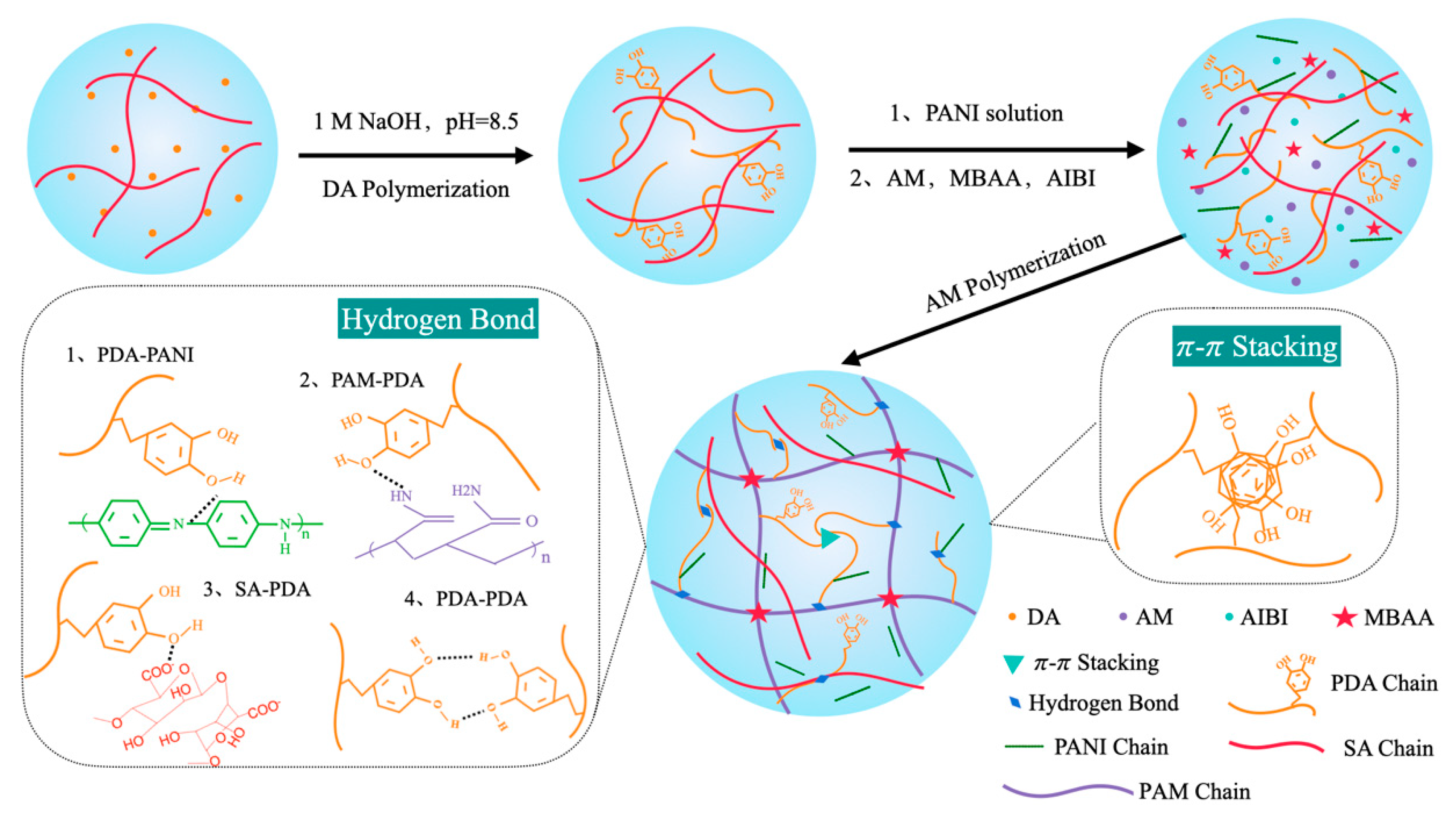
2.2.2. Preparation and Design Strategy of PAM/SA/PDA/PANI Hydrogel
2.3. Assembly of Hydrogel Sensor
2.4. General Characterizations
2.5. Mechanical Tests
2.6. Adhesion Property Tests
2.7. Sensing Performance Test
3. Results and Discussion
3.1. Characterization
3.2. Mechanical and Adhesive Properties
3.3. Encapsulation Effect on the Sensor’s Stability and Response Performance
3.4. The Influence of Material and Shape of Electrode on Sensor Performance
3.5. Theoretical Derivation and Simulation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, J.-A.; Ye, W.-H.; Su, Y.-W.; Hattori, Y.; Lee, W. Fractal design concepts for stretchable electronics. Nat. Commun. 2014, 5, 3266. [Google Scholar] [CrossRef] [PubMed]
- Lipomi, D.-J. Stretchable Figures of Merit in Deformable Electronics. Adv. Mater. 2016, 28, 4180–4183. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Ma, Z.-P.; Wang, L.; Li, H.; Mi, H.-Y.; Feng, P.-Y. Synthesis and Fabrication of Supramolecular Polydimethylsiloxane-Based Nanocomposite Elastomer for Versatile and Intelligent Sensing. Ind. Eng. Chem. Res. 2021, 60, 10419–10430. [Google Scholar] [CrossRef]
- Ma, Z.-P.; Li, H.; Jing, X.; Liu, Y.-J.; Mi, H.-Y. Recent advancements in self-healing composite elastomers for flexible strain sensors: Materials, healing systems, and features. Sens. Actuators A Phys. 2021, 329, 112800. [Google Scholar] [CrossRef]
- Kim, Y.; Parada, G.-A.; Liu, S.-D.; Zhao, X.-H. Ferromagnetic soft continuum robots. Sci. Robot. 2019, 4, eaax7329. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yuk, H.; Zhao, R.; Zhao, X.-H. Printing ferromagnetic domains for untethered fast-transforming soft materials. Nature 2018, 558, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; He, K.; Chen, G.; Leow, W.-R. Nature-Inspired Structural Materials for Flexible Electronic Devices. Chem. Rev. 2017, 117, 12893–12941. [Google Scholar] [CrossRef]
- Wang, S.; Oh, J.-Y.; Xu, J.; Tran, H. Skin-Inspired Electronics: An Emerging Paradigm. Acc. Chem. Res. 2018, 51, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, Z.; Chen, C.; Shi, K.; Zhang, L. Reduced Graphene Oxide-Containing Smart Hydrogels with Excellent Electro-Response and Mechanical Properties for Soft Actuators. ACS Appl. Mater. Interfaces 2017, 9, 15758–15767. [Google Scholar] [CrossRef] [PubMed]
- Forte, A.-E.; Galvan, S.; Manieri, F. A composite hydrogel for brain tissue phantoms. Mater. Des. 2016, 112, 227–238. [Google Scholar] [CrossRef]
- Oveissi, F.; Fletcher, D.-F.; Dehghani, F. Tough hydrogels for soft artificial muscles. Mater. Des. 2021, 203, 109609. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Liu, J.; Gu, J.-F.; Zhu, J.-D. High toughness multifunctional organic hydrogels for flexible strain and temperature sensor. J. Mater. Chem. A 2021, 9, 23243–23255. [Google Scholar] [CrossRef]
- Wei, Y.; Xiang, L.; Ou, H.; Li, F. MXene-Based Conductive Organohydrogels with Long-Term Environmental Stability and Multifunctionality. Adv. Funct. Mater. 2020, 30, 2005135. [Google Scholar] [CrossRef]
- Zou, J.; Jing, X.; Chen, Z.; Wang, S.-J.; Hu, X.-S.; Feng, P.-Y.; Liu, Y.-J. Multifunctional Organohydrogel with Ultralow-Hysteresis, Ultrafast-Response, and Whole-Strain-Range Linearity for Self-Powered Sensors. Adv. Funct. Mater. 2023, 33, 2213895. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Parada, G.-A.; Liu, X.-Y.; Zhao, X. Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 2016, 7, 12028. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ding, H.; Tao, K.; Wei, Y.-M.; Gui, X.-C. Ultrasensitive, Stretchable, and Fast-Response Temperature Sensors Based on Hydrogel Films for Wearable Applications. ACS Appl. Mater. Interfaces 2021, 13, 21854–21864. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cong, Y.; Fu, J. Stretchable and tough conductive hydrogels for flexible pressure and strain sensors. J. Mater. Chem. B 2020, 8, 3437–3459. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gu, S.; Jia, F.; Wang, Q.; Gao, G.-H. “All-in-one”hydrolyzed keratin protein-modified polyacrylamide composite hydrogel transducer. Chem. Eng. J. 2020, 398, 125555. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Wang, L. Flexible and wearable strain sensors based on tough and self-adhesive ion conducting hydrogels. J. Mater. Chem. B 2019, 7, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yan, L.; Wang, K.-F.; Fang, L.-M.; Zhang, H.-P. Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality. NPG Asia Mater. 2017, 9, e372. [Google Scholar] [CrossRef]
- Hu, H.; Tian, H.; Shao, J.; Li, X.-M.; Wang, Y. Discretely Supported Dry Adhesive Film Inspired by Biological Bending Behavior for Enhanced Performance on a Rough Surface. ACS Appl. Mater. Interfaces 2017, 9, 7752–7760. [Google Scholar] [CrossRef] [PubMed]
- Kank, M.-K.; Jeong, H.-E.; Suk, K.-Y. Rational Design and Enhanced Biocompatibility of a Dry Adhesive Medical Skin Patch. Adv. Mater. 2011, 23, 3949–3953. [Google Scholar]
- Liao, M.; Wan, P.; Wen, J.; Gong, M.; Wu, X.-X. Wearable, Healable, and Adhesive Epidermal Sensors Assembled from Mussel-Inspired Conductive Hybrid Hydrogel Framework. Adv. Funct. Mater. 2017, 27, 1703852. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Zhong, W. Mussel-inspired hydrogel tissue adhesives for wound closure. RSC Adv. 2017, 7, 47380–47396. [Google Scholar] [CrossRef]
- Xie, C.; Wang, X.; He, H. Mussel-Inspired Hydrogels for Self-Adhesive Bioelectronics. Adv. Funct. Mater. 2020, 30, 1909954. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Napiwocki, B.-N.; Peng, X.-F. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Xie, Z.-H.; Chen, Z.; Hu, X.-S.; Mi, H.-Y.; Zhou, J.; Li, H. Ultrastretchable, self-healable and adhesive composite organohydrogels with a fast response for human–machine interface applications. J. Mater. Chem. C 2022, 10, 8266–8277. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, G.; Ren, X. Graphene assisted ion-conductive hydrogel with super sensitivity for strain sensor. Polymer 2021, 215, 123340. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, H.; Lan, J.-N.; Wu, H.; Wang, L.-J.; Zhou, J.-P. Dual-network polyacrylamide/carboxymethyl chitosan-grafted-polyaniline conductive hydrogels for wearable strain sensors. Carbohydr. Polym. 2022, 295, 119848. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Wu, S.; Ma, Y. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Pi, M.-H.; Ran, R. High-strength, highly conductive and woven organic hydrogel fibers for flexible electronics. Chem. Eng. J. 2022, 428, 131172. [Google Scholar] [CrossRef]
- Pang, H.; Chen, C.; Zhang, Y.-C. The effect of electric field, annealing temperature and filler loading on the percolation threshold of polystyrene containing carbon nanotubes and graphene nanosheets. Carbon 2011, 49, 1980–1988. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Lee, K.-H.; Anjum, D.-H.; Jiang, Q.; Kim, H. MXenes stretch hydrogel sensor performance to new limits. Sci. Adv. 2018, 4, eaat0098. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Cho, K.-G.; Cho, D.-H.; Hong, K.; Lee, K.-H. Ultra-Sensitive and Stretchable Ionic Skins for High-Precision Motion Monitoring. Adv. Funct. Mater. 2021, 31, 2010199. [Google Scholar] [CrossRef]
- Hozumi, S.; Honda, S.; Arie, T.; Akita, S.; Takei, K. Multimodal Wearable Sensor Sheet for Health-Related Chemical and Physical Monitoring. ACS Sens. 2021, 6, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Su, G.; Yin, S.; Guo, Y. Balancing the mechanical, electronic, and self-healing properties in conductive self-healing hydrogel for wearable sensor applications. Mater. Horiz. 2021, 8, 1795–1804. [Google Scholar] [CrossRef]
- Wang, C.; Yu, H.-Y.; Miao, Z.-Y.; Ge, D.; Yao, J.-M. Interface Growth of PANI-ZnO Nanohybrids on a Self-Formed Grapefruit Peel Aerogel to Construct a Quick Self-Restored Gas Sensor. ACS Sustain. Chem. Eng. 2022, 10, 6573–6583. [Google Scholar] [CrossRef]
- Wang, S.-J.; Jing, X.; Chen, Z.; Hu, X.-S.; Zou, J.; Mi, H.-Y.; Zhang, Z. MXene reinforced organohydrogels with ultra-stability, high sensitivity and anti-freezing ability for flexible strain sensors. J. Mater. Chem. C 2022, 10, 11914–11923. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Li, Z.; Yue, M.-Q.; Qiang, X.-L.; Zhang, P.-X. PVA/SA/MXene dual-network conductive hydrogel for wearable sensor to monitor human motions. J. Appl. Polym. Sci. 2022, 139, 51627. [Google Scholar] [CrossRef]
- Jin, X.-H.; Wei, Z.; Heng, L.; Yu, X.-G.; Ding, S.; Wu, C.-W. An autonomous self-healing hydrogel with high polydopamine content for improved tensile strength. J. Mater. Sci. 2020, 55, 17255–17265. [Google Scholar]
- Li, Y.-Q.; Liu, X.-H.; Gong, Q. Facile preparation of stretchable and self-healable conductive hydrogels based on sodium alginate/polypyrrole nanofibers for use in flexible supercapacitor and strain sensors. Int. J. Biol. Macromol. 2021, 172, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Pana, L.-J.; Yu, G.-H.; Zhai, D.-Y. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-T.; He, W.-N.; Lu, Y. Self-crosslinked polyaniline hydrogel electrodes for electrochemical energy storage. Carbon 2015, 92, 133–141. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Liu, Y.-J.; Peng, X.-F.; Turng, L.-S. Highly Stretchable and Biocompatible Strain Sensors Based on Mussel-Inspired Super-Adhesive Self-Healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 20897–20909. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; He, Y.; An, R.; Wang, X.-D.; Zhang, Y.-L.; Shi, L.-Y. Mussel-inspired, robust and self-healing nanocomposite hydrogels: Effective reusable absorbents for removal both anionic and cationic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 569, 18–27. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, C.; Sun, X. Electrical Contact Resistance of Contact Bodies with Cambered Surface. IEEE Access 2020, 8, 93857–93867. [Google Scholar] [CrossRef]
- Slade, P.-G. Electrical Contacts: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2014; ISBN 1138077100. [Google Scholar]
- Ta, W.; Qiu, S.; Wang, Y.; Yuan, J.-Y.; Gao, Y.-W.; Zhao, Y.-H. Volumetric contact theory to electrical contact between random rough surfaces. Tribol. Int. 2021, 160, 107007. [Google Scholar] [CrossRef]
- Popov, V.-L. Contact Mechanics and Friction: Physical Principles and Applications; Springer: Cham, Switzerland, 2010; ISBN 978-3-642-10802-0. [Google Scholar]
- Sano, Y. Effect of Space Angle of Constriction Resistance and Contact Resistance for the Case of Line Contact. IEEE Trans. Compon. Hybrids Manuf. Technol. 1985, 8, 228–234. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Chen, Z.; Wang, S.-J.; Liu, Z.-H.; Liu, Y.-J.; Feng, P.-Y.; Jing, X. A Flexible Sensor with Excellent Environmental Stability Using Well-Designed Encapsulation Structure. Polymers 2023, 15, 2308. https://doi.org/10.3390/polym15102308
Zou J, Chen Z, Wang S-J, Liu Z-H, Liu Y-J, Feng P-Y, Jing X. A Flexible Sensor with Excellent Environmental Stability Using Well-Designed Encapsulation Structure. Polymers. 2023; 15(10):2308. https://doi.org/10.3390/polym15102308
Chicago/Turabian StyleZou, Jian, Zhuo Chen, Sheng-Ji Wang, Zi-Hao Liu, Yue-Jun Liu, Pei-Yong Feng, and Xin Jing. 2023. "A Flexible Sensor with Excellent Environmental Stability Using Well-Designed Encapsulation Structure" Polymers 15, no. 10: 2308. https://doi.org/10.3390/polym15102308




