Thermal Conductivity of Polyvinylidene Fluoride Films with a Multi-Scale Framework
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Processing
3. Characterizations
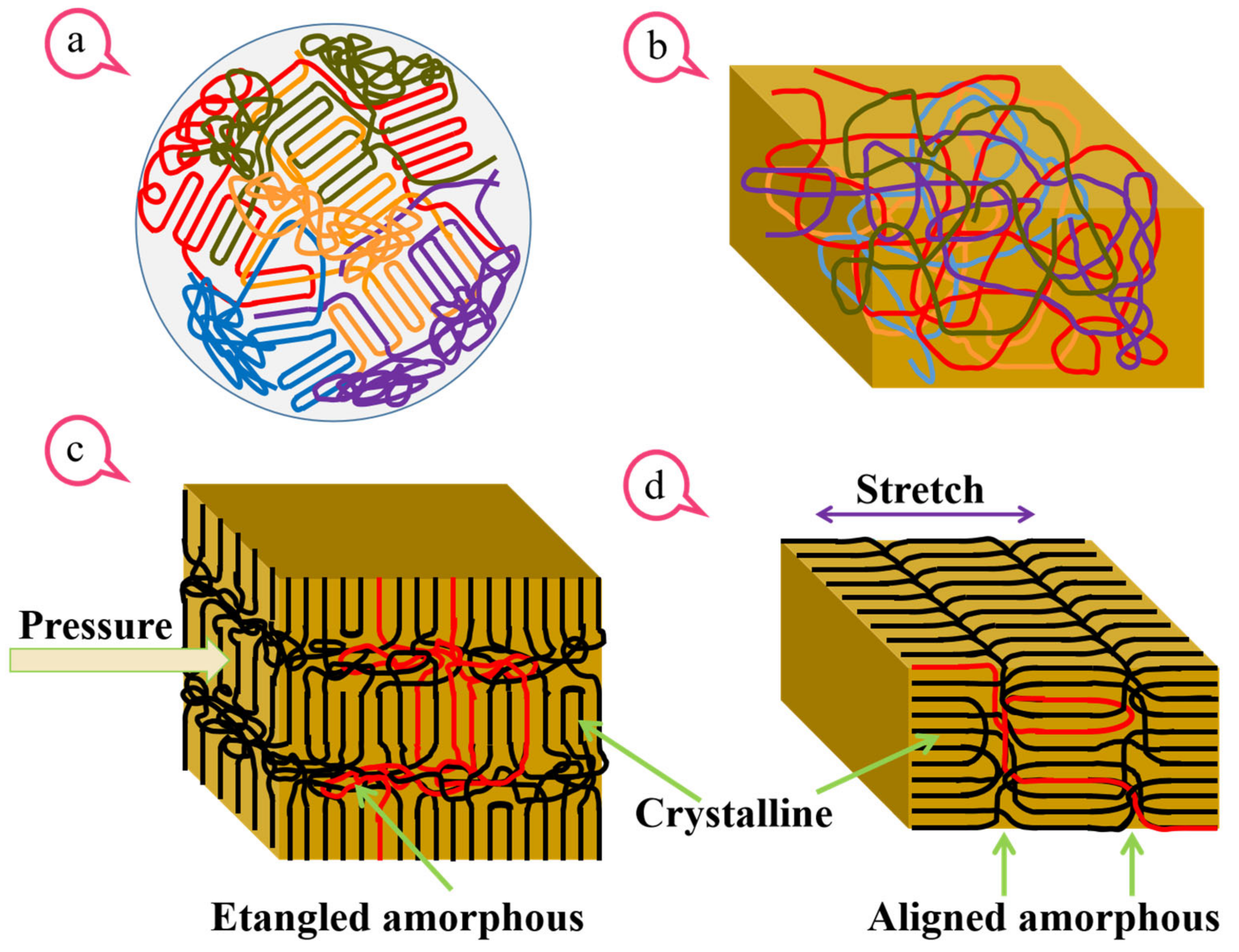
4. Results and Discussion of BONs
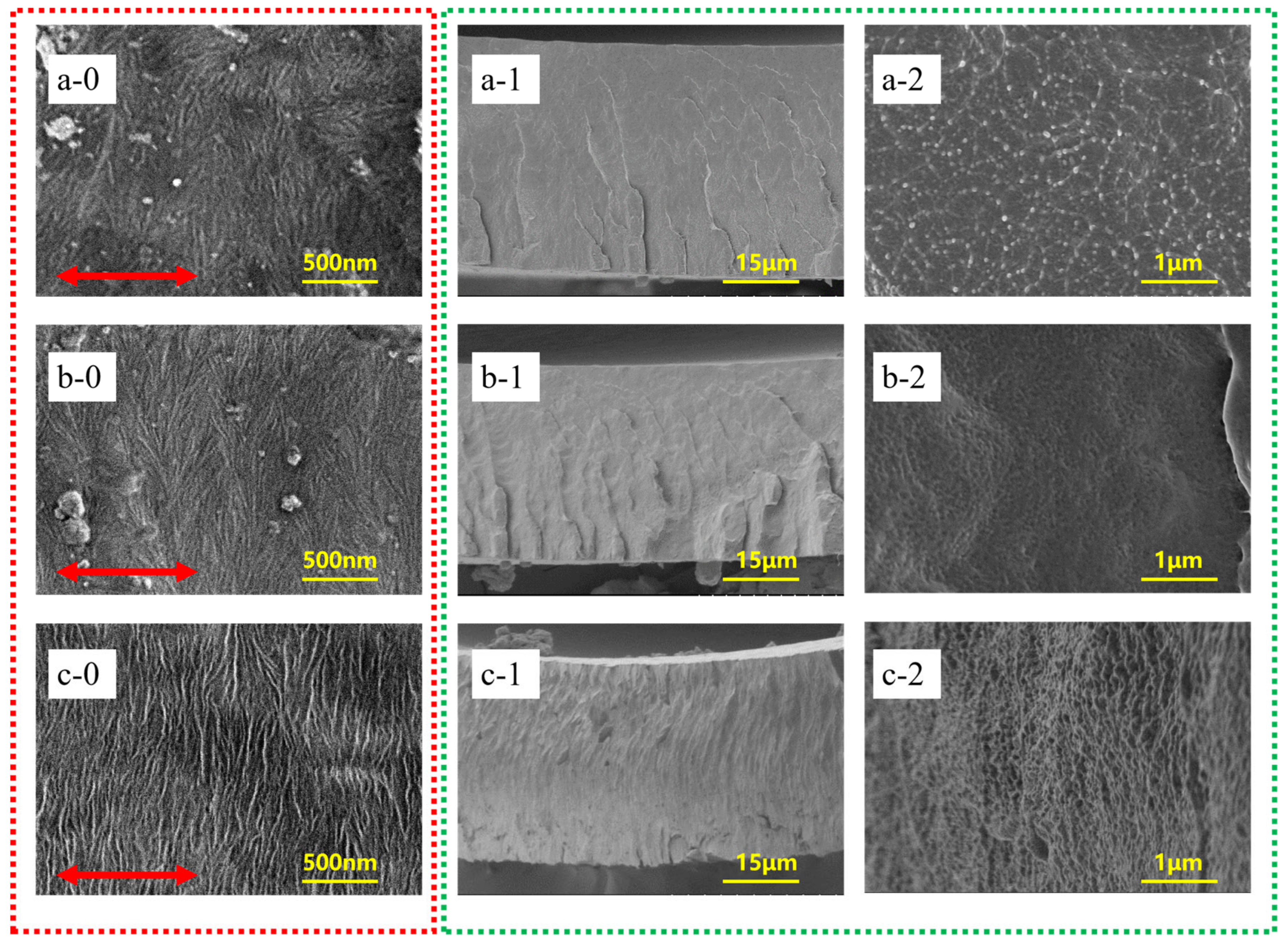
4.1. SEM Morphology of BONs Films
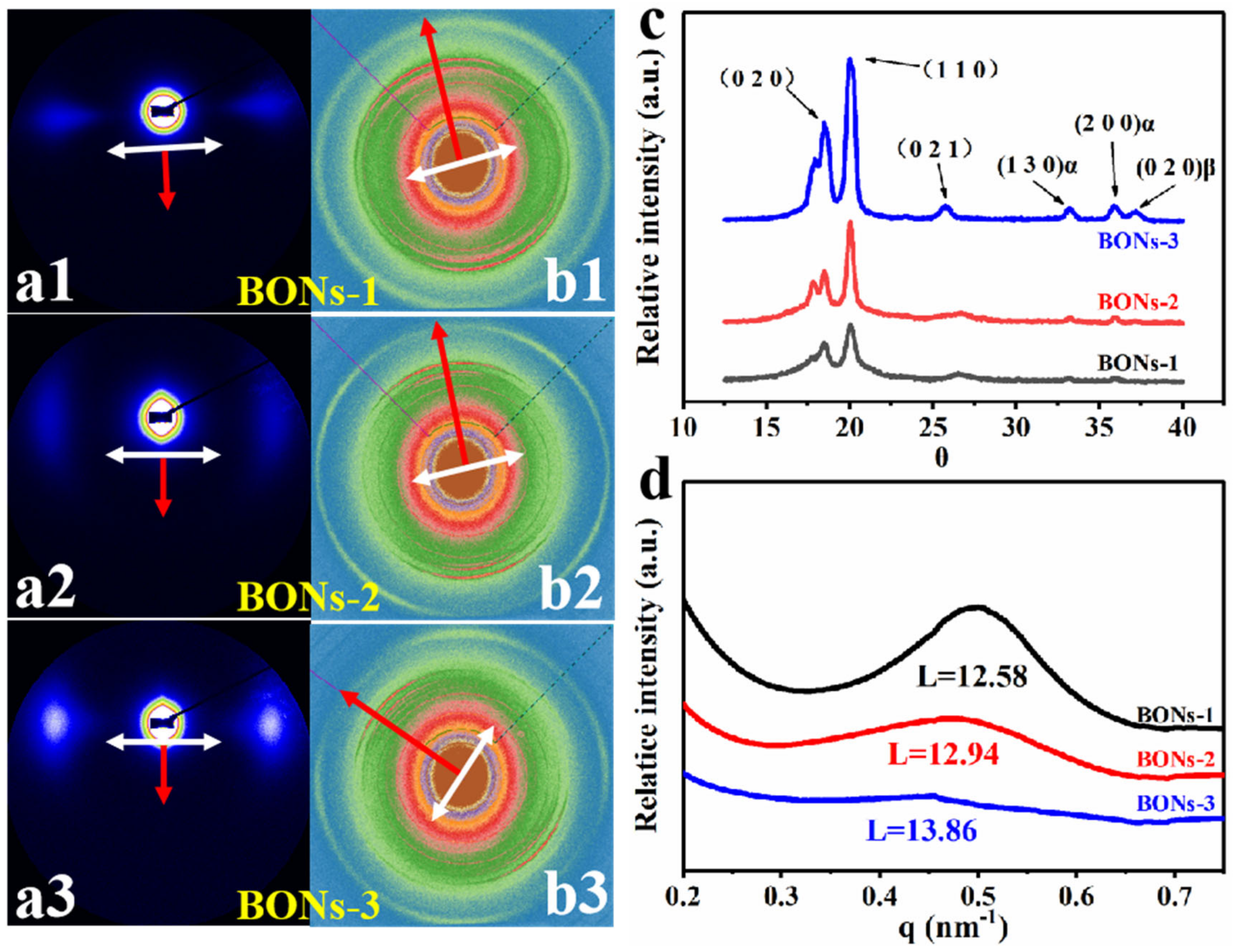
4.2. Atomic-Scale and Nanoscale Structure of BONs Films
4.3. Thermal Anisotropy Analysis of the Amorphous Region
4.4. Physical Properties of BONs Films
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wieme, T.; Tang, D.; Delva, L.; D’Hooge, D.R.; Cardon, L. The relevance of material and processing parameters on the thermal conductivity of thermoplastic composites. Polym. Eng. Sci. 2018, 58, 466–474. [Google Scholar] [CrossRef]
- Guo, H.; Hu, B.; Wang, Q.; Liu, J.; Li, M.; Li, B. Horizontally aligned graphene/silver heterostructure for anisotropically highly thermoconductive polymer-based composites by stress-induced assembly. Appl. Surf. Sci. 2023, 615, 156404. [Google Scholar] [CrossRef]
- Luo, S.; Zeng, Z.; Wang, H.; Xiong, W.; Song, B.; Zhou, C.; Duan, A.; Tan, X.; He, Q.; Zeng, G.; et al. Recent progress in conjugated microporous polymers for clean energy: Synthesis, modification, computer simulations, and applications. Prog. Polym. Sci. 2021, 115, 101374. [Google Scholar] [CrossRef]
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef]
- Pourhashem, S.; Seif, A.; Saba, F.; Nezhad, E.G.; Ji, X.; Zhou, Z.; Zhai, X.; Mirzaee, M.; Duan, J.; Rashidi, A.; et al. Antifouling nanocomposite polymer coatings for marine applications: A review on experiments, mechanisms, and theoretical studies. J. Mater. Sci. Technol. 2022, 118, 73–113. [Google Scholar] [CrossRef]
- Fei, F.; Kotak, P.; He, L.; Li, X.; Vanderhoef, C.; Lamuta, C.; Song, X. Cephalopod-Inspired Stretchable Self-Morphing Skin Via Embedded Printing and Twisted Spiral Artificial Muscles. Adv. Funct. Mater. 2021, 31, 2105528. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X.; Yang, Z.; Zhuang, X.; Guo, H.; Luo, X.; Chen, J.; Liang, Y.; Chen, Y. Effects of Chain Length, Stretching, and Molecular Groups on the Thermal Conductivity of Single Crosslinked Epoxy Resin Chains. J. Electron. Mater. 2023, 52, 2831–2842. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Lan, Z.; Li, C.; Yu, Y.; Wei, J. Colorless Semi-Alicyclic Copolyimides with High Thermal Stability and Solubility. Polymers 2019, 11, 1319. [Google Scholar] [CrossRef]
- Tan, X.; Yuan, Q.; Qiu, M.; Yu, J.; Jiang, N.; Lin, C.-T.; Dai, W. Rational design of graphene/polymer composites with excellent electromagnetic interference shielding effectiveness and high thermal conductivity: A mini review. J. Mater. Sci. Technol. 2022, 117, 238–250. [Google Scholar] [CrossRef]
- Rostom, S.; Dadmun, M.D. Improving heat transfer in fused deposition modeling with graphene enhances inter filament bonding. Polym. Chem. 2019, 10, 5967–5978. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.; Drummer, D.; Liu, C.; Shen, W.; Tomiak, F.; Schneider, K.; Liu, X.; Chen, Q. Highly thermally conductive polybenzoxazine composites based on boron nitride flakes deposited with copper particles. Mater. Des. 2020, 191, 108698. [Google Scholar] [CrossRef]
- Bhanushali, S.; Ghosh, P.C.; Simon, G.P.; Cheng, W. Copper Nanowire-Filled Soft Elastomer Composites for Applications as Thermal Interface Materials. Adv. Mater. Interfaces 2017, 4, 1700387. [Google Scholar] [CrossRef]
- Wang, L.; Han, D.; Luo, J.; Li, T.; Lin, Z.; Yao, Y. Highly Efficient Growth of Boron Nitride Nanotubes and the Thermal Conductivity of Their Polymer Composites. J. Phys. Chem. C 2018, 122, 1867–1873. [Google Scholar] [CrossRef]
- Ding, D.; Wang, H.; Wu, Z.; Chen, Y.; Zhang, Q. Highly Thermally Conductive Polyimide Composites via Constructing 3D Networks. Macromol. Rapid Commun. 2019, 40, e1800805. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, X.; Li, B.; Wang, J.; Wang, S. Thermal conductivity of graphene/poly(vinylidene fluoride) nanocomposite membrane. Mater. Des. 2017, 114, 355–363. [Google Scholar] [CrossRef]
- Park, K.-R.; Cho, H.-B.; Lim, M.; Jang, B.K.; Lee, J.; Jeon, B.; Choa, Y.-H. Through-plane high thermal conducting networks via incorporation of graphene nanoplatelets in nanocomposite film under electric field and avoiding breakdown voltage. Appl. Surf. Sci. 2021, 551, 149201. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, W.; Wang, Y.; Liu, C.; Liu, X.; Cui, S. Polyurethane-templated 3D BN network for enhanced thermally conductive property of epoxy composites. Polymer 2021, 235, 124239. [Google Scholar] [CrossRef]
- Su, Z.; Wang, H.; He, J.; Guo, Y.L.; Qu, Q.Q.; Tian, X.Y. Fabrication of Thermal Conductivity Enhanced Polymer Composites by Constructing an Oriented Three-dimensional Staggered Interconnected Network of Boron Nitride Platelets and Carbon Nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 36342–36351. [Google Scholar] [CrossRef]
- Li, Y.; Gong, C.; Li, C.; Ruan, K.; Liu, C.; Liu, H.; Gu, J. Liquid crystalline texture and hydrogen bond on the thermal conductivities of intrinsic thermal conductive polymer films. J. Mater. Sci. Technol. 2021, 82, 250–256. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, K.; Jiao, E.; Liu, Y.; Shi, J.; Lu, M. Self-assembled supramolecule for synthesizing highly thermally conductive Cellulose/Carbon nitride nanocomposites with improved flame retardancy. J. Colloid Interface Sci. 2022, 608, 2560–2570. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.; Chen, G. High Thermal Conductivity of Single Polyethylene Chains Using Molecular Dynamics Simulations. Phys. Rev. Lett. 2008, 101, 235502. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Xia, R.; Wu, B.; Chen, P.; Qian, J.-S.; Liang, H.-J. Effect of Chain Configuration on Thermal Conductivity of Polyethylene—A Molecular Dynamic Simulation Study. Chin. J. Polym. Sci. 2020, 38, 1418–1425. [Google Scholar] [CrossRef]
- Shen, S.; Henry, A.; Tong, J.; Zheng, R.; Chen, G. Polyethylene nanofibres with very high thermal conductivities. Nat. Nanotechnol. 2010, 5, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kraemer, D.; Song, B.; Jiang, Z.; Zhou, J.; Loomis, J.; Wang, J.; Li, M.; Ghasemi, H.; Huang, X.; et al. Nanostructured polymer films with metal-like thermal conductivity. Nat. Commun. 2019, 10, 1771. [Google Scholar] [CrossRef]
- Singh, V.; Bougher, T.L.; Weathers, A.; Cai, Y.; Bi, K.; Pettes, M.; McMenamin, S.A.; Lv, W.; Resler, D.P.; Gattuso, T.R.; et al. High thermal conductivity of chain-oriented amorphous polythiophene. Nat. Nanotechnol. 2014, 9, 384–390. [Google Scholar] [CrossRef]
- Lu, C.; Chiang, S.W.; Du, H.; Li, J.; Gan, L.; Zhang, X.; Chu, X.; Yao, Y.; Li, B.; Kang, F. Thermal conductivity of electrospinning chain-aligned polyethylene oxide (PEO). Polymer 2017, 115, 52–59. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, T. Morphology-influenced thermal conductivity of polyethylene single chains and crystalline fibers. J. Appl. Phys. 2012, 112, 094304. [Google Scholar] [CrossRef]
- Yu, J.; Sundqvist, B.; Tonpheng, B.; Andersson, O. Thermal conductivity of highly crystallized polyethylene. Polymer 2014, 55, 195–200. [Google Scholar] [CrossRef]
- Huang, Y.F.; Wang, Z.G.; Yu, W.C.; Ren, Y.; Lei, J.; Xu, J.Z.; Li, Z.M. Achieving high thermal conductivity and mechanical rein-forcement in ultrahigh molecular weight polyethylene bulk material. Polymer 2019, 180, 121760. [Google Scholar] [CrossRef]
- Ronca, S.; Igarashi, T.; Forte, G.; Rastogi, S. Metallic-like thermal conductivity in a lightweight insulator: Solid-state processed Ultra High Molecular Weight Polyethylene tapes and films. Polymer 2017, 123, 203–210. [Google Scholar] [CrossRef]
- Dai, W.; Lv, L.; Ma, T.; Wang, X.; Ying, J.; Yan, Q.; Tan, X.; Gao, J.; Xue, C.; Yu, J.; et al. Multiscale Structural Modulation of Anisotropic Graphene Framework for Polymer Composites Achieving Highly Efficient Thermal Energy Management. Adv. Sci. 2021, 8, 2003734. [Google Scholar] [CrossRef]
- Guo, H.; Li, X.; Wang, Z.; Li, B.; Wang, J.; Wang, S. Thermal conductivity of PVDF/PANI-nanofifiber composite membrane aligned in an electric field. Chin. J. Chem. Eng. 2018, 26, 1213–1218. [Google Scholar] [CrossRef]
- Long, J.; Songyuan, M.; Deng, W.L. Polarization-free high-crystallization β-PVDF piezoelectric nanogenerator toward self-powered 3D acceleration sensor. Nano Energy 2018, 50, 632–638. [Google Scholar]
- Castagnet, S.; Gacougnolle, J.-L.; Dang, P. Correlation between macroscopical viscoelastic behaviour and micromechanisms in strained α polyvinylidene fluoride (PVDF). Mater. Sci. Eng. A 2000, 276, 152–159. [Google Scholar] [CrossRef]
- Henry, A.; Chen, G.; Plimpton, S.J.; Thompson, A. 1D-to-3D transition of phonon heat conduction in polyethylene using mo-lecular dynamics simulations. Phys. Rev. B 2010, 82, 144308. [Google Scholar] [CrossRef]
- Yu, J.C.; Tonpheng, B.; Andersson, O. High-Pressure-Induced Microstructural Evolution and Enhancement of Thermal Prop-erties of Nylon-6. Macromolecules 2010, 43, 10512–10520. [Google Scholar] [CrossRef]
- Wu, C.-F.; Arifin, D.E.S.; Wang, C.-A.; Ruan, J. Coalescence and split of high-entropy polymer lamellar cocrystals. Polymer 2018, 138, 188–202. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Li, Y.; Xu, J.-T.; Fan, Z.-Q. Cooperative Effect of Electrospinning and Nanoclay on Formation of Polar Crystalline Phases in Poly(vinylidene fluoride). ACS Appl. Mater. Interfaces 2010, 2, 1759–1768. [Google Scholar] [CrossRef]
- He, L.; Sun, J.; Wang, X.; Wang, C.; Song, R.; Hao, Y. Facile and effective promotion of β crystalline phase in poly(vinylidene fluoride) via the incorporation of imidazolium ionic liquids. Polym. Int. 2013, 62, 638–646. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Samoila, P.; Olaru, N.; Bele, A.; Airinei, A. Novel electrospun membranes based on PVDF fibers embedding lanthanide doped ZnO for adsorption and photocatalytic degradationof dye organic pollutants. Mater. Res. Bull. 2021, 141, 111376. [Google Scholar] [CrossRef]
- Cai, X.M.; Lei, T.P.; Sun, D.H.; Lin, L.W. A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Q.; Liu, J.; Du, C.; Li, B. Improved interfacial properties for largely enhanced thermal conductivity of poly(vinylidene fluoride)-based nanocomposites via functionalized multi-wall carbon nanotubes. Appl. Surf. Sci. 2019, 487, 379–388. [Google Scholar] [CrossRef]
- Choy, C.L. Thermal conductivity of polymers. Polymer 1977, 18, 984–1003. [Google Scholar] [CrossRef]
- Choy, C.L.; Chen, F.C.; Luk, W.H. Thermal conductivity of oriented crystalline polymers. J. Polym. Sci. Polym. Phys. Ed. 1980, 18, 1187–1207. [Google Scholar] [CrossRef]
- Picken, S.J.; Aerts, J.; Visser, R.; Northolt, M.G. Structure and rheology of aramidsolutions: X-ray scattering measurements. Macromolecules 1990, 23, 3849–3854. [Google Scholar] [CrossRef]
- Choy, C.; Young, K. Thermal conductivity of semicrystalline polymers—A model. Polymer 1977, 18, 769–776. [Google Scholar] [CrossRef]
- Sasabe, H.; Saito, S.; Asahina, M.; Kakutani, H. Dielectric relaxations in poly(vinylidene fluoride). J. Polym. Sci. Part A-2 Polym. Phys. 1969, 7, 1405–1414. [Google Scholar] [CrossRef]
- Yano, S. Dielectric relaxation and molecular motion in poly(vinylidene fluoride). J. Polym. Sci. Part A-2 Polym. Phys. 1970, 8, 1057–1072. [Google Scholar] [CrossRef]
- Kishimoto, M.; Takenaka, M.; Iwabuki, H. Spatial Distribution of the Amorphous Region Constrained by Polymer Crystallites. Macromolecules 2023, 56, 207–214. [Google Scholar] [CrossRef]
- Chmelař, J.; Pokorný, R.; Schneider, P.; Smolná, K.; Bělský, P.; Kosek, J. Free and constrained amorphous phases in polyethylene: Interpretation of 1H NMR and SAXS data over a broad range of crystallinity. Polymer 2015, 58, 189–198. [Google Scholar] [CrossRef]
- Righetti, M.C.; Prevosto, D.; Tombari, E. Time and Temperature Evolution of the Rigid Amorphous Fraction and Differently Constrained Amorphous Fractions in PLLA. Macromol. Chem. Phys. 2016, 217, 2013–2026. [Google Scholar] [CrossRef]
- Dorp, E.R.-V.; Möginger, B.; Hausnerova, B. Thermal expansion of semi-crystalline polymers: Anisotropic thermal strain and crystallite orientation. Polymer 2020, 191, 122249. [Google Scholar] [CrossRef]
- Kim, G.-H.; Lee, D.; Shanker, A.; Shao, L.; Kwon, M.S.; Gidley, D.W.; Kim, J.; Pipe, K.P. High thermal conductivity in amorphous polymer blends by engineered interchain interactions. Nat. Mater. 2015, 14, 295–300. [Google Scholar] [CrossRef]
- Zi, Y.-C.; Wu, G.-J.; Pei, D.-X.; Guo, S.; Qi, S.-L.; Tian, G.-F.; Wu, D.-Z. Decreasing the CTE of Thermoplastic Polyimide by Blending with a Thermosetting System. Chin. J. Polym. Sci. 2023, 41, 1–6. [Google Scholar] [CrossRef]
- Buck, W.; Rudtsch, S. Thermal properties. In Springer Handbook of Materials Measurement Methods; Czichos, H., Saito, T., Smith, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 399–429. [Google Scholar]
- ASTM C177-13; Standard Test Method for Steady State Heat Flux Measurements and Thermal transmission Properties by Mean of the Guarded Hot Plate Apparatus. American Society for Testing and Materials: West Conshohocken, PA, USA, 2013; 23p.
- ISO 8302:1991; Determination of Steady-State Thermal Resistance and Related Properties – Guarded Hot Plate Apparatus. International Standard Organization: Genova, Switzerland, 2013; 47p.
- EN 12667: 2001; Thermal Performance of Building Materials and Products—Determination of Thermal Resistance by Means of Guarded Hot Plate and Heat Flflow Meter Methods—Products of High and Medium Thermal Resistance. European Committee for Standardization: Brussels, Belgium, 2001; 52p.
- Corsan, J.M. Axial heat flflow methods of thermal conductivity measurementfor good conducting materials. In Compendium of Thermophysical Property Measurement Methods. Recommended Measurement Techniques and Practices; Maglic, K.D., Cezairliyan, A., Peletsky, V.E., Eds.; Plenum Press: New York, NY, USA, 1992; Volume 2, pp. 3–31. [Google Scholar]
- Maglic, K.D.; Cezairliyan, A.; Peletsky, V.E. Compendium of Thermophysical Property Measurement Methods, Survey of Measurement Techniques; Plenum Press: New York, NY, USA, 1984; Volume 1, pp. 11–40. [Google Scholar]
- ASTM E1225-13; Standard Test Method for Thermal Conductivity of Solids Using the Guarded-Comparative-Longitudinal Heat Flflow Technique. American Society for Testing and Materials: West Conshohocken, PA, USA, 2013; 10p.
- ASTM C518-10; Standard Test Method for Steady-State Thermal Transmission Properties by Means of The Heat Flflow Meter Apparatus. American Society for Testing and Materials: West Conshohocken, PA, USA, 2010; 16p.
- ASTME1530-11; Standard Test Method for Evaluating the Resistance of Thermal Transmission of Materials by the Guarded Heat Flflow Meter Technique. American Society for Testing and Materials: West Conshohocken, PA, USA, 2011; 9p.
- ISO 8301:1991; Thermal Insulation—Determination of Steady-State Thermal Resistance and Related Properties – Heat Flflow Meter Apparatus. International Standard Organization: Genova, Switzerland, 2014; 38p.
- ISO 8497:1994; Thermal Insulation—Determination of Steady-State Thermal Transmission Properties of Thermal Insulation for Circular Pipes. International Standard Organization: Genova, Switzerland, 2013; 16p.
- ASTM E1461-13; Standard Test Method for Thermal Diffusivity by the Flash Method. American Society for Testing and Materials: West Conshohocken, PA, USA, 2013; 11p.
- ISO 22007-4:2008; Plastics—Determination of Thermal Conductivity and Thermal Diffusivity—Part 4; Laser Flash Method. International Standard Organization: Genova, Switzerland, 2011; 12p.
- ISO 18755:2005; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Thermal Diffusivity of Monolithic Ceramics by Laser Flflash Method. International Standard Organization: Genova, Switzerland, 2014; 31p.
- ASTM C1113/C1113M—09(2013); Standard Test Method for Thermal Conductivity of Refractories by Hot Wire (Platinum Resistance Thermometer Technique). American Society for Testing and Materials: West Conshohocken, PA, USA, 2013; 6p.
- ISO 8894-1:2010; Refractory Materials—Determination of Thermal Conductivity—Part 1; Hot-Wire Methods (Cross-Array and Resistance Thermometer). International Standard Organization: Genova, Switzerland, 2015; 19p.
- ISO 8894-2:2007; Refractory Materials—Determination for Thermal Conductivity—Part 2; Hot-Wire Method (Parallel). International Standard Organization: Genova, Switzerland, 2011; 13p.
- Vozar, L. A computer-controlled apparatus for thermal conductivity measurement by the transient hot wire method. J. Therm. Anal. 1996, 46, 495–505. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Lee, S. Precise measurement of thermal conductivity of liquid over a wide temperature range using a transient hot-wire technique by uncertainty analysis. Thermochim. Acta 2012, 542, 18–23. [Google Scholar] [CrossRef]
- Solorzano, E.; Rodriguez-Perez, M.A.; de Saja, J.A. Thermal conductivity of cellular metals measured by the transient plane source method. Adv. Eng. Mater. 2008, 10, 596–602. [Google Scholar] [CrossRef]
- Gustafsson, S.E. Transient plane source techniques for thermalconductivity and thermal-diffusivity measurements of solid materials. Rev. Sci. Instrum. 1991, 62, 797–804. [Google Scholar] [CrossRef]
- ISO 22007-2:2015; Plastics—Determination of Thermal Conductivity and Thermal Diffusivity—Part 2; Transient Plane Heat Source (Hot Disk) Method. International Standard Organization: Genova, Switzerland, 2015; 20p.
- Dos Santos, W.N.; Mummery, P.; Wallwork, A. Thermal diffusivity of polymers by the laser flflash technique. Polym. Test. 2005, 24, 628–634. [Google Scholar] [CrossRef]
- Parker, W.J.; Jenkins, R.J.; Butler, C.P.; Abbott, G.L. Flash method of determining thermal diffusivity, heat capacity and thermal conductivity. J. Appl. Phys. 1961, 23, 1679–1684. [Google Scholar] [CrossRef]

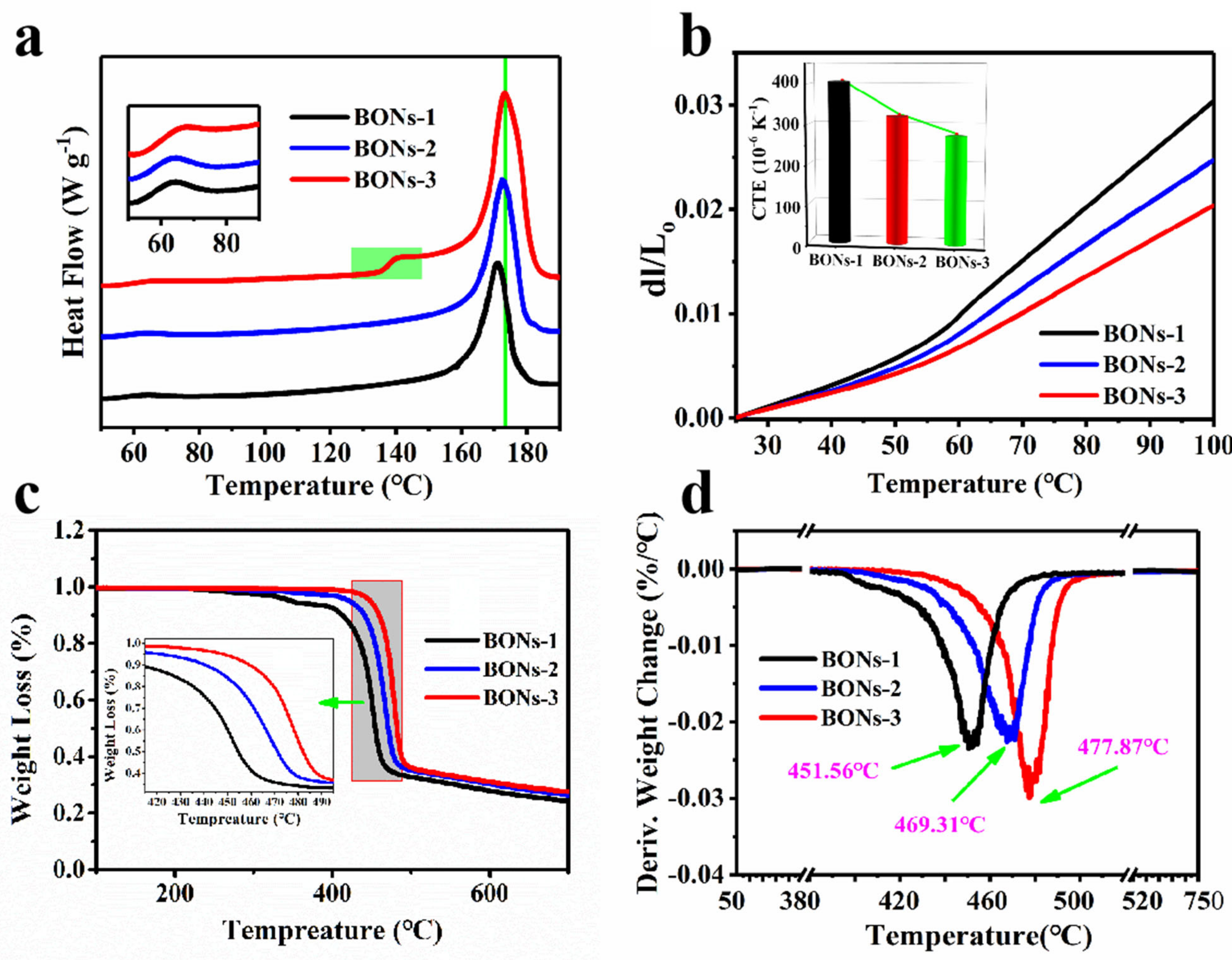
| Sample | Td5% (N2, °C) | Td10% (N2, °C) | Td50% (N2, °C) | Maximum Degradation Temperature (N2, °C) | Char Yield at 800 °C (N2, %) | CTE (10−6, K−1) |
|---|---|---|---|---|---|---|
| BONs-1 | 421.51 | 440.62 | 471.09 | 451.56 | 24.12 | 405.47 |
| BONs-2 | 440.35 | 453.11 | 478.22 | 469.31 | 23.52 | 320 |
| BONs-3 | 448.12 | 459.42 | 482.02 | 477.87 | 24.68 | 270 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Liu, S.; Guo, H.; Hu, B.; Li, Y.; Wang, J.; Li, B. Thermal Conductivity of Polyvinylidene Fluoride Films with a Multi-Scale Framework. Polymers 2023, 15, 2331. https://doi.org/10.3390/polym15102331
Wang Q, Liu S, Guo H, Hu B, Li Y, Wang J, Li B. Thermal Conductivity of Polyvinylidene Fluoride Films with a Multi-Scale Framework. Polymers. 2023; 15(10):2331. https://doi.org/10.3390/polym15102331
Chicago/Turabian StyleWang, Qin, Shixin Liu, Hong Guo, Boyang Hu, Yi Li, Jixiao Wang, and Baoan Li. 2023. "Thermal Conductivity of Polyvinylidene Fluoride Films with a Multi-Scale Framework" Polymers 15, no. 10: 2331. https://doi.org/10.3390/polym15102331





