Current Applications of Bionanocomposites in Food Processing and Packaging
Abstract
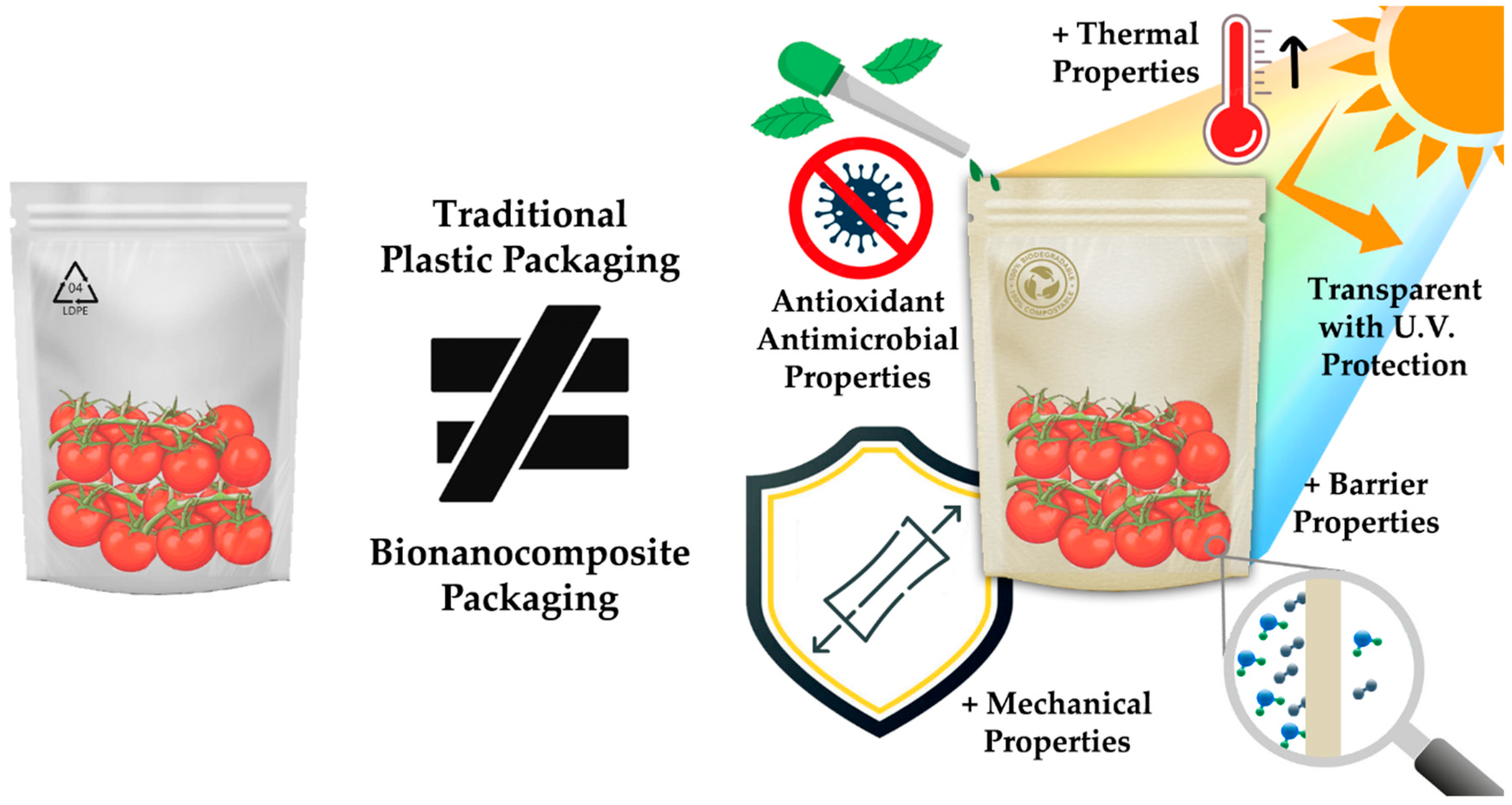
:1. Introduction
2. Bionanocomposites Preparation for Food Processing Applications
2.1. Emulsification Techniques
2.2. Drying Techniques
2.3. Extrusion Techniques
2.4. Electrohydrodynamic Process
2.5. Cryogelation
2.6. Complex Coacervation
3. Bionanocomposites and Nanoparticles for Food Packaging Applications
3.1. Nanoparticles Used in Food Packaging
3.1.1. Nanoclays
3.1.2. Metal and Metal Oxide Nanoparticles
3.1.3. Nanocellulose
3.1.4. Carbon Nanotubes
3.2. Incorporation Techniques
3.3. Examples of Bionanocomposites Application in Food Packaging
3.3.1. Examples of Bionanocomposites as Intelligent Packaging for Food Application
3.3.2. Safety Aspects Related to the Use of Bionanocomposite as Food Contact Materials
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Souza, V.G.L.; Mello, I.P.; Khalid, O.; Pires, J.R.A.; Rodrigues, C.; Alves, M.M.; Santos, C.; Fernando, A.L.; Coelhoso, I. Strategies to Improve the Barrier and Mechanical Properties of Pectin Films for Food Packaging: Comparing Nanocomposites with Bilayers. Coatings 2022, 12, 108. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Sanches-Silva, A. Application of encapsulated essential oils as antimicrobial agents in food packaging. Curr. Opin. Food Sci. 2017, 14, 78–84. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Fuciños, P.; Pastrana, L.M. Nanotechnology as a way for bio-based and biodegradable food packaging with enhanced properties. In Advances in Processing Technologies for Bio-Based Nanosystems in Food; Ramos, O.L., Pereira, R.N., Cerqueira, M.A., Teixeira, J.A., Vicente, A.A., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 233–258. ISBN 978-1-138-03730-4. [Google Scholar]
- Sharma, R.; Ghoshal, G. Emerging trends in food packaging. Nutr. Food Sci. 2018, 48, 764–779. [Google Scholar] [CrossRef]
- Imran, M.; Revol-Junelles, A.-M.; Martyn, A.; Tehrany, E.A.; Jacquot, M.; Linder, M.; Desobry, S. Active food packaging evolution: Transformation from micro- to nanotechnology. Crit. Rev. Food Sci. Nutr. 2010, 50, 799–821. [Google Scholar] [CrossRef]
- Ahari, H.; Soufiani, S.P. Smart and Active Food Packaging: Insights in Novel Food Packaging. Front. Microbiol. 2021, 12, 657233. [Google Scholar] [CrossRef] [PubMed]
- Ahari, H.; Golestan, L.; Anvar, S.A.A.; Cacciotti, I.; Garavand, F.; Rezaei, A.; Sani, M.A.; Jafari, S.M. Bio-nanocomposites as food packaging materials; the main production techniques and analytical parameters. Adv. Colloid Interface Sci. 2022, 310, 102806. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Souza, V.G.L.; Fuciños, P.; Pastrana, L.; Fernando, A.L. Methodologies to Assess the Biodegradability of Bio-Based Polymers—Current Knowledge and Existing Gaps. Polymers 2022, 14, 1359. [Google Scholar] [CrossRef]
- Ghoshal, G. Recent Trends in Active, Smart, and Intelligent Packaging for Food Products; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128115169. [Google Scholar]
- Pires, J.R.A.; Souza, V.G.L.; Fernando, A.L. Valorization of energy crops as a source for nanocellulose production—Current knowledge and future prospects. Ind. Crops Prod. 2019, 140, 111642. [Google Scholar] [CrossRef]
- Pascoal, A.; Quirantes-Piné, R.; Fernando, A.L.; Alexopoulou, E.; Segura-Carretero, A. Phenolic composition and antioxidant activity of kenaf leaves. Ind. Crops Prod. 2015, 78, 116–123. [Google Scholar] [CrossRef]
- Santos, J.C.P.; Sousa, R.C.S.; Otoni, C.G.; Moraes, A.R.F.; Souza, V.G.L.; Medeiros, E.A.A.; Espitia, P.J.P.; Pires, A.C.S.; Coimbra, J.S.R.; Soares, N.F.F. Nisin and other antimicrobial peptides: Production, mechanisms of action, and application in active food packaging. Innov. Food Sci. Emerg. Technol. 2018, 48, 179–194. [Google Scholar] [CrossRef]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Mahdi, S.J. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surfaces B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Giaconia, M.A.; dos Passos Ramos, S.; Pereira, C.F.; Lemes, A.C.; De Rosso, V.V.; Braga, A.R.C. Overcoming restrictions of bioactive compounds biological effects in food using nanometer-sized structures. Food Hydrocoll. 2020, 107, 105939. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, C.; Pires, J.R.A.; Coelhoso, I.; Fernando, A.L. Effectiveness and Release Studies of Bioactive Systems. In Releasing Systems in Active Food Packaging—Preparation and Aplication; Jafari, S.M., Sanches-Silva, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 223–251. ISBN 9783030902988. [Google Scholar]
- Saifullah, M.; Shishi, M.R.I.; Ferdowsi, R.; Rahman, M.R.T.; Vuong, Q. Van Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Sultana, M.; Chan, E.-S.; Pushpamalar, J.; Choo, W.S. Advances in extrusion-dripping encapsulation of probiotics and omega-3 rich oils. Trends Food Sci. Technol. 2022, 123, 69–86. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Wei, C. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Pires, J.; de Paula, C.D.; Souza, V.G.L.; Fernando, A.L.; Coelhoso, I. Understanding the Barrier and Mechanical Behavior of Different Nanofillers in Chitosan Films for Food Packaging. Polymers 2021, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- Marques Mandaji, C.; da Silva Pena, R.; Campos Chisté, R. Encapsulation of bioactive compounds extracted from plants of genus Hibiscus: A review of selected techniques and applications. Food Res. Int. 2022, 151, 110820. [Google Scholar] [CrossRef]
- More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Hougaard Bennekou, S.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: Human and animal health. EFSA J. 2021, 19, e06768. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of Bioplastics: Opportunities and Challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef]
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and implications of nanotechnologies for the food sector. Food Addit. Contam. Part A Chem. Anal. Control. Expo Risk Assess. 2008, 25, 241–258. [Google Scholar] [CrossRef]
- Miteluț, A.C.; Popa, E.E.; Popescu, P.A.; Rapa, M.; Popa, M.E. Soil Ecotoxicity Assessment After Biodegradation of Some Polymeric Materials. Agron. Ser. Sci. Res. 2019, 62, 538–543. [Google Scholar]
- Chew, S.C.; Tan, C.H.; Pui, L.P.; Chong, P.N.; Gunasekaran, B.; Lin, N.K. Encapsulation technologies: A tool for functional foods development. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 154–160. [Google Scholar]
- Rezaei, A.; Fathi, M.; Mahdi, S. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Niu, B.; Shao, P.; Luo, Y.; Sun, P. Recent advances of electrosprayed particles as encapsulation systems of bioactives for food application. Food Hydrocoll. 2020, 99, 105376. [Google Scholar] [CrossRef]
- Khorasani, S.; Yazdi, A.P.G.; Taghavi, E.; Abadi, M.A.A.; Ghobadi, H.; Zihayat, B.; Rasti, B.; Mozafari, M.R. Recent Trends in the Nanoencapsulation Processes for Food and Nutraceutical Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780081005965. [Google Scholar]
- Taheri, A.; Jafari, S.M. Gum-based nanocarriers for the protection and delivery of food bioactive compounds. Adv. Colloid Interface Sci. 2019, 269, 277–295. [Google Scholar] [CrossRef]
- Sharma, S.; Cheng, S.; Bhattacharya, B.; Chakkaravarthi, S. Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: Special emphasis on nanoemulsion-based encapsulation. Trends Food Sci. Technol. 2019, 91, 305–318. [Google Scholar] [CrossRef]
- Charcosset, C.; Limayem, I.; Fessi, H. The membrane emulsification process—A review. J. Chem. Technol. Biotechnol. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Vladisavljević, G.T. Preparation of microemulsions and nanoemulsions by membrane emulsification. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 579, 123709. [Google Scholar] [CrossRef]
- Charcosset, C. Preparation of emulsions and particles by membrane emulsification for the food processing industry. J. Food Eng. 2009, 92, 241–249. [Google Scholar] [CrossRef]
- Kaade, W.; Ferrando, M.; Khanmohammed, A.; Torras, C.; De Lamo-Castellví, S.; Güell, C. Low-energy high-throughput emulsification with nickel micro-sieves for essential oils encapsulation. J. Food Eng. 2019, 263, 326–336. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Zou, Y.; Liang, X.; Peng, Y.; Julian, D.; Hu, K. Encapsulation of resveratrol in zein/pectin core-shell nanoparticles: Stability, bioaccessibility, and antioxidant capacity after simulated gastrointestinal digestion. Food Hydrocoll. 2019, 93, 261–269. [Google Scholar] [CrossRef]
- Tavares, L.; Pelayo, C.; Noreña, Z. Encapsulation of garlic extract using complex coacervation with whey protein isolate and chitosan as wall materials followed by spray drying. Food Hydrocoll. 2018, 89, 360–369. [Google Scholar] [CrossRef]
- Wang, B.; Adhikari, B.; Mathesh, M.; Yang, W.; Barrow, J. Anchovy oil microcapsule powders prepared using two-step complex coacervation between gelatin and sodium hexametaphosphate followed by spray drying. Powder Technol. 2018, 358, 68–78. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Barbosa-Pereira, L.; Vicente, A.A.; Cerqueira, M.A.; Pastrana, L. Dehydration of protein lactoferrin-glycomacropeptide nanohydrogels. Food Hydrocoll. 2020, 101, 105550. [Google Scholar] [CrossRef]
- Nunes, R.; Pereira, B.D.A.; Cerqueira, M.A.; Silva, P.; Pastrana, L.M.; Vicente, A.A.; Martins, J.T.; Bourbon, A.I. Lactoferrin-based nanoemulsions to improve the physical and chemical stability of omega-3 fatty acids. Food Funct. 2020, 11, 1966–1981. [Google Scholar] [CrossRef]
- Pedrozo, R.C.; Antônio, E.; Khalil, N.M.; Mainardes, R.M. Bovine serum albumin–based nanoparticles containing the flavonoid rutin produced by nano spray drying. Braz. J. Pharm. Sci. 2020, 56, 1–8. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, S.; Fleming, E.; Lee, J.Y.; Luo, Y. Chitosan-caseinate-dextran ternary complex nanoparticles for potential oral delivery of astaxanthin with significantly improved bioactivity. Int. J. Biol. Macromol. 2020, 151, 747–756. [Google Scholar] [CrossRef]
- Luo, L.; Lai, J.; Chou, S.; Hsueh, Y.; Ma, D.H. Development of gelatin/ascorbic acid cryogels for potential use in corneal stromal tissue engineering. Acta Biomater. 2018, 65, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Nwabor, O.F.; Singh, S.; Marlina, D.; Voravuthikunchai, S.P. Chemical characterization, release, and bioactivity of Eucalyptus camaldulensis polyphenols from freeze-dried sodium alginate and sodium carboxymethyl cellulose matrix. Food Qual. Saf. 2020, 4, 203–212. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Fabra, M.J.; López-rubio, A. Development of gelatin-coated ι-carrageenan hydrogel capsules by electric field-aided extrusion. Impact of phenolic compounds on their performance. Food Hydrocoll. 2019, 90, 523–533. [Google Scholar] [CrossRef]
- Horuz, T.İ.; Belibağlı, K.B. Nanoencapsulation by electrospinning to improve stability and water solubility of carotenoids extracted from tomato peels. Food Chem. 2018, 268, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Bayat, S.; Pishavar, E.; Kalalinia, F.; Mova, J. Bromelain-loaded chitosan nano fibers prepared by electrospinning method for burn wound healing in animal models. Life Sci. 2019, 229, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Soleimanifar, M.; Jafari, S.M.; Assadpour, E. Encapsulation of olive leaf phenolics within electrosprayed whey protein nanoparticles; production and characterization. Food Hydrocoll. 2020, 101, 105572. [Google Scholar] [CrossRef]
- Schmatz, D.A.; Costa, J.A.V.; de Morais, M.G.; Alberto, J.; Costa, V.; Greque, M.; Morais, D. A novel nanocomposite for food packaging developed by electrospinning and electrospraying. Food Packag. Shelf Life 2019, 20, 100314. [Google Scholar] [CrossRef]
- Benjemaa, M.; Neves, M.A.; Falleh, H.; Isoda, H.; Ksouri, R. Nanoencapsulation of Thymus capitatus essential oil: Formulation process, physical stability characterization and antibacterial efficiency monitoring. Ind. Crops Prod. 2018, 113, 414–421. [Google Scholar] [CrossRef]
- De Campo, C.; Dick, M.; Pereira, P.; Maria, T.; Costa, H.; Paese, K.; Stanisçuaski, S.; De Oliveira, A.; Hickmann, S. Zeaxanthin nanoencapsulation with Opuntia monacantha mucilage as structuring material: Characterization and stability evaluation under different temperatures. Colloids Surf. A 2018, 558, 410–421. [Google Scholar] [CrossRef]
- de Oliveira Cavalcanti Medeiros, A.K.; de Carvalho Gomes, C.; de Araújo Amaral, M.L.Q.; de Medeiros, L.D.G.; Medeiros, I.; Porto, D.L.; Aragão, C.F.S.; Bruna Maciel, L.L.; de Araujo Morais, A.H.; Passos, T.S. Nanoencapsulation improved water solubility and color stability of carotenoids extracted from Cantaloupe melon (Cucumis melo L.). Food Chem. 2019, 270, 562–572. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Cristina, I.; Moraes, F.; Favaro-trindade, C.S. Reducing carotenoid loss during storage by co-encapsulation of pequi and buriti oils in oil-in-water emulsions followed by freeze-drying: Use of heated and unheated whey protein isolates as emulsi fi ers. Food Res. Int. 2020, 130, 108901. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- Rajabi, H.; Mahdi, S.; Rajabzadeh, G.; Sarfarazi, M. Chitosan-gum Arabic complex nanocarriers for encapsulation of saffron bioactive components. Colloids Surf. A 2019, 578, 123644. [Google Scholar] [CrossRef]
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S.M. Nano Spray Drying for Encapsulation of Pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Arpagaus, C.; Cerqueira, M.A.; Samborska, K. Nano spray drying of food ingredients; materials, processing and applications. Trends Food Sci. Technol. 2021, 109, 632–646. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhur, U.; Chakraborty, R. An overview of encapsulation of active compoundsused in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C. Spray-Freeze-Drying of Coffee; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 10, ISBN 9780128158647. [Google Scholar]
- Vishali, D.A.; Monisha, J.; Sivakamasundari, S.K.; Moses, J.A.; Anandharamakrishnan, C. Spray freeze drying: Emerging applications in drug delivery. J. Control. Release 2019, 300, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Elik, A.; Koçak Yanık, D.; Göğüş, F. A comparative study of encapsulation of carotenoid enriched-flaxseed oil and flaxseed oil by spray freeze-drying and spray drying techniques. LWT 2021, 143, 111153. [Google Scholar] [CrossRef]
- Arredondo-Ochoa, T.; Regalado-González, C.; Martín-Belloso, O. Current processing methods in the development of micro- and nanoencapsulation from edible polymers. In Polymers for Food Applications; Springer: Berlin/Heidelberg, Germany, 2018; pp. 423–445. ISBN 9783319946252. [Google Scholar]
- Pimentel-Moral, S.; Verardo, V.; Robert, P.; Segura-Carretero, A.; Martínez-Férez, A. Nanoencapsulation strategies applied to maximize target delivery of intact polyphenols. In Encapsulations; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 559–595. ISBN 9780128043073. [Google Scholar]
- Topuz, F.; Uyar, T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2019, 130, 108927. [Google Scholar] [CrossRef]
- Rogers, Z.J.; Bencherif, S.A. Cryogelation and cryogels. Gels 2019, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.R.; Damasceno, S.; Tatiana, E.; de Carvalho, S.Y.B.; de Carvalho, G.S.; Gontijo, L.A.P.; de Lima Guimarães, L.G. Chitosan nanogels condensed to ferulic acid for the essential oil of Lippia origanoides Kunth encapsulation. Carbohydr. Polym. 2018, 188, 268–275. [Google Scholar] [CrossRef]
- Sengel, S.B.; Sahiner, N. Poly (vinyl phosphonic acid) nanogels with tailored properties and their use for biomedical and environmental applications. Eur. Polym. J. 2016, 75, 264–275. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, B. Introduction to polymeric gels. In Polymeric Gels: Characterization, Properties and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–27. ISBN 9780081021798. [Google Scholar]
- Mohanty, F.; Swain, S.K. Bionanocomposites for Food Packaging Applications. In Nanotechnology Applications in Food: Flavor, Stability, Nutrition and Safety; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 363–379. ISBN 9780128119433. [Google Scholar]
- Genovese, L.; Lotti, N.; Gazzano, M.; Siracusa, V.; Dalla Rosa, M.; Munari, A. Novel biodegradable aliphatic copolyesters based on poly(butylene succinate) containing thioether-linkages for sustainable food packaging applications. Polym. Degrad. Stab. 2016, 132, 191–201. [Google Scholar] [CrossRef]
- Galvis-Sánchez, A.C.; Castro, M.C.R.; Biernacki, K.; Gonçalves, M.P.; Souza, H.K.S. Natural deep eutectic solvents as green plasticizers for chitosan thermoplastic production with controlled/desired mechanical and barrier properties. Food Hydrocoll. 2018, 82, 478–489. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C. De Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.; Fernando, A.L. Novel Approaches for Chitin/Chitosan Composites in the Packaging Industry. In Chitin- and Chitosan-Based Biocomposites for Food Packaging Applications; Jacob, J., Loganathan, S., Thomas, S., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 87–96. ISBN 9780429299605. [Google Scholar]
- Kausar, A. A review of high performance polymer nanocomposites for packaging applications in electronics and food industries. J. Plast. Film Sheeting 2020, 36, 94–112. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Fernandes, F.M.; Wicklein, B.; Alcântara, A.C.S.; Aranda, P. Fibrous clays based bionanocomposites. Prog. Polym. Sci. 2013, 38, 1392–1414. [Google Scholar] [CrossRef]
- Sanches, A.O.; Ricco, L.H.S.; Malmonge, L.F.; Da Silva, M.J.; Sakamoto, W.K.; Malmonge, J.A. Influence of cellulose nanofibrils on soft and hard segments of polyurethane/cellulose nanocomposites and effect of humidity on their mechanical properties. Polym. Test. 2014, 40, 99–105. [Google Scholar] [CrossRef]
- Azevedo, V.M.; Dias, M.V.; Borges, S.V.; Costa, A.L.R.; Silva, E.K.; Medeiros, É.A.A.; Soares, N.D.F.F. Development of whey protein isolate bio-nanocomposites: Effect of montmorillonite and citric acid on structural, thermal, morphological and mechanical properties. Food Hydrocoll. 2015, 48, 179–188. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Sadiku, E.R.; Mochane, M.J.; Ray, S.S.; John, M.J.; Mtibe, A. Mechanical properties of cellulose nanofibril papers and their bionanocomposites: A review. Carbohydr. Polym. 2021, 273, 118507. [Google Scholar] [CrossRef]
- Wu, Q.; Miao, W.; Zhang, Y.; Gao, H.; Hui, D. Mechanical properties of nanomaterials: A review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Jafari, S.M. Impact of metal nanoparticles on the mechanical, barrier, optical and thermal properties of biodegradable food packaging materials. Crit. Rev. Food Sci. Nutr. 2021, 61, 2640–2658. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.G.; Sam, S.T.; bin Abdullah, M.F.; Omar, M.F. Thermal properties of nanocellulose-reinforced composites: A review. J. Appl. Polym. Sci. 2020, 137, 48544. [Google Scholar] [CrossRef]
- Jiménez, A.; Vargas, M.; Chiralt, A. Antimicrobial nanocomposites for food packaging applications: Novel approaches. In Novel Approaches of Nanotechnology in Food; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 347–386. ISBN 9780128043080. [Google Scholar]
- Bagal-Kestwal, D.R.; Pan, M.H.; Chiang, B.-H. Processing Methods for Bionanocomposites. In Bio Monomers Green Polymeric Composite Materials; Wiley and Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 25–55. ISBN 9781119301646. [Google Scholar] [CrossRef]
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Chitosan/montmorillonite bionanocomposites incorporated with rosemary and ginger essential oil as packaging for fresh poultry meat. Food Packag. Shelf Life 2018, 17, 142–149. [Google Scholar] [CrossRef]
- Vilarinho, F.; Vaz, M.F.; Silva, A.S. The use of Montmorillonite (MMT) in Food Nanocomposites: Methods of Incorporation, Characterization of MMT/Polymer Nanocomposites and Main Consequences in the Properties. Recent Pat. Food Nutr. Agric. 2019, 11, 13–26. [Google Scholar] [CrossRef]
- Shankar, S.; Kasapis, S.; Rhim, J.W. Alginate-based nanocomposite films reinforced with halloysite nanotubes functionalized by alkali treatment and zinc oxide nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1824–1832. [Google Scholar] [CrossRef]
- Rezić, I.; Haramina, T.; Rezić, T. Metal nanoparticles and carbon nanotubes—Perfect antimicrobial nano-fillers in polymer based food packaging materials. In Food Packaging; Academic Press: New York, NY, USA, 2017; pp. 497–532. ISBN 978-0-12-804302-8. [Google Scholar]
- Souza, V.G.L.; Alves, M.M.; Santos, C.F.; Ribeiro, I.A.C.; Rodrigues, C.; Coelhoso, I.; Fernando, A.L. Biodegradable chitosan films with ZnO nanoparticles synthesized using food industry by-products—Production and characterization. Coatings 2021, 11, 646. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Singh, S.; Paosen, S.; Vongkamjan, K.; Voravuthikunchai, S.P. Enhancement of food shelf life with polyvinyl alcohol-chitosan nanocomposite films from bioactive Eucalyptus leaf extracts. Food Biosci. 2020, 36, 100609. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Singh, S.; Ontong, J.C.; Vongkamjan, K.; Voravuthikunchai, S.P. Valorization of Wastepaper Through Antimicrobial Functionalization with Biogenic Silver Nanoparticles, a Sustainable Packaging Composite. Waste Biomass Valorization 2021, 12, 3287–3301. [Google Scholar] [CrossRef]
- Marvizadeh, M.M.; Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Jokar, M. Preparation and characterization of bionanocomposite film based on tapioca starch/bovine gelatin/nanorod zinc oxide. Int. J. Biol. Macromol. 2017, 99, 1–7. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; de Fátima Ferreira Soares, N.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, C.; Valente, S.; Pimenta, C.; Pires, J.R.A.; Alves, M.M.; Santos, C.F.; Coelhoso, I.M.; Fernando, A.L.L. Eco-Friendly ZnO/Chitosan Bionanocomposites Films for Packaging of Fresh Poultry Meat. Coatings 2020, 10, 110. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A.A. Modification of physicochemical and thermal properties of starch films by incorporation of TiO2 nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef]
- Alves, M.M.; Andrade, S.M.; Grenho, L.; Fernandes, M.H.; Santos, C.; Montemor, M.F. Influence of apple phytochemicals in ZnO nanoparticles formation, photoluminescence and biocompatibility for biomedical applications. Mater. Sci. Eng. C 2019, 101, 76–87. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Preethi, S.; Abarna, K.; Nithyasri, M.; Kishore, P.; Deepika, K.; Ranjithkumar, R.; Bhuvaneshwari, V.; Bharathi, D. Synthesis and characterization of chitosan/zinc oxide nanocomposite for antibacterial activity onto cotton fabrics and dye degradation applications. Int. J. Biol. Macromol. 2020, 164, 2779–2787. [Google Scholar] [CrossRef]
- Bharimalla, A.K.; Deshmukh, S.P.; Vigneshwaran, N.; Patil, P.G.; Prasad, V.; Deshmukh, S.P.; Vigneshwaran, N.; Patil, P.G.; Prasad, V. Nanocellulose-Polymer Composites for Applications in Food Packaging: Current Status, Future Prospects and Challenges Nanocellulose-Polymer Composites for Applications in Food Packaging: Current. Polym. Plast. Technol. Eng. 2017, 56, 805–823. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P. The effect of nanofillers on the functional properties of biopolymer-based films: A review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Souza, V.G.L.; Fernando, A.L. Production of nanocellulose from lignocellulosic biomass wastes: Prospects and limitations. In Innovation, Engineering and Entrepreneurship; Machado, J., Soares, F., Veiga, G., Eds.; Lecture Notes in Electrical Engineering; Springer International Publishing: Cham, Switzerland, 2019; Volume 505, pp. 719–725. ISBN 978-3-319-91333-9. [Google Scholar]
- Pires, J.R.A.; Souza, V.G.L.; Gomes, L.A.; Coelhoso, I.M.; Godinho, M.H.; Fernando, A.L. Micro and nanocellulose extracted from energy crops as reinforcement agents in chitosan films. Ind. Crops Prod. 2022, 186, 115247. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Niamsap, T.; Lam, N.T.; Sukyai, P. Production of hydroxyapatite-bacterial nanocellulose scaffold with assist of cellulose nanocrystals. Carbohydr. Polym. 2019, 205, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Madenci, E.; Özkılıç, Y.O.; Aksoylu, C.; Asyraf, M.R.M.; Syamsir, A.; Supian, A.B.M.; Mamaev, N. Buckling Analysis of CNT-Reinforced Polymer Composite Beam Using Experimental and Analytical Methods. Materials 2023, 16, 614. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Padua, G.W. Review: Nanocomposites in food packaging. J. Food Sci. 2010, 75, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.M.D.; Ferreira, G.M.D.; Almeida, G.W.R.; Soares, N.F.F.; Pires, A.C.S.; Silva, L.H.M. Thermodynamics of multi-walled carbon nanotube biofunctionalization using nisin: The effect of peptide structure. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123611. [Google Scholar] [CrossRef]
- Avella, M.; De Vlieger, J.J.; Errico, M.E.; Fischer, S.; Vacca, P.; Volpe, M.G. Biodegradable starch/clay nanocomposite films for food packaging applications. Food Chem. 2005, 93, 467–474. [Google Scholar] [CrossRef]
- Uddin, F. Clays, nanoclays, and montmorillonite minerals. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2008, 39, 2804–2814. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Kaczmarek, B.; Furtos, G. Characterization of chitosan composites with various clays. Int. J. Biol. Macromol. 2014, 65, 534–541. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B.; Duarte, M.P.; Coelhoso, I.M.; Fernando, A.L. Bionanocomposites of chitosan/montmorillonite incorporated with Rosmarinus officinalis essential oil: Development and physical characterization. Food Packag. Shelf Life 2018, 16, 148–156. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Mattoso, L.H.C.; McHugh, T.H. Nanocomposites in Food Packaging—A Review. In Advances in Diverse Industrial Applications of Nanocomposites; Reddy, B., Ed.; InTech: London, UK, 2011; p. 550. ISBN 978-953-307-202-9. [Google Scholar]
- Alexandre, E.M.C.; Lourenço, R.V.; Bittante, A.M.Q.B.; Moraes, I.C.F.; Sobral, P.J. do A. Gelatin-based films reinforced with montmorillonite and activated with nanoemulsion of ginger essential oil for food packaging applications. Food Packag. Shelf Life 2016, 10, 87–96. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Santos, T.A.; Cabral, B.R.; de Oliveira, A.C.S.; Dias, M.V.; de Oliveira, C.R.; Borges, S.V. Release of papain incorporated in chitosan films reinforced with cellulose nanofibers. J. Food Process. Preserv. 2021, 45, e15900. [Google Scholar] [CrossRef]
- El Achaby, M.; El Miri, N.; Aboulkas, A.; Zahouily, M.; Bilal, E.; Barakat, A.; Solhy, A. Processing and properties of eco-friendly bio-nanocomposite films filled with cellulose nanocrystals from sugarcane bagasse. Int. J. Biol. Macromol. 2017, 96, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Swaroop, C.; Shukla, M. Nano-magnesium oxide reinforced polylactic acid bio fi lms for food packaging applications. Int. J. Biol. Macromol. 2018, 113, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Wang, L.F.; Rhim, J.W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef]
- Xie, Y.; Niu, X.; Yang, J.; Fan, R.; Shi, J.; Ullah, N.; Feng, X.; Chen, L. Active biodegradable films based on the whole potato peel incorporated with bacterial cellulose and curcumin. Int. J. Biol. Macromol. 2020, 150, 480–491. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Development of emulsion films based on bovine gelatin-nano chitin-nano ZnO for cake packaging. Food Sci. Nutr. 2020, 8, 1303–1312. [Google Scholar] [CrossRef]
- Li, W.; Zheng, K.; Chen, H.; Feng, S.; Wang, W.; Qin, C. Influence of nano titanium dioxide and clove oil on chitosan-starch film characteristics. Polymers 2019, 11, 1418. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.V.; Azevedo, V.M.; Ferreira, L.F.; Oliveira, A.C.S.; Borges, S.V.; Fátima Ferreira Soares, N.; Medeiros, E.A.A.; Deus Souza Carneiro, J. Chitosan-nanocomposites as a food active packaging: Effect of addition of tocopherol and modified montmorillonite. J. Food Process Eng. 2021, 44, e13843. [Google Scholar] [CrossRef]
- Pattarasiriroj, K.; Kaewprachu, P.; Rawdkuen, S. Properties of rice flour-gelatine-nanoclay film with catechin-lysozyme and its use for pork belly wrapping. Food Hydrocoll. 2020, 107, 105951. [Google Scholar] [CrossRef]
- Arezoo, E.; Mohammadreza, E.; Maryam, M.; Abdorreza, M.N. The synergistic effects of cinnamon essential oil and nano TiO2 on antimicrobial and functional properties of sago starch films. Int. J. Biol. Macromol. 2019, 157, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Teymourpour, S.; Abdorreza, M.N.; Nahidi, F. Functional, thermal, and antimicrobial properties of soluble soybean polysaccharide biocomposites reinforced by nano TiO2. Carbohydr. Polym. 2015, 134, 726–731. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Hassan, E.A.; Abou-Zeid, R.E.; Dufresne, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Famá, L.M.; Pettarin, V.; Goyanes, S.N.; Bernal, C.R. Starch/multi-walled carbon nanotubes composites with improved mechanical properties. Carbohydr. Polym. 2011, 83, 1226–1231. [Google Scholar] [CrossRef]
- Dias, M.V.; De Fátima, F.; Soares, N.; Borges, S.V.; De Sousa, M.M.; Nunes, C.A.; De Oliveira, I.R.N.; Medeiros, E.A. a Use of allyl isothiocyanate and carbon nanotubes in an antimicrobial film to package shredded, cooked chicken meat. Food Chem. 2013, 141, 3160–3166. [Google Scholar] [CrossRef]
- Tangsatianpan, V.; Torgbo, S.; Sukyai, P. Release Kinetic Model and Antimicrobial Activity of Freeze-Dried Curcumin-loaded Bacterial Nanocellulose Composite. Polym. Sci.—Ser. A 2020, 62, 218–227. [Google Scholar] [CrossRef]
- Gonçalves, I.S.; Lima, L.R.; Berretta, A.A.; Amorim, N.A.; Pratavieira, S.; Corrêa, T.Q.; Nogueira, F.A.R.; Barud, H.S. Antimicrobial Formulation of a Bacterial Nanocellulose/Propolis-Containing Photosensitizer for Biomedical Applications. Polymers 2023, 15, 987. [Google Scholar] [CrossRef]
- dos Santos, C.A.; dos Santos, G.R.; Soeiro, V.S.; dos Santos, J.R.; de Araujo Rebelo, M.; Chaud, M.V.; Gerenutti, M.; Grotto, D.; Pandit, R.; Rai, M.; et al. Bacterial nanocellulose membranes combined with nisin: A strategy to prevent microbial growth. Cellulose 2018, 25, 6681–6689. [Google Scholar] [CrossRef]
- Pirsa, S.; Sani, I.K.; Mirtalebi, S.S. Nano-biocomposite based color sensors: Investigation of structure, function, and applications in intelligent food packaging. Food Packag. Shelf Life 2022, 31, 100789. [Google Scholar] [CrossRef]
- Singh, S.; Nwabor, O.F.; Syukri, D.M.; Voravuthikunchai, S.P. Chitosan-poly(vinyl alcohol) intelligent films fortified with anthocyanins isolated from Clitoria ternatea and Carissa carandas for monitoring beverage freshness. Int. J. Biol. Macromol. 2021, 182, 1015–1025. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; Julian McClements, D.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent advances in intelligent food packaging materials: Principles, preparation and applications. Food Chem. 2022, 375, 131738. [Google Scholar] [CrossRef] [PubMed]
- Sobhan, A.; Muthukumarappan, K.; Wei, L. Biosensors and biopolymer-based nanocomposites for smart food packaging: Challenges and opportunities. Food Packag. Shelf Life 2021, 30, 100745. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Shi, J.; Huang, X.; Sun, Z.; Zhang, D.; Zou, X.; Sun, Y.; Zhang, J.; Holmes, M.; et al. A colorimetric hydrogen sulfide sensor based on gellan gum-silver nanoparticles bionanocomposite for monitoring of meat spoilage in intelligent packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Shi, S.; Li, M.; Zhang, L.; Yang, C.; Du, T.; Wang, S.; Nie, H.; Sun, J.; Zhang, W.; et al. Visible light responsive, self-activated bionanocomposite films with sustained antimicrobial activity for food packaging. Food Chem. 2021, 362, 130201. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zou, Z.; Huang, Y.; Liang, S.; Li, H.; Xu, L. Novel ammonia-responsive carboxymethyl cellulose/Co-MOF multifunctional films for real-time visual monitoring of seafood freshness. Int. J. Biol. Macromol. 2023, 230, 123129. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Wu, H.; Tong, C.; Pang, J.; Wu, C. Multifunctional bionanocomposite films based on konjac glucomannan/chitosan with nano-ZnO and mulberry anthocyanin extract for active food packaging. Food Hydrocoll. 2020, 107, 105942. [Google Scholar] [CrossRef]
- Cao, G.; Bu, N.; Zeng, T.; Sun, R.; Mu, R.; Pang, J.; Wang, L. Development of pH-responsive konjac glucomannan/pullulan films incorporated with acai berry extract to monitor fish freshness. Int. J. Biol. Macromol. 2022, 219, 897–906. [Google Scholar] [CrossRef]
- Fathi, M.; Samadi, M.; Abbaszadeh, S.; Nourani, M.R. Fabrication and characterization of multifunctional bio-safe films based on Carboxymethyl Chitosan and Saffron Petal Anthocyanin Reinforced with Copper Oxide Nanoparticles for sensing the meat freshness. J. Polym. Environ. 2022, 30, 4538–4549. [Google Scholar] [CrossRef]
- Liu, J.; Li, K.; Chen, Y.; Ding, H.; Wu, H.; Gao, Y.; Huang, S.; Wu, H.; Kong, D.; Yang, Z.; et al. Active and smart biomass film containing cinnamon oil and curcumin for meat preservation and freshness indicator. Food Hydrocoll. 2022, 133, 107979. [Google Scholar] [CrossRef]
- Spada, A.; Conte, A.; Del Nobile, M.A. The influence of shelf life on food waste: A model-based approach by empirical market evidence. J. Clean. Prod. 2018, 172, 3410–3414. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Wang, Y.; Kapronezai, K.; Lorente, L.R.; Zhang, R.; Pyrgiotakis, G.; Konduru, N.V.; Ericsson, M.; White, J.C.; De La Torre-Roche, R.; et al. An integrated methodology for assessing the impact of food matrix and gastrointestinal effects on the biokinetics and cellular toxicity of ingested engineered nanomaterials. Part. Fibre Toxicol. 2017, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Enescu, D.; Cerqueira, M.A.; Fucinos, P.; Pastrana, L.M. Recent advances and challenges on applications of nanotechnology in food packaging. A literature review. Food Chem. Toxicol. 2019, 134, 110814. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Bott, J.; Störmer, A.; Franz, R. A model study into the migration potential of nanoparticles from plastics nanocomposites for food contact. Food Packag. Shelf Life 2014, 2, 73–80. [Google Scholar] [CrossRef]
- Bradley, E.L.; Castle, L.; Chaudhry, Q. Applications of nanomaterials in food packaging with a consideration of opportunities for developing countries. Trends Food Sci. Technol. 2011, 22, 604–610. [Google Scholar] [CrossRef]
- Šimon, P.; Chaudhry, Q.; Bakoš, D. Migration of engineered nanoparticles from polymer packaging to food—A physicochemical view. J. Food Nutr. Res. 2008, 47, 105–113. [Google Scholar]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- Bott, J.; Störmer, A.; Franz, R. Migration of nanoparticles from plastic packaging materials containing carbon black into foodstuffs. Food Addit. Contam. Part A 2014, 31, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the safety evaluation of the substance zinc oxide, nanoparticles, uncoated and coated with [3-(methacryloxy)propyl] trimethoxysilane, for use in food contact materials. EFSA J. 2015, 13, 40–63. [Google Scholar] [CrossRef]
- Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Brüschweiler, B.J.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; et al. Safety assessment of the substance, montmorillonite clay modified with hexadecyltrimethylammonium bromide, for use in food contact materials. EFSA J. 2019, 17, 5552. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Safety assessment of the substance zinc oxide, nanoparticles, for use in food contact materials. EFSA J. 2016, 14, 4408. [Google Scholar] [CrossRef]
| Nanocapsule—Wall Material | Bioactive Compound | Encapsulation Technique | Ref |
|---|---|---|---|
| Zein-Pectin | Resveratrol | Antisolvent precipitation and electrostatic deposition | [30] |
| Whey protein and chitosan | Garlic extract | Complex coacervation and spray-drying | [31] |
| Gelatin and sodium hexametaphosphate | Anchovy oil crosslinked with fucoidan extract | Complex coacervation and spray-drying | [32] |
| Lactoferrin-glycomacropeptide nanohydrogels | Nanohydrogels formed | Thermal gelation and nano spray-drying | [33] |
| Bovine lactoferrin | Omega-3 fatty acids | Nano spray-drying of the emulsion | [34] |
| Bovine serum albumin | Flavonoid rutin | Nano spray-drying | [35] |
| Stearic acid-chitosan conjugate and sodium caseinate | Astaxanthin | Ionic gelation and nano spray-drying | [36] |
| Gelatin | Ascorbic acid | Cryogelation | [37] |
| Sodium alginate and sodium carboxymethyl cellulose | Eucalyptus camaldulensis polyphenols | Freeze-drying | [38] |
| Gelatin-carrageenan | Gallic acid, catechin, chlorogenic acid, and tannic acid | Electric field-aided extrusion | [39] |
| Gelatin | Tomato peel carotenoids | Electro-spinning | [40] |
| Chitosan | Bromelain | Electro-spinning | [41] |
| Whey protein | Olive oil phenolic | Electro-spraying | [42] |
| Polyvinyl alcohol | Phycocyanin | Electro-spraying and electro-spinning | [43] |
| Oil with Tween 20 or Sodium dodecyl sulfate | Thyme essential oil | Emulsification | [44] |
| Cactus cladode mucilage | Zeaxanthin | Emulsification | [45] |
| Gelatin or whey protein | Melon carotenoids | Emulsification | [46] |
| Whey protein | Buriti and pequi carotenoids | Emulsification and freeze-drying | [47] |
| Chitosan | Clove essential oil | Emulsion and ionic gelation | [48] |
| Chitosan-Gum Arabic | Saffron Extract | Ionic Gelation | [49] |
| Polymer | Nanoparticles (NPs) | Food Applied | Major Results | Ref |
|---|---|---|---|---|
| Gelatin/ginger essential oil | Montmorillonite (MMT) | - | MMT increases the thickness of the films. MMT decreases water solubility, moisture content, and superficial hydrophobicity. | [116] |
| Chitosan/rosemary essential oil | MMT | Fresh poultry meat | MMT improves barrier properties. MMT traps the phenolic compounds present in REO, avoiding its release into poultry meat. | [117] |
| Chitosan/papain | Cellulose nanofibers (CNF) | - | Papain impairs the dispersion of the CNF in the polymer matrix and increases crystallinity; however, the films show good transparency. CNF reduces the proteolytic activity of the films and retards the release of papain, due to changes in bionanocomposite crystallinity. | [118] |
| Polyvinyl alcohol/carboxymethyl cellulose (PVA/CMC) | Cellulose nanocrystals (CNC) | - | CNC decreases water vapor permeability. Tensile modulus and tensile strength increase with the addition of CNC to PVA/CMC films. | [119] |
| Chitosan | ZnO | Fresh poultry meat | Enhancement of the antimicrobial and antioxidant properties of the chitosan film. Films decrease the deterioration speed of fresh poultry meat. | [95] |
| Polylactic acid (PLA) | ZnO | - | ZnO addition increases mechanical properties. Antimicrobial activity against E. coli. | [120] |
| PLA | MgO | - | Good mechanical and gas barrier properties. Efficient antibacterial activity. | [121] |
| PLA | ZnO | Minced fish paste | Mechanical and water vapor improvement. Films applied in minced fish paste increase the shelf life of the product and have a strong antibacterial effect. | [122] |
| Potato peel/curcumin | Bacterial cellulose | Fresh pork | Bacterial cellulose is effective in the reduction of water vapor and oxygen permeability. | [123] |
| Starch | MMT | Lettuce and spinach | Improvement of mechanical properties. No migration to the vegetables. | [110] |
| Bovine gelatin/nano-chitin | ZnO | Cake | ZnO NPs improve barrier properties. Bilayer film prevents fungal growth on the cake. | [124] |
| Chitosan/starch/clove oil | TiO2 | - | Improvement of tensile strength and antioxidant activity. | [125] |
| Chitosan/tocopherol | MMT | - | Incorporation of MMT improves the mechanical strength and thermal stability of the films. Incorporation of tocopherol provides antioxidant activity, improves the films’ thermal stability, and decreases tensile strength and elastic modulus, thus acting as a plasticizer. | [126] |
| Rice flour/gelatin/catechin-lysozyme | MMT | Fresh pork belly | Prevention of lipid oxidation and microbial growth. Increase in mechanical properties and film solubility. | [127] |
| Starch/cinnamon oil | TiO2 | - | Mechanical and barrier properties improved. | [128] |
| Soluble soybean polysaccharide | TiO2 | - | Mechanical and barrier properties improved. | [129] |
| Wheat gluten | TiO2 and CNC | - | Tensile strength and water resistance improvement. TiO2 exhibits good antimicrobial activity. | [130] |
| Whey protein/citric acid | MMT | - | Good mechanical, thermal, morphological, and structural properties. | [79] |
| Starch | Multi-walled carbon nanotubes | - | Mechanical properties improved. | [131] |
| Cellulose/allyl isothiocyanate | Carbon nanotubes (CNT) | Shredded and cooked chicken meat | Microbial contamination reduced. Improvement of the gas barrier. | [132] |
| Biomaterial | Bioactive Compound | Food Application | Function | Major Results | Ref |
|---|---|---|---|---|---|
| Gellan gum/Silver nanoparticles | - | Fresh chicken breast and silver carp | Intelligent packaging | The hydrogel is successfully produced to act as hydrogen sulfide (H2S) detector. When exposed to H2S, the hydrogel changes its color, providing information about the status of spoilage chicken and fish samples. | [140] |
| Chitosan/Graphitic carbon nitride | - | Tangerines | Active packaging | Films with chitosan and graphitic carbon nitride are successful. The addition of graphitic carbon nitride improves the mechanical, thermal, and barrier properties. When applied in tangerines (chitosan with graphitic carbon nitride (30%), antibacterial activity is produced, maintaining fruit freshness for 24 days. | [141] |
| Carboxymethyl cellulose/Cobalt-based metal-organic framework (Co-MOF) | - | Shrimp | Intelligent and active packaging | Films show promising results in terms of antibacterial and sensitivity to ammonia as well as color-stability to act as intelligent and active film. In addition, the application of shrimp and its freshness monitorization has been achieved. | [142] |
| Konjac glucomannan/Chitosan/Zinc oxide nanoparticles | Anthocyanins from Mulberry extract | - | Intelligent and active packaging | The addition of nanoparticles and anthocyanins to the film matrix improves mechanical, gas, thermal, and light-barrier properties. Moreover, films have been demonstrated to be pH-sensitive, by changing color according to the pH of the environment. Films also show good antioxidant and antibacterial properties. | [143] |
| Konjac glucomannan/Pullulan | Anthocyanins from Açai berry extract | Grass carps | Intelligent packaging | Pullulan has been used as a reinforcing agent to enhance the properties of films. The addition of açai berry extract to films improves several properties, namely water barrier, antioxidant, and antibacterial. In addition, films exhibit a color response when applied to fish, changing color according to degradation evolution due to pH alterations. | [144] |
| Carboxymethyl chitosan/Copper oxide nanoparticles | Anthocyanin from Saffron Petal | Lamb | Intelligent and Active packaging | The use of anthocyanins from saffron petals and nanoparticles enhances films in terms of their mechanical, thermal, antioxidant, and antibacterial properties. Moreover, anthocyanins also act as plasticizers. When in contact with lamb meat, films demonstrate freshness indicator ability. | [145] |
| Gelatin/Chitosan/Oxidized cellulose nanofiber | Curcumin and cinnamon oil | Pork | Intelligent and active packaging | Films have antioxidant features and increased UV-light barrier compared to pristine films. The use of bioactive compounds also allows a visual color change when pork degradation occurs. | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.; Fernando, A.L.; Souza, V.G.L. Current Applications of Bionanocomposites in Food Processing and Packaging. Polymers 2023, 15, 2336. https://doi.org/10.3390/polym15102336
Pires JRA, Rodrigues C, Coelhoso I, Fernando AL, Souza VGL. Current Applications of Bionanocomposites in Food Processing and Packaging. Polymers. 2023; 15(10):2336. https://doi.org/10.3390/polym15102336
Chicago/Turabian StylePires, João Ricardo Afonso, Carolina Rodrigues, Isabel Coelhoso, Ana Luisa Fernando, and Victor Gomes Lauriano Souza. 2023. "Current Applications of Bionanocomposites in Food Processing and Packaging" Polymers 15, no. 10: 2336. https://doi.org/10.3390/polym15102336








