Lignin from Brewers’ Spent Grain: Structural and Thermal Evaluations
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Lignin from BSG
2.2. Lignin Characterization
2.2.1. Fourier Transform Infrared Spectroscopy
2.2.2. Thermogravimetric Analysis (TGA)
2.2.3. X-ray Diffraction (XRD)
2.2.4. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
3.1. Identification of Functional Groups and Hydrogen-Bond Interactions
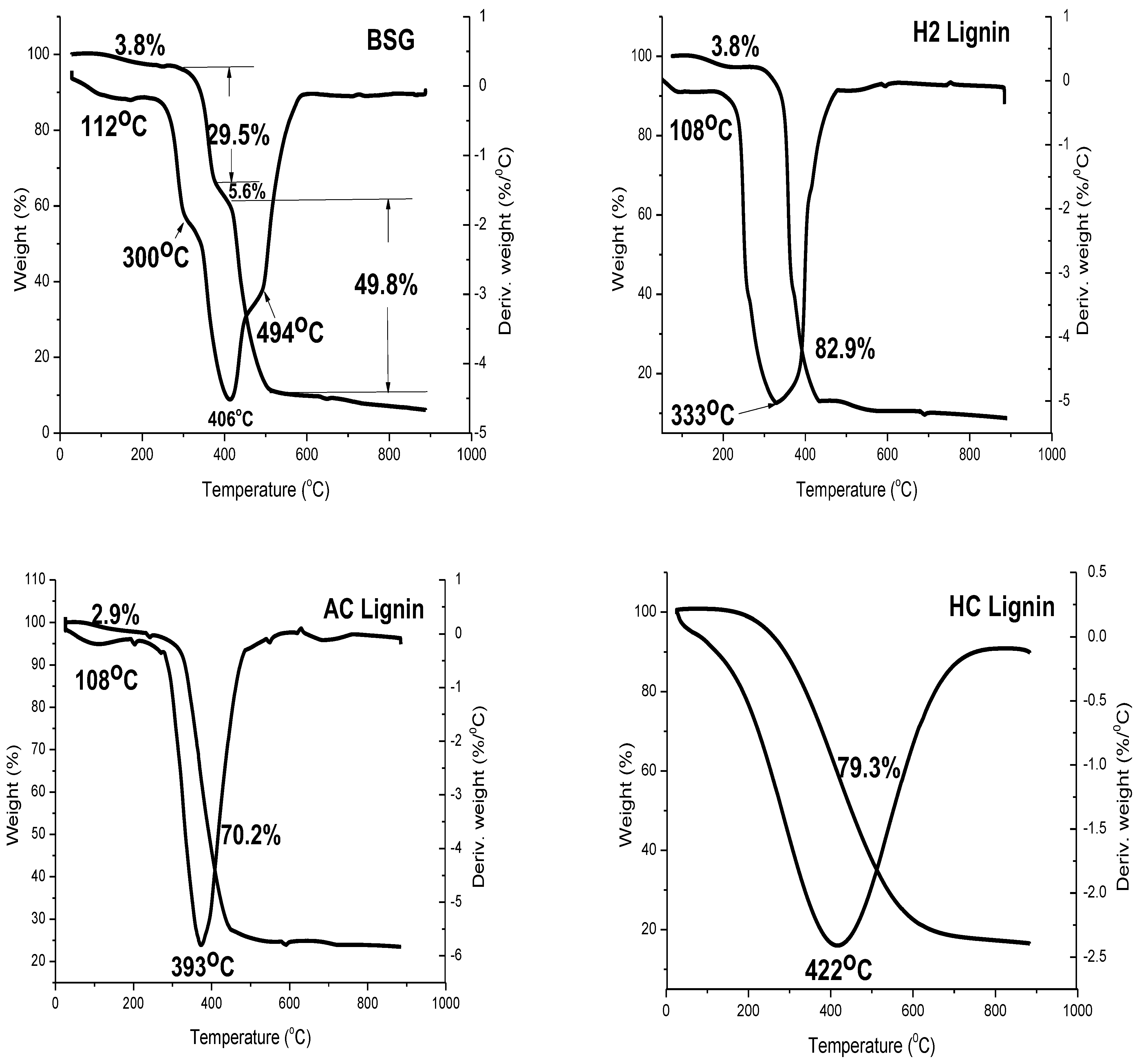
3.2. Thermal Profiles of BSG and Lignin Samples
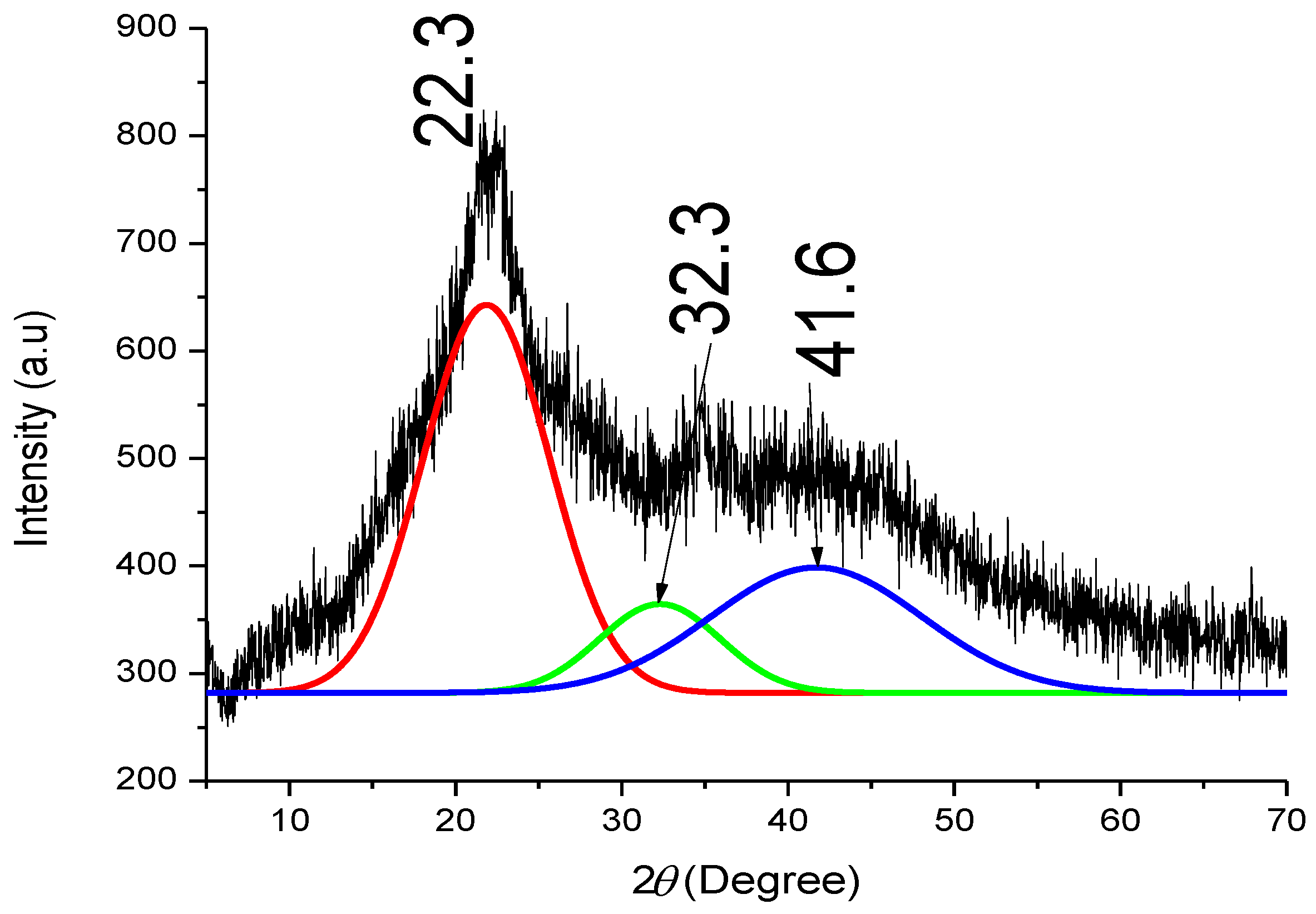
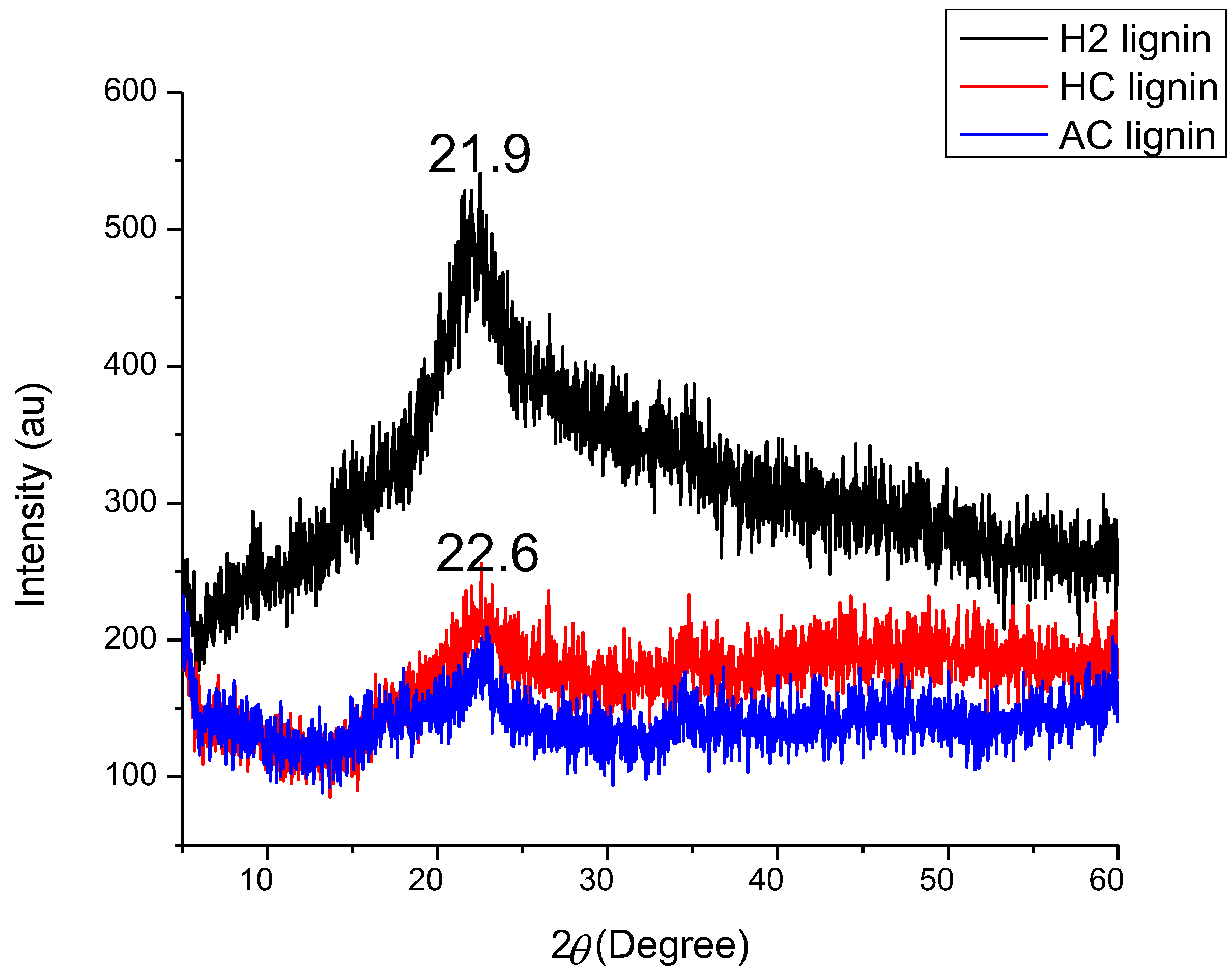
3.3. Crystallographic Structure of BSG and Its Lignin Samples
3.4. Glass Transition Temperatures and Reaction Enthalpies of Lignin Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Har, N.P.; Irzaman; Irmansyah. Crystallinity and electrical properties of silicon dioxide (SiO2) from rice straw. In Proceedings of the International Conference on Science and Applied Science (ICSAS), Surakarta, Indonesia, 20 July 2019; AIP Publishing LLC: College Park, MD, USA, 2019; pp. 1–7. [Google Scholar]
- Mansor, A.M.; Lim, J.S.; Ani, F.N.; Hashim, H.; Ho, W.S. Characteristics of cellulose, hemicellulose and lignin of MD2 pineapple biomass. Chem. Eng. Trans. 2019, 72, 79–84. [Google Scholar]
- Brethauer, S.; Shahab, R.L.; Studer, M.H. Impacts of biofilms on the conversion of cellulose. Appl. Mic.Bio. Biotechnol. 2020, 104, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.; Nypelö, T.; Abreu, C.R.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Coll. Int. Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Lee, M.H.; Jeon, H.S.; Kim, S.H.; Chung, J.H.; Roppolo, D.; Lee, H.J.; Cho, H.J.; Tobimatsu, Y.; Ralph, J.; Park, O.K. ArticleLignin-based barrier restricts pathogens to theinfection site and confers resistance in plants. EMBO J. 2019, 38, e101948. [Google Scholar] [CrossRef] [PubMed]
- Norgren, M.; Edlund, H. Lignin: Recent advances and emerging applications. Curr. Opin. Coll. Int. Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- Podkościelna, B.; Goliszek, M.; Sevastyanova, O. New approach in the application of lignin for the synthesis of hybrid materials. Pure Appl. Chem. 2017, 89, 161–171. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R. Nano-structured lignin as green antioxidant and uv shielding ingredient for sunscreen applications. Antioxidants 2021, 10, 274. [Google Scholar] [CrossRef]
- Capecchi, E.; Tomaino, E.; Piccinino, D.; Kidibule, P.E.; Lobato, M.F.; Spinelli, D.; Pogni, R.; Cabado, A.G.; Lago, J.; Saladino, R. Nanoparticles of lignins and saccharides from fishery wastes as sustainable uv-shielding, antioxidant, and antimicrobial biofillers. Biomacromolecules 2022, 23, 3154–3164. [Google Scholar] [CrossRef]
- Tomaino, E.; Capecchi, E.; Piccinino, D.; Saladino, R. Lignin Nanoparticles support lipase-tyrosinase enzymatic cascade in the synthesis of lipophilic hydroxytyrosol ester derivatives. ChemCatChem 2022, 14, e202200380. [Google Scholar] [CrossRef]
- Sippone, M.; Farooq, M.; Koivisto, J.; Pellis, A.; Seitsonen, J.; Österberg, M. Spatially confined lignin nanospheres for biocatalytic ester synthesis in aqueous media. Nat. Commun. 2018, 9, 2300. [Google Scholar] [CrossRef] [PubMed]
- Tortolini, C.; Capecchi, E.; Tasca, F.; Pofi, R.; Venneri, M.A.; Saladino, R.; Antiochia, R. Novel nanoarchitectures based on lignin nanoparticles for electrochemical eco-Friendly biosensing development. Nanomaterials 2021, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, L.; Bulletti, L.; Capecchi, E.; La Viola, A.; Piccinino, D.; Piscopo, V. Perspectives of using lignin as additive to improve the permeability of in-Situ soils for barrier materials in landfills. Sustainability 2020, 12, 519. [Google Scholar] [CrossRef]
- Xiao, B.; Sun, X.F.; Sun, R.C. Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym. Degrad. Stab. 2011, 74, 307–319. [Google Scholar] [CrossRef]
- Minu, K.; Jiby, K.K.; Kishore, V.V.N. Isolation and purification of lignin and silica from the black liquor generated during the production of bioethanol from rice straw. Biomass Bioenergy 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; pp. 1–7. [Google Scholar]
- Motaung, T.E.; Mtibe, A. Alkali treatment and cellulose nanowhiskers extracted from maize stalk residues. Mater. Sci. Appl. 2015, 6, 1022–1032. [Google Scholar] [CrossRef]
- Abdul-Rahman, N.H.; Chieng, B.W.; Ibrahim, N.A.; Abdul Rahman, N. Extraction and characterization of cellulose nanocrystals from tea leaf waste fibers. Polymers 2017, 9, 588. [Google Scholar] [CrossRef]
- Dai, Z.; Shi, X.; Liu, H.; Li, H.; Han, Y.; Zhou, J. High-strength lignin-based carbon fibers via a low energy method. Roy. Soc. Chem. 2018, 8, 1218–1224. [Google Scholar] [CrossRef]
- Ajani, A.O.; Agarry, S.E.; Agbede, O.O. A comparative kinetic study of acidic hydrolysis of wastes cellulose from agricultural derived biomass. J. Appl. Sci. Environ. Manag. 2011, 15, 531–537. [Google Scholar]
- De, S.; Mishra, S.; Poonguzhali, E.; Rajesh, M.; Tamilarasan, K. Fabrication and characterization of lignin from rice waste straw: Biomass surface chemical composition analysis. Int. J. Biol. Macromol. 2020, 145, 795–803. [Google Scholar] [CrossRef]
- Taleb, F.; Ammar, M.; Mosbah, M.; Salem, R.; Moussaoui, Y. Chemical modification of lignin derived from spent coffee grounds for methylene blue adsorption. Sci. Rep. 2020, 10, 11048. [Google Scholar] [CrossRef] [PubMed]
- Xiros, C.; Topakas, E.; Katapodis, P.; Christakopoulos, P. Hydrolysis and fermentation of brewer’s spent grain by Neurospora crassa. Bioresour. Technol. 2008, 99, 5427–5435. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Paliwal, J.; Cenkowsk, S. Issues with utilization of brewer’s spent grain. Stewart Postharvest Rev. 2010, 4, 1–8. [Google Scholar]
- Ikram, S.; Huang, L.Y.; Zhang, H.; Wang, J.; Yin, M. Composition and nutrient value proposition of brewers spent grain. J. Food. Sci. 2017, 88, 2232–2242. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Viganó, J.; Castro, L.E.N.; Maciel-Silva, F.W.; Rostagno, M.A.; Mussatto, S.I.; Carneiro, T.F. Recovery of sugars and amino acids from brewers’ spent grains using subcritical water hydrolysis in a single and two sequential semi-continuous flow-through reactors. Food Res. Int. 2022, 157, 111470. [Google Scholar] [CrossRef]
- Terefe, G. Preservation techniques and their effecton nutritional values and microbial population of brewer’s spent grain: A review. CABI Agric. Biosci. 2022, 3, 51. [Google Scholar] [CrossRef]
- Karlsen, F.; Skov, P.V. Review—Potentials and limitations of using brewer’s spent grain as a protein source in aquaculture feeds. J. Clean. Prod. 2022, 357, 131986. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Liu, J.; Wang, C.; Chen, G. Hydrodeoxygenation of lignin-derived phenols to produce hydrocarbons over Ni/Al-SBA-15 prepared with different impregnants. Fuel 2019, 243, 314–321. [Google Scholar] [CrossRef]
- Vu, N.D.; Tran, H.T.; Bui, N.D.; Vu, C.D.; Tran, H.V. Lignin and cellulose extraction from vietnam’s rice straw using ultrasound-assisted alkaline treatment method. Int. J. Polym. Sci. 2017, 2017, 1063695. [Google Scholar]
- Kubo, S.; Kadila, J.F. Hydrogen bonding in lignin: A fourier transform infrared model compound study. Biomacromolecules 2005, 6, 2815–2821. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J. Materials produced from plant biomass. part III: Degradation kinetics and hydrogen bonding in lignin. Mater. Res. 2013, 16, 1065–1070. [Google Scholar] [CrossRef]
- Pimentel, G.C.; Sederholm, C.H. Correlation of infrared stretching frequencies and hydrogen bond distances in crystals. J. Chem. Phys. 1955, 24, 639–641. [Google Scholar] [CrossRef]
- Lin, B.J.; Chen, W.H. Sugarcane bagasse pyrolysis in a carbon dioxide atmosphere with conventional and microwave-assisted heating. Front. Energy Res. 2015, 3, 4. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Chen, W.H.; Ye, S.C.; Sheen, H.K. Hydrothermal carbonization of sugarcane bagasse via wet torrefaction in association with microwave heating. Bioresour. Technol. 2012, 118, 195–203. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin- a review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Rodrigues, J.; Graça, J.; Pereira, H. Influence of tree eccentric growth on syringyl/guaiacyl ratio in Eucalyptus globulus wood lignin assessed by analytical pyrolysis. J. Anal. Appl. Phys. 2001, 58, 481–489. [Google Scholar] [CrossRef]
- Reddy, P.M.; Mahammadunnisa, S.; Ramaraju, B.; Sreedhar, B.; Subrahmanyam, C. Low-cost adsorbents from bio-waste for the removal of dyes from aqueous solution. Environ. Sci. Pollut. Res. 2012, 20, 4111–4124. [Google Scholar] [CrossRef]
- Alzagameem, A.; Hansen, B.E.; Büchne, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic biomass as source for lignin-based environmentally benign antioxidants. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef]
- Li, Y.; Cui, D.; Tong, Y.; Xu, L. Study on structure and thermal stability properties of lignin during thermostabilization and carbonization. Int. J. Biol. Macromol. 2013, 62, 663–669. [Google Scholar] [CrossRef]
- Costa, E.S.; Perlatti, B.; Silva, E.M.; Matos, A.P.; Silva, M.F.G.F.; Fernandes, J.B.; Zuin, V.G.; Silva, C.M.P.; Forim, M.R. Use of lignins from sugarcane bagasse for assembling microparticles loaded with azadirachta indica extracts for use as neem-based organic insecticides. J. Braz. Chem. Soc. 2017, 28, 126–135. [Google Scholar]
- Santos, P.; Erdocia, X.; Gatto, D.A.; Labidi, J. Characterisation of kraft lignin separated by gradient acid precipitation. Ind. Crops Prod. 2014, 55, 149–154. [Google Scholar] [CrossRef]
- Hansen, B.; Kamm, B.; Schulze, M. Qualitative and quantitative analysis of lignin produced from beech wood by different conditions of the organosolv process. J. Polym. Environ. 2016, 24, 85–97. [Google Scholar] [CrossRef]
- Chollet, B.; Lopez-Cuesta, J.M.; Laoutid, F.; Ferry, L. Lignin nanoparticles as a promising way for enhancing lignin flame retardant effect in polylactide. Materials 2019, 12, 2132. [Google Scholar] [CrossRef] [PubMed]
- Lavarda, G.; Morales-delaRosa, S.; Centomo, P.; Campos-Martin, J.M.; Zecca, M.; Fierro, J.L.G. Gel-type and macroporous cross-linked copolymers functionalized with acid groups for the hydrolysis of wheat straw pretreated with an ionic liquid. Catalysts 2019, 9, 675. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Osabumwenre, F.O.; Adeosun, S.O. Structural, mechanical and thermal properties of low-density polyethylene/biomass composite: Effects of particle size. Kufa J. Engin. 2020, 11, 67–83. [Google Scholar]
- Gbenebor, O.P.; Adeosun, S.O.; Lawal, G.I.; Jun, S.; Olaleye, S.A. Acetylation, crystalline and morphological properties of structural polysaccharide from shrimp exoskeleton. Eng. Sci. Technol. Int. J. 2017, 20, 1155–1165. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Ochulor, E.F.; Shogunwa, A.A.; Adeosun, S.O. Evaluation studies of hydrogen bond, crystallinity and water propensity of treated Bambusa vulgaris cellulose particles. Nig. Res. J. Engin. Environ. Sci. 2022, 7, 260–272. [Google Scholar]
- Mission, E.G.; Agutaya, J.K.C.N.; Quitain, A.T.; Sasaki, M.; Kida, T. Carbocatalysed hydrolytic cleaving of the glycosidic bond in fucoidan under microwave irradiation. RSC Adv. 2019, 9, 30325–30334. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Zhang, H.; Li, Y.; Wang, F.; Xie, H.; Su, L.; Song, A. Biodegradation of Gramineous Lignocellulose by Locusta migratoria manilensis (Orthoptera: Acridoidea). Front. BioEng. BioTechnol. 2022, 10, 943692. [Google Scholar] [CrossRef]
- Guodarzi, A.; Lin, L.; Ko, F.K. X-Ray diffraction analysis of kraft lignins and lignin-derived carbon nanofibers. J. Nanotechnol. Eng. Med. 2014, 5, 021006. [Google Scholar] [CrossRef]
- Gomide, R.A.C.; Oliveira, A.C.S.; Rodrigues, D.A.C.; Oliveira, C.R.; Assis, O.B.G.; Dias, M.V.; Borges, S.V. Development and characterization of lignin microparticles for physical and antioxidant enhancement of biodegradable polymers. J. Polym. Environ. 2020, 28, 1326–1334. [Google Scholar] [CrossRef]
- Dallmeyer, J.I. Preparation and Characterization of Lignin Nanofibre- Based Materials Obtained by Electrostatic Spinning. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2013; pp. 1–160. [Google Scholar]
- Feldman, D.; Banu, D.; Campanelli, J.; Zhu, H. Blends of vinylic copolymer with plasticized lignin: Thermal and mechanical properties. J. Appl. Polym. Sci. 2001, 81, 861–874. [Google Scholar] [CrossRef]
- Buranov, A.U.; Ross, K.A.; Mazza, G. Isolation and characterization of lignins extracted from flax shives using pressurized aqueous ethanol. Bioresour. Technol. 2010, 101, 7446–7455. [Google Scholar] [CrossRef]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass sources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
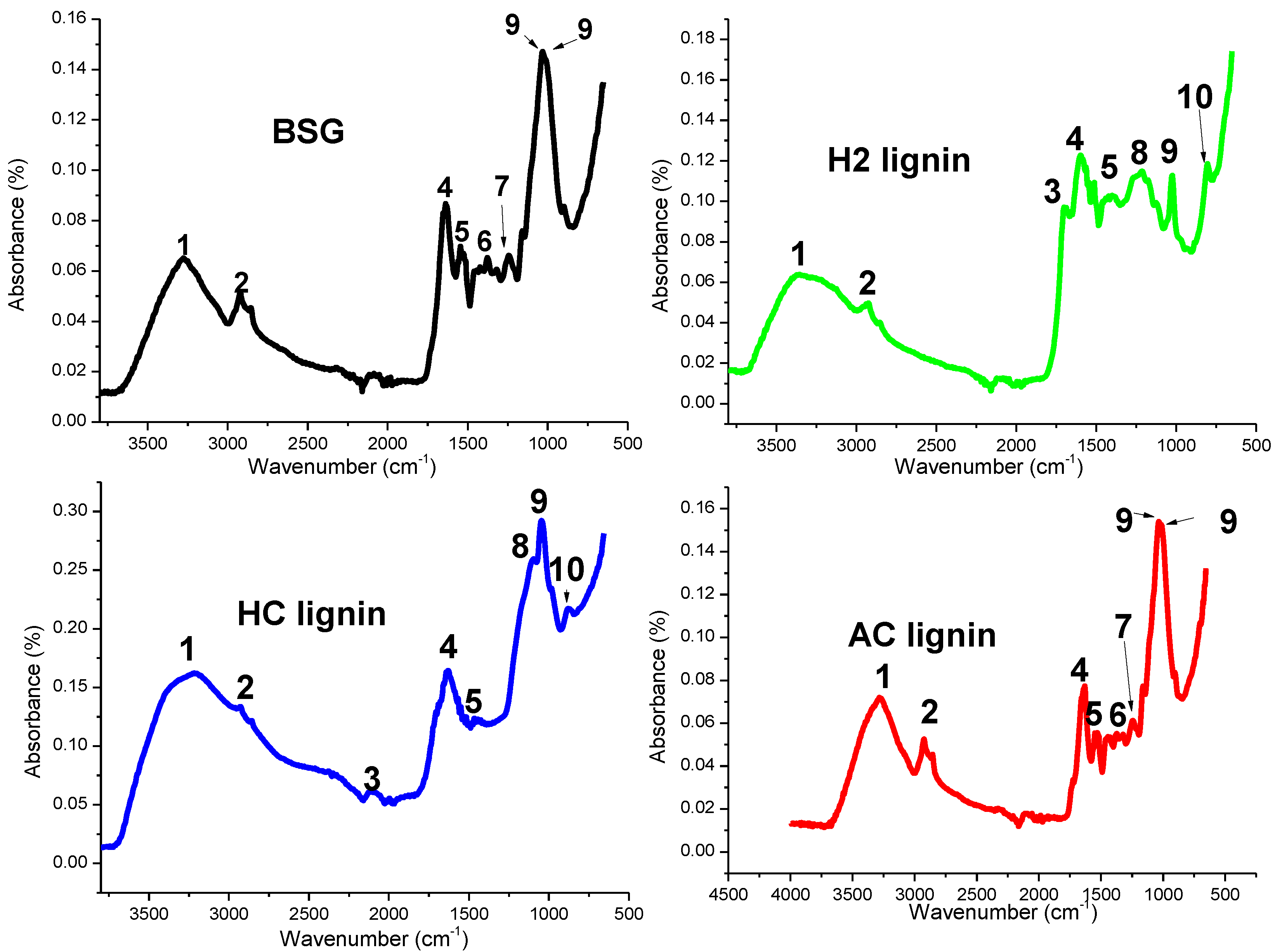

| Functional Groups | Wavenumber (cm−1) | |||
|---|---|---|---|---|
| BSG | AC Lignin | HC Lignin | H2 Lignin | |
| 1. Free OH groups in alcoholic compounds | 3292 | 3269 | 3349 | 3367 |
| 2. CH stretching | 2923 | 2932 | 2916 | 2920 |
| 3. Unconjugated C=O stretching | -- | -- | 1700 | 1700 |
| 4. Lignin aromatic ring stretching | 1515 | 1515 | 1513 | 1580 |
| 5. Asymmetric CH deformation | 1465 | 1465 | 1467 | 1470 |
| 6. CH2 bending in cellulose and hemicellulose | 1370 | 1370 | -- | -- |
| 7. Syringyl ring in lignin | 1226 | 1226 | -- | -- |
| 8. Aromatic C-H deformation in the syringyl ring | -- | -- | 1112 | 1109 |
| 9. Aromatic ring and primary alcohol | 1039, 1003 | 1039, 1003 | 1026 | 1041 |
| 10. Aromatic ring | -- | -- | 804 | 860 |
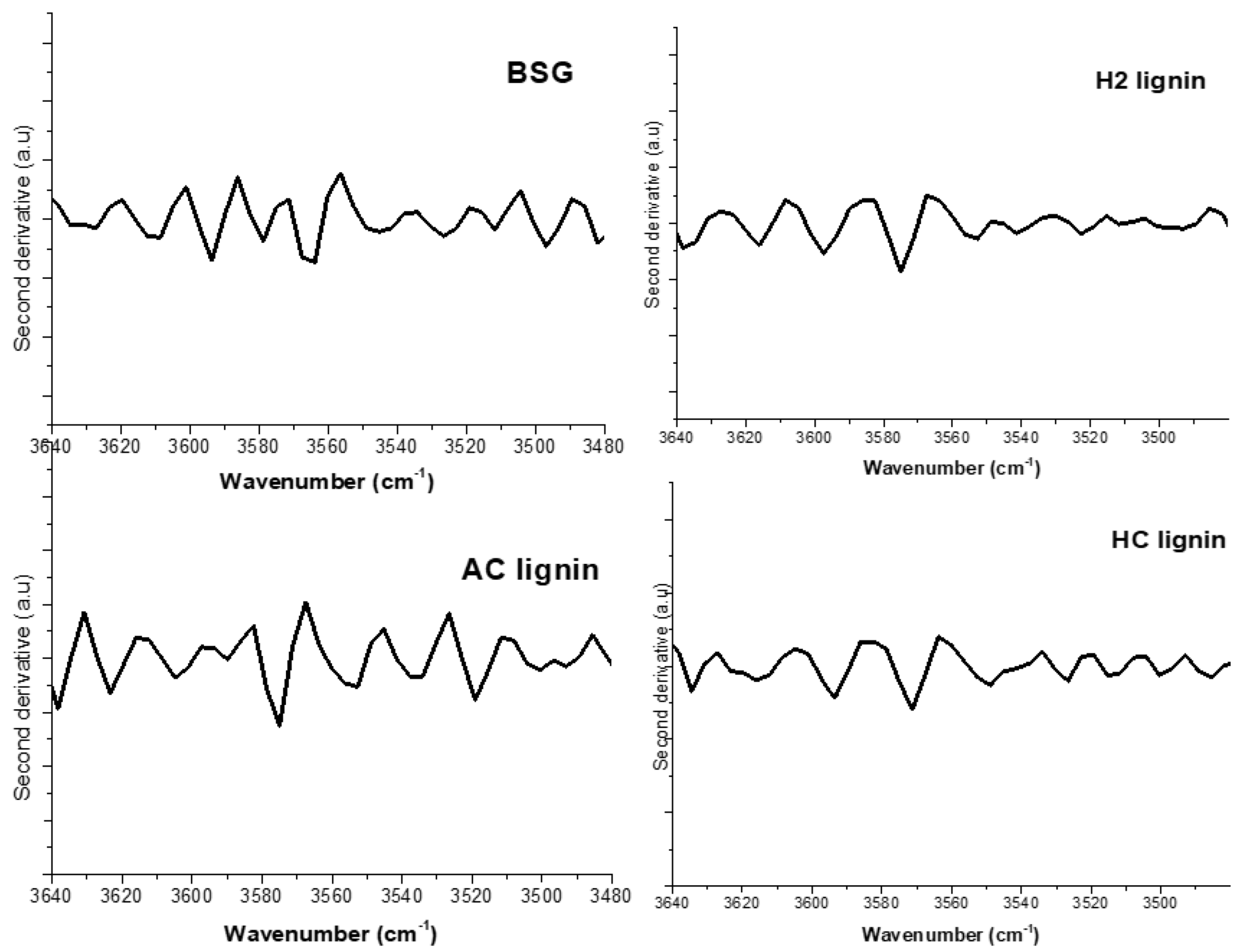
| Sample Wavenumber (cm−1) | |||||||
|---|---|---|---|---|---|---|---|
| BSG | H2 | HC | AC | BSG | H2 | HC | AC |
| 3635 | 3634 | 3637 | 3637 | 343 | 386 | 366 | 358 |
| 3610 | 3614 | 3616 | 3622 | 318 | 363 | 345 | 343 |
| 3579 | 3570 | 3574 | 3574 | 287 | 317 | 303 | 295 |
| 3545 | 3549 | 3542 | 3536 | 253 | 298 | 271 | 257 |
| 3526 | 3526 | 3521 | 3519 | 234 | 275 | 250 | 240 |
| Lignin Samples | Bands Assigned to Phenolic Groups (cm−1) | H (kCal/mol) | R(Á) |
|---|---|---|---|
| H2 | 3570 | 5.73 | 2.882 |
| 3549 | 5.34 | 2.876 | |
| HC | 3574 | 5.48 | 2.884 |
| 3542 | 4.97 | 2.876 | |
| AC | 3574 | 5.35 | 2.884 |
| 3536 | 4.74 | 2.877 | |
| BSG | 3579 | 5.22 | 2.885 |
| 3563 | 4.68 | 2.878 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gbenebor, O.P.; Olanrewaju, O.A.; Usman, M.A.; Adeosun, S.O. Lignin from Brewers’ Spent Grain: Structural and Thermal Evaluations. Polymers 2023, 15, 2346. https://doi.org/10.3390/polym15102346
Gbenebor OP, Olanrewaju OA, Usman MA, Adeosun SO. Lignin from Brewers’ Spent Grain: Structural and Thermal Evaluations. Polymers. 2023; 15(10):2346. https://doi.org/10.3390/polym15102346
Chicago/Turabian StyleGbenebor, Oluwashina Philips, Oludolapo Akanni Olanrewaju, Mohammed Awwalu Usman, and Samson Oluropo Adeosun. 2023. "Lignin from Brewers’ Spent Grain: Structural and Thermal Evaluations" Polymers 15, no. 10: 2346. https://doi.org/10.3390/polym15102346
APA StyleGbenebor, O. P., Olanrewaju, O. A., Usman, M. A., & Adeosun, S. O. (2023). Lignin from Brewers’ Spent Grain: Structural and Thermal Evaluations. Polymers, 15(10), 2346. https://doi.org/10.3390/polym15102346







