Thermal and Oxidative Aging Effects of Polyamide-11 Powder Used in Multi-Jet Fusion
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Material and Aging Procedure
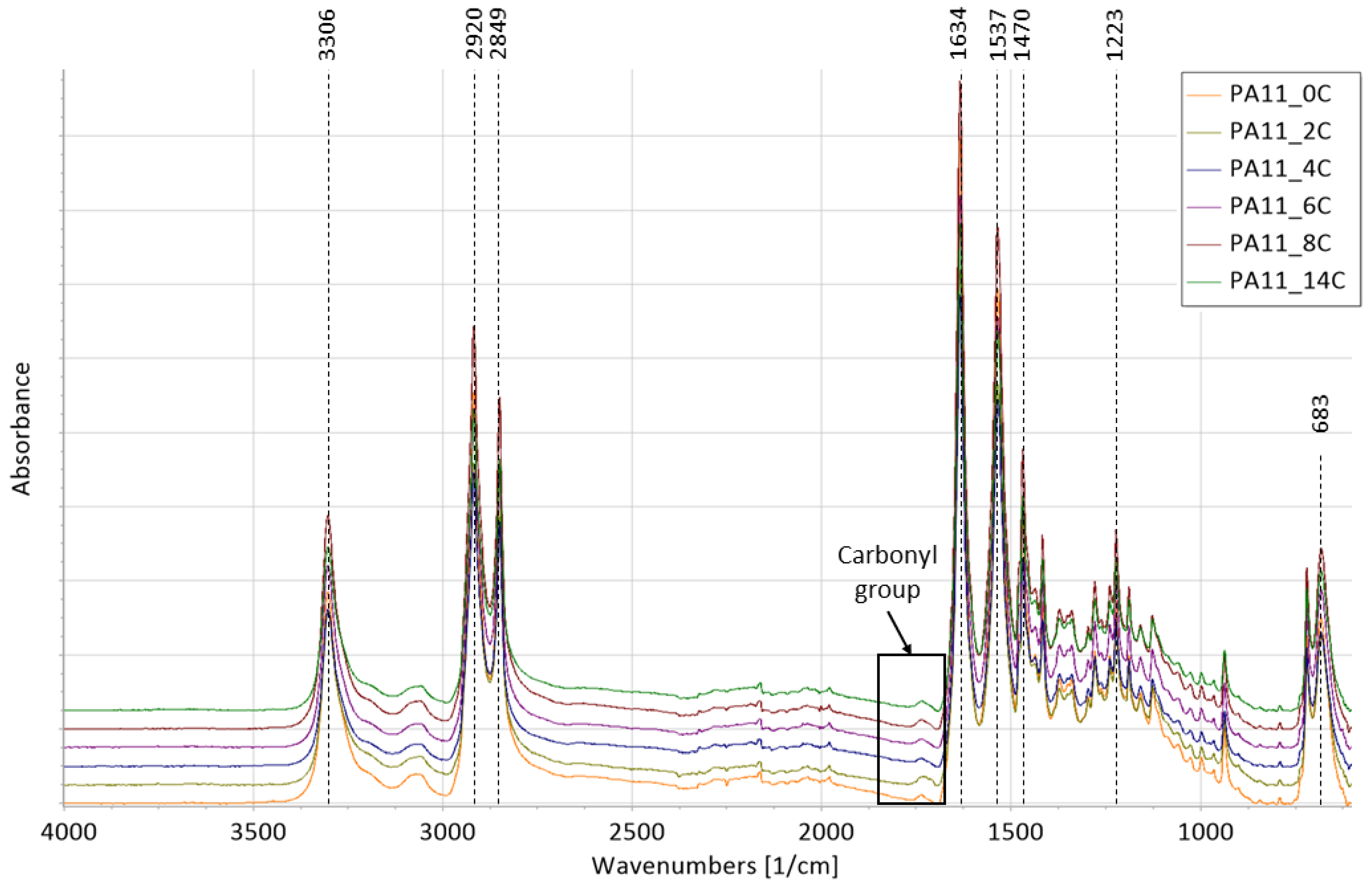
2.2. Fourier-Transform Infrared Spectroscopy
2.3. X-ray Photoelectron Spectroscopy
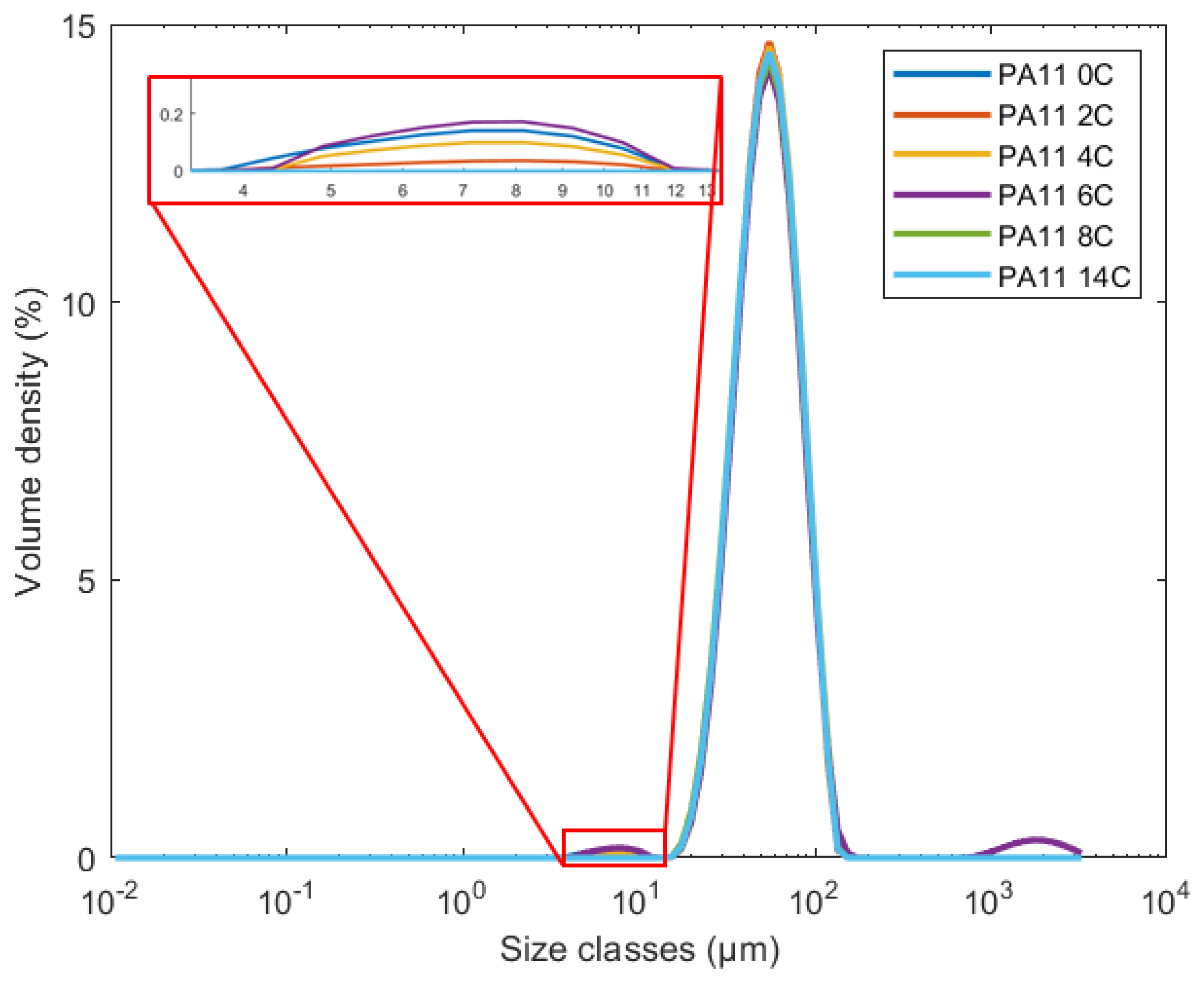
2.4. Powder Size Distribution
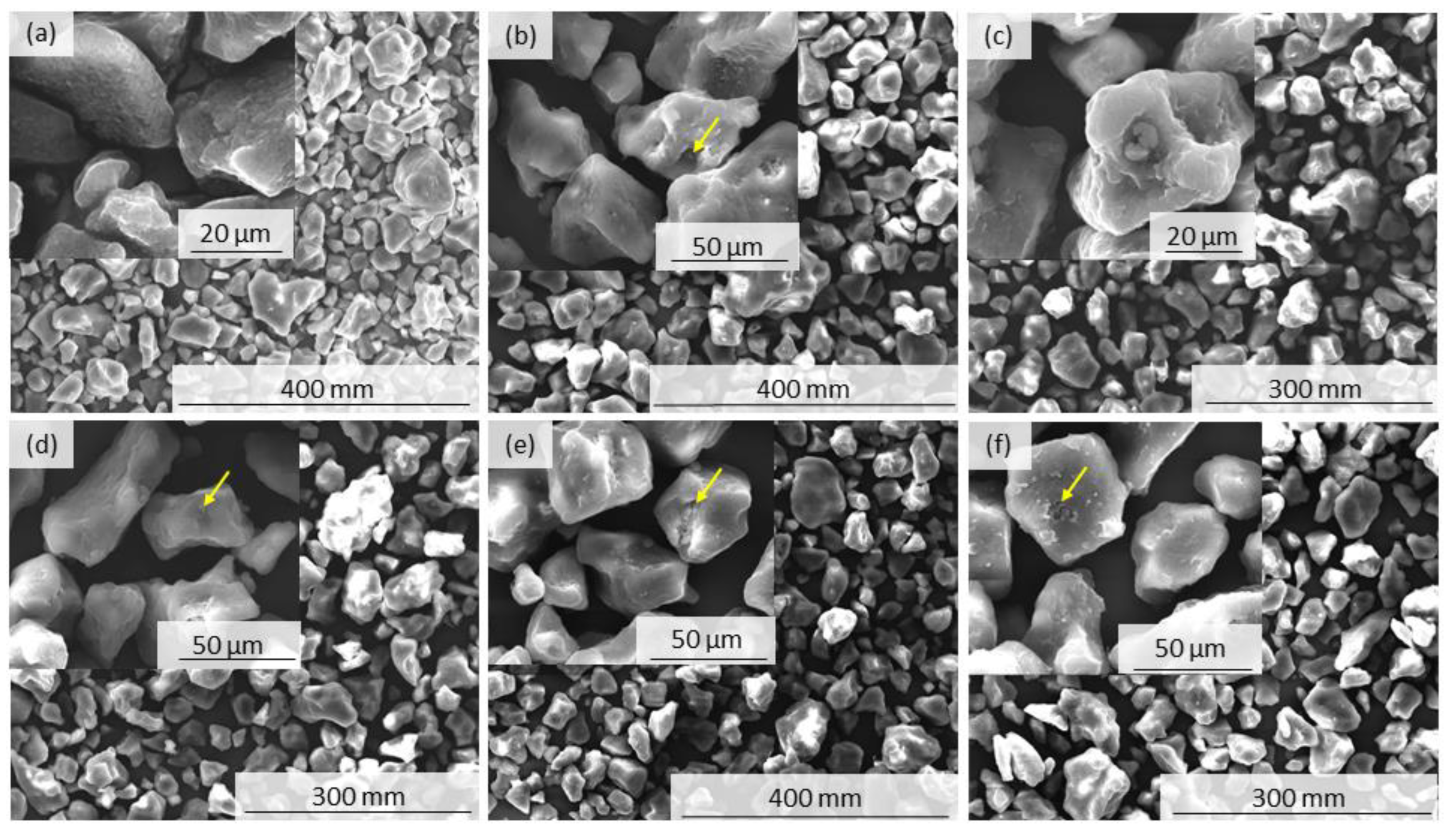
2.5. Scanning Electron Microscopy
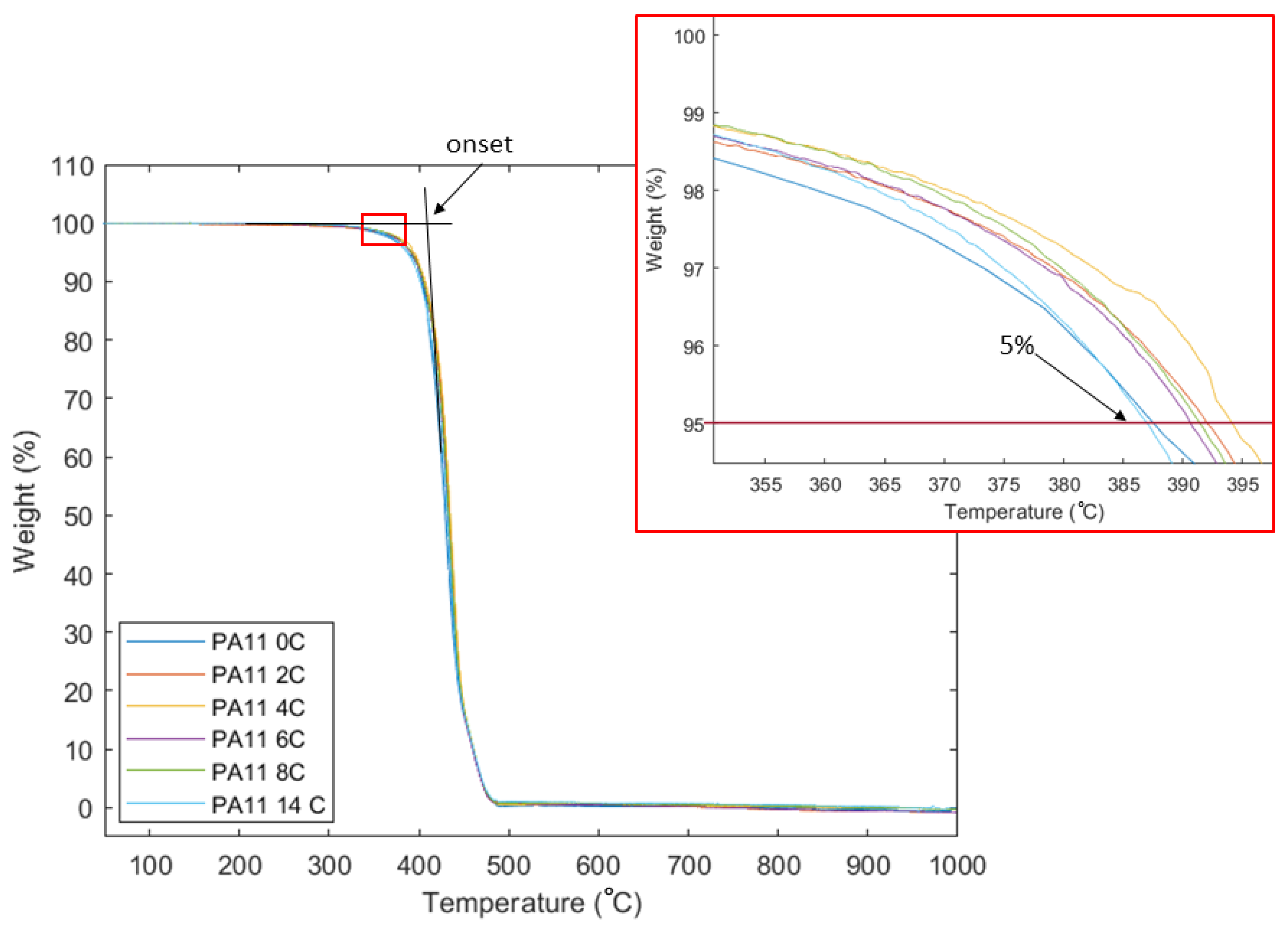
2.6. Thermogravimetric Analysis
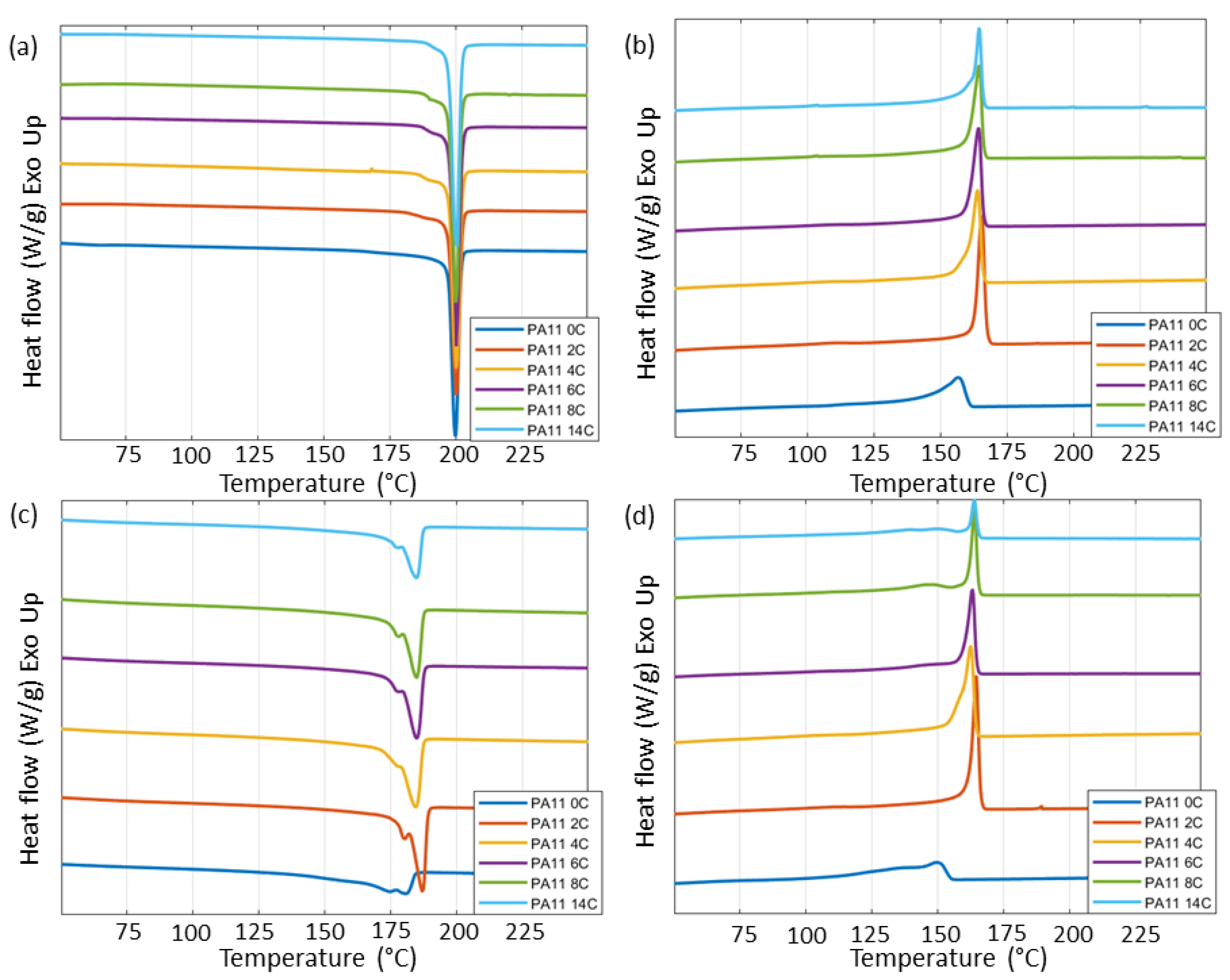
2.7. Differential Scanning Calorimetry
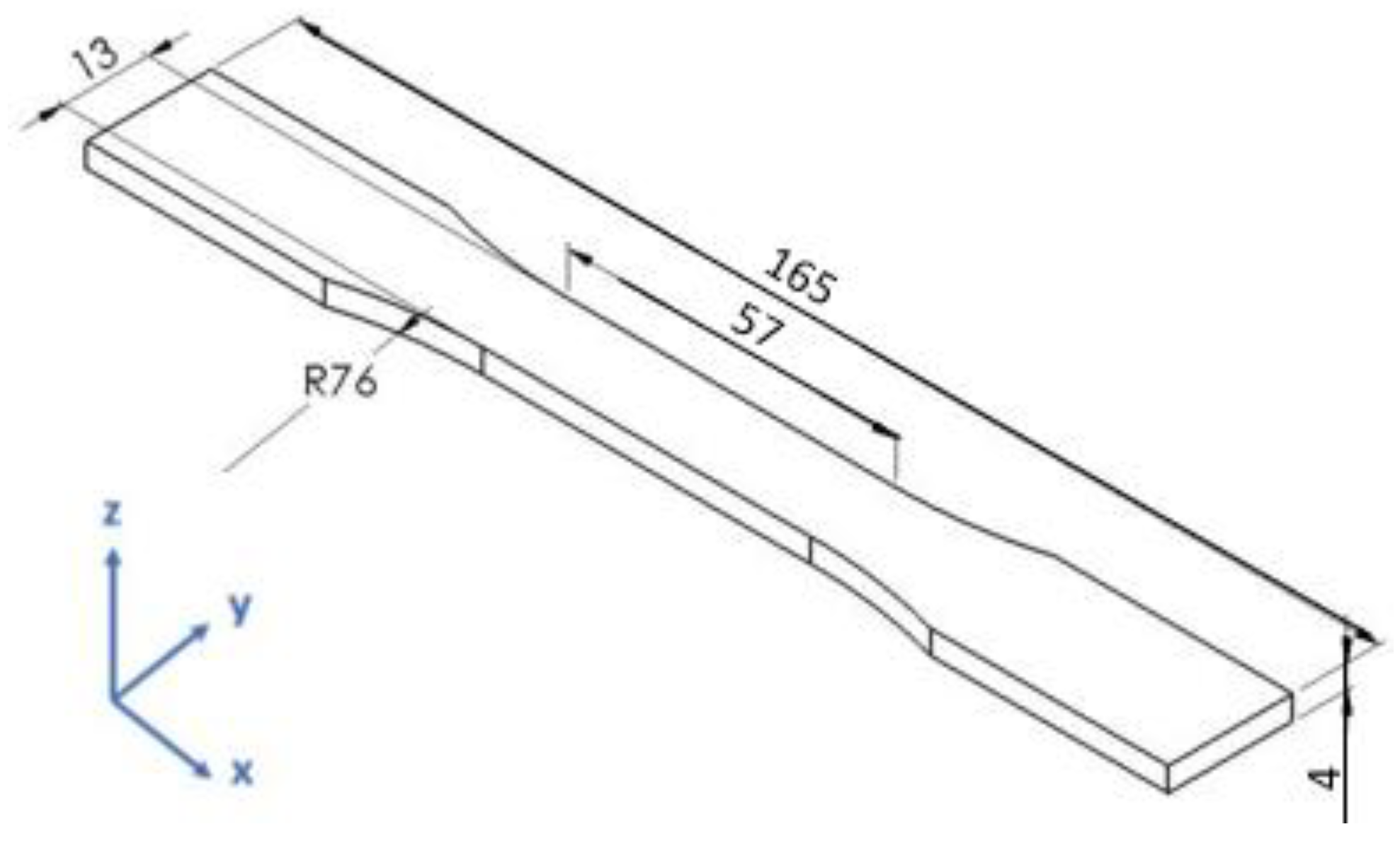
2.8. Specimen Fabrication
2.9. Parallel Plate Rheology
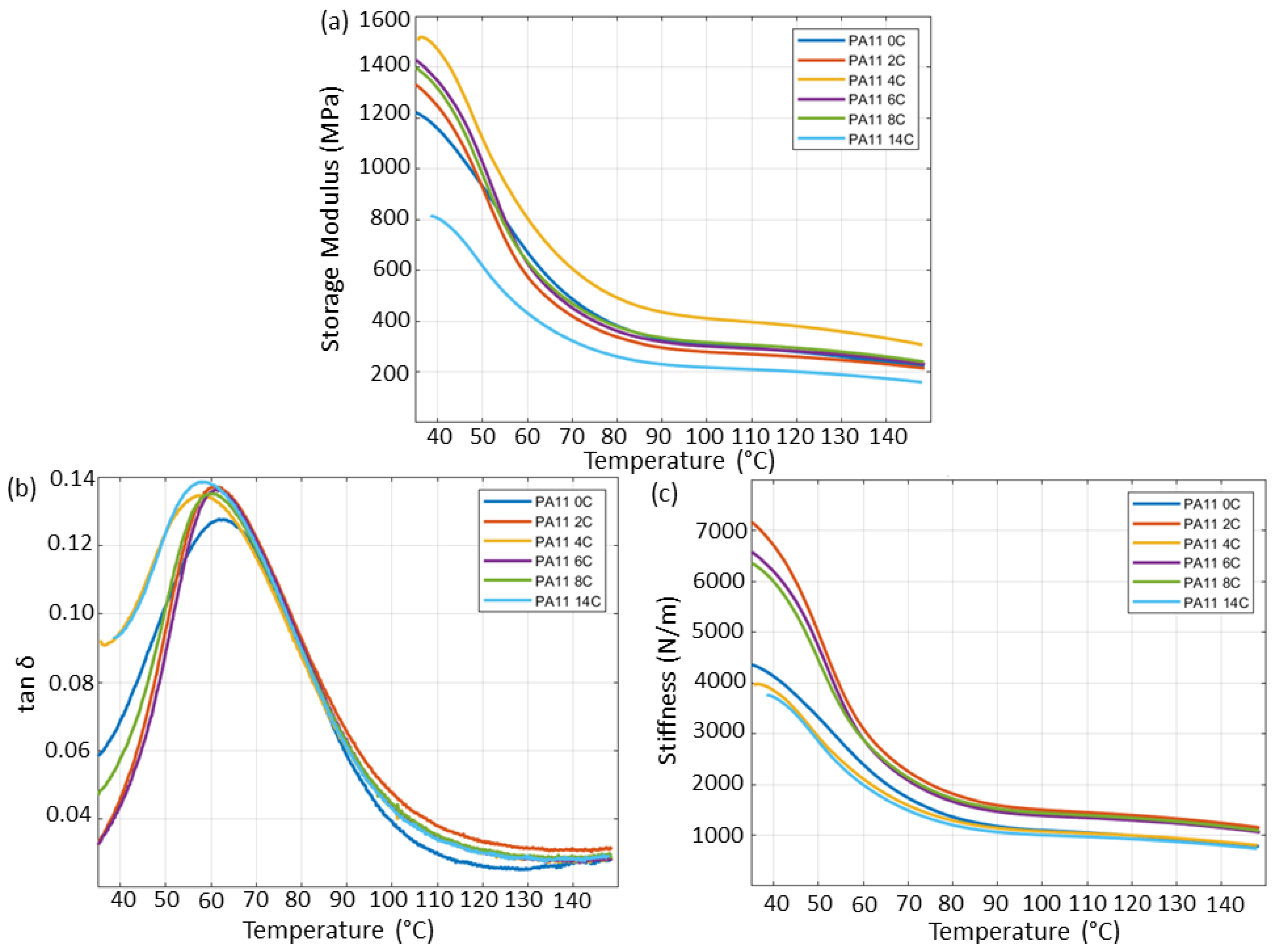
2.10. Dynamic Mechanical Analysis
2.11. Tensile Testing
3. Results and Discussion
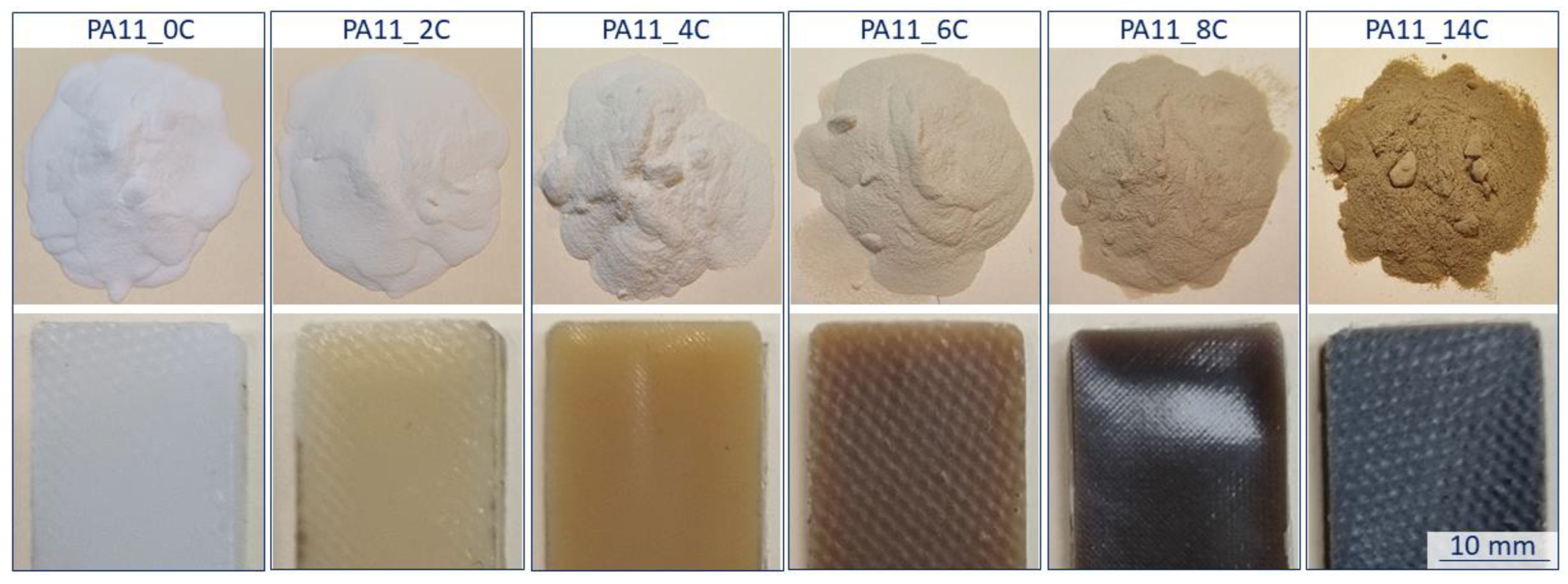
3.1. Physicochemical Observations
- -
- The absorbance at 3306 cm−1 reflects N-H stretching,
- -
- Asymmetric and symmetric stretching of CH2 is found at 2920 and 2849 cm−1, respectively;
- -
- The absorbance at 1634 cm−1 indicates C=O stretching (Amide I);
- -
- The absorbance at 1537 cm−1 may be assigned to C-N stretching and C=O in-plane bending;
- -
- The absorbance at 1470 cm−1 can be assigned to C=O and N-vicinal CH2 bending
- -
- C-N stretching is found at 1223 cm−1;
- -
- The absorbance at 683 cm−1 may be assigned to CONH out-of-plane deformation (Amide V).
| x10.0 (μm) | x50.0 (μm) | x80.0 (μm) | |
|---|---|---|---|
| PA11_0C | 31.2 ± 0.1 | 53.4 ± 0.2 | 87.6 ± 0.4 |
| PA11_2C | 31.7 ± 0.0 | 53.8 ± 0.1 | 75.1 ± 0.3 |
| PA11_4C | 31.7 ± 0.1 | 54.2 ± 0.2 | 75.8 ± 0.3 |
| PA11_6C | 31.4 ± 0.2 | 54.2 ± 0.9 | 77.0 ± 2.7 |
| PA11_8C | 31.2 ± 0.2 | 53.5 ± 0.1 | 75.2 ± 0.3 |
| PA11_14C | 31.5 ± 0.1 | 53.7 ± 0.1 | 75.3 ± 0.2 |
3.2. Thermal Analysis
| Tg (°C) | Tm (°C) | Tc (°C) | Td (°C) | Crystallinity (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 1st | 2nd | 5% | Onset | 1st | 2nd | ||
| PA11_0C | 63 | 200 | 179 | 164 | 154 | 385 | 411 | 63 | 26 |
| PA11_2C | 61 | 200 | 184 | 163 | 160 | 391 | 416 | 57 | 27 |
| PA11_4C | 57 | 200 | 184 | 163 | 161 | 394 | 417 | 60 | 31 |
| PA11_6C | 61 | 200 | 185 | 164 | 163 | 389 | 416 | 56 | 31 |
| PA11_8C | 60 | 200 | 185 | 165 | 164 | 390 | 413 | 61 | 32 |
| PA11_14C | 58 | 200 | 185 | 164 | 163 | 387 | 413 | 57 | 24 |
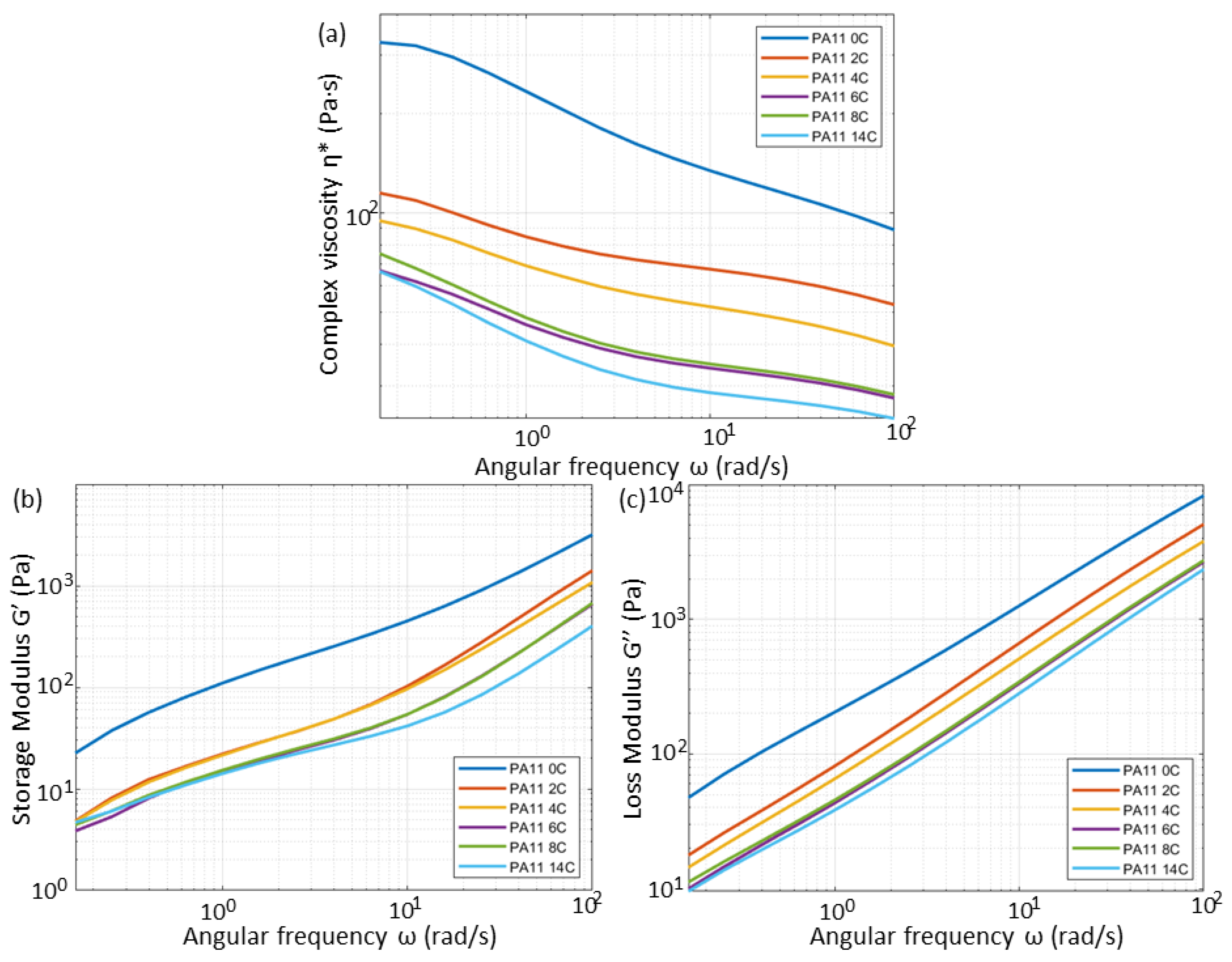
3.3. Rheological Properties
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Mazry, C.; Ben Hassine, M.; Correc, O.; Colin, X. Thermal oxidation kinetics of additive free polyamide 6-6. Polym. Degrad. Stab. 2013, 98, 22–36. [Google Scholar] [CrossRef]
- Okamba-Diogo, O.; Richaud, E.; Verdu, J.; Fernagut, F.; Guilment, J.; Fayolle, B. Molecular and macromolecular structure changes in polyamide 11 during thermal oxidation—Kinetic modeling. Polym. Degrad. Stab. 2015, 120, 76–87. [Google Scholar] [CrossRef]
- Do, C.H.; Pearce, E.M.; Bulkin, B.J.; Reimschuessel, H.K. FT–IR spectroscopic study on the thermal and thermal oxidative degradation of nylons. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 2409–2424. [Google Scholar] [CrossRef]
- Mazan, T.; Berggren, R.; Jørgensen, J.K.; Echtermeyer, A. Aging of polyamide 11. Part 1: Evaluating degradation by thermal, mechanical, and viscometric analysis. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Okamba-Diogo, O.; Richaud, E.; Verdu, J.; Fernagut, F.; Guilment, J.; Fayolle, B. Investigation of polyamide 11 embrittlement during oxidative degradation. Polymer 2016, 82, 49–56. [Google Scholar] [CrossRef]
- Valko, E.I.; Chiklis, C.K. Effects of thermal exposure on the physicochemical properties of polyamides. J. Appl. Polym. Sci. 1965, 9, 2855–2877. [Google Scholar] [CrossRef]
- Wudy, K.; Drummer, D.; Kuhnlein, F.; Drexler, M. Influence of degradation behavior of polyamide 12 powders in laser sintering process on produced parts. In Proceedings of the PPS-29: The 29th International Conference of the Polymer Processing Society—Conference Papers, Nuremberg, Germany, 15–19 July 2013; Volume 1593, pp. 691–695. [Google Scholar]
- De Leon, A.C.; Chen, Q.; Palaganas, N.B.; Palaganas, J.O.; Manapat, J.; Advincula, R.C. High performance polymer nanocomposites for additive manufacturing applications. React. Funct. Polym. 2016, 103, 141–155. [Google Scholar] [CrossRef]
- Pandelidi, C.; Lee, K.P.M.; Kajtaz, M. Effects of polyamide-11 powder refresh ratios in multi-jet fusion: A comparison of new and used powder. Addit. Manuf. 2021, 40, 101933. [Google Scholar] [CrossRef]
- Pham, D.T.; Dotchev, K.D.; Yusoff, W.A.Y. Deterioration of polyamide powder properties in the laser sintering process. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2008, 222, 2163–2176. [Google Scholar] [CrossRef]
- Dadbakhsh, S.; Verbelen, L.; Verkinderen, O.; Strobbe, D.; Van Puyvelde, P.; Kruth, J.-P. Effect of PA12 powder reuse on coalescence behaviour and microstructure of SLS parts. Eur. Polym. J. 2017, 92, 250–262. [Google Scholar] [CrossRef]
- Josupeit, S.; Lohn, J.; Hermann, E.; Gessler, M.; Tenbrink, S.; Schmid, H.J. Material properties of laser sintered polyamide 12 as function of build cycles using low refresh rates. In Proceedings of the 2015 International Solid Freeform Fabrication Symposium, Austin, TX, USA, 10–12 August 2015. [Google Scholar]
- Morales-Planas, S.; Minguella-Canela, J.; Lluma-Fuentes, J.; Travieso-Rodriguez, J.A.; García-Granada, A.-A. Multi Jet Fusion PA12 Manufacturing Parameters for Watertightness, Strength and Tolerances. Materials 2018, 11, 1472. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, H.J.; Dickson, A.N.; Dowling, D.P. Evaluation of the mechanical performance of polymer parts fabricated using a production scale multi jet fusion printing process. Addit. Manuf. 2018, 22, 381–387. [Google Scholar] [CrossRef]
- Riedelbauch, J.; Rietzel, D.; Witt, G. Analysis of material aging and the influence on the mechanical properties of polyamide 12 in the Multi Jet Fusion process. Addit. Manuf. 2019, 27, 259–266. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Wu, D.; Ananth, K.P.; Bai, J. The process and performance comparison of polyamide 12 manufactured by multi jet fusion and selective laser sintering. J. Manuf. Process. 2019, 47, 419–426. [Google Scholar] [CrossRef]
- Duddleston, L.; Puck, A.T.; Harris, A.; Doll, N.; Osswald, T. Differential Scanning Calorimetry (DSC) quantification of Polyamide 12 (Nylon 12) degradation during the Selective Laser Sintering (SLS) process. In Proceedings of the Society of Plastics Engineers—ANTEC, Indianapolis, IN, USA, 23–25 May 2016. [Google Scholar]
- Sanders, B.; Cant, E.; Amel, H.; Jenkins, M. The Effect of Physical Aging and Degradation on the Re-Use of Polyamide 12 in Powder Bed Fusion. Polymers 2022, 14, 2682. [Google Scholar] [CrossRef] [PubMed]
- Wudy, K.; Drummer, D. Aging effects of polyamide 12 in selective laser sintering: Molecular weight distribution and thermal properties. Addit. Manuf. 2018, 25, 1–9. [Google Scholar] [CrossRef]
- Maïza, S.; Lefebvre, X.; Brusselle-Dupend, N.; Klopffer, M.-H.; Cangémi, L.; Castagnet, S.; Grandidier, J.-C. Physicochemical and mechanical degradation of polyamide 11 induced by hydrolysis and thermal aging. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Okamba-Diogo, O.; Richaud, E.; Verdu, J.; Fernagut, F.; Guilment, J.; Fayolle, B. Molecular and macromolecular structure changes in polyamide 11 during thermal oxidation. Polym. Degrad. Stab. 2014, 108, 123–132. [Google Scholar] [CrossRef]
- Lee, K.P.M.; Pandelidi, C.; Kajtaz, M. Build orientation effects on mechanical properties and porosity of polyamide-11 fabricated via multi jet fusion. Addit. Manuf. 2020, 36, 101533. [Google Scholar] [CrossRef]
- Eriksson, P.A.; Boydell, P.; Eriksson, K.; Månson, J.A.; Albertsson, A.C. Effect of thermal-oxidative aging on mechanical, chemical, and thermal properties of recycled polyamide 66. J. Appl. Polym. Sci. 1997, 65, 1619–1630. [Google Scholar] [CrossRef]
- Zhang, J.; Adams, A. Understanding thermal aging of non-stabilized and stabilized polyamide 12 using 1H solid-state NMR. Polym. Degrad. Stab. 2016, 134, 169–178. [Google Scholar] [CrossRef]
- Latko, P.; Kolbuk, D.; Kozera, R.; Boczkowska, A. Microstructural Characterization and Mechanical Properties of PA11 Nanocomposite Fibers. J. Mater. Eng. Perform. 2015, 25, 68–75. [Google Scholar] [CrossRef]
- D638-15; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2015.
- Neiman, M.B. Aging and Stabilization of Polymers, 1st ed.; Springer: New York, NY, USA, 1965. [Google Scholar]
- White, J.R. Polymer ageing: Physics, chemistry or engineering? Time to reflect. Comptes Rendus Chim. 2006, 9, 1396–1408. [Google Scholar] [CrossRef]
- Li, R.; Hu, X. Study on discoloration mechanism of polyamide 6 during thermo-oxidative degradation. Polym. Degrad. Stab. 1998, 62, 523–528. [Google Scholar] [CrossRef]
- Sang, L.; Wang, C.; Wang, Y.; Wei, Z. Thermo-oxidative ageing effect on mechanical properties and morphology of short fibre reinforced polyamide composites—Comparison of carbon and glass fibres. RSC Adv. 2017, 7, 43334–43344. [Google Scholar] [CrossRef]
- Pliquet, M.; Rapeaux, M.; Delange, F.; Bourgogne, D.; Gardette, J.; Therias, S.; Bussiere, P. Multiscale analysis of thermal degradation of polyamide 6,6—Influence of temperature on oxygen diffusion-limited oxidation profiles. Polym. Degrad. Stab. 2021, 192, 109695. [Google Scholar] [CrossRef]
- Forsström, D.; Terselius, B. Thermo oxidative stability of polyamide 6 films I. Mechanical and chemical characterisation. Polym. Degrad. Stab. 2000, 67, 69–78. [Google Scholar] [CrossRef]
- Tey, W.S.; Cai, C.; Zhou, K. A Comprehensive Investigation on 3D Printing of Polyamide 11 and Thermoplastic Polyurethane via Multi Jet Fusion. Polymers 2021, 13, 2139. [Google Scholar] [CrossRef]
- Pliquet, M.; Rapeaux, M.; Delange, F.; Bussiere, P.; Therias, S.; Gardette, J. Multiscale analysis of the thermal degradation of polyamide 6,6: Correlating chemical structure to mechanical properties. Polym. Degrad. Stab. 2021, 185, 109496. [Google Scholar] [CrossRef]
- Ghosh, S.; Khastgir, D.; Bhowmick, A.K.; Mukunda, P. Thermal degradation and ageing of segmented polyamides. Polym. Degrad. Stab. 2000, 67, 427–436. [Google Scholar] [CrossRef]
- Biswas, N.; Datta, A. Polymer entanglement—A barrier to nanoparticles aggregation. Chem. Phys. Lett. 2012, 531, 177–182. [Google Scholar] [CrossRef]
- Prut, E.V.; Medintseva, T.I.; Kuznetsova, O.P. The effect of PP molecular weight on the complex viscosity of PP/EPDM blends. Express Polym. Lett. 2021, 15, 166–176. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Singh, B.P.; Malik, R.S.; Verma, P.; Choudhary, V. Detailed dynamic mechanical analysis of thermomechanically stable melt-processed PEK-MWCNT nanocomposites. Polym. Compos. 2016, 39, 2587–2596. [Google Scholar] [CrossRef]
- Billmeyer, F.W.J. Textbook of Polymer Science; John Wiley and Sons: New York, NY, USA; London, UK, 1962. [Google Scholar]
- Bessell, T.J.; Hull, D.; Shortall, J.B. The effect of polymerization conditions and crystallinity on the mechanical properties and fracture of spherulitic nylon 6. J. Mater. Sci. 1975, 10, 1127–1136. [Google Scholar] [CrossRef]
- Callister, W.D.J. Επιστήμη Και Τεχνολογια Των Υλικών, Materials Science and Engineering: An Introduction, 5th ed.; Εκδόσεις Τζιόλα: Thessaloniki, Greece, 2008; p. 1065. [Google Scholar]
- Yao, B.; Li, Z.; Zhu, F. Effect of powder recycling on anisotropic tensile properties of selective laser sintered PA2200 polyamide. Eur. Polym. J. 2020, 141, 110093. [Google Scholar] [CrossRef]
- Ding, L.; Davidchack, R.L.; Pan, J. A molecular dynamics study of Young’s modulus change of semi-crystalline polymers during degradation by chain scissions. J. Mech. Behav. Biomed. Mater. 2012, 5, 224–230. [Google Scholar] [CrossRef] [PubMed]
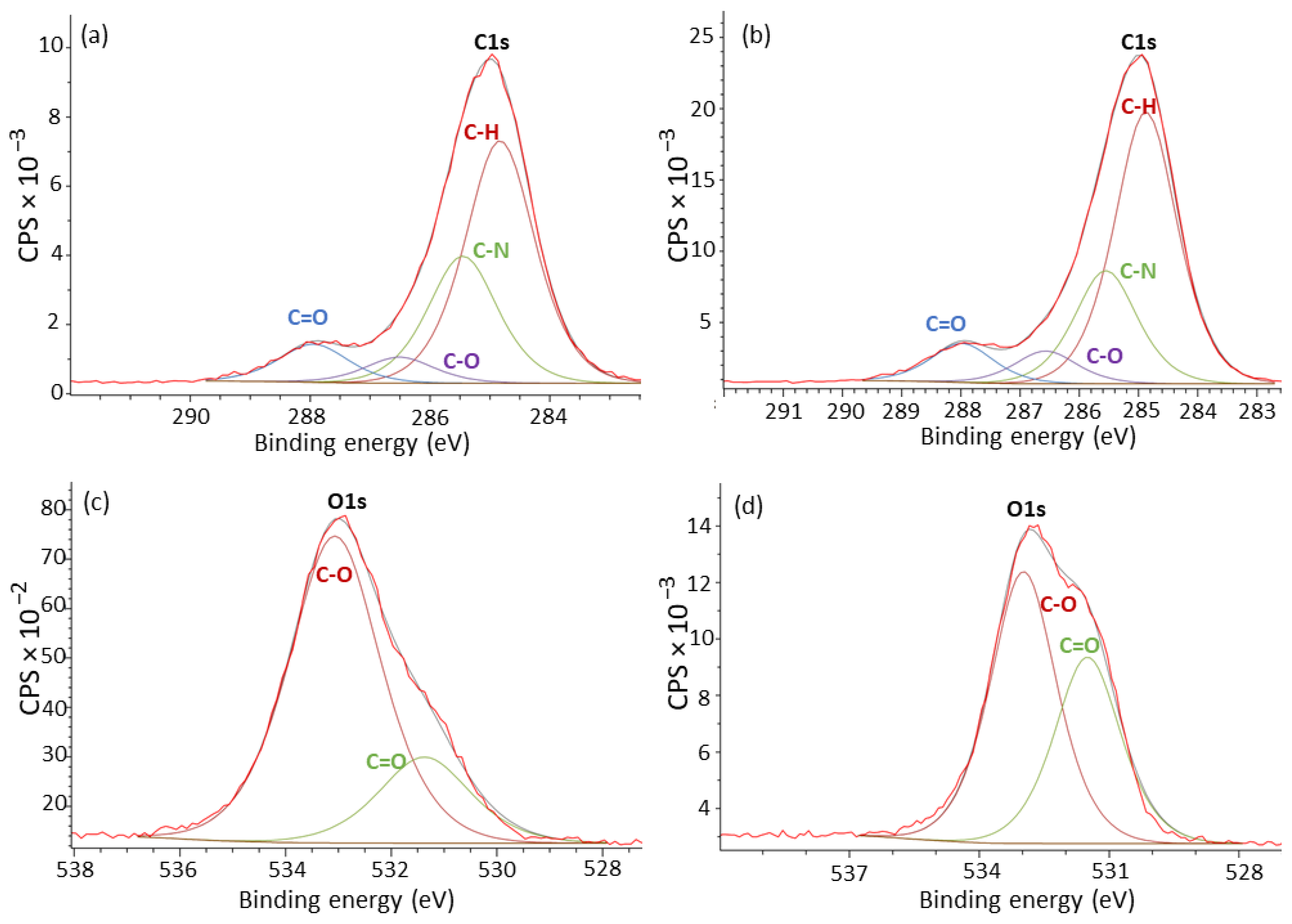

| C1s (at%) | O1s (at%) | C-N (at%)/C=O (at%) | Carbonyl Ratio (A1718/A1193) | |||||
|---|---|---|---|---|---|---|---|---|
| C-H | C-N | C=O | C-O | C=O | C-O | |||
| PA11_0C | 55.89 | 29.29 | 8.81 | 6.01 | 21.92 | 78.08 | 1.3362 | 0.03427 |
| PA11_2C | 63.99 | 21.52 | 8.58 | 5.91 | 24.60 | 75.40 | 0.8748 | 0.05150 |
| PA11_4C | 65.40 | 20.52 | 8.45 | 5.63 | 31.06 | 68.94 | 0.6606 | 0.04943 |
| PA11_6C | 66.16 | 19.30 | 8.65 | 5.90 | 32.74 | 67.26 | 0.5895 | 0.05229 |
| PA11_8C | 62.98 | 21.92 | 8.51 | 6.59 | 34.42 | 65.58 | 0.6368 | 0.06694 |
| PA11_14C | 59.46 | 24.79 | 8.56 | 7.18 | 40.67 | 59.33 | 0.6095 | 0.06746 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandelidi, C.; Blakis, R.; Lee, K.P.M.; Bateman, S.; Brandt, M.; Kajtaz, M. Thermal and Oxidative Aging Effects of Polyamide-11 Powder Used in Multi-Jet Fusion. Polymers 2023, 15, 2395. https://doi.org/10.3390/polym15102395
Pandelidi C, Blakis R, Lee KPM, Bateman S, Brandt M, Kajtaz M. Thermal and Oxidative Aging Effects of Polyamide-11 Powder Used in Multi-Jet Fusion. Polymers. 2023; 15(10):2395. https://doi.org/10.3390/polym15102395
Chicago/Turabian StylePandelidi, Chrysoula, Ryan Blakis, Kok Peng Marcian Lee, Stuart Bateman, Milan Brandt, and Mladenko Kajtaz. 2023. "Thermal and Oxidative Aging Effects of Polyamide-11 Powder Used in Multi-Jet Fusion" Polymers 15, no. 10: 2395. https://doi.org/10.3390/polym15102395
APA StylePandelidi, C., Blakis, R., Lee, K. P. M., Bateman, S., Brandt, M., & Kajtaz, M. (2023). Thermal and Oxidative Aging Effects of Polyamide-11 Powder Used in Multi-Jet Fusion. Polymers, 15(10), 2395. https://doi.org/10.3390/polym15102395







