Development of a New Clay-Based Aerogel Composite from Ball Clay from Piauí, Brazil and Polysaccharides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
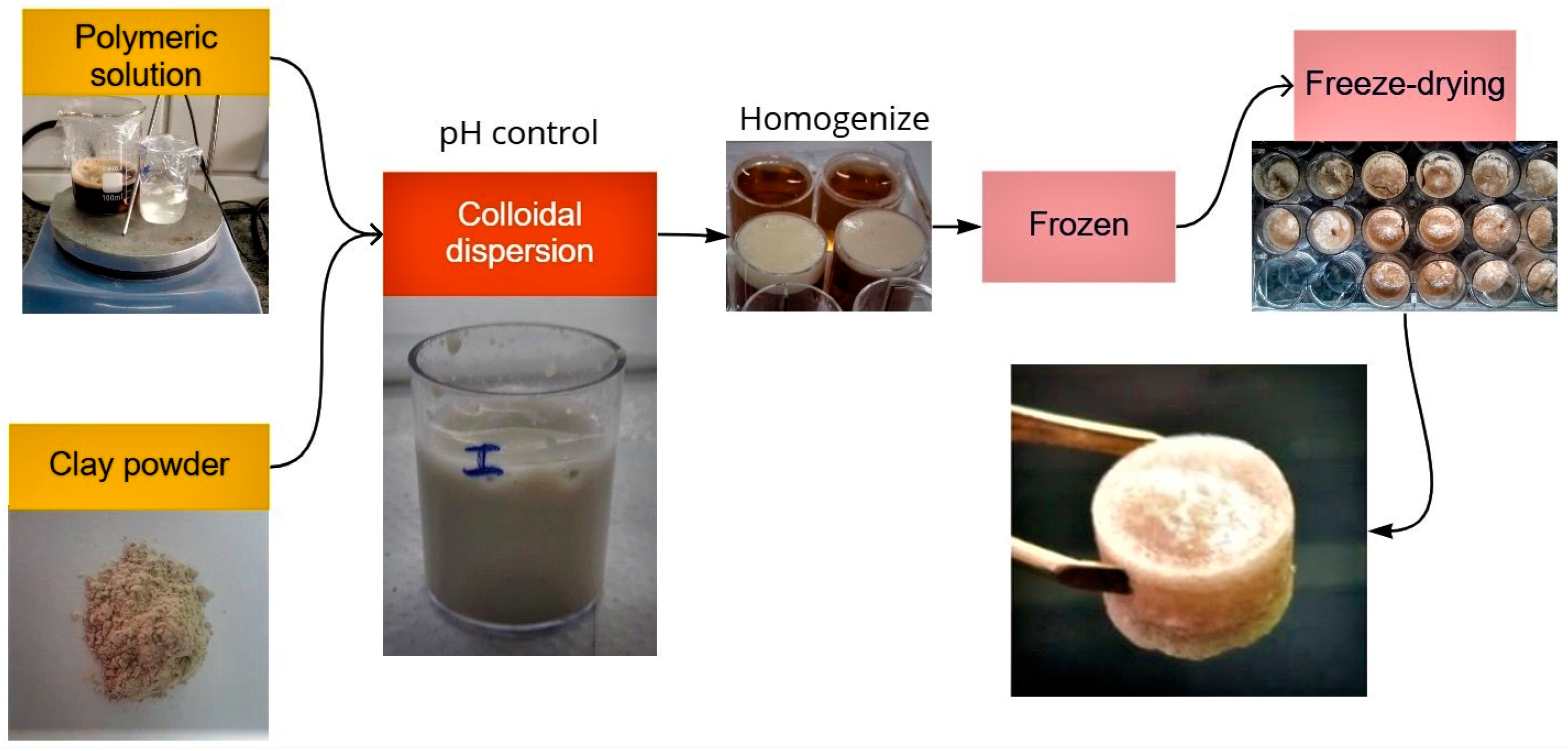
2.2. Preparation of Aerogel
2.3. Characterization
2.3.1. Mechanical Performance
2.3.2. Scanning Electron Microscope (SEM)
2.3.3. Apparent Density
2.3.4. X-ray Diffraction
Microstructure
Degree of Relative Crystallinity
2.3.5. Fourier Transform Infrared Spectroscopy–FTIR
2.3.6. Thermogravimetric Analysis (TGA)
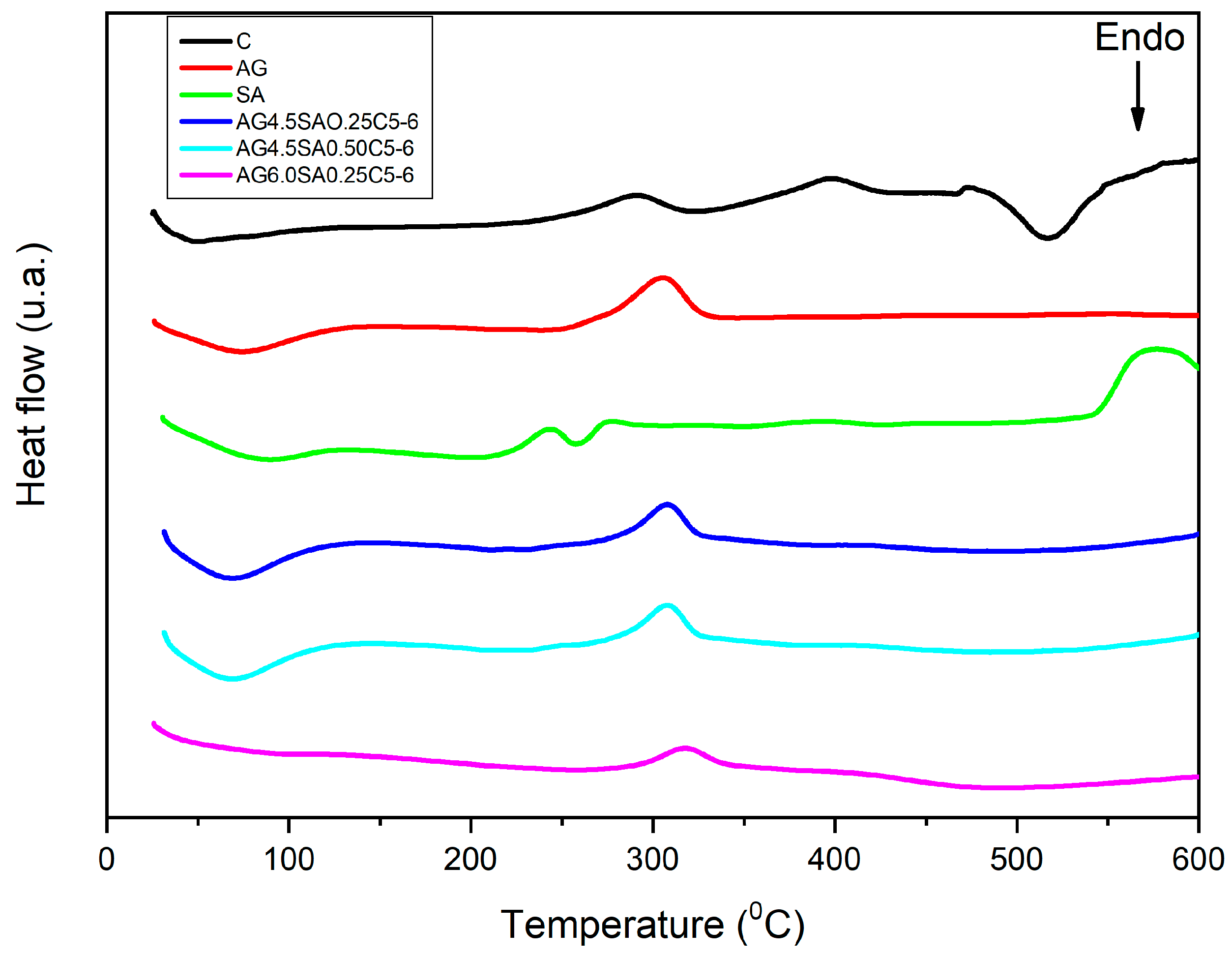
2.3.7. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
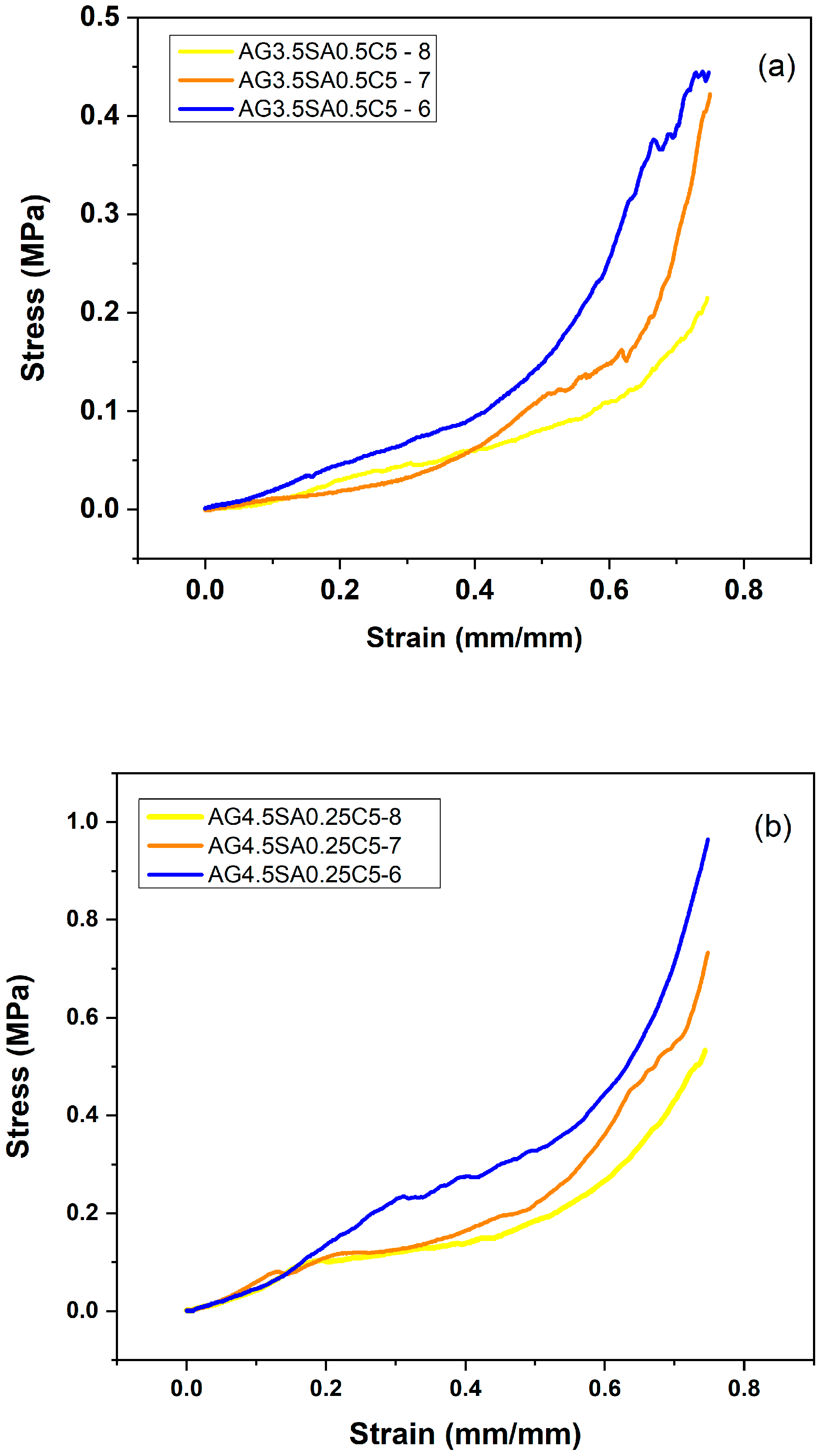
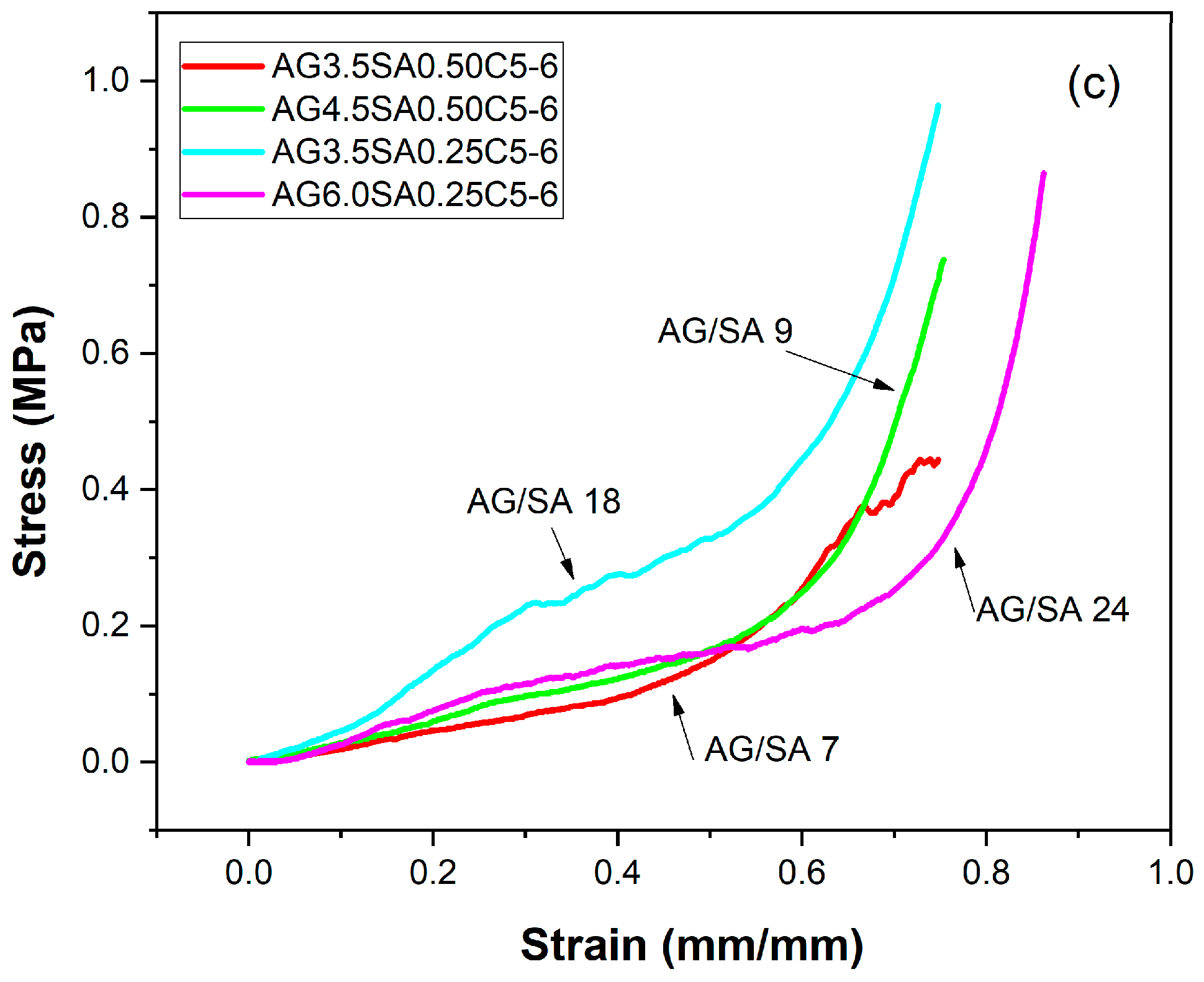
3.1. Mechanical Properties
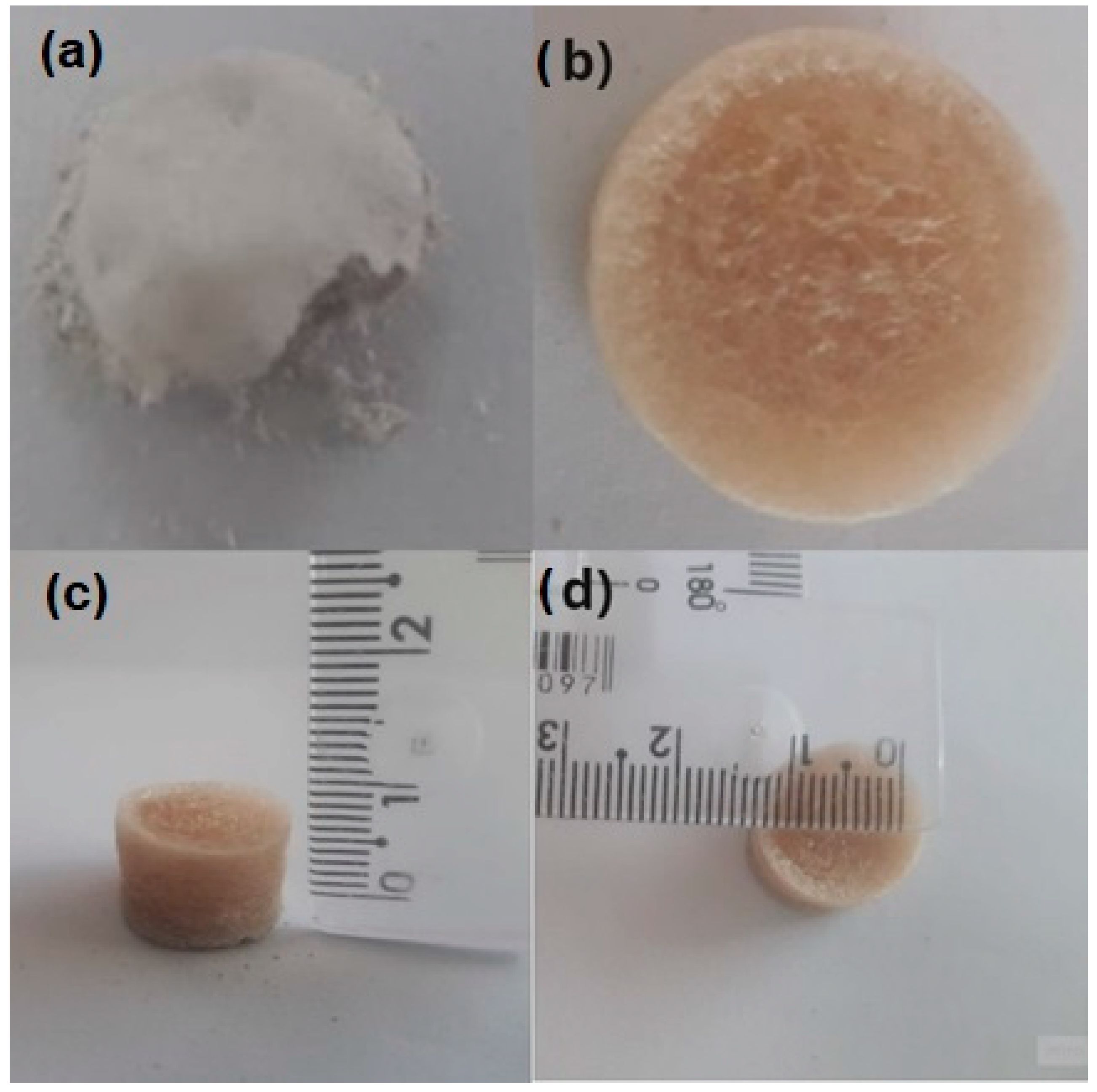
3.2. Morphological Structure of Aerogels with pH Variation
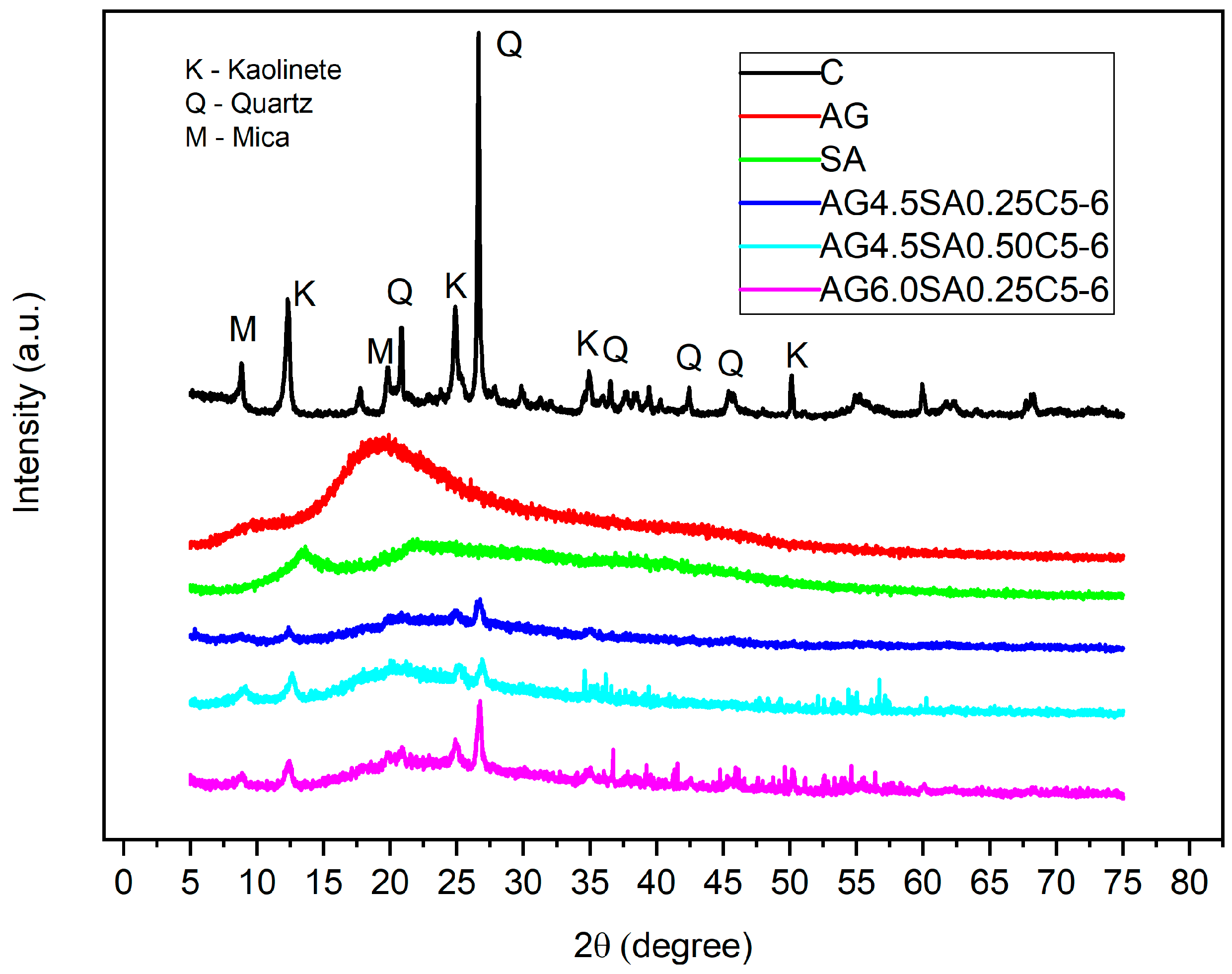
3.3. Analysis of Crystalline Phases and Microstructure
3.4. Chemical Structure
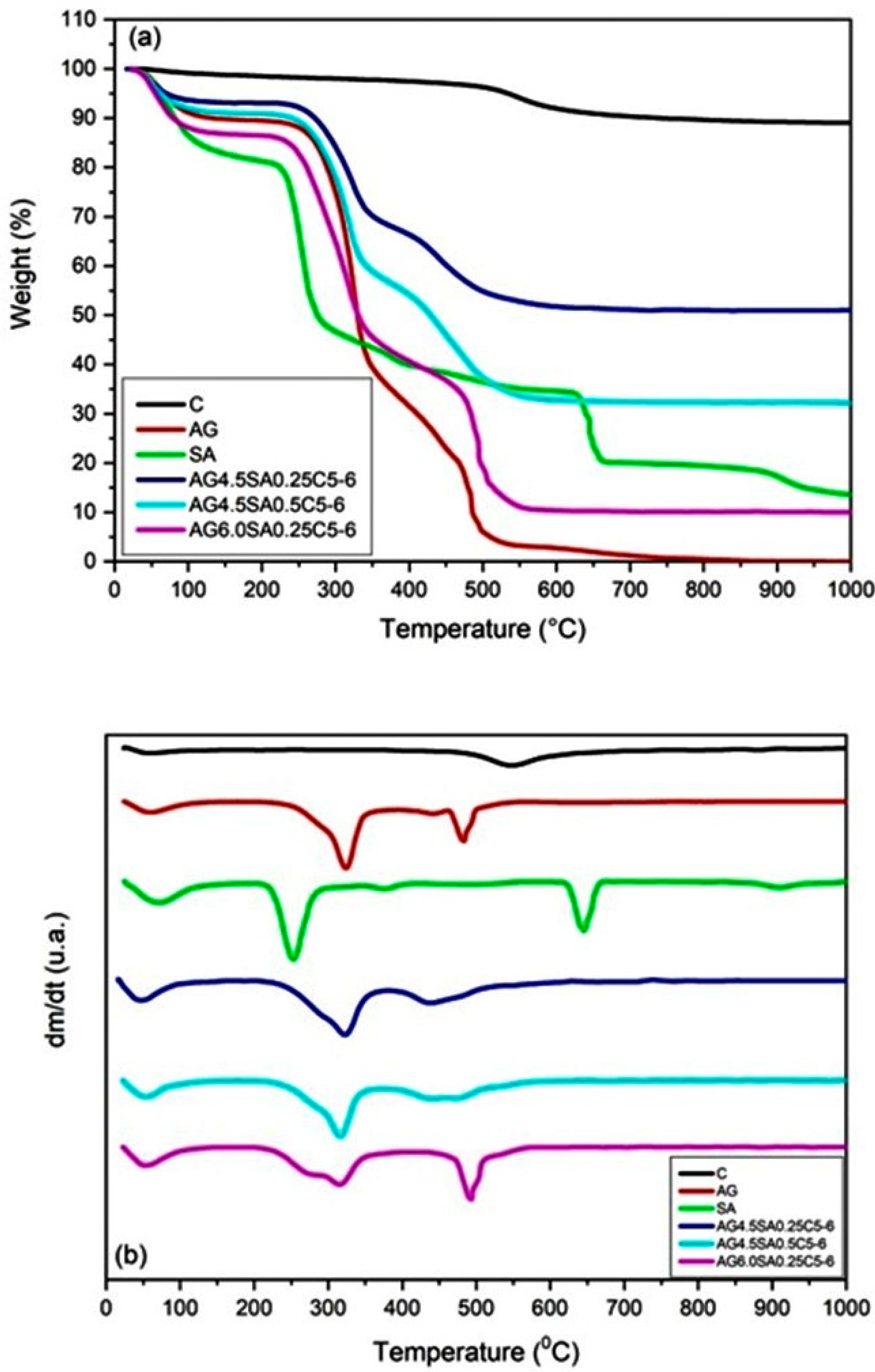
3.5. Thermal Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of Chitosan Aerogels as Promising Carriers for Drug Delivery: A Review. Carbohydr. Polym. 2020, 231, 115744. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhang, Y.; Zhang, L.; Miao, Y.E.; Fan, W.; Liu, T. Polymer/Carbon-Based Hybrid Aerogels: Preparation, Properties and Applications. Materials 2015, 8, 6806–6848. [Google Scholar] [CrossRef]
- Liang, W.; Wang, R.; Wang, C.; Jia, J.; Sun, H.; Zhang, J.; Yang, Y. Facile Preparation of Attapulgite-Based Aerogels with Excellent Fl Ame Retardancy and Better Thermal Insulation Properties. J. Appl. Polym. Sci. 2019, 136, 47849. [Google Scholar] [CrossRef]
- Pröbstle, H.; Wiener, M.; Fricke, J. Carbon Aerogels for Electrochemical Double Layer Capacitors. J. Porous Mater. 2003, 10, 213–222. [Google Scholar] [CrossRef]
- Ye, D.; Wang, T.; Liao, W.; Wang, H.; Zhao, H.; Wang, Y.; Xu, S.; Wang, Y. Ultrahigh-Temperature Insulating and Fire-Resistant Aerogels from Cationic Amylopectin and Clay via a Facile Route. ACS Sustain. Chem. Eng. 2019, 7, 11582–11592. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4265. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Z.; Li, K.; Zhao, S.; Fei, Z.; Zhang, Z. A Flexible Silica Aerogel with Good Thermal and Acoustic Insulation Prepared via Water Solvent System. J. Sol Gel Sci. Technol. 2019, 92, 652–661. [Google Scholar] [CrossRef]
- Li, D.; Tian, X.; Wang, Z.; Guan, Z.; Li, X.; Qiao, H.; Ke, H.; Luo, L.; Wei, Q. Multifunctional Adsorbent Based on Metal-Organic Framework Modified Bacterial Cellulose/Chitosan Composite Aerogel for High Efficient Removal of Heavy Metal Ion and Organic Pollutant. Chem. Eng. J. 2019, 383, 123127. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Chen, H. Facile Fabrication of Mechanically-Strong and Flame Retardant Alginate/Clay Aerogels. Compos. Part B Eng. 2018, 164, 18–25. [Google Scholar] [CrossRef]
- Gupta, P.; Verma, C.; Maji, P.K. Flame Retardant and Thermally Insulating Clay Based Aerogel Facilitated by Cellulose Nanofibers. J. Supercrit. Fluids 2019, 152, 104537. [Google Scholar] [CrossRef]
- Chen, H.B.; Shen, P.; Chen, M.J.; Zhao, H.B.; Schiraldi, D.A. Highly Efficient Flame Retardant Polyurethane Foam with Alginate/Clay Aerogel Coating. ACS Appl. Mater. Interfaces 2016, 8, 32557–32564. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B.; Schiraldi, D.A. Flammability of Polymer/Clay Aerogel Composites: An Overview. Polym. Rev. 2019, 59, 1–24. [Google Scholar] [CrossRef]
- Liu, A.; Medina, L.; Berglund, L.A. High-Strength Nanocomposite Aerogels of Ternary Composition: Poly(Vinyl Alcohol), Clay, and Cellulose Nanofibrils. ACS Appl. Mater. Interfaces 2017, 9, 6453–6461. [Google Scholar] [CrossRef]
- Laursen, A.; Santana, L.N.L.; Menezes, R.R. Characterization of Brazilian Northeastern Plastic Clays. Ceramica 2019, 65, 578–584. [Google Scholar] [CrossRef]
- Ali, R.; Rao, K.; Kashifuddin, M. Adsorption Studies of Cd (II) on Ball Clay: Comparison with Other Natural Clays. Arab. J. Chem. 2016, 9, S1233–S1241. [Google Scholar] [CrossRef]
- Menezes, R.R.; Ferreira, H.S.; Neves GD, A.; Ferreira, H.C. Caracterização de Argilas Plásticas Do Tipo “Ball Clay” Do Litoral Paraibano. Cerâmica 2003, 49, 120–127. [Google Scholar] [CrossRef]
- Cartaxo, J.M.; Bastos PD, M.; Santana LN, L.; Menezes, R.R.; Neves, G.A.; Ferreira, H.C. Estudo de Novas Ocorrências de Argilas Plásticas (Ball Clays) Do Nordeste Do Brasil Para Uso Em Cerâmicas Refratárias. Cerâmica 2016, 62, 338–344. [Google Scholar] [CrossRef]
- da Silva, A.G.; Rodrigues, J.F.; de Paula, R.C.M. Composição e Propriedades Reológicas Da Goma Do Angico (Anadenanthera Macrocarpa Benth). Polímeros 1998, 8, 34–40. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Biomaterials Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Xu, Y.; Qu, L.; Liu, Y.; Zhu, P. An Overview of Alginates as Flame-Retardant Materials: Pyrolysis Behaviors, Flame Retardancy, and Applications. Carbohydr. Polym. 2021, 260, 117827. [Google Scholar] [CrossRef] [PubMed]
- Shang, K.; Liao, W.; Wang, J.; Wang, Y.T.; Wang, Y.Z.; Schiraldi, D.A. Nonflammable Alginate Nanocomposite Aerogels Prepared by a Simple Freeze-Drying and Post-Cross-Linking Method. ACS Appl. Mater. Interfaces 2016, 8, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.H.; Wan, P.; Heng, S.; Chan, L.W. Alginates as a Useful Natural Polymer for Microencapsulation and Therapeutic Applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Khan, M.N.; Chowdhury, M.; Rahman, M.M. Biobased Amphoteric Aerogel Derived from Amine-Modified Clay-Enriched Chitosan/Alginate for Adsorption of Organic Dyes and Chromium (VI) Ions from Aqueous Solution. Mater. Today Sustain. 2021, 13, 100077. [Google Scholar] [CrossRef]
- Wang, L.; Schiraldi, D.A.; Sánchez-Soto, M. Foamlike Xanthan Gum/Clay Aerogel Composites and Tailoring Properties by Blending with Agar. Ind. Eng. Chem. Res. 2014, 53, 7680–7687. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, W.; Zhao, X.; Liu, Y.; Gao, W. Study on the Morphology, Crystalline Structure and Thermal Properties of Yellow Ginger Starch Acetates with Different Degrees of Substitution. Thermochim. Acta 2009, 495, 57–62. [Google Scholar] [CrossRef]
- Bandi, S.; Schiraldi, D.A. Glass Transition Behavior of Clay Aerogel/Poly(Vinyl Alcohol) Composites. Macromolecules 2006, 39, 6537–6545. [Google Scholar] [CrossRef]
- Madyan, O.A.; Fan, M.; Huang, Z. Functional Clay Aerogel Composites through Hydrophobic Modification and Architecture of Layered Clays. Appl. Clay Sci. 2017, 141, 64–71. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, H.; Xu, T.; Wang, J.; Zhu, Y.; Ding, S.; Hu, T.; Yun, S.; Chen, J. Excellent Flame Retardant and Thermal Insulated Palygorskite/Wood Fiber Composite Aerogels with Improved Mechanical Properties. Appl. Clay Sci. 2020, 184, 105402. [Google Scholar] [CrossRef]
- Wu, S.; Du, A.; Xiang, Y.; Liu, M.; Li, T.; Shen, J.; Zhang, Z.; Li, C.; Zhou, B. Silica-Aerogel-Powders “Jammed” Polyimide Aerogels with Excellent Hydrophobicity and Conversion to Ultra-Light Polyimide Aerogel. RSC Adv. 2016, 6, 58268–58278. [Google Scholar] [CrossRef]
- Chen, H.B.; Wang, Y.Z.; Sánchez-Soto, M.; Schiraldi, D.A. Low Flammability, Foam-like Materials Based on Ammonium Alginate and Sodium Montmorillonite Clay. Polymer 2012, 53, 5825–5831. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S. Chitosan/Polybenzoxazine/Clay Mixed Matrix Composite Aerogels: Preparation, Physical Properties, and Water Absorbency. Appl. Clay Sci. 2020, 184, 105403. [Google Scholar] [CrossRef]
- Cao, S.; Song, D.; Deng, Y.; Ragauskas, A. Preparation of Starch-Fatty Acid Modified Clay and Its Application in Packaging Papers. Ind. Eng. Chem. Res. 2011, 50, 5628–5633. [Google Scholar] [CrossRef]
- Liao, W.; Wang, G.; Liu, Z.; Xu, S.; Wang, Y.Z. Rheological Premonitory of Nanoclay Morphology on the Mechanical Characteristics of Composite Aerogels. Compos. Part B Eng. 2019, 173, 106889. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Herbert, M.M.; Alhassan, S.M.; Ishida, H.; Qutubuddin, S.; Schiraldi, D.A. Laponite/Multigraphene Hybrid-Reinforced Poly (Vinyl Alcohol) Aerogels. Polymer 2016, 91, 180–186. [Google Scholar] [CrossRef]
- de Oliveira, T.C.; de Jesus Oliveira, A.C.; Patriota, Y.B.G.; Chaves, L.L.; Ribeiro, F.d.O.S.; de Paula, R.C.; Silva-Filho, E.C.; da Silva, D.A.; de La Roca Soares, M.F.; Soares-Sobrinho, J.L. Eco-Friendly Synthesis of Phthalate Angico Gum towards Nanoparticles Engineering Using Quality by Design (QbD) Approach. Int. J. Biol. Macromol. 2021, 190, 801–809. [Google Scholar] [CrossRef]
- Nair, R.M.; Bindhu, B.; VL, R. A Polymer Blend from Gum Arabic and Sodium Alginate—Preparation and Characterization. J. Polym. Res. 2020, 27, 154. [Google Scholar] [CrossRef]
- Laurienzo, P.; Malinconico, M.; Motta, A.; Vicinanza, A. Synthesis and Characterization of a Novel Alginate-Poly(Ethylene Glycol) Graft Copolymer. Carbohydr. Polym. 2005, 62, 274–282. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Q.; Yang, J.; Ma, S.; Frost, R.L. The Thermal Behavior of Kaolinite Intercalation Complexes—A Review. Thermochim. Acta 2012, 545, 1–13. [Google Scholar] [CrossRef]
- De Lemos, E.; Silva, V.; Carla, A.; Oliveira, D.J.; Basílio, Y.; Patriota, G.; José, A.; Veiga, F.; Hallwass, F.; Silva-filho, E.C.; et al. Solvent-Free Synthesis of Acetylated Cashew Gum for Oral Delivery System of Insulin. Carbohydr. Polym. 2019, 207, 601–608. [Google Scholar] [CrossRef]
- Gomes, C. Thermal Stability of the PBAT Biofilms with Cellulose Nanostructures/Essential Oils for Active Packaging. J. Therm. Anal. Calorim. 2019, 138, 2375–2386. [Google Scholar] [CrossRef]
- Da Silva Abreu, F.O.M.; Costa, E.F.; Cardial, M.R.L.; André, W.P.P. Polymeric Nanoemulsions Enriched with Eucalyptus Citriodora Essential Oil. Polimeros 2020, 30, e2020024. [Google Scholar] [CrossRef]
- Hao, R.; Li, X.; Xu, P.; Liu, Q. Thermal Activation and Structural Transformation Mechanism of Kaolinitic Coal Gangue from Jungar Coalfield, Inner Mongolia, China. Appl. Clay Sci. 2022, 223, 106508. [Google Scholar] [CrossRef]
- Wu, T.; Dong, J.; Xu, G.; Gan, F.; Zhao, X.; Zhang, Q. Attapulgite-Reinforced Polyimide Hybrid Aerogels with High Dimensional Stability and Excellent Thermal Insulation Property. Polymer 2019, 176, 196–205. [Google Scholar] [CrossRef]
- Silva, M.; Santana, G. Caulinita: Estrutura Cristalina, Técnicas Físicas e Estudo de Adsorção. Sci. Amaz. 2013, 2, 54–70. [Google Scholar]
- Braz, E.M.A.; Silva, S.C.C.C.; Brito, C.A.R.S.; Carvalho, F.A.A.; Alves, M.M.M.; Barreto, H.M.; Silva, D.A.; Magalhães, R.; Oliveira, A.L.; Silva-Filho, E.C. Modified Chicha Gum by Acetylation for Antimicrobial and Antiparasitic Applications: Characterization and Biological Properties. Int. J. Biol. Macromol. 2020, 160, 1177–1188. [Google Scholar] [CrossRef]
- Zhu, W.; Wan, L.; Zhang, C.; Gao, Y.; Zheng, X.; Jiang, T.; Wang, S. Exploitation of 3D Face-Centered Cubic Mesoporous Silica as a Carrier for a Poorly Water Soluble Drug: In Fl Uence of Pore Size on Release Rate. Mater. Sci. Eng. C 2014, 34, 78–85. [Google Scholar] [CrossRef]
- Wattanasiriwech, S.; Wattanasiriwech, D. Examination of Ball Clays Derived from a New Seam in Northern Thailand. IOP Conf. Ser. Earth Environ. Sci. 2019, 351, 012006. [Google Scholar] [CrossRef]
- Adauto, A.; Khan, S.; Augusto da Silva, M.; Gomes Neto, J.A.; Picasso, G.; Del Pilar Taboada Sotomayor, M. Synthesis, Characterization and Application of a Novel Ion Hybrid Imprinted Polymer to Adsorb Cd(II) in Different Samples. Environ. Res. 2020, 187, 109669. [Google Scholar] [CrossRef]


| Mass Composition for Every 100 mL of Water | |||||
|---|---|---|---|---|---|
| Samples | pH | Angico Gum—AG (g) | Sodium Alginate—SA (g) | Ball Clay—C (g) | Distilled Water (mL) |
| C-6 | 6 | 0 | 0 | 5 | 100 |
| AG3.5SA0.5C5-8 | 8 | 3.5 | 0.5 | 5 | 100 |
| AG3.5SA0.5C5-7 | 7 | 3.5 | 0.5 | 5 | 100 |
| AG3.5SA0.5C5-6 | 6 | 3.5 | 0.5 | 5 | 100 |
| AG4.5SA0.25C5-8 | 8 | 4.5 | 0.25 | 5 | 100 |
| AG4.5SA0.25C5-7 | 7 | 4.5 | 0.25 | 5 | 100 |
| AG4.5SA0.25C5-6 | 6 | 4.5 | 0.25 | 5 | 100 |
| AG4.5SA0.50C5-6 | 6 | 4.5 | 0.50 | 5 | 100 |
| AG6.0SA0.25C5-6 | 6 | 6.0 | 0.25 | 5 | 100 |
| Samples | AG/SA Ratio | Apparent Density (g/cm3) | Compressive Strength (MPa) | Specific Moduli (MPa cm3/g) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|
| AG3.5SA0.5C5-8 | 7 | 0.089 ± 0.08 | 0.55 ± 0.15 | 6.17 | 0.205 ± 0.002 |
| AG3.5SA0.5C5-7 | 7 | 0.090 ± 0.02 | 0.42 ± 0.08 | 4.65 | 0.323 ± 0.005 |
| AG3.5SA0.5C5-6 | 7 | 0.077 ± 0.05 | 0.79 ± 0.14 | 10.25 | 0.464 ± 0.006 |
| AG4.5SA0.25C5-8 | 18 | 0.094 ± 0.01 | 0.53 ± 0.02 | 5.64 | 0.505 ± 0.005 |
| AG4.5SA0.25C5-7 | 18 | 0.012 ± 0.12 | 0.73 ± 0.03 | 60.83 | 0.688 ± 0.008 |
| AG4.5SA0.25C5-6 | 18 | 0.012 ± 0.15 | 0.96 ± 0.12 | 80.00 | 0.934 ± 0.009 |
| AG4.5SA0.50C5-6 | 9 | 0.096 ± 0.06 | 0.74 ± 0.50 | 7.71 | 0.605 ± 0.020 |
| AG6.0SA0.25C5-6 | 24 | 0.097 ± 0.08 | 1.44 ± 0.12 | 14.84 | 0.574 ± 0.060 |
| Clay | Angico Gum | Alginate | AG4.5SA0.25C5-6 | AG4.5SA0.5C5-6 | AG6.0SA0.25C5-6 | |
|---|---|---|---|---|---|---|
| Crystallinity (%) | 47.69 | 10.76 | 20.89 | 10.98 | 21.77 | 21.38 |
| Samples | Decomposition Temperature | Residues (%) | |||
|---|---|---|---|---|---|
| Td 5% (°C) | Td 20% (°C) | Td 30% (°C) | Td 40% (°C) | ||
| C | 533 | - | - | - | 90 |
| AG | 56 | 287 | 307 | 320 | 0.5 |
| SA | 62 | 224 | 246 | 257 | 20 |
| AG4.5SA0.25C5-6 | 70 | 314 | 351 | 451 | 51 |
| AG4.5SA0.50C5-6 | 55 | 261 | 287 | 309 | 10 |
| AG6.0SA0.25C5-6 | 64 | 294 | 315 | 341 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, W.C.; Brito, F.M.; Neto, F.E.; Araújo, A.R.; Leite, R.C.; Viana, V.G.F.; Silva-Filho, E.C.; Silva, D.A. Development of a New Clay-Based Aerogel Composite from Ball Clay from Piauí, Brazil and Polysaccharides. Polymers 2023, 15, 2412. https://doi.org/10.3390/polym15112412
Lopes WC, Brito FM, Neto FE, Araújo AR, Leite RC, Viana VGF, Silva-Filho EC, Silva DA. Development of a New Clay-Based Aerogel Composite from Ball Clay from Piauí, Brazil and Polysaccharides. Polymers. 2023; 15(11):2412. https://doi.org/10.3390/polym15112412
Chicago/Turabian StyleLopes, Wilton C., Francisco M. Brito, Francisco E. Neto, Alyne R. Araújo, Rodolpho C. Leite, Vicente G. Freitas Viana, Edson C. Silva-Filho, and Durcilene A. Silva. 2023. "Development of a New Clay-Based Aerogel Composite from Ball Clay from Piauí, Brazil and Polysaccharides" Polymers 15, no. 11: 2412. https://doi.org/10.3390/polym15112412
APA StyleLopes, W. C., Brito, F. M., Neto, F. E., Araújo, A. R., Leite, R. C., Viana, V. G. F., Silva-Filho, E. C., & Silva, D. A. (2023). Development of a New Clay-Based Aerogel Composite from Ball Clay from Piauí, Brazil and Polysaccharides. Polymers, 15(11), 2412. https://doi.org/10.3390/polym15112412









